Circadian Phase Determines Tissue-Specific Adaptations to Long-Term Exercise in Obese Mice
Abstract
1. Introduction
1.1. Research Background and Theoretical Foundation
1.2. Previous Research and Evidence
1.3. Research Gaps and Objectives
2. Materials and Methods
2.1. Experimental Approach
2.2. Animals and Circadian Phase Manipulation
2.3. Exercise Intervention Protocol
2.4. Blood Glucose Measurement
2.5. Hepatic Lipid Extraction and TG Measurement
2.6. Assessment of Plasma Biochemical Markers
2.7. Oil Red O Staining
2.8. Quantitative Real-Time PCR (qPCR)
2.9. Statistical Analysis
3. Results
3.1. Circadian and Metabolic Phenotypes During HFD and Exercise Intervention
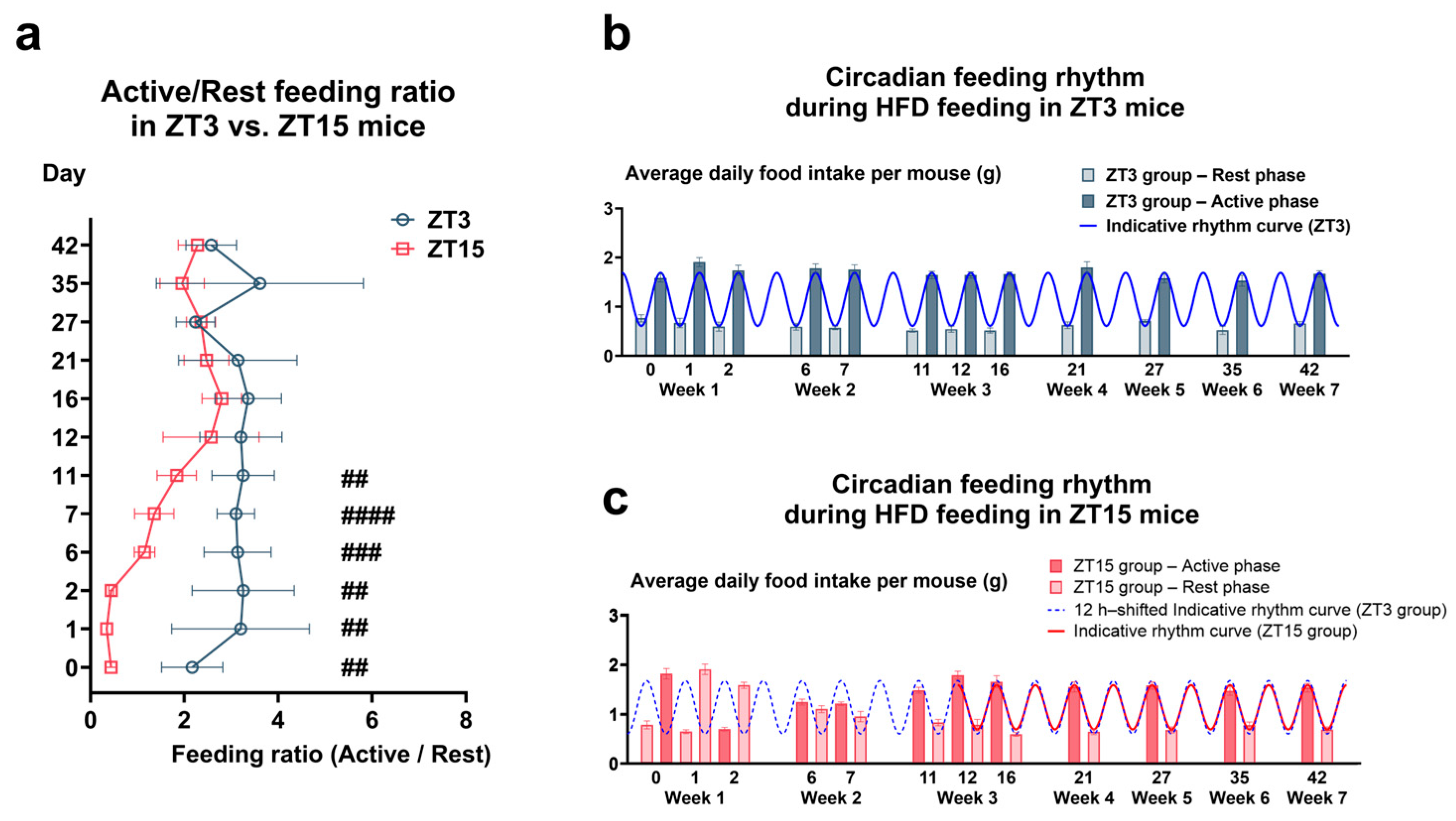
3.1.1. Circadian Regulation of Feeding Under HFD and Exercise Training
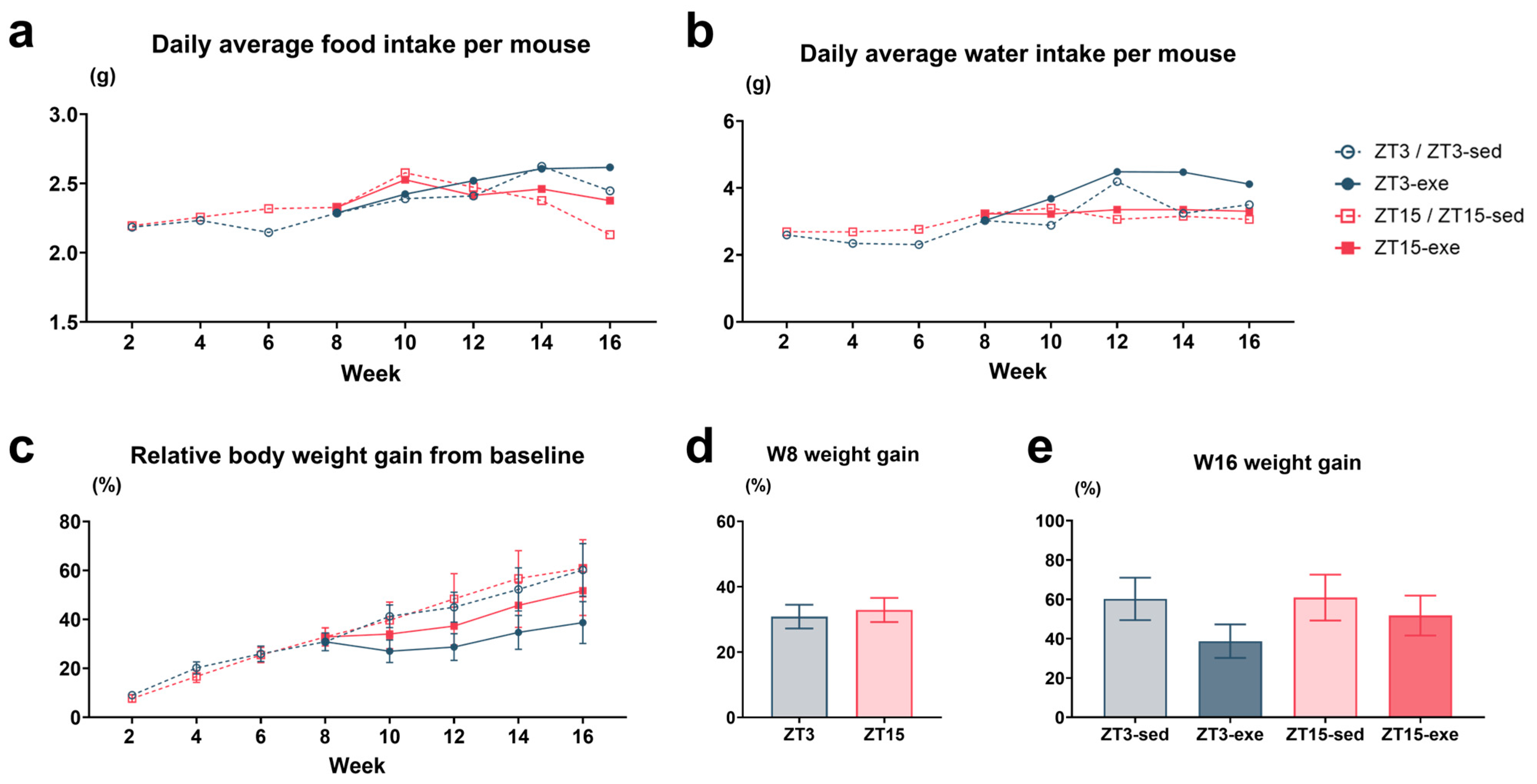
3.1.2. Food and Water Intake and Body Weight Gain During HFD and Exercise Intervention
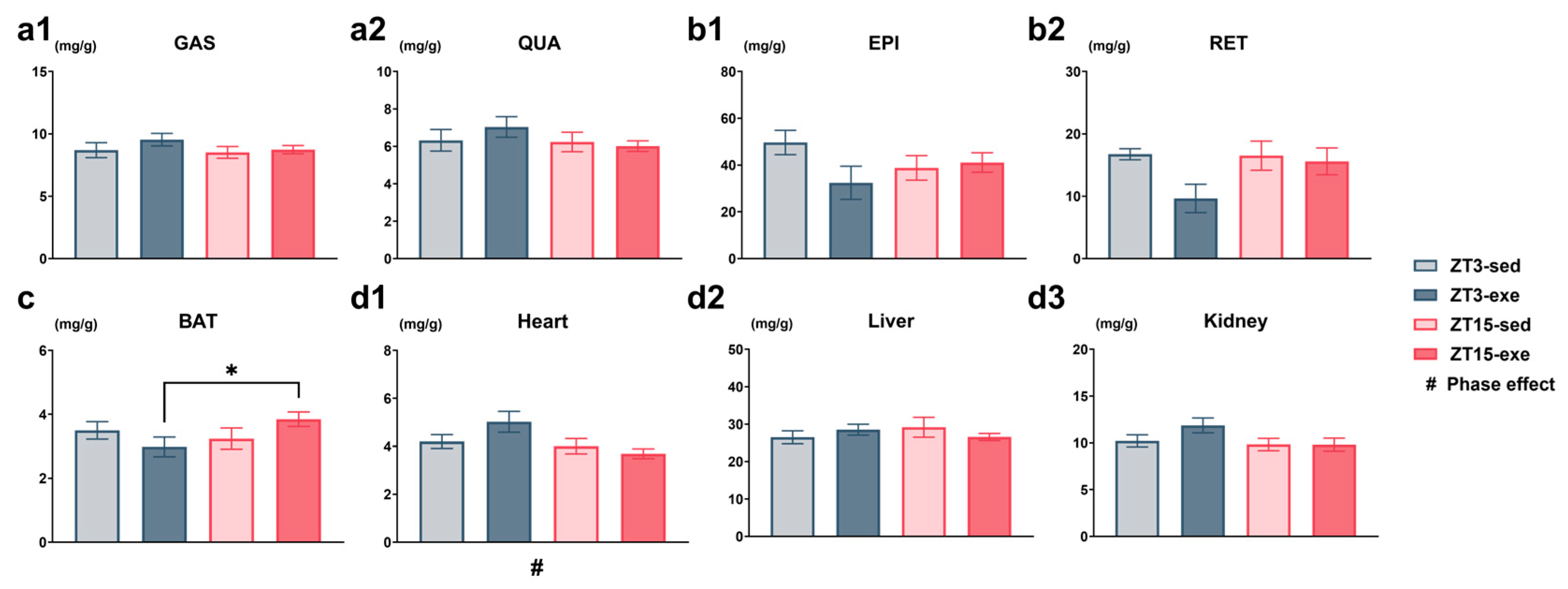
3.1.3. Effects of Exercise Timing on Relative and Absolute Tissue Weights
3.2. Primary Endpoint: Plasma TG and Other Metabolic Parameters
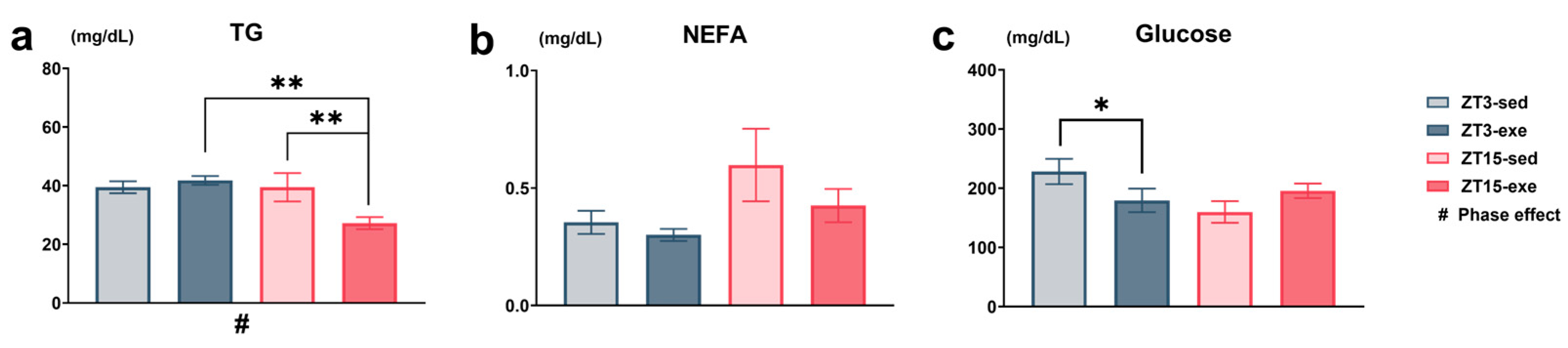
3.2.1. Plasma TGs (Primary Endpoint), NEFAs, and Blood Glucose Concentrations After Exercise Intervention
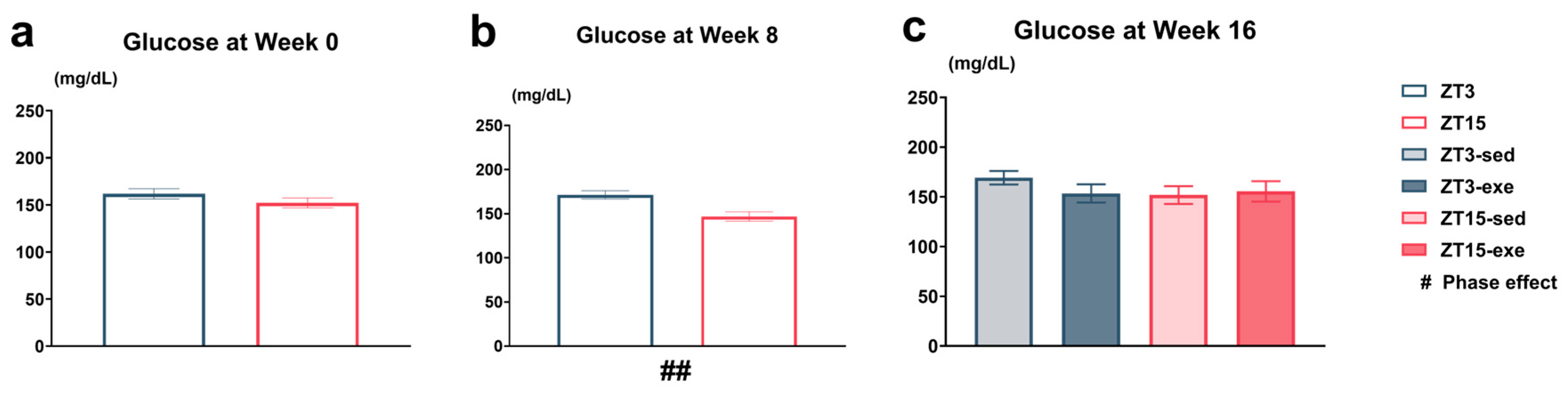
3.2.2. Circadian Variation in Tail-Tip Capillary Blood Glucose
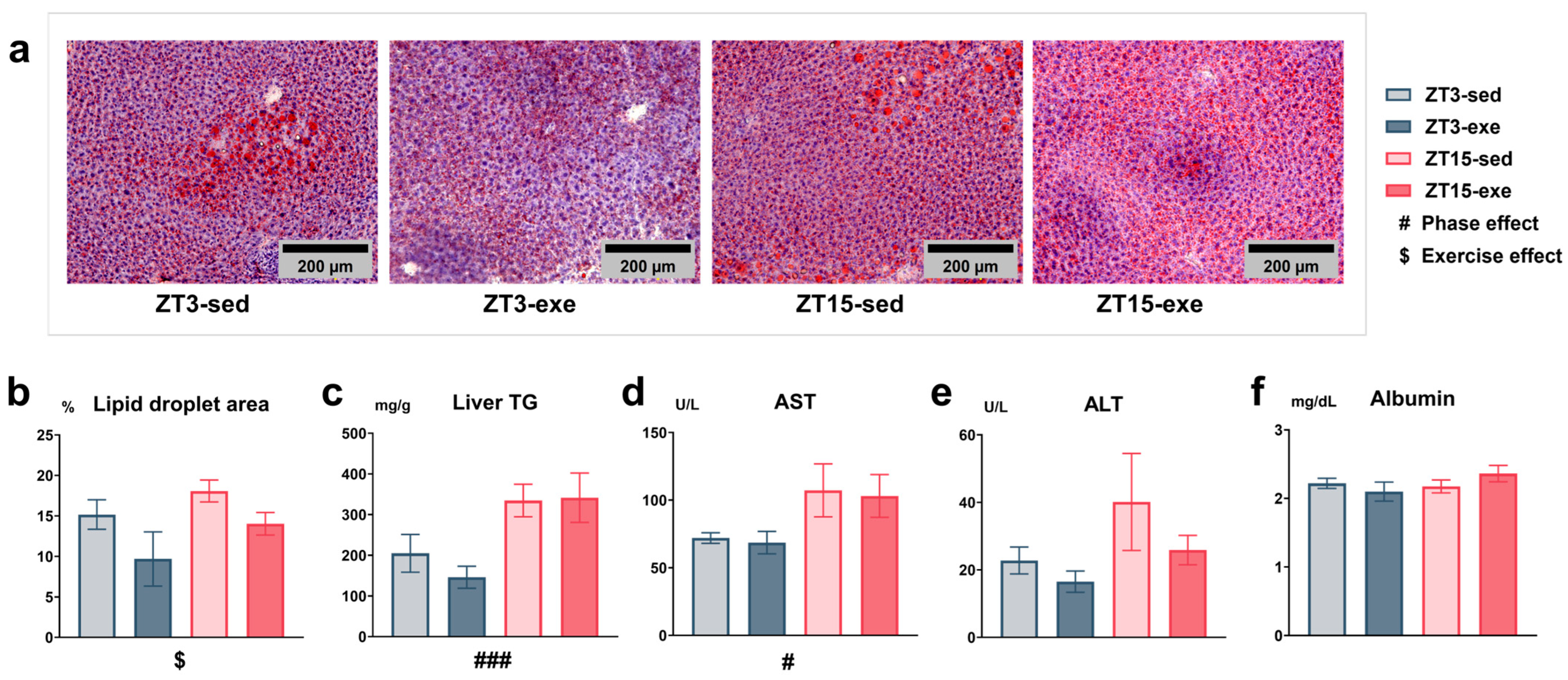
3.3. Effects of Exercise and Circadian Phase on Hepatic Lipid Accumulation and Plasma Biochemical Parameters
3.4. Expression of Clock and Metabolic Genes in the Liver
3.4.1. Core Clock Regulation (Figure 9a)
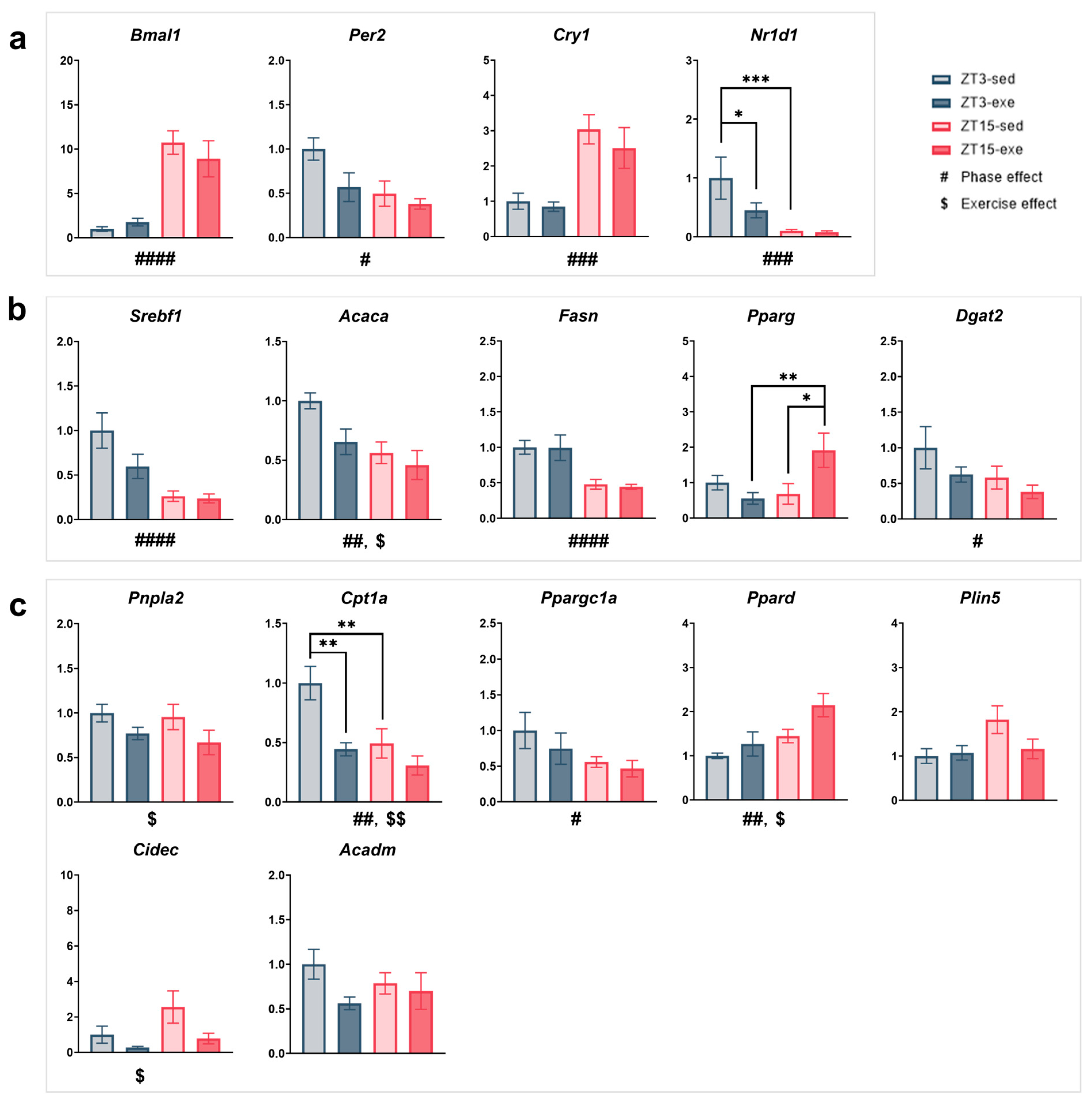
3.4.2. Lipid Synthesis and Transcriptional Control (Figure 9b)
3.4.3. Lipid Mobilization and Oxidation (Figure 9c)
3.4.4. Other Metabolic Processes (Supplementary Figure S3c,d)
3.5. Expression of Clock and Metabolic Genes in EPI
3.5.1. Core Clock Regulation (Figure 10a)
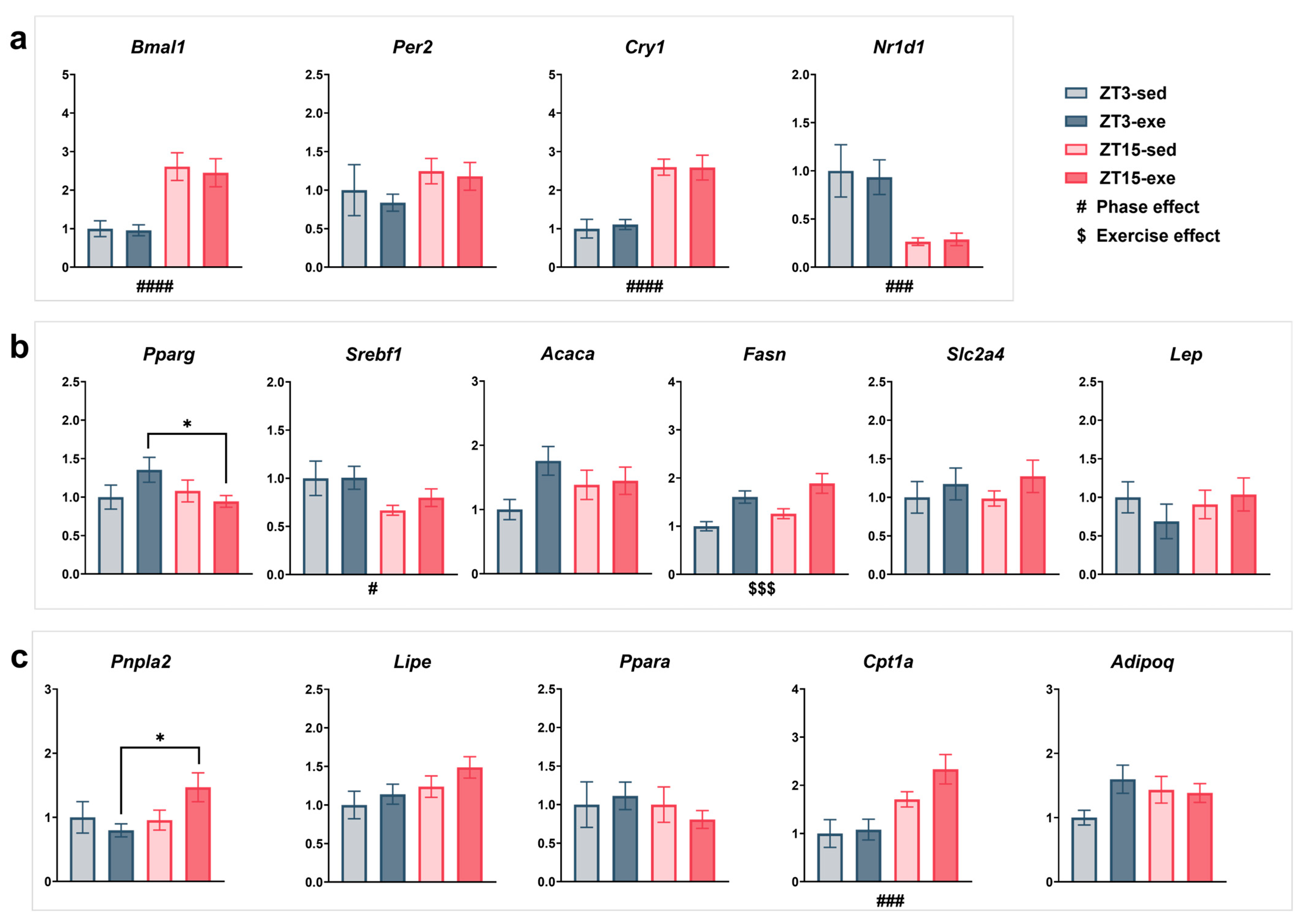
3.5.2. Lipogenesis and Energy Metabolism (Figure 10b)
3.5.3. Lipolysis and Fatty Acid Oxidation (Figure 10c)
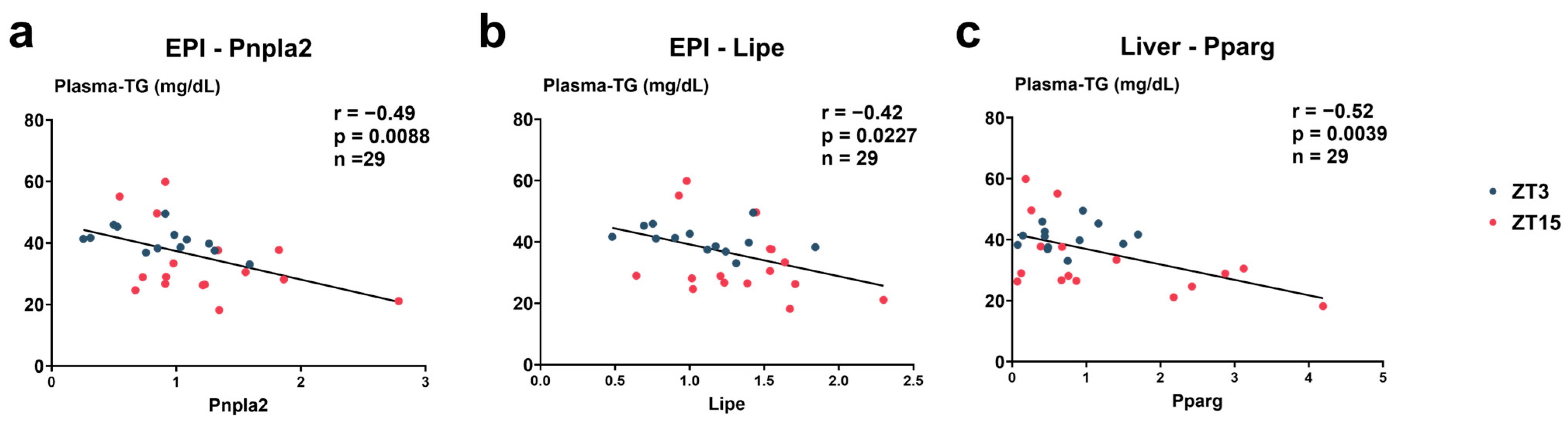
3.6. Correlations Between Plasma TG and Gene Expression Levels (Figure 11)
4. Discussion
4.1. Main Findings
4.2. Behavioral and Systemic Phenotypes
4.3. Hepatic Mechanisms
4.4. Adipose Tissue Mechanisms
4.5. Summary Model
4.6. Translational Implications
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A/R | Active/Rest ratio |
| Acaca | Acetyl-Coenzyme A carboxylase alpha |
| Acadm | Acyl-Coenzyme A dehydrogenase, medium chain |
| Acox1 | Acyl-Coenzyme A oxidase 1, palmitoyl |
| Adipoq | Adiponectin, C1Q and collagen domain containing |
| ALT | Alanine aminotransferase |
| ANCOVA | Analysis of covariance |
| ANOVA | Analysis of variance |
| Apob | Apolipoprotein B |
| AST | Aspartate aminotransferase |
| BAT | Brown adipose tissue |
| Bmal1 | Basic helix-loop-helix ARNT like 1 |
| BUN | Blood urea nitrogen |
| Cd36 | CD36 molecule |
| cDNA | Complementary deoxyribonucleic acid |
| CI | Confidence interval |
| Cidec | Cell death-inducing DFFA-like effector c |
| CK | Creatine kinase |
| Cpt1a | Carnitine palmitoyltransferase 1a, liver |
| Cr | Creatinine |
| Cry1 | Cryptochrome circadian regulator 1 |
| Dgat2 | Diacylglycerol O-acyltransferase 2 |
| EPI | Epididymal white adipose tissue |
| Fasn | Fatty acid synthase |
| FDR | False discovery rate |
| G6pc1 | Glucose-6-phosphatase catalytic subunit 1 |
| gDNA | Genomic deoxyribonucleic Acid |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase |
| GAS | Gastrocnemius |
| Gpam | Glycerol-3-phosphate acyltransferase, mitochondrial |
| HDL-C | High-density lipoprotein cholesterol |
| HFD | High-fat diet |
| LDH | Lactate dehydrogenase |
| LDL-C | Low-density lipoprotein cholesterol |
| Lep | Leptin |
| Lipe | Lipase, hormone sensitive |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| Mgll | Monoglyceride lipase |
| Mlxipl | MLX interacting protein-like |
| Mttp | MLX interacting protein-like |
| NAFLD | Non-alcoholic fatty liver disease |
| NEFAs | Non-esterified fatty acids |
| Nr1d1 | Nuclear receptor subfamily 1, group D, member 1 |
| PBS | Phosphate-buffered saline |
| Per2 | Period circadian clock 2 |
| Plin2 | Perilipin 2 |
| Plin5 | Perilipin 5 |
| Pnpla2 | Patatin-like phospholipase domain containing 2 |
| Ppara | Peroxisome proliferator activator receptor alpha |
| Ppard | Peroxisome proliferator activator receptor delta |
| Pparg | Peroxisome proliferator activated receptor gamma |
| Ppargc1a | Peroxisome proliferative activated receptor, gamma, coactivator 1 alpha |
| qPCR | Quantitative real-time PCR |
| QUA | Quadriceps |
| RET | Retroperitoneal white adipose tissue |
| RNA | Ribonucleic acid |
| Rps18 | Ribosomal protein S18 |
| SEM | Standard error of the mean |
| Slc27a2 | Solute carrier family 27 (fatty acid transporter), member 2 |
| Slc27a5 | Solute carrier family 27 (fatty acid transporter), member 5 |
| Slc2a2 | Solute carrier family 2 |
| Slc2a4 | Solute carrier family 4 |
| Srebf1 | Sterol regulatory element binding transcription factor 1 |
| T-Cho | Total cholesterol |
| TGs | Triglycerides |
| UA | Uric acid |
| VLDL | Very-low-density lipoprotein |
| VO2 max | Maximal oxygen uptake; |
| WAT | White adipose tissue |
| ZT | Zeitgeber time |
References
- Koliaki, C.; Dalamaga, M.; Liatis, S. Update on the Obesity Epidemic: After the Sudden Rise, Is the Upward Trajectory Beginning to Flatten? Curr. Obes. Rep. 2023, 12, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.; Rust, K.F.; Fradkin, J.; Cheng, Y.J.; Cowie, C.C. Associations between Trends in Race/Ethnicity, Aging, and Body Mass Index with Diabetes Prevalence in the United States: A Series of Cross-Sectional Studies. Ann. Intern. Med. 2014, 161, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Yatsuya, H.; Folsom, A.R.; Yamagishi, K.; North, K.E.; Brancati, F.L.; Stevens, J. Race- and Sex-Specific Associations of Obesity Measures with Ischemic Stroke Incidence in the Atherosclerosis Risk in Communities (ARIC) Study. Stroke 2010, 41, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatol. Baltim. Md. 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy Balance and Obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef]
- Neumark-Sztainer, D.; Wall, M.M.; Chen, C.; Larson, N.I.; Christoph, M.J.; Sherwood, N.E. Eating, Activity, and Weight-Related Problems From Adolescence to Adulthood. Am. J. Prev. Med. 2018, 55, 133–141. [Google Scholar] [CrossRef]
- Bensimhon, D.R.; Kraus, W.E.; Donahue, M.P. Obesity and Physical Activity: A Review. Am. Heart J. 2006, 151, 598–603. [Google Scholar] [CrossRef]
- Faienza, M.F.; Lassandro, G.; Chiarito, M.; Valente, F.; Ciaccia, L.; Giordano, P. How Physical Activity across the Lifespan Can Reduce the Impact of Bone Ageing: A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 1862. [Google Scholar] [CrossRef]
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A.J. Circadian Clock Proteins and Immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef]
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian Rhythms, Sleep, and Metabolism. J. Clin. Investig. 2011, 121, 2133–2141. [Google Scholar] [CrossRef]
- Kadowaki, T. Adiponectin and Adiponectin Receptors in Insulin Resistance, Diabetes, and the Metabolic Syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.; Sassone-Corsi, P. Metabolism and the Circadian Clock Converge. Physiol. Rev. 2013, 93, 107–135. [Google Scholar] [CrossRef]
- Dyar, K.A.; Lutter, D.; Artati, A.; Ceglia, N.J.; Liu, Y.; Armenta, D.; Jastroch, M.; Schneider, S.; De Mateo, S.; Cervantes, M.; et al. Atlas of Circadian Metabolism Reveals System-Wide Coordination and Communication between Clocks. Cell 2018, 174, 1571–1585.e11. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Xiong, Y.; Borck, P.C.; Jang, C.; Doulias, P.-T.; Papazyan, R.; Fang, B.; Jiang, C.; Zhang, Y.; Briggs, E.R.; et al. Diet-Induced Circadian Enhancer Remodeling Synchronizes Opposing Hepatic Lipid Metabolic Processes. Cell 2018, 174, 831–842.e12. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Mahan, K.L.; Patel, V.R.; de Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the Circadian Clock by Nutritional Challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef]
- Lee, K.; Hong, K.-S.; Park, J.; Park, W. Readjustment of Circadian Clocks by Exercise Intervention Is a Potential Therapeutic Target for Sleep Disorders: A Narrative Review. Phys. Act. Nutr. 2024, 28, 35–42. [Google Scholar] [CrossRef]
- Negri, M.; Pivonello, C.; Amatrudo, F.; Cimmino, F.; Trinchese, G.; Vetrani, C.; Iaccarino, G.; Pivonello, R.; Mollica, M.P.; Colao, A. Effects of Chrono-Exercise and Chrono-Nutrition on Muscle Health: Understanding the Molecular Mechanisms Activated by Timed Exercise and Consumption of Proteins and Carbohydrates. Nutr. Rev. 2025, 83, 1571–1593. [Google Scholar] [CrossRef]
- Sato, S.; Dyar, K.A.; Treebak, J.T.; Jepsen, S.L.; Ehrlich, A.M.; Ashcroft, S.P.; Trost, K.; Kunzke, T.; Prade, V.M.; Small, L.; et al. Atlas of Exercise Metabolism Reveals Time-Dependent Signatures of Metabolic Homeostasis. Cell Metab. 2022, 34, 329–345.e8. [Google Scholar] [CrossRef]
- Pendergrast, L.A.; Lundell, L.S.; Ehrlich, A.M.; Ashcroft, S.P.; Schönke, M.; Basse, A.L.; Krook, A.; Treebak, J.T.; Dollet, L.; Zierath, J.R. Time of Day Determines Postexercise Metabolism in Mouse Adipose Tissue. Proc. Natl. Acad. Sci. USA 2023, 120, e2218510120. [Google Scholar] [CrossRef]
- Pendergrast, L.A.; Ashcroft, S.P.; Ehrlich, A.M.; Treebak, J.T.; Krook, A.; Dollet, L.; Zierath, J.R. Metabolic Plasticity and Obesity-Associated Changes in Diurnal Postexercise Metabolism in Mice. Metabolism 2024, 155, 155834. [Google Scholar] [CrossRef]
- Yasumoto, Y.; Nakao, R.; Oishi, K. Free Access to a Running-Wheel Advances the Phase of Behavioral and Physiological Circadian Rhythms and Peripheral Molecular Clocks in Mice. PLoS ONE 2015, 10, e0116476. [Google Scholar] [CrossRef]
- Casanova-Vallve, N.; Duglan, D.; Vaughan, M.E.; Pariollaud, M.; Handzlik, M.K.; Fan, W.; Yu, R.T.; Liddle, C.; Downes, M.; Delezie, J.; et al. Daily Running Enhances Molecular and Physiological Circadian Rhythms in Skeletal Muscle. Mol. Metab. 2022, 61, 101504. [Google Scholar] [CrossRef] [PubMed]
- Adamovich, Y.; Dandavate, V.; Ezagouri, S.; Manella, G.; Zwighaft, Z.; Sobel, J.; Kuperman, Y.; Golik, M.; Auerbach, A.; Itkin, M.; et al. Clock Proteins and Training Modify Exercise Capacity in a Daytime-Dependent Manner. Proc. Natl. Acad. Sci. USA 2021, 118, e2101115118. [Google Scholar] [CrossRef]
- Kovynev, A.; Ying, Z.; Zhang, S.; Olgiati, E.; Lambooij, J.M.; Visentin, C.; Guigas, B.; Ducarmon, Q.R.; Rensen, P.C.N.; Schönke, M. Timing Matters: Late, but Not Early, Exercise Training Ameliorates MASLD in Part by Modulating the Gut--Liver Axis in Mice. J. Pineal Res. 2024, 76, e70003. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.J.; Jimerson, D.C.; Ozaki, Y.; Post, R.M.; Bunney, W.E.; Wurtman, R.J. Entrainment of Rhythmic Melatonin Secretion in Man to a 12-Hour Phase Shift in the Light/Dark Cycle. Life Sci. 1978, 23, 1557–1563. [Google Scholar] [CrossRef]
- Sudo, A.; Miki, K. Circadian Rhythm of Catecholamine Excretion in Rats after Phase Shift of Light-Dark Cycle. Ind. Health 1995, 33, 57–66. [Google Scholar] [CrossRef]
- Gehrke, N.; Biedenbach, J.; Huber, Y.; Straub, B.K.; Galle, P.R.; Simon, P.; Schattenberg, J.M. Voluntary Exercise in Mice Fed an Obesogenic Diet Alters the Hepatic Immune Phenotype and Improves Metabolic Parameters-an Animal Model of Life Style Intervention in NAFLD. Sci. Rep. 2019, 9, 4007. [Google Scholar] [CrossRef]
- Cho, J.; Johnson, B.D.; Watt, K.D.; Niven, A.S.; Yeo, D.; Kim, C.-H. Exercise Training Attenuates Pulmonary Inflammation and Mitochondrial Dysfunction in a Mouse Model of High-Fat High-Carbohydrate-Induced NAFLD. BMC Med. 2022, 20, 429. [Google Scholar] [CrossRef]
- Xu, Z.; Qin, Y.; Lv, B.; Tian, Z.; Zhang, B. Effects of Moderate-Intensity Continuous Training and High-Intensity Interval Training on Testicular Oxidative Stress, Apoptosis and m6A Methylation in Obese Male Mice. Antioxidants 2022, 11, 1874. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Telfser, A.J.; Olzomer, E.M.; Vancuylenberg, C.S.; Zhou, M.; Beretta, M.; Li, C.; Alexopoulos, S.J.; Turner, N.; Byrne, F.L.; et al. Beneficial Effects of Simultaneously Targeting Calorie Intake and Calorie Efficiency in Diet-Induced Obese Mice. Clin. Sci. 2024, 138, 173–187. [Google Scholar] [CrossRef]
- Omar, A.M.; Zhang, Q. Evaluation of Lipid Extraction Protocols for Untargeted Analysis of Mouse Tissue Lipidome. Metabolites 2023, 13, 1002. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sáinz, N.; Félix-Soriano, E.; Gil-Iturbe, E.; Castilla-Madrigal, R.; Fernández-Galilea, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Effects of Long-Term DHA Supplementation and Physical Exercise on Non-Alcoholic Fatty Liver Development in Obese Aged Female Mice. Nutrients 2021, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A Circadian Gene Expression Atlas in Mammals: Implications for Biology and Medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the Past Two Decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Sato, S.; Basse, A.L.; Schönke, M.; Chen, S.; Samad, M.; Altıntaş, A.; Laker, R.C.; Dalbram, E.; Barrès, R.; Baldi, P.; et al. Time of Exercise Specifies the Impact on Muscle Metabolic Pathways and Systemic Energy Homeostasis. Cell Metab. 2019, 30, 92–110.e4. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Hashimoto, S.; Takasu, N.N.; Tanahashi, Y.; Nishide, S.; Honma, S.; Honma, K. Morning and Evening Physical Exercise Differentially Regulate the Autonomic Nervous System during Nocturnal Sleep in Humans. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 309, R1112–R1121. [Google Scholar] [CrossRef]
- Lan, H.; Wu, K.; Deng, C.; Wang, S. Morning vs. Evening: The Role of Exercise Timing in Enhancing Fat Oxidation in Young Men. Front. Physiol. 2025, 16, 1574757. [Google Scholar] [CrossRef]
- Moholdt, T.; Parr, E.B.; Devlin, B.L.; Debik, J.; Giskeødegård, G.; Hawley, J.A. The Effect of Morning vs Evening Exercise Training on Glycaemic Control and Serum Metabolites in Overweight/Obese Men: A Randomised Trial. Diabetologia 2021, 64, 2061–2076. [Google Scholar] [CrossRef]
- Zambon, A.C.; McDearmon, E.L.; Salomonis, N.; Vranizan, K.M.; Johansen, K.L.; Adey, D.; Takahashi, J.S.; Schambelan, M.; Conklin, B.R. Time- and Exercise-Dependent Gene Regulation in Human Skeletal Muscle. Genome Biol. 2003, 4, R61. [Google Scholar] [CrossRef]
- Mądra-Gackowska, K.; Szewczyk-Golec, K.; Gackowski, M.; Woźniak, A.; Kędziora-Kornatowska, K. Evaluation of Selected Parameters of Oxidative Stress and Adipokine Levels in Hospitalized Older Patients with Diverse Nutritional Status. Antioxidants 2023, 12, 569. [Google Scholar] [CrossRef]


| Gene | Forward (5′-3′) | Reverse (3′-5′) |
|---|---|---|
| Acaca | GGCTCGTGTGTGGAAGTGGATG | GTGGTGTAACTGCTGCCGTCAT |
| Acadm | TGTTAATCGGTGAAGGAGCAG | CTATCCAGGGCATACTTCGTG |
| Acox1 | GGGGAACATCATCACAGGGG | ATCATAGCGGCCGAGAACAG |
| Adipoq | GCCGCTTATGTGTATCGCTCAG | CTTGCCAGTGCTGCCGTCATA |
| Apob | GCCTTCCAGTTGGCAACACAGT | GGCAGGTCAACATCGGCAATCA |
| Bmal1 | GGACTTCGCCTCTACCTGTT | CCTCGTTGTCTGGCTCATTG |
| Cd36 | TGCGACATGATTAATGGCACAGAC | TCCGAACACAGCGTAGATAGACCT |
| Cidec | TGTCGTGTTAGCACCGCAGAT | GCCATCTTCCTCCAGCACCA |
| Cpt1a | CAAGCCAGACGAAGAACA | TGACCATAGCCATCCAGAT |
| Cry1 | GCTGGCGTGGAAGTCATCGT | ATGGTGTCTGCTGGCATCTCC |
| Dgat2 | GCACCCGACCCAGAAAGACATC | AGTTCACCTCCAGCACCTCAGT |
| Fasn | ACTCAAGTGGCTGATGTG | TGCTGTCGTCTGTAGTCT |
| G6pc1 | GTCGTGGCTGGAGTCTTGT | CGGAGGCTGGCATTGTAGA |
| Gapdh | TCTCCTGCGACTTCAACA | TGTAGCCGTATTCATTGTCA |
| Gpam | TGAGCAGCAGCAGAGTCCAAGA | GTTCAACTCCGCAGCCACTTCA |
| Lep | TTCACACACGCAGTCGGTATCC | AGGCTGGTGAGGACCTGTTGAT |
| Lipe | CTGAGATTGAGGTGCTGTC | GGTGAGATGGTAACTGTGAG |
| Mgll | TGATTTCACCTCTGGTCCTTG | GTCAACCTCCGACTTGTTCC |
| Mlxipl | GCAACCACGCTTCAGAAGACAG | GCTGCTGGCACAAGTTGATGG |
| Mttp | CAGCGTCCACATACAGCCTTGA | TCCTCAGAATGCCAGAGCCAGA |
| Nr1d1 | ACGGCAAGGCAACACCAA | GCGGCTCAGGAACATCACT |
| Per2 | GCTGCGGATGCTCGTGGAAT | GGTTGTGCTCTGCCTCTGTCAT |
| Plin2 | GCAACAGAGCGTGGTGATGAGA | CGGAGGACACAAGGTCGTAGGT |
| Plin5 | ACCGCTTCCTGCCCATGACT | TTGCTGCCTCTGCTCCTCCA |
| Pnpla2 | TTCAGACAACTTGCCACTT | CGGTAGAGATTGCGAAGG |
| Ppara | ACGATGCTGTCCTCCTTGAT | AACGGCTTCCTCAGGTTCTTA |
| Ppard | GCTGCTGCAGAAGATGGCA | CACTGCATCATCTGGGCATG |
| Pparg | CGTGAAGCCCATCGAGGACATC | TGGAGCACCTTGGCGAACAG |
| Ppargc1a | GTCGTGGCTGGAGTCTTGT | CGGAGGCTGGCATTGTAGA |
| Rps18 | TTCTGGCCAACGGTCTAGACAAC | CCAGTGGTCTTGGTGTGCTGA |
| Slc27a2 | ACCACAGAAGTCGCTGACATCG | GCACAGGCACGCCATACACAT |
| Slc27a5 | CCTTGTGCTGCTTGGCTTGG | GGTGGCTGTAGAGGCAATAGGA |
| Slc2a2 | TTGACTGGAGCCCTCTTGATGG | CTGAGTGTGGTTGGAGCGATCT |
| Slc2a4 | GCTGGTGTGGTCAATACGGTCT | GCAGAGCCACGGTCATCAAGAT |
| Srebf1 | GCCATCGACTACATCCGCTTCT | TGCCTCCTCCACTGCCACAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, Z.; Huang, J.; Tong, Y.; Wu, C.; Kobori, H.; Ma, S.; Suzuki, K. Circadian Phase Determines Tissue-Specific Adaptations to Long-Term Exercise in Obese Mice. Nutrients 2025, 17, 3281. https://doi.org/10.3390/nu17203281
Wang S, Zhang Z, Huang J, Tong Y, Wu C, Kobori H, Ma S, Suzuki K. Circadian Phase Determines Tissue-Specific Adaptations to Long-Term Exercise in Obese Mice. Nutrients. 2025; 17(20):3281. https://doi.org/10.3390/nu17203281
Chicago/Turabian StyleWang, Shuo, Ziwei Zhang, Jiapeng Huang, Yishan Tong, Cong Wu, Haruki Kobori, Sihui Ma, and Katsuhiko Suzuki. 2025. "Circadian Phase Determines Tissue-Specific Adaptations to Long-Term Exercise in Obese Mice" Nutrients 17, no. 20: 3281. https://doi.org/10.3390/nu17203281
APA StyleWang, S., Zhang, Z., Huang, J., Tong, Y., Wu, C., Kobori, H., Ma, S., & Suzuki, K. (2025). Circadian Phase Determines Tissue-Specific Adaptations to Long-Term Exercise in Obese Mice. Nutrients, 17(20), 3281. https://doi.org/10.3390/nu17203281











