Dietary Branched-Chain Amino Acids and Hyper-LDL-Cholesterolemia: A Case–Control Study Using Interpretable Machine-Learning Models in Chinese Children and Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurement and Definitions
2.3. Statistics
3. Results
3.1. Characteristics of Participants
3.2. Association Between BCAA Intake and Hyper-LDL-Cholesterolemia Prevalence Risk
3.3. Machine Learning: LightGBM Algorithm
3.4. Interaction Analysis
3.5. Dose–Response Relationship Analysis
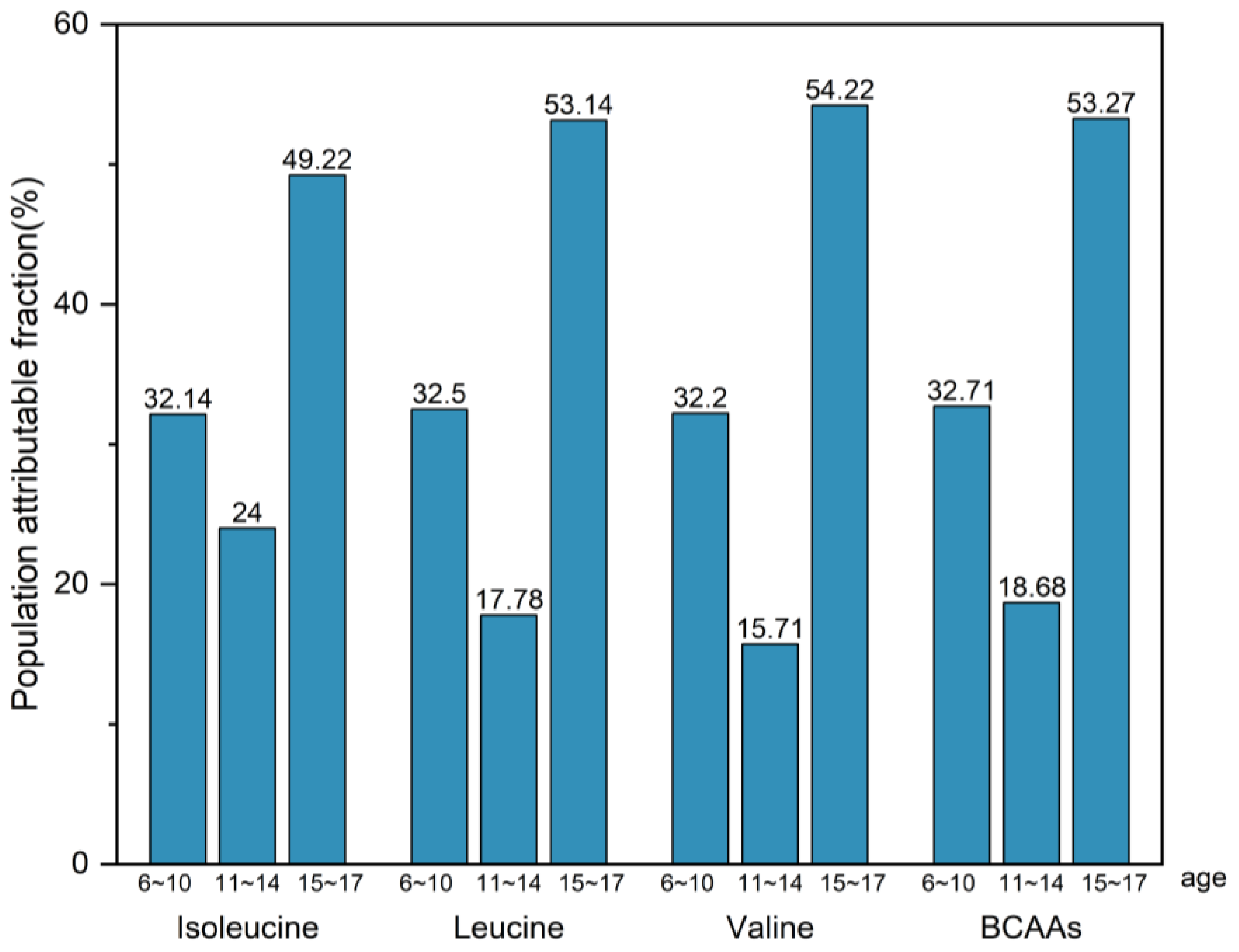
3.6. Population Attributable Fraction (PAF)
3.7. Sensitivity Analysis
3.8. Mendelian Randomization for Mediation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| ROC | Receiver Operator Characteristic |
| RCS | Restricted Cubic Splines |
| OR | Odds Ratios |
| BCAA | Branched-Chain Amino Acids |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| CVD | Cardiovascular Disease |
| PAF | Population Attributable Fraction |
References
- Mortensen, M.B.; Dzaye, O.; Bøtker, H.E.; Jensen, J.M.; Maeng, M.; Bentzon, J.F.; Kanstrup, H.; Sørensen, H.T.; Leipsic, J.; Blankstein, R.; et al. Low-Density Lipoprotein Cholesterol Is Predominantly Associated with Atherosclerotic Cardiovascular Disease Events in Patients with Evidence of Coronary Atherosclerosis: The Western Denmark Heart Registry. Circulation 2023, 147, 1053–1063. [Google Scholar] [CrossRef]
- Global Burden of Disease: High LDL Cholesterol-Level 2 Risk. 2021. Available online: https://www.healthdata.org/research-analysis/diseases-injuries-risks/factsheets/2021-high-ldl-cholesterol-level-2-risk (accessed on 18 August 2025).
- Kartiosuo, N.; Raitakari, O.T.; Juonala, M.; Viikari, J.S.A.; Sinaiko, A.R.; Venn, A.J.; Jacobs, D.R., Jr.; Urbina, E.M.; Woo, J.G.; Steinberger, J.; et al. Cardiovascular Risk Factors in Childhood and Adulthood and Cardiovascular Disease in Middle Age. JAMA Netw. Open 2024, 7, e2418148. [Google Scholar] [CrossRef]
- Wu, F.; Juonala, M.; Jacobs, D.R., Jr.; Daniels, S.R.; Kähönen, M.; Woo, J.G.; Sinaiko, A.R.; Viikari, J.S.A.; Bazzano, L.A.; Burns, T.L.; et al. Childhood Non-HDL Cholesterol and LDL Cholesterol and Adult Atherosclerotic Cardiovascular Events. Circulation 2024, 149, 217–226. [Google Scholar] [CrossRef]
- Rousseau, M.; Guénard, F.; Garneau, V.; Allam-Ndoul, B.; Lemieux, S.; Pérusse, L.; Vohl, M.C. Associations Between Dietary Protein Sources, Plasma BCAA and Short-Chain Acylcarnitine Levels in Adults. Nutrients 2019, 11, 173. [Google Scholar] [CrossRef]
- White, P.J.; McGarrah, R.W.; Herman, M.A.; Bain, J.R.; Shah, S.H.; Newgard, C.B. Insulin action, type 2 diabetes, and branched-chain amino acids: A two-way street. Mol. Metab. 2021, 52, 101261. [Google Scholar] [CrossRef]
- White, P.J.; Newgard, C.B. Branched-chain amino acids in disease. Science 2019, 363, 582–583. [Google Scholar] [CrossRef]
- McGarrah, R.W.; White, P.J. Branched-chain amino acids in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 77–89. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, G.; Chen, X.; Zhang, L.; Guo, L.; Li, C.; Zhao, H.; Cui, Z.; Guo, X.; Sun, F.; et al. Excessive branched-chain amino acid accumulation restricts mesenchymal stem cell-based therapy efficacy in myocardial infarction. Signal Transduct. Target. Ther. 2022, 7, 171. [Google Scholar] [CrossRef]
- Yang, R.; Dong, J.; Zhao, H.; Li, H.; Guo, H.; Wang, S.; Zhang, C.; Wang, S.; Wang, M.; Yu, S.; et al. Association of branched-chain amino acids with carotid intima-media thickness and coronary artery disease risk factors. PLoS ONE 2014, 9, e99598. [Google Scholar] [CrossRef]
- Rahimi, M.H.; Shab-Bidar, S.; Mollahosseini, M.; Djafarian, K. Branched-chain amino acid supplementation and exercise-induced muscle damage in exercise recovery: A meta-analysis of randomized clinical trials. Nutrition 2017, 42, 30–36. [Google Scholar] [CrossRef]
- Bai, G.H.; Tsai, M.C.; Tsai, H.W.; Chang, C.C.; Hou, W.H. Effects of branched-chain amino acid-rich supplementation on EWGSOP2 criteria for sarcopenia in older adults: A systematic review and meta-analysis. Eur. J. Nutr. 2022, 61, 637–651. [Google Scholar] [CrossRef]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.E.; Cheng, S.; Mora, S. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women. Circ. Genom. Precis. Med. 2018, 11, e002157. [Google Scholar] [CrossRef]
- Yang, P.; Hu, W.; Fu, Z.; Sun, L.; Zhou, Y.; Gong, Y.; Yang, T.; Zhou, H. The positive association of branched-chain amino acids and metabolic dyslipidemia in Chinese Han population. Lipids Health Dis. 2016, 15, 120. [Google Scholar] [CrossRef]
- Burcham, S.; Liu, Y.; Merianos, A.L.; Mendy, A. Outliers in nutrient intake data for U.S. adults: National health and nutrition examination survey 2017–2018. Epidemiol. Methods 2023, 12, 20230018. [Google Scholar] [CrossRef]
- Lee, M.S.; Carcone, A.I.; Ko, L.; Kulik, N.; Ellis, D.A.; Naar, S. Managing Outliers in Adolescent Food Frequency Questionnaire Data. J. Nutr. Educ. Behav. 2021, 53, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhu, W.-L.; Zhang, M.; Ma, G.-S. Interpretation on dietary guidelines for Chinese school-aged children. Chin. J. Sch. Health 2022, 43, 805–808. [Google Scholar]
- WS/T 424-2013; Anthropometric Measurements Method in Health Surveillance. Health Industry Standards of the People’s Republic of China: Beijing, China, 2013.
- WS/T 586-2018; Screening for Overweight and Obesity Among School-Age Children and Adolescents. Health Industry Standards of the People’s Republic of China: Beijing, China, 2018.
- Fan, H.; Yan, Y.; Mi, J. Updating blood pressure references for Chinese children aged 3–17 years. Chin. J. Hypertens. 2017, 25, 428–435. [Google Scholar]
- WS/T 610-2018; Reference of Screening for Elevated Blood Pressure Among Children and Adolescents Aged 7~18 Years. Health Industry Standards of the People’s Republic of China: Beijing, China, 2018.
- Bamba, V. Update on screening, etiology, and treatment of dyslipidemia in children. J. Clin. Endocrinol. Metab. 2014, 99, 3093–3102. [Google Scholar] [CrossRef]
- Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [CrossRef]
- WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2007; pp. 1–265. ISSN 0512-3054. [Google Scholar]
- Bar-Peled, L.; Sabatini, D.M. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014, 24, 400–406. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Hagve, M.; Simbo, S.Y.; Ruebush, L.E.; Engelen, M.; Gutierrez-Osuna, R.; Mortazavi, B.J.; Cote, G.L.; Deutz, N.E.P. Postprandial concentration of circulating branched chain amino acids are able to predict the carbohydrate content of the ingested mixed meal. Clin. Nutr. 2021, 40, 5020–5029. [Google Scholar] [CrossRef]
- Dimou, A.; Tsimihodimos, V.; Bairaktari, E. The Critical Role of the Branched Chain Amino Acids (BCAAs) Catabolism-Regulating Enzymes, Branched-Chain Aminotransferase (BCAT) and Branched-Chain α-Keto Acid Dehydrogenase (BCKD), in Human Pathophysiology. Int. J. Mol. Sci. 2022, 23, 4022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Ni, Y.; Yu, Y.; Guo, F.; Lu, Y.; Wang, X.; Hao, H.; Li, S.; Wei, P.; et al. Branched-chain amino acids promote occurrence and development of cardiovascular disease dependent on triglyceride metabolism via activation of the mTOR/SREBP-1/betatrophin pathway. Mol. Cell. Endocrinol. 2024, 584, 112164. [Google Scholar] [CrossRef]
- Tanase, D.M.; Valasciuc, E.; Costea, C.F.; Scripcariu, D.V.; Ouatu, A.; Hurjui, L.L.; Tarniceriu, C.C.; Floria, D.E.; Ciocoiu, M.; Baroi, L.G.; et al. Duality of Branched-Chain Amino Acids in Chronic Cardiovascular Disease: Potential Biomarkers versus Active Pathophysiological Promoters. Nutrients 2024, 16, 1972. [Google Scholar] [CrossRef]
- Huang, H.; Chen, H.; Yao, Y.; Lou, X. Branched-chain amino acids supplementation induces insulin resistance and pro-inflammatory macrophage polarization via INFGR1/JAK1/STAT1 signal pathway. Mol. Med. 2024, 30, 149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pu, L.; Zhao, T.; Wang, L.; Shu, C.; Xu, S.; Sun, J.; Zhang, R.; Han, L. Global Burden of Cardiovascular Disease from 1990 to 2019 Attributable to Dietary Factors. J. Nutr. 2023, 153, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Brodowski, J.; Pokorska-Niewiada, K.; Szczuko, M. The Change in the Content of Nutrients in Diets Eliminating Products of Animal Origin in Comparison to a Regular Diet from the Area of Middle-Eastern Europe. Nutrients 2020, 12, 2986. [Google Scholar] [CrossRef]
- Ko, G.J.; Rhee, C.M.; Kalantar-Zadeh, K.; Joshi, S. The Effects of High-Protein Diets on Kidney Health and Longevity. J. Am. Soc. Nephrol. 2020, 31, 1667–1679. [Google Scholar] [CrossRef]
- Park, J.E.; Miller, M.; Rhyne, J.; Wang, Z.; Hazen, S.L. Differential effect of short-term popular diets on TMAO and other cardio-metabolic risk markers. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 513–517. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.P.; Brandon, A.E.; et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019, 1, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, N.L.; Garry, J.; Shi, X.; Gerszten, R.E.; Anderson, E.J.; Walford, G.A. A pilot, short-term dietary manipulation of branched chain amino acids has modest influence on fasting levels of branched chain amino acids. Food Nutr. Res. 2016, 60, 28592. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, I.; Taylor, M.; Phillips, B.; Wilkinson, D.; Smith, K.; Hession, K.; Idris, I.; Atherton, P. A Novel Dietary Intervention Reduces Circulatory Branched-Chain Amino Acids by 50%: A Pilot Study of Relevance for Obesity and Diabetes. Nutrients 2020, 13, 95. [Google Scholar] [CrossRef] [PubMed]
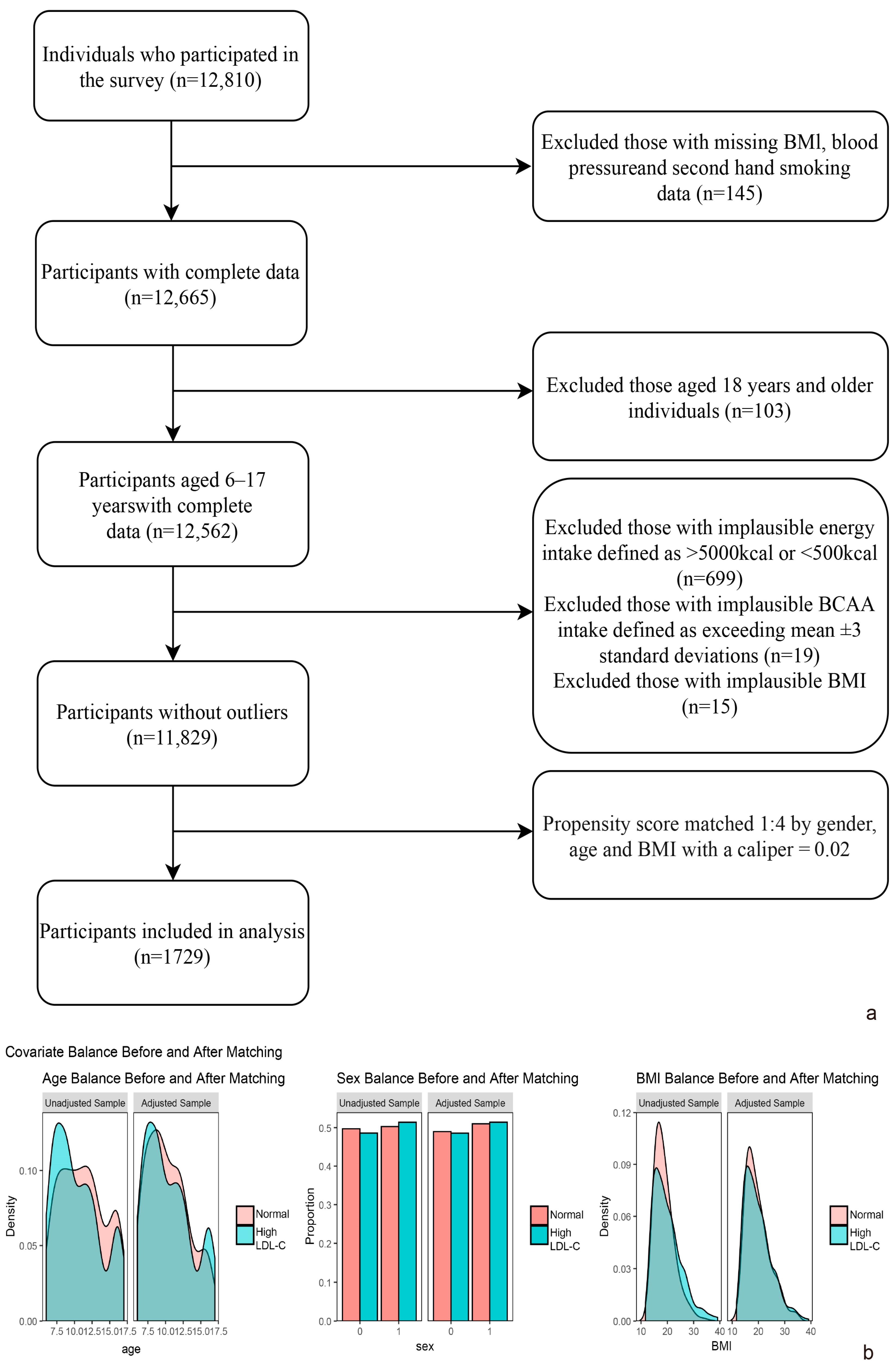



| Total (n = 1729) | Normal | High Blood LDL-C | χ2/t | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Gender (n, %) | 0.006 | 0.938 | ||||||
| Male | 843 (48.8) | 673 (38.9) | 170 (9.8) | |||||
| Female | 886 (51.2) | 706 (40.8) | 180 (10.4) | |||||
| Age (n, %) | 10.4 | 2.99 | 10.39 | 2.94 | 10.46 | 3.20 | −0.390 | 0.697 |
| 6~10 years | 962 (55.6) | 768 (44.4) | 194 (11.2) | |||||
| 11~14 years | 545 (31.5) | 443 (25.6) | 102 (5.9) | |||||
| 15~17 years | 222 (12.8) | 168 (9.7) | 54 (3.1) | |||||
| BMI [kg/m2, (n, %)] | 19.66 | 4.61 | 19.61 | 4.48 | 19.85 | 5.07 | −0.826 | 0.409 |
| Non-obesity | 1364 (78.9) | 1095 (63.3) | 269 (15.6) | |||||
| Obesity | 365 (21.1) | 284 (16.4) | 81 (4.7) | |||||
| Blood pressure (n, %) | 0.047 | 0.827 | ||||||
| Normal | 1248 (72.2) | 997 (57.7) | 251 (14.5) | |||||
| Elevated blood pressure | 481 (27.8) | 382 (22.1) | 99 (5.7) | |||||
| Secondhand smoking (n, %) | 0.004 | 0.950 | ||||||
| No | 1035 (59.9) | 826 (47.8) | 209 (12.1) | |||||
| Yes | 694 (40.1) | 553 (32.0) | 141 (8.2) | |||||
| Drinker (n, %) | 0.645 | 0.422 | ||||||
| No | 1589 (91.9) | 1271 (73.5) | 318 (18.4) | |||||
| Yes | 140 (8.1) | 108 (6.2) | 32 (1.9) | |||||
| Lack of physical activity (n, %) | 0.087 | 0.768 | ||||||
| Yes | 1187 (68.7) | 949 (54.9) | 238 (13.8) | |||||
| No | 542 (31.3) | 430 (24.9) | 112 (6.5) | |||||
| Energy intake (kcal/day) | 1940.39 | 923.21 | 1932.48 | 922.68 | 1971.56 | 925.97 | −0.707 | 0.480 |
| Isoleucine intake (g/day) | 4.20 | 2.15 | 4.12 | 2.10 | 4.51 | 2.32 | −2.812 | 0.005 |
| Leucine intake (g/day) | 8.51 | 4.45 | 8.35 | 4.35 | 9.13 | 4.77 | −2.948 | 0.003 |
| Valine intake (g/day) | 5.68 | 3.02 | 5.56 | 2.95 | 6.12 | 3.25 | −2.926 | 0.004 |
| BCAAs intake (g/day) | 18.38 | 9.58 | 18.03 | 9.36 | 19.76 | 10.31 | −3.017 | 0.003 |
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | OR | 95%CI | p-Value | |
| Conditional logistic regression model | |||||||||
| Isoleucine | 1.08 | (1.03, 1.14) | 0.003 | 1.08 | (1.03, 1.14) | 0.003 | 1.30 | (1.17, 1.45) | <0.001 |
| Leucine | 1.04 | (1.01, 1.06) | 0.003 | 1.04 | (1.01, 1.06) | 0.003 | 1.11 | (1.06, 1.17) | <0.001 |
| Valine | 1.06 | (1.02, 1.10) | 0.002 | 1.06 | (1.02, 1.10) | 0.002 | 1.16 | (1.09, 1.24) | <0.001 |
| BCAAs | 1.02 | (1.01, 1.03) | 0.003 | 1.02 | (1.01, 1.03) | 0.003 | 1.05 | (1.03, 1.08) | <0.001 |
| Logistic regression mixed effects model | |||||||||
| Isoleucine | 1.09 | (1.02, 1.17) | 0.009 | 1.09 | (1.02, 1.16) | 0.009 | 1.34 | (1.17, 1.54) | <0.001 |
| Leucine | 1.04 | (1.01, 1.08) | 0.010 | 1.04 | (1.01, 1.08) | 0.010 | 1.13 | (1.06, 1.20) | <0.001 |
| Valine | 1.07 | (1.02, 1.12) | 0.007 | 1.07 | (1.02, 1.12) | 0.007 | 1.18 | (1.09, 1.29) | <0.001 |
| BCAAs | 1.02 | (1.01, 1.04) | 0.009 | 1.02 | (1.01, 1.04) | 0.009 | 1.06 | (1.03, 1.09) | <0.001 |
| Isoleucine | p for Interaction | Leucine | p for Interaction | Valine | p for Interaction | BCAAs | p for Interaction | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | OR | 95%CI | OR | 95%CI | |||||
| Blood pressure | 0.233 | 0.408 | 0.449 | 0.372 | ||||||||
| Normal | 1.25 | (1.11, 1.42) | 1.09 | (1.03, 1.15) | 1.12 | (1.04, 1.21) | 1.04 | (1.02, 1.07) | ||||
| Elevated blood pressure | 1.51 | (1.21, 1.89) | 1.22 | (1.11, 1.35) | 1.32 | (1.16, 1.50) | 1.10 | (1.05, 1.15) | ||||
| Secondhand smoking | 0.589 | 0.756 | 0.763 | 0.710 | ||||||||
| No | 1.20 | (1.04, 1.40) | 1.07 | (1.01, 1.15) | 1.11 | (1.02, 1.21) | 1.04 | (1.01, 1.07) | ||||
| Yes | 1.45 | (1.24, 1.70) | 1.17 | (1.09, 1.25) | 1.24 | (1.13, 1.37) | 1.08 | (1.04, 1.11) | ||||
| Drinker | 0.884 | 0.884 | 0.965 | 0.903 | ||||||||
| No | 1.30 | (1.16, 1.46) | 1.11 | (1.06, 1.17) | 1.16 | (1.08, 1.24) | 1.05 | (1.03, 1.08) | ||||
| Yes | 1.29 | (0.98, 1.69) | 1.12 | (0.99, 1.26) | 1.18 | (0.99, 1.41) | 1.05 | (0.99, 1.12) | ||||
| Lack of physical activity | 0.847 | 0.732 | 0.645 | 0.722 | ||||||||
| No | 1.36 | (1.11, 1.66) | 1.12 | (1.03, 1.21) | 1.16 | (1.04, 1.30) | 1.06 | (1.02, 1.10) | ||||
| Yes | 1.27 | (1.12, 1.44) | 1.11 | (1.05, 1.18) | 1.16 | (1.08, 1.26) | 1.05 | (1.02, 1.08) | ||||
| Method | Total Effect OR (95%CI) | p-Value | Indirect Effect OR (95%CI) | p-Value | Direct Effect OR (95%CI) | p-Value | Mediated Proportion (%) |
|---|---|---|---|---|---|---|---|
| MR Egger | 0.99 (0.92, 1.07) | 0.817 | 0.99 (0.98, 1.01) | 0.903 | 0.99 (0.92, 1.07) | 0.829 | 10.5 |
| Weighted median | 1.03 (1.01, 1.05) | 0.003 | 1.01 (1.00, 1.01) | 0.003 | 1.02 (1.00, 1.04) | 0.022 | 24.4 |
| Inverse variance weighted | 1.06 (1.02, 1.11) | 0.005 | 1.02 (1.00, 1.02) | 0.026 | 1.05 (1.01, 1.09) | 0.017 | 18.0 |
| Simple mode | 1.04 (1.00, 1.09) | 0.070 | 1.02 (1.00, 1.04) | 0.042 | 1.02 (0.98, 1.06) | 0.255 | 48.7 |
| Weighted mode | 1.03 (1.01, 1.05) | 0.003 | 1.00 (0.99, 1.01) | 0.067 | 1.03 (1.01, 1.04) | <0.001 | 13.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, Z.; Zhang, S.; Liu, C.; Liu, Y.; Tian, M.; Luo, X.; Zhu, Q.; Liu, L.; Yu, L. Dietary Branched-Chain Amino Acids and Hyper-LDL-Cholesterolemia: A Case–Control Study Using Interpretable Machine-Learning Models in Chinese Children and Adolescents. Nutrients 2025, 17, 3280. https://doi.org/10.3390/nu17203280
Zang Z, Zhang S, Liu C, Liu Y, Tian M, Luo X, Zhu Q, Liu L, Yu L. Dietary Branched-Chain Amino Acids and Hyper-LDL-Cholesterolemia: A Case–Control Study Using Interpretable Machine-Learning Models in Chinese Children and Adolescents. Nutrients. 2025; 17(20):3280. https://doi.org/10.3390/nu17203280
Chicago/Turabian StyleZang, Zeping, Shixiu Zhang, Changqing Liu, Yiya Liu, Meina Tian, Xiaoyan Luo, Qianrang Zhu, Lei Liu, and Lianlong Yu. 2025. "Dietary Branched-Chain Amino Acids and Hyper-LDL-Cholesterolemia: A Case–Control Study Using Interpretable Machine-Learning Models in Chinese Children and Adolescents" Nutrients 17, no. 20: 3280. https://doi.org/10.3390/nu17203280
APA StyleZang, Z., Zhang, S., Liu, C., Liu, Y., Tian, M., Luo, X., Zhu, Q., Liu, L., & Yu, L. (2025). Dietary Branched-Chain Amino Acids and Hyper-LDL-Cholesterolemia: A Case–Control Study Using Interpretable Machine-Learning Models in Chinese Children and Adolescents. Nutrients, 17(20), 3280. https://doi.org/10.3390/nu17203280





