The Impact of a High-Fat Diet on Eye Health
Abstract
1. Introduction
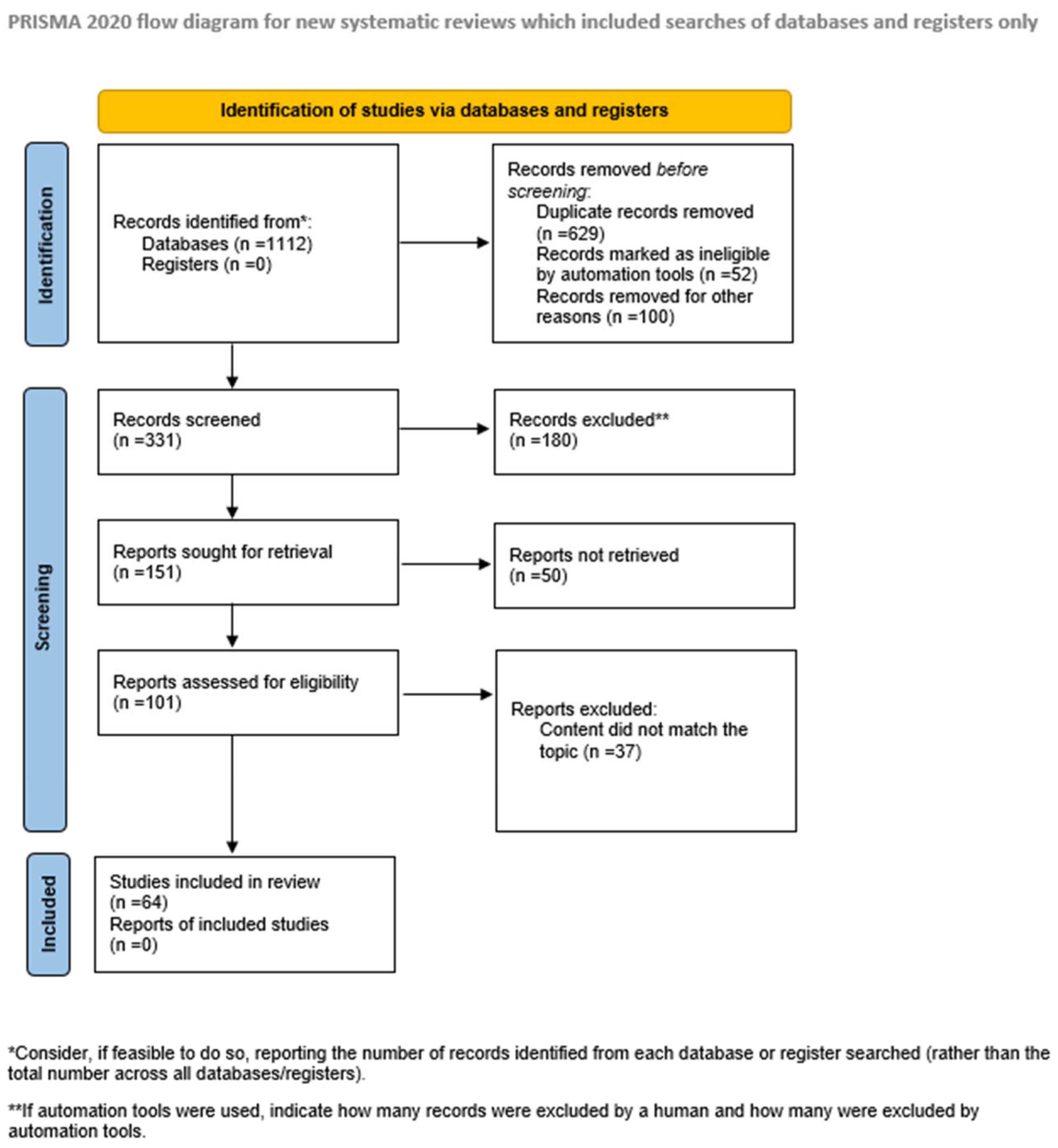
2. Materials and Methods
3. Impact of High-Fat Diet on Retinal Structure and Function
3.1. Retinal Structure
- Inner Limiting Membrane (ILM)—The ILM constitutes the basal boundary of the retina, interfacing with the vitreous body. It primarily comprises the endfeet of Müller glial cells, which contribute to retinal homeostasis by maintaining structural organization and supporting neuronal function.
- Retinal Nerve Fiber Layer (RNFL)—This layer consists of unmyelinated axons of retinal ganglion cells (RGCs), interspersed with astrocytes and Müller cell processes. The ILM serves as the basement membrane for this layer, providing structural anchoring.
- Ganglion Cell Layer (GCL)—Contains the somata of RGCs, whose axons converge to form the optic nerve, facilitating the transmission of visual information to central targets.
- Inner Plexiform Layer (IPL)—A synaptic layer where bipolar cell axons contact ganglion cell dendrites. Amacrine cells also form synapses here, playing a critical role in modulating signal transmission through lateral inhibition and temporal filtering.
- Inner Nuclear Layer (INL)—Composed of the cell bodies of bipolar, horizontal, and amacrine cells. Bipolar cells act as intermediaries, relaying signals from photoreceptors to ganglion cells, while horizontal and amacrine cells provide lateral modulation of synaptic input.
- Outer Plexiform Layer (OPL)—The site of synaptic interactions between photoreceptor terminals and the dendrites of bipolar and horizontal cells, enabling vertical and horizontal signal integration.
- Outer Nuclear Layer (ONL)—Contains the nuclei of rod and cone photoreceptors, which are responsible for phototransduction.
- External Limiting Membrane (ELM)—Composed of adherens and gap junctions between photoreceptors and Müller cells. This layer demarcates the boundary between the nuclear components of photoreceptors and their inner segments.
- Photoreceptor Layer (PRL)—Comprises the inner and outer segments of rods and cones. The outer segments contain stacks of membranous discs enriched with opsins (e.g., rhodopsin), which is essential for light absorption, while the inner segments house mitochondria that support the high metabolic demands of phototransduction.
- Retinal Pigment Epithelium (RPE)—A monolayer of pigmented epithelial cells located between the neural retina and Bruch’s membrane. The RPE performs multiple functions, including forming part of the blood-retinal barrier (in concert with retinal endothelial cells), recycling visual pigments (conversion of all-trans-retinal to 11-cis-retinal), phagocytosing shed photoreceptor outer segments, and secreting trophic factors essential for retinal and choroidal homeostasis [6].
3.2. High-Fat Diet and Inflammatory Pathways in Retina (Figure 3)
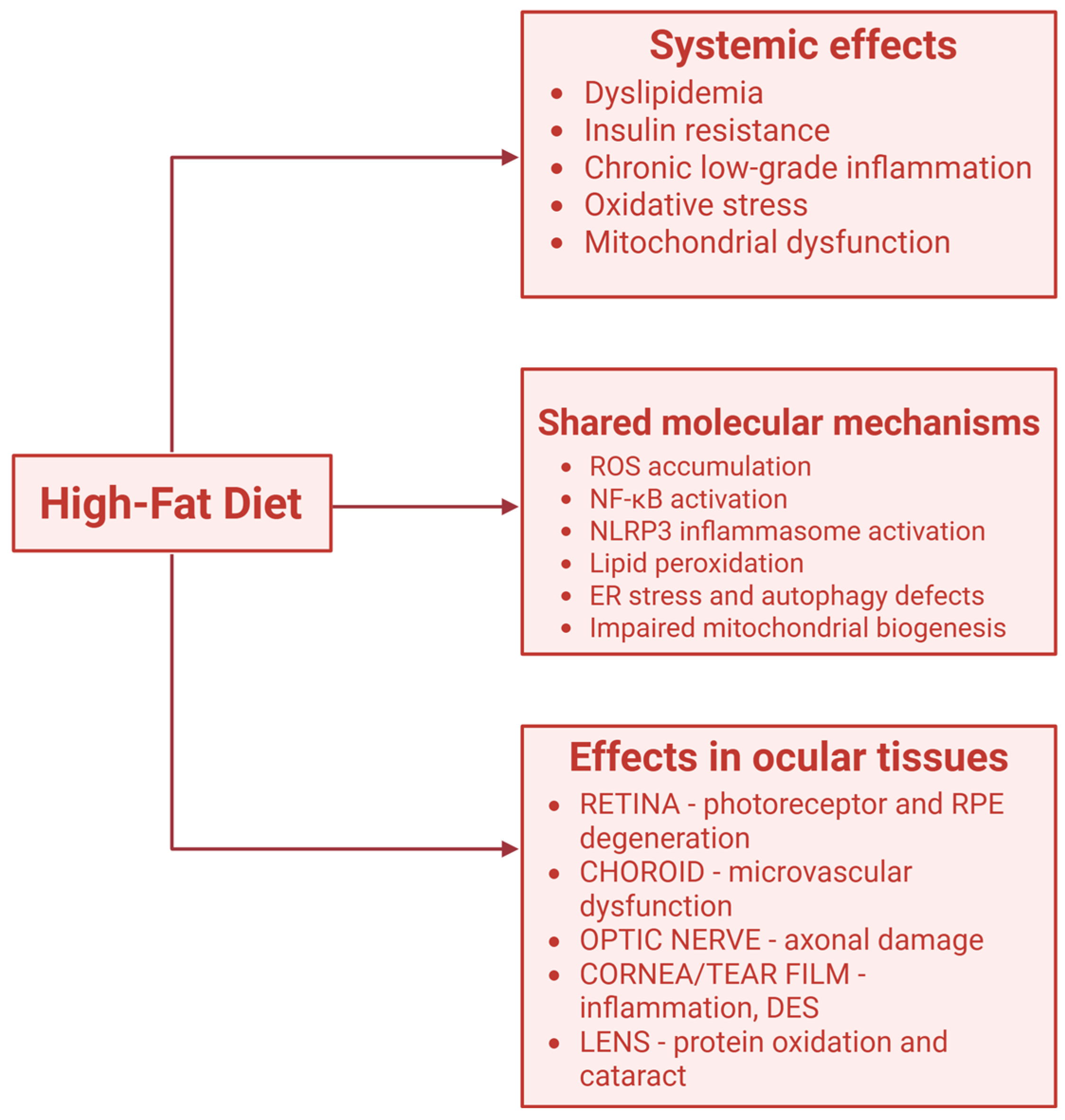
4. The Role of Fats in Myopia
5. Dry Eye Syndrome and High Fat Diet
6. The Impact of Lipids on Age-Related Macular Degeneration (AMD)
7. The Connection Between Cataract and HFD
8. The Association Between Glaucoma and HFD
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Ibrahim, K.S.; Baran, M.R.; Tchivelekete, G.M.; Zhou, X.; Wu, Y.; Reilly, J.; Tan, Z.; He, Z.; Shu, X. Traditional Chinese medicine, Ziyin-Mingmu decoction, regulates cholesterol metabolism, oxidative stress, inflammation and gut microbiota in age-related macular degeneration models. Pharm. Res. 2025, 42, 1101–1118. [Google Scholar] [CrossRef]
- Li, Z.; Heber, D. Ketogenic diets. JAMA 2020, 323, 386. [Google Scholar] [CrossRef]
- Kennedy, A.; Martinez, K.; Chuang, C.C.; LaPoint, K.; McIntosh, M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: Mechanisms of action and implications. J. Nutr. 2009, 139, 1–4. [Google Scholar] [CrossRef]
- Kim, H.K.; Kang, E.Y.; Go, G.W. Recent insights into dietary ω-6 fatty acid health implications using a systematic review. Food Sci. Biotechnol. 2022, 31, 1365–1376. [Google Scholar] [CrossRef]
- Schunck, W.H.; Konkel, A.; Fischer, R.; Weylandt, K.H. Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol. Ther. 2018, 183, 177–204. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Patel, B.C.; Tadi, P. Anatomy, Head and Neck: Eye Retina. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542332/ (accessed on 20 September 2025).
- Zou, R.; Cai, J.; Chen, T.; Mo, W.; Qian, H.; Zhu, X.; Zhang, L. High-fat diet alters retinal lipid composition and gene expression networks in mice. BMC Biol. 2025, 23, 103. [Google Scholar] [CrossRef]
- Jiang, S.; Xia, N.; Buonfiglio, F.; Böhm, E.W.; Tang, Q.; Pfeiffer, N.; Olinger, D.; Li, H.; Gericke, A. High-fat diet causes endothelial dysfunction in the mouse ophthalmic artery. Exp. Eye Res. 2024, 238, 109727. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, S.; Ng, T.K.; Liang, J.; Huang, S.; Deng, M.; Wu, Z.; Sun, Y.; Fu, C.; Pang, C.P.; et al. Lipid metabolism disorder promoting retinal structural and functional damage in ApoE−/− mice with age superposition. Acta Neuropathol. Commun. 2025, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Taskintuna, K.; Hum, J.; Gulati, J.; Olaya, S.; Steinman, J.; Golestaneh, N. PGC-1α repression dysregulates lipid metabolism and induces lipid droplet accumulation in retinal pigment epithelium. Cell Death Dis. 2024, 15, 385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chu, Y.; Mowery, J.; Konkel, B.; Galli, S.; Theos, A.C.; Golestaneh, N. Pgc-1α repression and high-fat diet induce age-related macular degeneration-like phenotypes in mice. Dis. Model Mech. 2018, 11, dmm032698. [Google Scholar] [CrossRef]
- Lim, L.S.; Gazzard, G.; Low, Y.L.; Choo, R.; Tan, D.T.; Tong, L.; Yin Wong, T.; Saw, S.M. Dietary factors, myopia, and axial dimensions in children. Ophthalmology 2010, 117, 993–997.e4. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Choi, Y.J. Nutritional intake, environmental factors, and their impact on myopia prevalence in Korean children aged 5–12 years. J. Health Popul. Nutr. 2024, 43, 14. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Zhao, F.; Xie, B.; Wu, H.; Zhang, S.; Ye, C.; Guan, Z.; Kang, L.; Zhang, Y.; Zhou, X.; et al. Dietary ω-3 polyunsaturated fatty acids are protective for myopia. Proc. Natl. Acad. Sci. USA 2021, 118, e2104689118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Zhang, Y.; Zhang, Y.J.; Yu, J.; Tang, F.Y.; Li, Y.; Yeung, S.; Kam, K.W.; Agrawal, K.; Loh, N.C.; et al. Dietary omega-3 polyunsaturated fatty acids as a protective factor of myopia: The Hong Kong Children Eye Study. Br. J. Ophthalmol. 2025, bjo-2024-326872. [Google Scholar] [CrossRef]
- Xue, C.C.; Li, H.; Dong, X.X.; Yu, M.; Soh, Z.D.; Chong, C.C.Y.; Jiang, C.; Choquet, H.; Zebardast, N.; Zekavat, S.M.; et al. Omega-3 polyunsaturated fatty acids as a protective factor for myopia. Am. J. Ophthalmol. 2024, 268, 368–377. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Z.; Wang, Y.; Wen, S.; Shi, Y.; Qu, J.; Lu, F.; Hu, L. Association between omega-3 polyunsaturated fatty acids and myopia: Results from the two-sample and multi-tissue genomic Mendelian randomization study and KNHANES. Food Sci. Nutr. 2025, 13, e70552. [Google Scholar] [CrossRef]
- Chun, Y.H.; Kim, H.R.; Han, K.; Park, Y.G.; Song, H.J.; Na, K.S. Total cholesterol and lipoprotein composition are associated with dry eye disease in Korean women. Lipids Health Dis. 2013, 12, 84. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Bu, J.; Tang, L.; Yang, Y.; Ouyang, W.; Lin, X.; Liu, Z.; Huang, C.; Quantock, A.J.; et al. High-fat diet induces dry eye-like ocular surface damages in murine. Ocul. Surf. 2020, 18, 267–276. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, X.; Yang, C.; Jiang, Y.; Chen, Y. Melatonin alleviates high-fat-diet-induced dry eye by regulating macrophage polarization via IFT27 and lowering ERK/JNK phosphorylation. iScience 2024, 27, 110367. [Google Scholar] [CrossRef]
- Walter, S.D.; Gronert, K.; McClellan, A.L.; Levitt, R.C.; Sarantopoulos, K.D.; Galor, A. ω-3 tear film lipids correlate with clinical measures of dry eye. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2472–2478. [Google Scholar] [CrossRef]
- Liu, Y.; Kam, W.R.; Sullivan, D.A. Influence of omega 3 and 6 fatty acids on human meibomian gland epithelial cells. Cornea 2016, 35, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Vazquez, J. Omega-3 fatty acid supplementation improves dry eye symptoms in patients with glaucoma: Results of a prospective multicenter study. Clin. Ophthalmol. 2016, 10, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Oleñik, A.; Jiménez-Alfaro, I.; Alejandre-Alba, N.; Mahillo-Fernández, I. A randomized, double-masked study to evaluate the effect of omega-3 fatty acids supplementation in meibomian gland dysfunction. Clin. Interv. Aging 2013, 8, 1133–1138. [Google Scholar] [CrossRef]
- Cortina, M.S.; He, J.; Li, N.; Bazan, N.G.; Bazan, H.E. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Investig. Ophthalmol. Vis. Sci. 2010, 51, 804–810. [Google Scholar] [CrossRef]
- Kenchegowda, S.; He, J.; Bazan, H.E. Involvement of pigment epithelium-derived factor, docosahexaenoic acid and neuroprotectin D1 in corneal inflammation and nerve integrity after refractive surgery. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 27–31. [Google Scholar] [CrossRef]
- Brignole-Baudouin, F.; Baudouin, C.; Aragona, P.; Rolando, M.; Labetoulle, M.; Pisella, P.J.; Barabino, S.; Siou-Mermet, R.; Creuzot-Garcher, C. A multicentre, double-masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega-6 fatty acids on a conjunctival inflammatory marker in dry eye patients. Acta Ophthalmol. 2011, 89, e591–e597. [Google Scholar] [CrossRef]
- Tsubota, K.; Fukagawa, K.; Fujihara, T.; Shimmura, S.; Saito, I.; Saito, K.; Takeuchi, T. Regulation of human leukocyte antigen expression in human conjunctival epithelium. Investig. Ophthalmol. Vis. Sci. 1999, 40, 28–34. [Google Scholar]
- Bhargava, R.; Pandey, K.; Ranjan, S.; Mehta, B.; Malik, A. Omega-3 fatty acids supplements for dry eye—Are they effective or ineffective? Indian J. Ophthalmol. 2023, 71, 1619–1625. [Google Scholar] [CrossRef]
- Park, J.; Yoo, Y.S.; Shin, E.; Han, G.; Shin, K.; Lim, D.H.; Chung, T.Y. Effects of the re-esterified triglyceride (rTG) form of omega-3 supplements on dry eye following cataract surgery. Br. J. Ophthalmol. 2021, 105, 1504–1509. [Google Scholar] [CrossRef]
- Hong, S.; Woo, M.; Eom, Y.; Kim, H.K.; Yoon, K.C.; Na, K.S.; Cho, K.J.; Lee, H.K.; Song, J.S. A multicenter, randomized, clinical trial assessing the effect of rTG-omega 3 supplementation on meibomian gland dysfunction patients after cataract surgery. J. Ocul. Pharmacol. Ther. 2025, 41, 65–74. [Google Scholar] [CrossRef]
- Goyal, P.; Jain, A.K.; Malhotra, C. Oral omega-3 fatty acid supplementation for laser in situ keratomileusis-associated dry eye. Cornea 2017, 36, 169–175. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Brodzka, S.; Baszyński, J.; Rektor, K.; Hołderna-Bona, K.; Stanek, E.; Kurhaluk, N.; Tkaczenko, H.; Malukiewicz, G.; Woźniak, A.; Kamiński, P. Immunogenetic and environmental factors in age-related macular disease. Int. J. Mol. Sci. 2024, 25, 6567. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Jiao, Z.; Chen, X.; Li, H.; Pan, M.; Liu, G. Association between body roundness index and age-related macular degeneration: A cross-sectional analysis of NHANES 2005–2008. Medicine 2025, 104, e44875. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, K.; Sandoval, H.; Yamamoto, S.; Jaiswal, M.; Sanz, E.; Li, Z.; Hui, J.; Graham, B.H.; Quintana, A.; et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 2015, 160, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, J.; Zheng, H.; Dong, Z. Correction: High-fat diet activates pyroptosis of retinal pigment epithelial cells in aged TgAPPswePS1 transgenic mice. Eur. J. Med. Res. 2025, 30, 724. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, Z.; Chen, Y.; Cen, X.; Zhang, H.; Chen, D. A high-fat plus high-sucrose diet induces age-related macular degeneration in an experimental rabbit model. Dis. Model Mech. 2024, 17, dmm052015. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Liang, Y.; Huang, J.; Fu, Y.; Chen, N.; Lu, B.; Zhao, C. Clearance of lipid droplets by chimeric autophagy-tethering compound ameliorates the age-related macular degeneration phenotype in mice lacking APOE. Autophagy 2023, 19, 2668–2681. [Google Scholar] [CrossRef]
- Gabrielle, P.H. Lipid metabolism and retinal diseases. Acta Ophthalmol. 2022, 100 (Suppl. S269), 3–43. [Google Scholar] [CrossRef]
- Tan, J.S.; Wang, J.J.; Flood, V.; Mitchell, P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: The Blue Mountains Eye Study. Arch. Ophthalmol. 2009, 127, 656–665. [Google Scholar] [CrossRef]
- Wu, J.; Cho, E.; Giovannucci, E.L.; Rosner, B.A.; Sastry, S.M.; Willett, W.C.; Schaumberg, D.A. Dietary intakes of eicosapentaenoic acid and docosahexaenoic acid and risk of age-related macular degeneration. Ophthalmology 2017, 124, 634–643. [Google Scholar] [CrossRef]
- Li, S.; Li, D.; Zhang, Y.; Teng, J.; Shao, M.; Cao, W. Association between serum lipids concentration and patients with age-related cataract in China: A cross-sectional, case-control study. BMJ Open 2018, 8, e021496. [Google Scholar] [CrossRef]
- Theodoropoulou, S.; Samoli, E.; Theodossiadis, P.G.; Papathanassiou, M.; Lagiou, A.; Lagiou, P.; Tzonou, A. Diet and cataract: A case-control study. Int. Ophthalmol. 2014, 34, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ghanavati, M.; Behrooz, M.; Rashidkhani, B.; Ashtray-Larky, D.; Zameni, S.D.; Alipour, M. Healthy eating index in patients with cataract: A case-control study. Iran. Red Crescent Med. J. 2015, 17, e22490. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Taylor, A.; Chylack, L.T.; Rogers, G.; Hankinson, S.E.; Willett, W.C.; Jacques, P.F. Dietary fat intake and early age-related lens opacities. Am. J. Clin. Nutr. 2005, 81, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Shengnan, Z.; Tao, W.; Yanan, Z.; Chao, S. Exploring the impact of diet, sleep, and metabolomic pathways on glaucoma sub-types: Insights from Mendelian randomization and cross-sectional analyses. Nutr. Metab. 2025, 22, 74. [Google Scholar] [CrossRef]
- López-Gil, J.F.; Fernandez-Montero, A.; Bes-Rastrollo, M.; Moreno-Galarraga, L.; Kales, S.N.; Martínez-González, M.Á.; Moreno-Montañés, J. Is ultra-processed food intake associated with a higher risk of glaucoma? A prospective cohort study including 19,255 participants from the SUN project. Nutrients 2024, 16, 1053. [Google Scholar] [CrossRef]
- Mehta, R.; Ray, R.M.; Tussing-Humphreys, L.M.; Pasquale, L.R.; Maki, P.; Haan, M.N.; Jackson, R.; Vajaranant, T.S. Effect of low-fat dietary modification on incident open-angle glaucoma. Ophthalmology 2023, 130, 565–574. [Google Scholar] [CrossRef]
- Coleman, A.L.; Stone, K.L.; Kodjebacheva, G.; Yu, F.; Pedula, K.L.; Ensrud, K.E.; Cauley, J.A.; Hochberg, M.C.; Topouzis, F.; Badala, F.; et al. Glaucoma risk and the consumption of fruits and vegetables among older women in the study of osteoporotic fractures. Am. J. Ophthalmol. 2008, 145, 1081–1089. [Google Scholar] [CrossRef]
- Mylona, I.; Chourdakis, M.; Makedou, K.; Tzamalis, A.; Dermenoudi, M.; Tsinopoulos, I. The role of nutrition in primary open angle glaucoma: A multivariate analysis. J. Am. Coll. Nutr. 2020, 39, 438–442. [Google Scholar] [CrossRef]
- Pérez de Arcelus, M.; Toledo, E.; Martínez-González, M.Á.; Sayón-Orea, C.; Gea, A.; Moreno-Montañés, J. Omega 3:6 ratio intake and incidence of glaucoma: The SUN cohort. Clin. Nutr. 2014, 33, 1041–1045. [Google Scholar] [CrossRef]
- Kang, J.H.; Pasquale, L.R.; Willett, W.C.; Rosner, B.A.; Egan, K.M.; Faberowski, N.; Hankinson, S.E. Dietary fat consumption and primary open-angle glaucoma. Am. J. Clin. Nutr. 2004, 79, 755–764. [Google Scholar] [CrossRef]
- Edokpa, G.D.; McFarlane, S.R. Glaucoma and dietary intake: A scoping review. Front. Nutr. 2024, 11, 1497366. [Google Scholar] [CrossRef]
- Ramel, A.; Martinez, J.A.; Kiely, M.; Bandarra, N.M.; Thorsdottir, I. Effects of weight loss and seafood consumption on inflammation parameters in young, overweight and obese European men and women during 8 weeks of energy restriction. Eur. J. Clin. Nutr. 2010, 64, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Kinouchi, R.; Ishiko, S.; Hanada, K.; Hayashi, H.; Mikami, D.; Tani, T.; Zenimaru, T.; Kawai, M.; Nakabayashi, S.; Kinouchi, M.; et al. A low meat diet increases the risk of open-angle glaucoma in women—The results of population-based, cross-sectional study in Japan. PLoS ONE 2018, 13, e0204955. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.P.; Rouland, J.F.; Bron, A.; Sellem, E.; Nordmann, J.P.; Baudouin, C.; Denis, P.; Villain, M.; Chaine, G.; Colin, J.; et al. Nutritional, lifestyle and environmental factors in ocular hypertension and primary open-angle glaucoma: An exploratory case-control study. Acta Ophthalmol. 2013, 91, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.E.; Tseng, V.L.; Yu, F.; Caprioli, J.; Coleman, A.L. Association of dietary fatty acid intake with glaucoma in the United States. JAMA Ophthalmol. 2018, 136, 141–147. [Google Scholar] [CrossRef]
- Downie, L.E.; Vingrys, A.J. Oral omega-3 supplementation lowers intraocular pressure in normotensive adults. Transl. Vis. Sci. Technol. 2018, 7, 1. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Yang, Y.J. Comparison of food and nutrient intake according to the presence of glaucoma among Korean older adults. Nutr. Res. Pract. 2024, 18, 701–710. [Google Scholar] [CrossRef]
- Kalogerou, M.; Kolovos, P.; Prokopiou, E.; Papagregoriou, G.; Deltas, C.; Malas, S.; Georgiou, T. Omega-3 fatty acids protect retinal neurons in the DBA/2J hereditary glaucoma mouse model. Exp. Eye Res. 2018, 167, 128–139. [Google Scholar] [CrossRef]
- Luo, J.; Tu, S.; Li, K.; Yang, R.; Lin, Y.; Deng, J.; Chen, X.; Ge, J. Preliminary evaluation on the effect of oral omega-3 supplementation from herring caviar oil in primary open-angle glaucoma patients. Int. Ophthalmol. 2025, 45, 305. [Google Scholar] [CrossRef]
- Tourtas, T.; Birke, M.T.; Kruse, F.E.; Welge-Lüssen, U.C.; Birke, K. Preventive effects of omega-3 and omega-6 fatty acids on peroxide mediated oxidative stress responses in primary human trabecular meshwork cells. PLoS ONE 2012, 7, e31340. [Google Scholar] [CrossRef]
- Ren, H.; Magulike, N.; Ghebremeskel, K.; Crawford, M. Primary open-angle glaucoma patients have reduced levels of blood docosahexaenoic and eicosapentaenoic acids. Prostaglandins Leukot. Essent. Fatty Acids 2006, 74, 157–163. [Google Scholar] [CrossRef] [PubMed]
| Eye Condition | Study (Year) | Poulation/Design | Formulation and Dose | Duration | Primary Outcome | Main Findings |
|---|---|---|---|---|---|---|
| DES | Tellez-Vazquez (2016) [23] | Glaucoma patients with dry eye (n = 536), multicenter prospective trial | 1680 mg EPA + 560 mg DHA per day (fish oil) | 3 months | OSDI score, tear breakup time (TBUT) | Significant improvement in symptoms and TBUT |
| Brignole-Baudouin et al. (2011) [27] | Dry eye (n = 145), double-masked RCT | Omega-3/6 blend (2 g/day) | 6 months | Conjunctival HLA-DR expression | Reduced inflammatory markers | |
| Bhargava et al. (2023) [29] | Dry eye (n = 300), RCT | 1000 mg omega-3/day | 12 weeks | Schirmer test, OSDI | No significant clinical improvement | |
| Park et al. (2021) [30] | Post-cataract patients (n = 102), RCT | Re-esterified TG (rTG) omega-3, 2000 mg/day | 8 weeks | TBUT, corneal staining | Improved tear stability and corneal integrity | |
| Hong et al. (2025) [31] | MGD after cataract surgery (n = 120), RCT | rTG omega-3 (1680 mg EPA + 560 mg DHA) | 12 weeks | Lipid layer thickness, MG function | Improved meibomian gland function | |
| Myopia | Pan et al. (2021) [14] | Experimental & clinical models | Dietary ω-3 PUFA | Variable | Axial length | ω-3 intake protective against myopia progression |
| Zhang et al. (2025) [15] | Hong Kong Children Eye Study (n ≈ 4000), cross-sectional | Dietary ω-3 intake | - | Refractive error, axial length | Higher ω-3 intake associated with lower myopia risk | |
| Xue et al. (2024) [16] | Genomic MR and epidemiologic data | Genetic instrument for ω-3 PUFA | - | Myopia risk | ω-3 inversely associated with myopia prevalence | |
| Glaucoma | Downie & Vingrys (2018) [59] | Healthy adults (n = 105), RCT | 3000 mg omega-3/day | 3 months | Intraocular pressure (IOP) | Reduced IOP by ~8% |
| Luo et al. (2025) [62] | POAG patients (n = 80), RCT | Herring caviar oil (2000 mg EPA + DHA/day) | 6 months | IOP, visual field | Modest IOP reduction and visual field trend | |
| Pérez de Arcelus et al. (2014) [52] | SUN cohort (n = 19,255), prospective | Dietary ω-3:ω-6 ratio | 6.5 years | Incident glaucoma | Higher ω-3:ω-6 ratio linked to lower glaucoma risk | |
| Age-Related Macular Degeneration/Retinal Health | Wu et al. (2017) [42] | Prospective cohort (n = 75,889) | Dietary EPA + DHA intake | 12 years | AMD incidence | Higher intake associated with reduced AMD risk |
| Schunck et al. (2018) [5] | Mechanistic review | - | - | Mechanistic insights | ω-3 metabolites (EETs) modulate inflammation, vascular tone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieńczykowska, K.; Bryl, A.; Mrugacz, M. The Impact of a High-Fat Diet on Eye Health. Nutrients 2025, 17, 3271. https://doi.org/10.3390/nu17203271
Pieńczykowska K, Bryl A, Mrugacz M. The Impact of a High-Fat Diet on Eye Health. Nutrients. 2025; 17(20):3271. https://doi.org/10.3390/nu17203271
Chicago/Turabian StylePieńczykowska, Kamila, Anna Bryl, and Małgorzata Mrugacz. 2025. "The Impact of a High-Fat Diet on Eye Health" Nutrients 17, no. 20: 3271. https://doi.org/10.3390/nu17203271
APA StylePieńczykowska, K., Bryl, A., & Mrugacz, M. (2025). The Impact of a High-Fat Diet on Eye Health. Nutrients, 17(20), 3271. https://doi.org/10.3390/nu17203271






