The Anti-Digestive Characteristics, Effects of Prebiotic Properties on NC and T2DM Mice of Achyranthes bidentata Polysaccharide, and the Hypoglycemic Effect of Its Fermentation Products
Abstract
1. Introduction
1.1. The Pharmacological Relevance of ABP
1.2. The Importance of Digestive Characteristics for Bioactivity
1.3. The Role of the Gut Microbiota in Polysaccharide Metabolism
1.4. The Objectives of This Study
2. Materials and Methods
2.1. Materials and Reagents
2.2. ABP Extraction
2.3. In Vitro Digestion
2.3.1. Saliva Digestion
2.3.2. Gastric Digestion
2.3.3. Small Intestine Digestion
2.4. In Vitro Fermentation
2.4.1. Fermentation Medium
2.4.2. In Vitro Fermentation Experiment
2.5. Animal Experiments
2.5.1. Effect of ABP on Gut Microbiota in NC Mice
2.5.2. Effect of ABP on Gut Microbiota in T2DM Mice
2.6. Cell Experiments
2.6.1. Caco-2 Cell Cultures
2.6.2. Cell Proliferation
2.6.3. Effect on Glucose Uptake and of α-Glucosidase Activity
2.7. Related Indexes Detection
2.7.1. Physicochemical Properties of ABP
2.7.2. Determination of SCFAs
2.7.3. Gut Microbiota Analysis
2.8. Statistical Analysis
3. Results
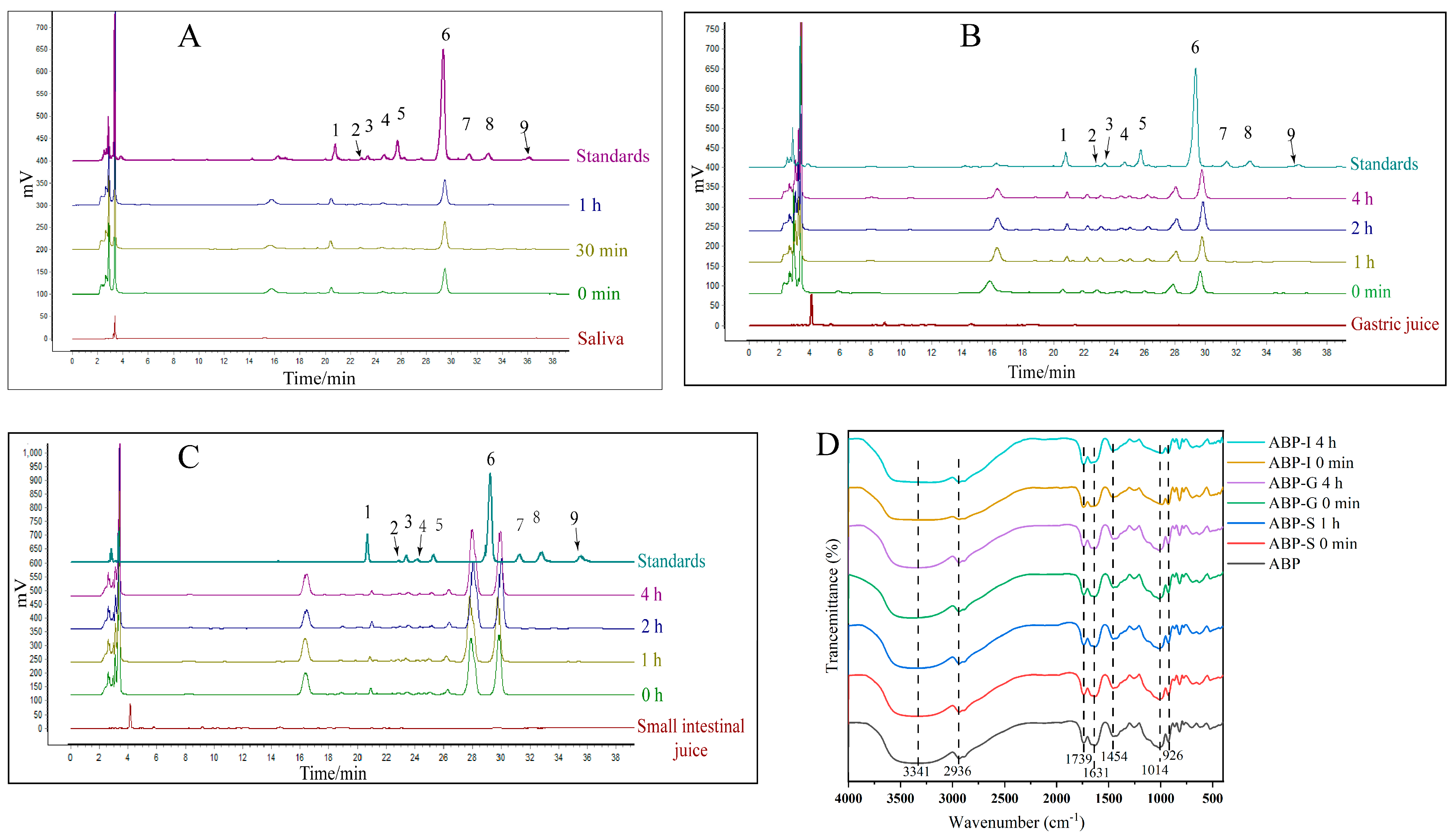
3.1. In Vitro Digestion of ABP
3.1.1. Saliva Digestion
3.1.2. Gastric Digestion
3.1.3. Small Intestine Digestion
3.2. In Vitro Fermentation of ABP
3.2.1. Relative MW and Total Carbohydrate Contents
3.2.2. pH and SCFA Contents
3.3. The Effects of ABP on Regulating Gut Microbiota
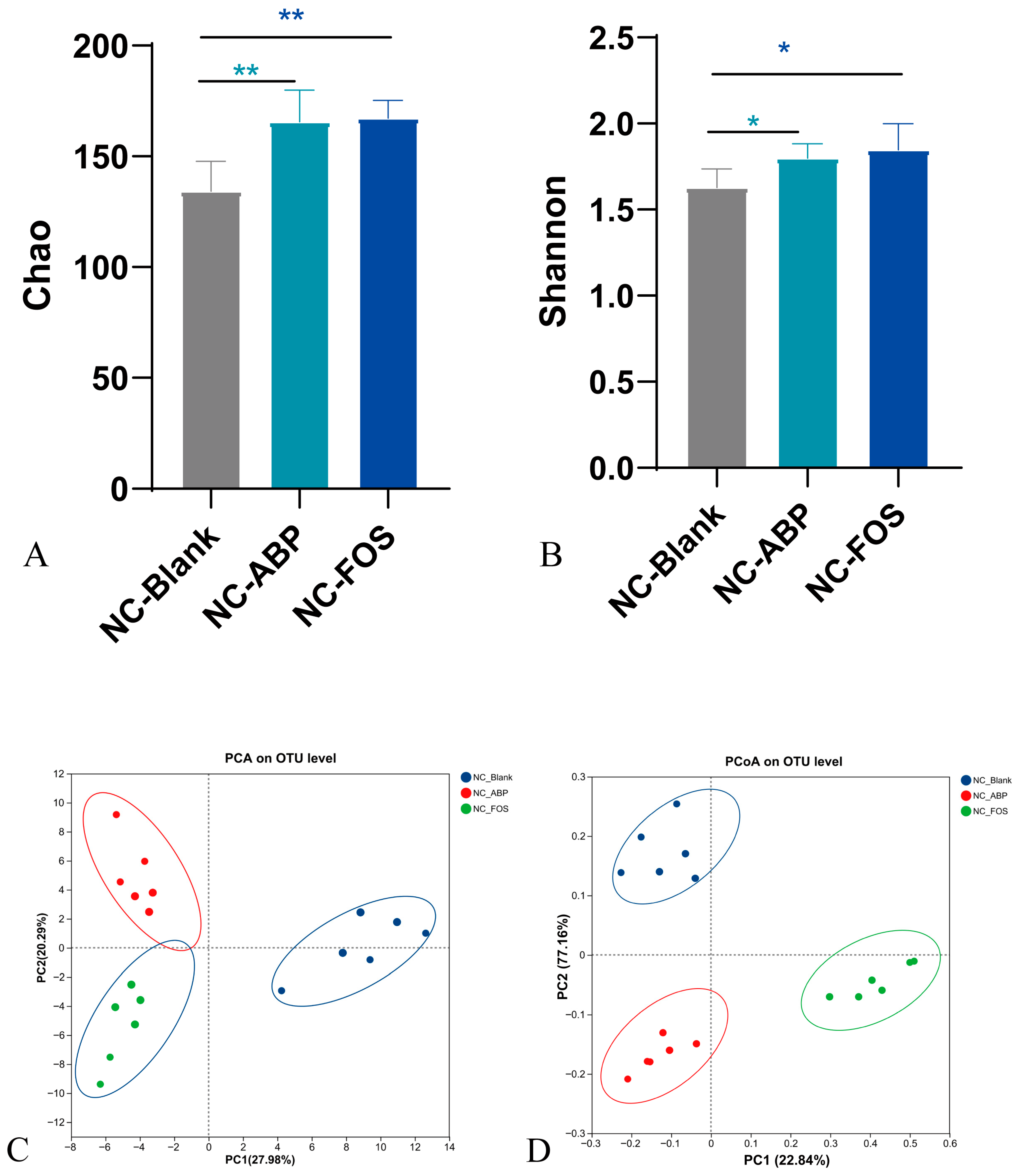
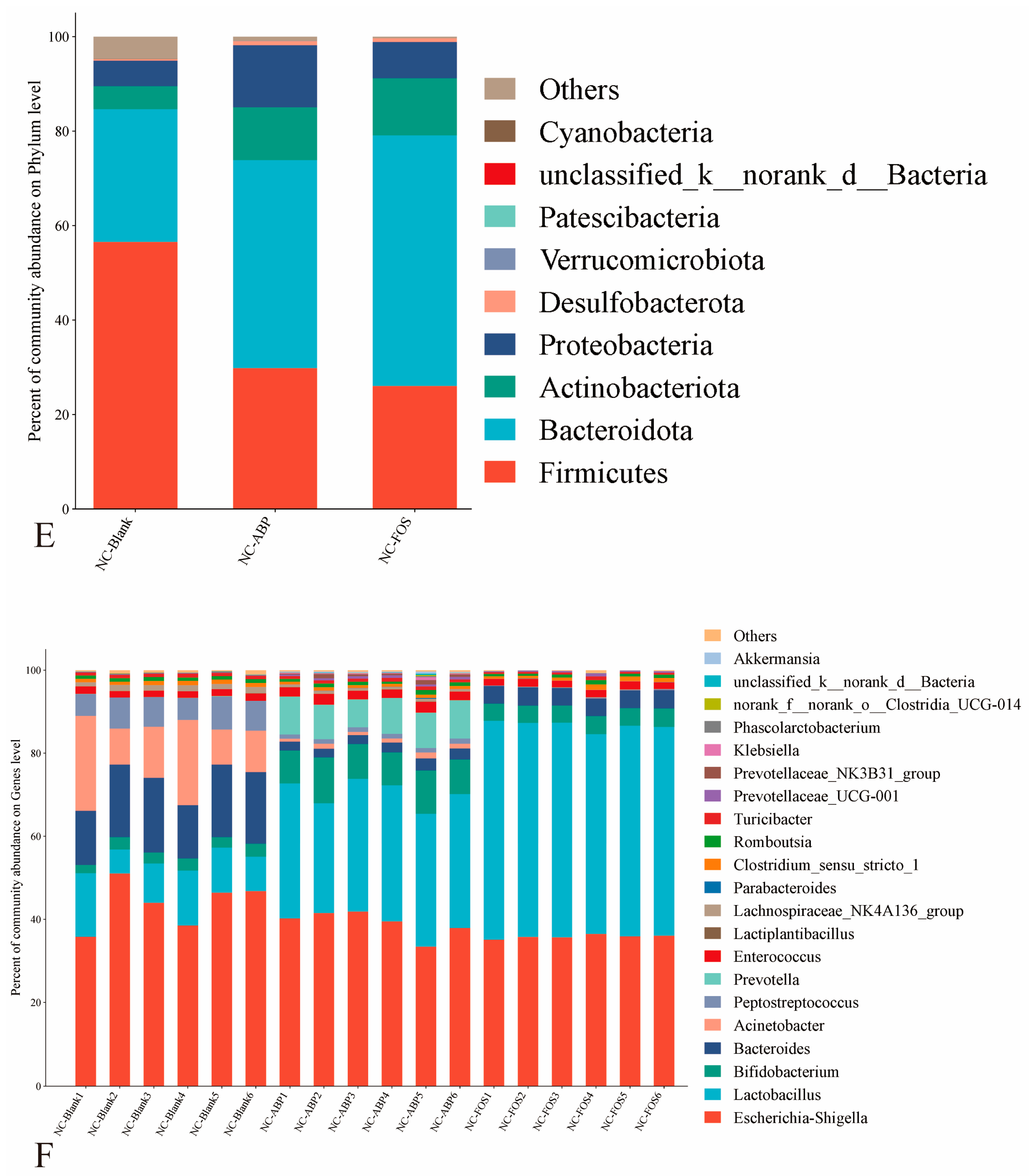
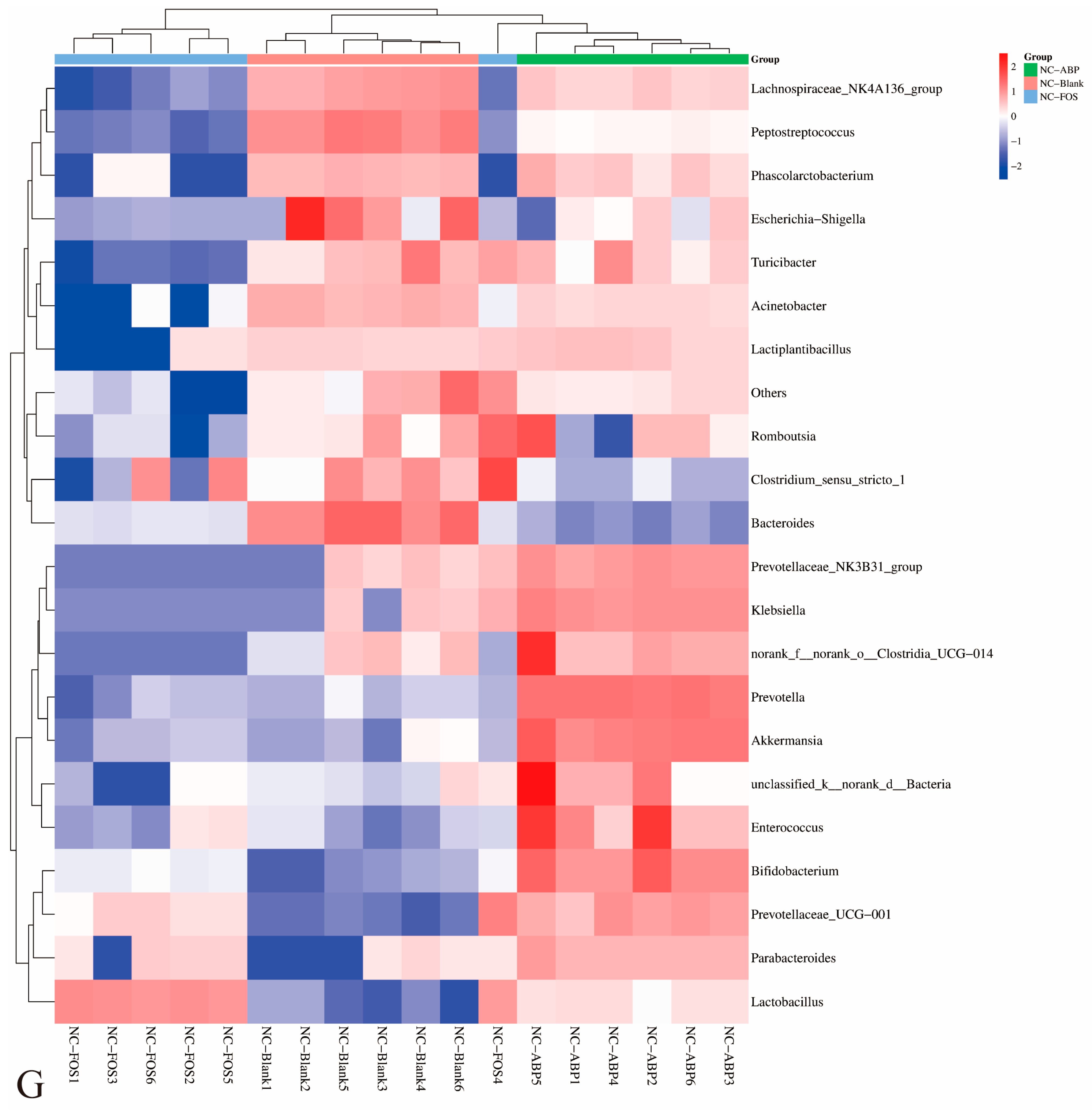
3.3.1. The Effect of ABP on the Gut Microbiota of NC Mice
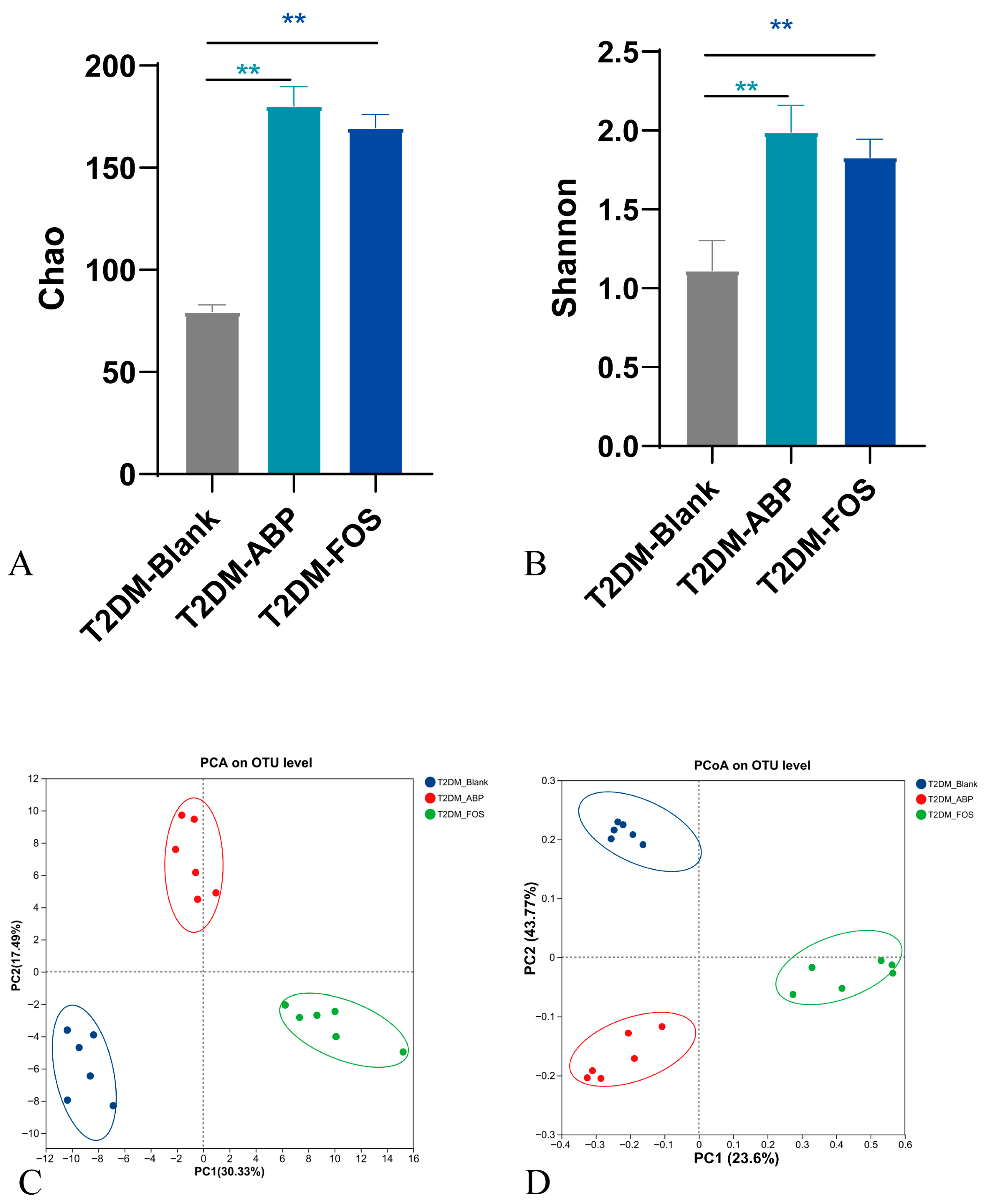
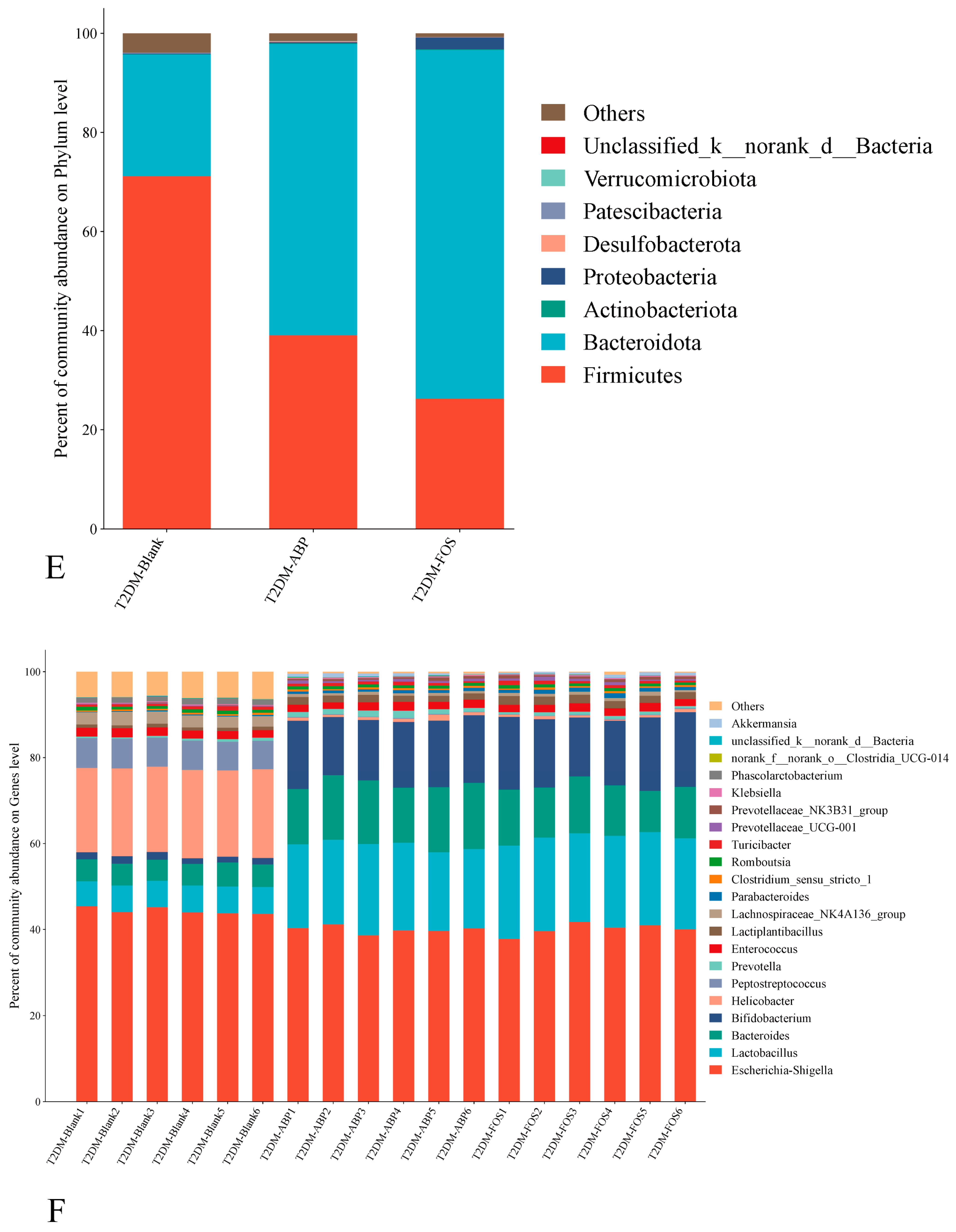
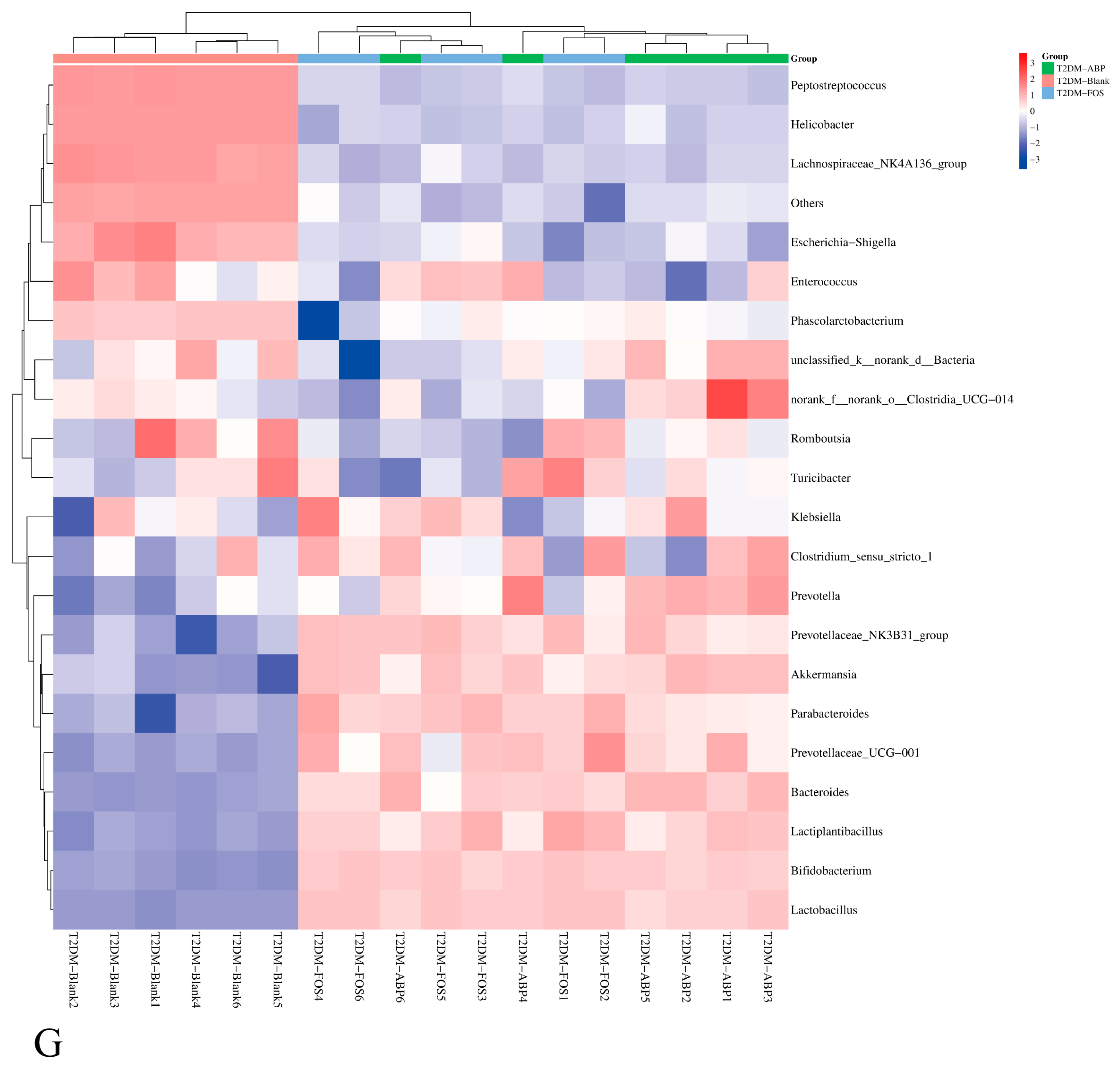
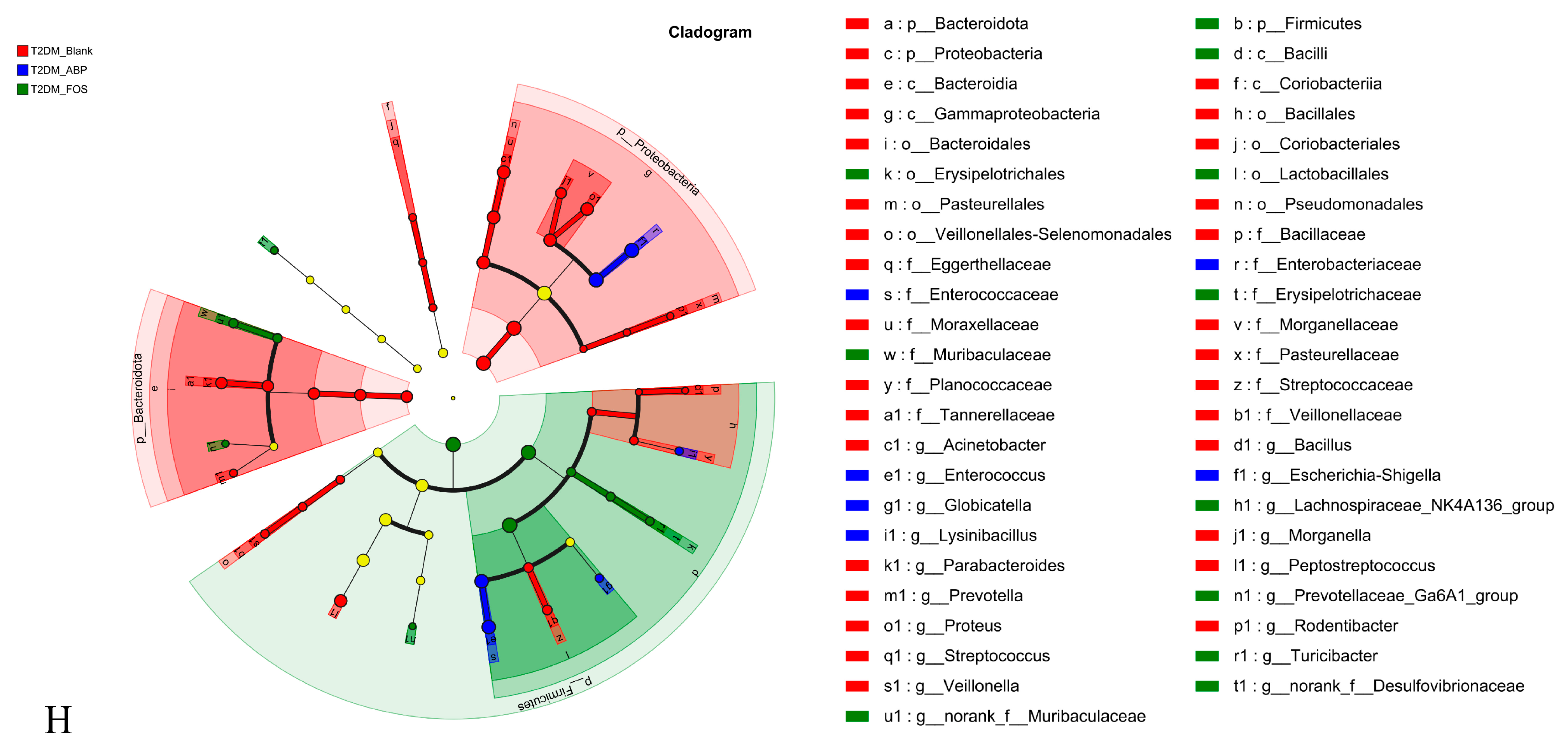
3.3.2. The Effect of ABP on the Gut Microbiota of T2DM Mice
3.3.3. Comparison of the Effects of ABP on Gut Microbiota and SCFAs in NC and T2DM Mice
3.4. Hypoglycemic Effect of ABPF on Caco-2 Cells
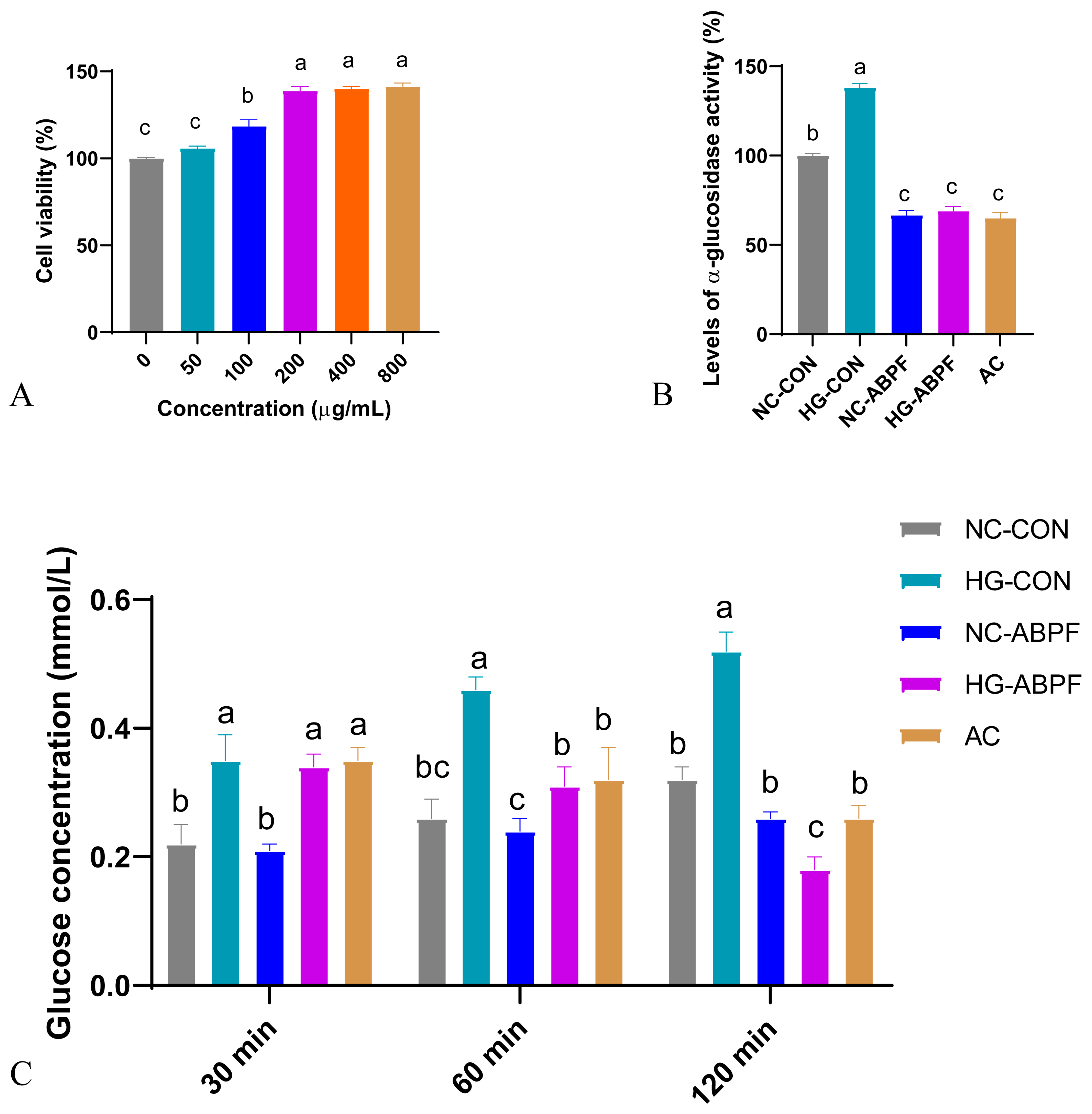
3.4.1. Effect of ABPF on Cell Viability
3.4.2. Effect of ABPF on Glucose Uptake and α-Glucosidase Activity
4. Discussion
4.1. Digestive Resistance of ABP
4.2. Microbial Fermentation and SCFAs
4.3. Gut Microbiota Modulation
4.4. Comparison Between NC and T2DM Mice
4.5. Implications for Glucose Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 16S rRNA | 16S ribosomal RNA |
| PMP | 1-phenyl-3 methyl-5-pyrazolone |
| ABPF | ABP fermentation samples |
| T2DM-ABP group | ABP group of T2DM mice |
| AC | Acarbose |
| ABP | Achyrantha bidentata polysaccharide |
| AB | Achyranthes bidentata |
| NC-Blank group | Blank group of NC mice |
| T2DM-Blank group | Blank group of T2DM mice |
| FBG | Fasting blood sugar |
| T2DM-FOS group | FOS group of T2DM mice |
| FT-IR | Fourier transform infrared spectroscopy |
| FOS | Fructooligosaccharides |
| GC-MS | Gas chromatography-mass spectrometry |
| ABP-G | Gastric-digested ABP |
| HG-Caco-2 | High-glucose Caco-2 cells |
| HG-CON | HG-Caco-2 cells treated with standard medium |
| HG-ABPF | HG- Caco-2 cells treated with ABPF medium |
| HSFD | High-sugar and high-fat diet |
| MW | Molecular weight |
| NC | Normal control |
| NC- Caco-2 | Normal Caco-2 cells |
| NC-CON | NC-Caco-2 cells treated with standard medium |
| NC-ABPF | NC-Caco-2 cells treated with ABPF medium |
| NCVP | Nostoc Commune Vauch polysaccharides |
| PBS | Phosphate buffer saline |
| PCA | Principal component analysis |
| PCoA | Principal coordinate analysis |
| ABP-S | Saliva-digested ABP |
| SCFAs | Short-chain fatty acids |
| ABP-I | Small intestinal-digested ABP |
| TCA | Trichloroacetic acid |
| T2DM | Type 2 diabetes mellitus |
References
- Fu, C.; Qiu, Z.; Huang, Y.; Lin, Q.; Jin, L.; Tu, H.; Ye, J.; Zheng, C.; Zhong, W.; Ma, D. Achyranthes bidentata polysaccharides alleviate endoplasmic reticulum stress in osteoarthritis via lncRNA NEAT1/miR-377-3p pathway. Biomed. Pharmacother. 2022, 154, 113551. [Google Scholar] [CrossRef]
- Lei, Y.Y.; Ye, Y.H.; Liu, Y.; Xu, J.L.; Zhang, C.L.; Lyu, C.M.; Feng, C.G.; Jiang, Y.; Yang, Y.; Ke, Y. Achyranthes bidentata polysaccharides improve cyclophosphamide-induced adverse reactions by regulating the balance of cytokines in helper T cells. Int. J. Biol. Macromol. 2024, 265 Pt 2, 130736. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Chen, Y.; Hu, D.; Yao, S.; Yang, J.; Wen, X. A graminan type fructan from Achyranthes bidentata prevents the kidney injury in diabetic mice by regulating gut microbiota. Carbohydr. Polym. 2024, 339, 122275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Zhang, Q.; Wang, C.; Zhang, D.; Wan, J.B.; Yan, C. UPLC/Q-TOF-MS-based metabolomics study of the anti-osteoporosis effects of Achyranthes bidentata polysaccharides in ovariectomized rats. Int. J. Biol. Macromol. 2018, 112, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; He, W.; Luo, Z.Y.; Wang, K.X.; Tan, X.M. Achyranthes bidentata polysaccharide ameliorates type 2 diabetes mellitus by gut microbiota-derived short-chain fatty acids-induced activation of the GLP-1/GLP-1R/cAMP/PKA/CREB/INS pathway. Int. J. Biol. Macromol. 2024, 270 Pt 2, 132256. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular modification of polysaccharides and resulting bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef]
- Yu, Y.H.; Wu, L.B.; Liu, X.; Zhao, L.C.; Li, L.Q.; Jin, M.Y.; Yu, X.; Liu, F.; Li, Y.; Li, L.; et al. In vitro simulated digestion and fermentation characteristics of pectic polysaccharides from fresh passion fruit (Passiflora edulis f. Flavicarpa L.) peel. Food Chem. 2024, 452, 139606. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Liu, Y.; Li, W.; Niu, A.; Ren, P.; Liu, Y.; Jiang, C.; Inam, M.; Guan, L. Effects of in vitro digestion and fermentation of Nostoc commune Vauch. Polysaccharides on properties and gut microbiota. Carbohydr. Polym. 2022, 281, 119055. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Z.; Lin, Y.; Zheng, B.; Zhang, Y.; Pan, L. In vitro dynamic digestion properties and fecal fermentation of Dictyophora indusiata polysaccharide: Structural characterization and gut microbiota. Int. J. Biol. Macromol. 2024, 282, 136713. [Google Scholar] [CrossRef]
- Fan, L.; Xia, Y.; Wang, Y.; Han, D.; Liu, Y.; Li, J.; Fu, J.; Wang, L.; Gan, Z.; Liu, B.; et al. Gut microbiota bridges dietary nutrients and host immunity. Sci. China Life Sci. 2023, 66, 2466–2514. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Cheng, H.; Wang, H.; Tan, Y.; Feng, W.; Peng, C. Interactions between polysaccharides and gut microbiota: A metabolomic and microbial review. Food Res. Int. 2022, 160, 111653. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef]
- Xu, N.; Zhou, Y.; Lu, X.; Chang, Y. Auricularia auricula-judae (Bull.) polysaccharides improve type 2 diabetes in HFD/STZ-induced mice by regulating the AKT/AMPK signaling pathways and the gut microbiota. J. Food Sci. 2021, 86, 5479–5494. [Google Scholar] [CrossRef] [PubMed]
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. In vitro digestion by saliva, simulated gastric and small intestinal juices and fermentation by human fecal microbiota of sulfated polysaccharides from Gracilaria rubra. J. Funct. Foods 2018, 40, 18–27. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Q.; Fu, X.; Liu, R.H. In vitro fermentation of mulberry fruit polysaccharides by human fecal inocula and impact on microbiota. Food Funct. 2016, 7, 4637–4643. [Google Scholar] [CrossRef]
- Jiao, W.; Sang, Y.; Wang, X.; Wang, S. Effects of 6-Shogaol on glucose uptake and intestinal barrier integrity in caco-2 cells. Foods 2023, 12, 503. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, M.; Liu, L.; Li, D.; Zhao, L.; Wu, Z.; Zhou, M.; Jia, L.; Yang, F. Cordyceps militaris polysaccharide alleviates diabetic symptoms by regulating gut microbiota against TLR4/NF-kappaB pathway. Int. J. Biol. Macromol. 2023, 230, 123241. [Google Scholar] [CrossRef]
- Yang, Q.; Chang, S.L.; Tian, Y.M.; Li, W.; Ren, J.L. Glucan polysaccharides isolated from Lactarius hatsudake Tanaka mushroom: Structural characterization and in vitro bioactivities. Carbohydr. Polym. 2024, 337, 122171. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, C.; Zhang, P.; Ai, C.; Song, S. Digestion characteristics of polysaccharides from Gracilaria lemaneiformis and its interaction with the human gut microbiota. Int. J. Biol. Macromol. 2022, 213, 305–316. [Google Scholar] [CrossRef]
- Hu, J.L.; Nie, S.P.; Min, F.F.; Xie, M.Y. Artificial simulated saliva, gastric and intestinal digestion of polysaccharide from the seeds of Plantago asiatica L. Carbohydr. Polym. 2013, 92, 1143–1150. [Google Scholar]
- Li, X.; Guo, R.; Wu, X.; Liu, X.; Ai, L.; Sheng, Y.; Song, Z.; Wu, Y. Dynamic digestion of tamarind seed polysaccharide: Indigestibility in gastrointestinal simulations and gut microbiota changes in vitro. Carbohydr. Polym. 2020, 239, 116194. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Ye, K.; Ma, S.; Du, H.; Chen, S.; Liu, D.; Ma, G.; Xiao, H. Simulated gastrointestinal digestion and gut microbiota fermentation of polysaccharides from Agaricus bisporus. Food Chem. 2023, 418, 135849. [Google Scholar] [CrossRef]
- Hays, K.E.; Pfaffinger, J.M.; Ryznar, R. The interplay between gut microbiota, short-chain fatty acids, and implications for host health and disease. Gut Microbes 2024, 16, 2393270. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cooper, D.E.; Cluntun, A.A.; Warmoes, M.O.; Zhao, S.; Reid, M.A.; Liu, J.; Lund, P.J.; Lopes, M.; Garcia, B.A.; et al. Acetate production from glucose and coupling to mitochondrial metabolism in mammals. Cell 2018, 175, 502–513. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, M.; Liu, Y.; Wei, F.; Li, X.; Feng, Y.; Jin, X.; Liu, D.; Guo, Y.; Hu, Y. Inulin-enriched Megamonas funiformis ameliorates metabolic dysfunction-associated fatty liver disease by producing propionic acid. npj Biofilms Microbiomes 2023, 9, 84. [Google Scholar] [CrossRef]
- Mei, X.; Li, Y.; Zhang, X.; Zhai, X.; Yang, Y.; Li, Z.; Li, L. Maternal phlorizin intake protects offspring from maternal Obesity-Induced metabolic disorders in mice via targeting gut microbiota to activate the SCFA-GPR43 pathway. J. Agric. Food Chem. 2024, 72, 4703–4725. [Google Scholar] [CrossRef]
- Luo, J.; Liang, S.; Jin, F. Gut microbiota and healthy longevity. Sci. China Life Sci. 2024, 67, 2590–2602. [Google Scholar] [CrossRef]
- Feng, J.; Qian, Y.; Zhou, Z.; Ertmer, S.; Vivas, E.I.; Lan, F.; Hamilton, J.J.; Rey, F.E.; Anantharaman, K.; Venturelli, O.S. Polysaccharide utilization loci in Bacteroides determine population fitness and community-level interactions. Cell Host Microbe 2022, 30, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Lacombe-Harvey, M.E.; Brzezinski, R.; Beaulieu, C. Chitinolytic functions in actinobacteria: Ecology, enzymes, and evolution. Appl. Microbiol. Biotechnol. 2018, 102, 7219–7230. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Fan, C.J.; Wang, Y.J.; Liu, Z.L.; Wang, J.Q.; Nie, S.P. Structural characterization and in vitro fermentation of the polysaccharide from fruits of Gardenia jasminoides. Int. J. Biol. Macromol. 2025, 309 Pt 1, 142678. [Google Scholar] [CrossRef]
- Fu, Q.; Tian, M.; Yang, Y.; Zhu, Y.; Zhou, H.; Tan, J.; Wang, J.; Huang, Q. Paotianxiong polysaccharides potential prebiotics: Structural analysis and prebiotic properties. Food Chem. 2024, 451, 139499. [Google Scholar] [CrossRef]
- Chen, S.; Han, P.; Zhang, Q.; Liu, P.; Liu, J.; Zhao, L.; Guo, L.; Li, J. Lactobacillus brevis alleviates the progress of hepatocellular carcinoma and type 2 diabetes in mice model via interplay of gut microflora, bile acid and NOTCH 1 signaling. Front. Immunol. 2023, 14, 1179014. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, F.; Meng, M.; Chen, Q.; Yang, Y.; Wang, W.; Xie, H.; Li, X.; Gu, W.; Yu, J. Effects of the synbiotic composed of mangiferin and Lactobacillus reuteri 1-12 on type 2 diabetes mellitus rats. Front. Microbiol. 2023, 14, 1158652. [Google Scholar] [CrossRef]
- Zhu, X.X.; Zhao, C.Y.; Meng, X.Y.; Yu, X.Y.; Ma, L.C.; Chen, T.X.; Chang, C.; Chen, X.Y.; Zhang, Y.; Hou, B.; et al. Bacteroides uniformis Ameliorates Carbohydrate and Lipid Metabolism Disorders in Diabetic Mice by Regulating Bile Acid Metabolism via the Gut-Liver Axis. Pharmaceuticals 2024, 17, 1015. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Neyrinck, A.M.; Backhed, F.; Cani, P.D. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nat. Rev. Endocrinol. 2011, 7, 639–646. [Google Scholar] [CrossRef]
- Niu, H.; Zhou, M.; Ji, A.; Zogona, D.; Wu, T.; Xu, X. Molecular Mechanism of Pasteurized Akkermansia muciniphila in Alleviating Type 2 Diabetes Symptoms. J. Agric. Food Chem. 2024, 72, 13083–13098. [Google Scholar] [CrossRef]
- Dong, L.; Xu, Z.; Huang, G.; Zhang, R.; Deng, M.; Huang, F.; Su, D. Lychee Pulp-Derived dietary Fiber-Bound phenolic complex upregulates the SCFAs-GPRs-ENS pathway and aquaporins in Loperamide-Induced constipated mice by reshaping gut microbiome. J. Agric. Food Chem. 2023, 71, 15087–15096. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Liang, B.; Wang, Y.; Gao, H.; Fang, S.; Xie, K.; Tan, J. The regulatory effect of polysaccharides on the gut microbiota and their effect on human health: A review. Int. J. Biol. Macromol. 2024, 270 Pt 2, 132170. [Google Scholar] [CrossRef] [PubMed]



| Reagents | Saliva | Gastric Fluid | Small Intestinal Fluid |
|---|---|---|---|
| NaCl | 12.7 mg | 310.0 mg | 0.2 g |
| KCl | 6.5 g | 110.0 mg | 25.0 mg |
| NaHCO3 | 6.2 g | 60.0 mg | - |
| NaSO4 | 4.1 g | - | - |
| Na2HPO4 | 6.5 g | - | - |
| KSCN | 1.5 g | - | - |
| CaCl2·2H2O | - | 15.0 mg | 12.7.0 mg |
| pH | 7.0 | 3.0 | 7.0 |
| Distilled water | 100 mL | 100 mL | 100 mL |
| Others | Urea: 1.8 g; α-amylase: 21.1 mg | Pepsase: 23.6 mg; Gastric lipase: 25.0 mg | Pancreatin: 2.7 g; Bile salt: 3.1 g |
| Process | Digestion Time (h) | MW (Da) | Sugar Content (mg/mL) |
|---|---|---|---|
| Saliva digestion | 0 | 41,083 ± 25 a | 0.08 ± 0.005 a |
| 0.5 | 41,003 ± 16 a | 0.10 ± 0.008 ab | |
| 1 | 40,662 ± 43 b | 0.13 ± 0.010 bc | |
| Gastric digestion | 0 | 40,642 ± 40 b | 0.13 ± 0.011 bc |
| 1 | 40,405 ± 19 c | 0.16 ± 0.008 cd | |
| 2 | 39,592 ± 32 d | 0.19 ± 0.015 d | |
| 4 | 39,562 ± 48 d | 0.18 ± 0.010 d | |
| Small intestinal digestion | 0 | 39,511 ± 32 d | 0.19 ± 0.009 d |
| 1 | 39,538 ± 35 d | 0.19 ± 0.012 d | |
| 2 | 39,525 ± 27 d | 0.18 ± 0.015 d | |
| 4 | 39,573 ± 28 d | 0.19 ± 0.011 d |
| Fermentation Time | MW (Da) | Residual Carbohydrate (%) | |
|---|---|---|---|
| ABP | FOS | ||
| 0 h | 39,573 ± 32 a | 100 a | 100 a |
| 6 h | 20,378 ± 31 b | 71.8 ± 2.3 b | 69.8 ± 1.6 b |
| 12 h | 15,842 ± 25 c | 68.4 ± 2.2 bc | 65.3 ± 2.2 bc |
| 24 h | 12,258 ± 22 d | 66.3 ± 2.7 bc | 61.3 ± 2.5 c |
| 48 h | 10,983 ± 24 e | 63.1 ± 1.5 c | 54.1 ± 6.2 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, T.; Liu, Z.; Ding, W.; Deng, L.; Ning, X.; Feng, J. The Anti-Digestive Characteristics, Effects of Prebiotic Properties on NC and T2DM Mice of Achyranthes bidentata Polysaccharide, and the Hypoglycemic Effect of Its Fermentation Products. Nutrients 2025, 17, 3249. https://doi.org/10.3390/nu17203249
Xia T, Liu Z, Ding W, Deng L, Ning X, Feng J. The Anti-Digestive Characteristics, Effects of Prebiotic Properties on NC and T2DM Mice of Achyranthes bidentata Polysaccharide, and the Hypoglycemic Effect of Its Fermentation Products. Nutrients. 2025; 17(20):3249. https://doi.org/10.3390/nu17203249
Chicago/Turabian StyleXia, Ting, Zhenjie Liu, Wenya Ding, Liting Deng, Xinyang Ning, and Jianfang Feng. 2025. "The Anti-Digestive Characteristics, Effects of Prebiotic Properties on NC and T2DM Mice of Achyranthes bidentata Polysaccharide, and the Hypoglycemic Effect of Its Fermentation Products" Nutrients 17, no. 20: 3249. https://doi.org/10.3390/nu17203249
APA StyleXia, T., Liu, Z., Ding, W., Deng, L., Ning, X., & Feng, J. (2025). The Anti-Digestive Characteristics, Effects of Prebiotic Properties on NC and T2DM Mice of Achyranthes bidentata Polysaccharide, and the Hypoglycemic Effect of Its Fermentation Products. Nutrients, 17(20), 3249. https://doi.org/10.3390/nu17203249






