Sesaminol Inhibits Adipogenesis by Suppressing Mitotic Clonal Expansion and Activating the Nrf2-ARE Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sesaminol from Defatted Sesame Cake
2.2. Cell Culture
2.3. Cell Viability Assay (Neutral Red Method)
2.4. Measurement of Triacylglycerol (TG) Accumulation
2.5. Analysis of Glycerol-3-Phosphate Dehydrogenase (GPDH) Activity
2.6. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.7. Western Blotting
2.8. Measurement of Intracellular ROS Production
2.9. Measurement of Nuclear Translocation of Nrf2
2.10. Immunofluorescence Microscopy
2.11. DNA Content Measurement
2.12. Statistical Analysis
3. Results
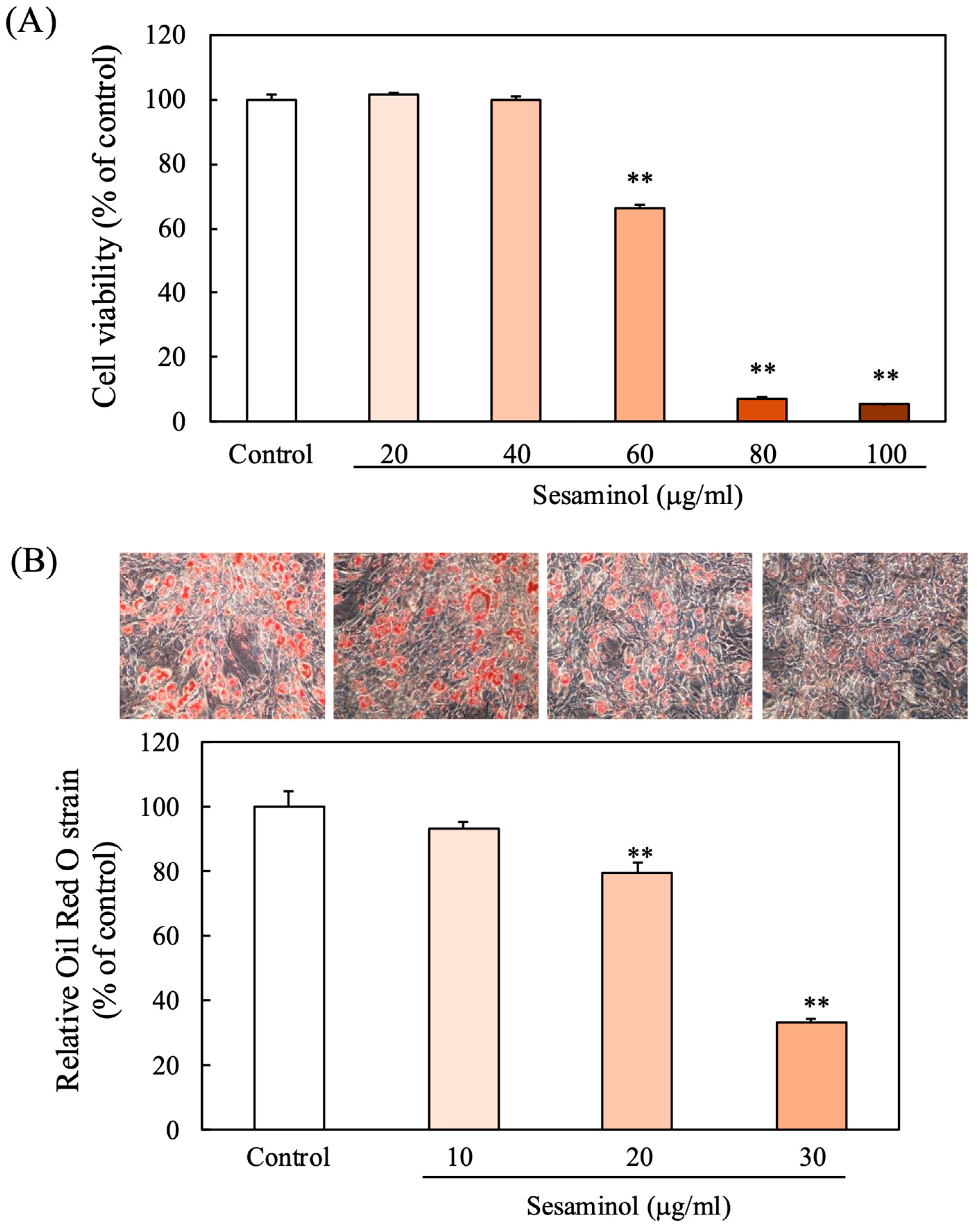
3.1. Effect of Sesaminol on the Viability of 3T3-L1 Preadipocytes
3.2. Effect of Sesaminol on Lipid Accumulation in 3T3-L1 Preadipocytes
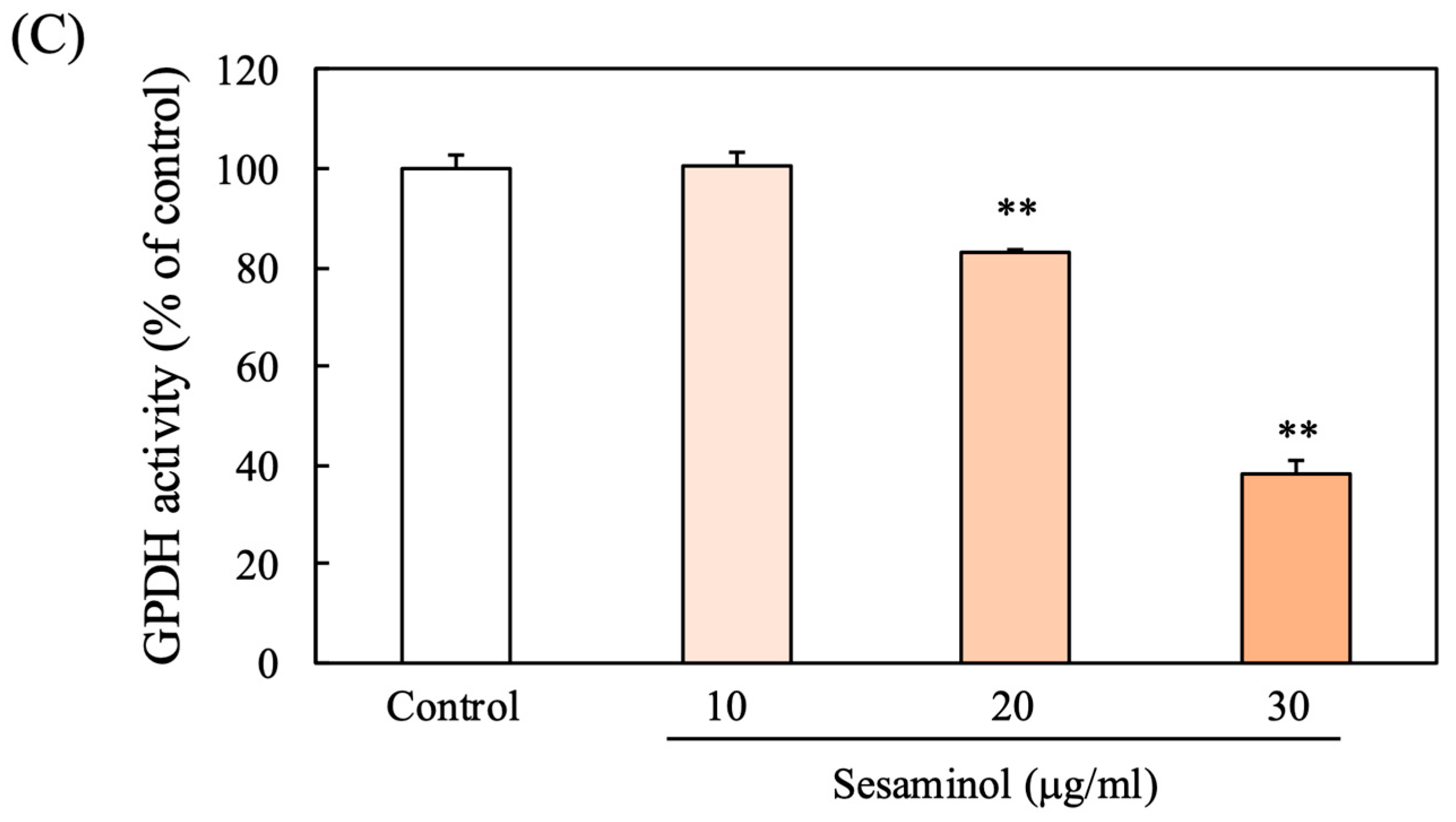
3.3. Effect of Sesaminol on GPDH Activity in 3T3-L1 Preadipocytes
3.4. Effect of Sesaminol at Different Stages of Adipogenesis
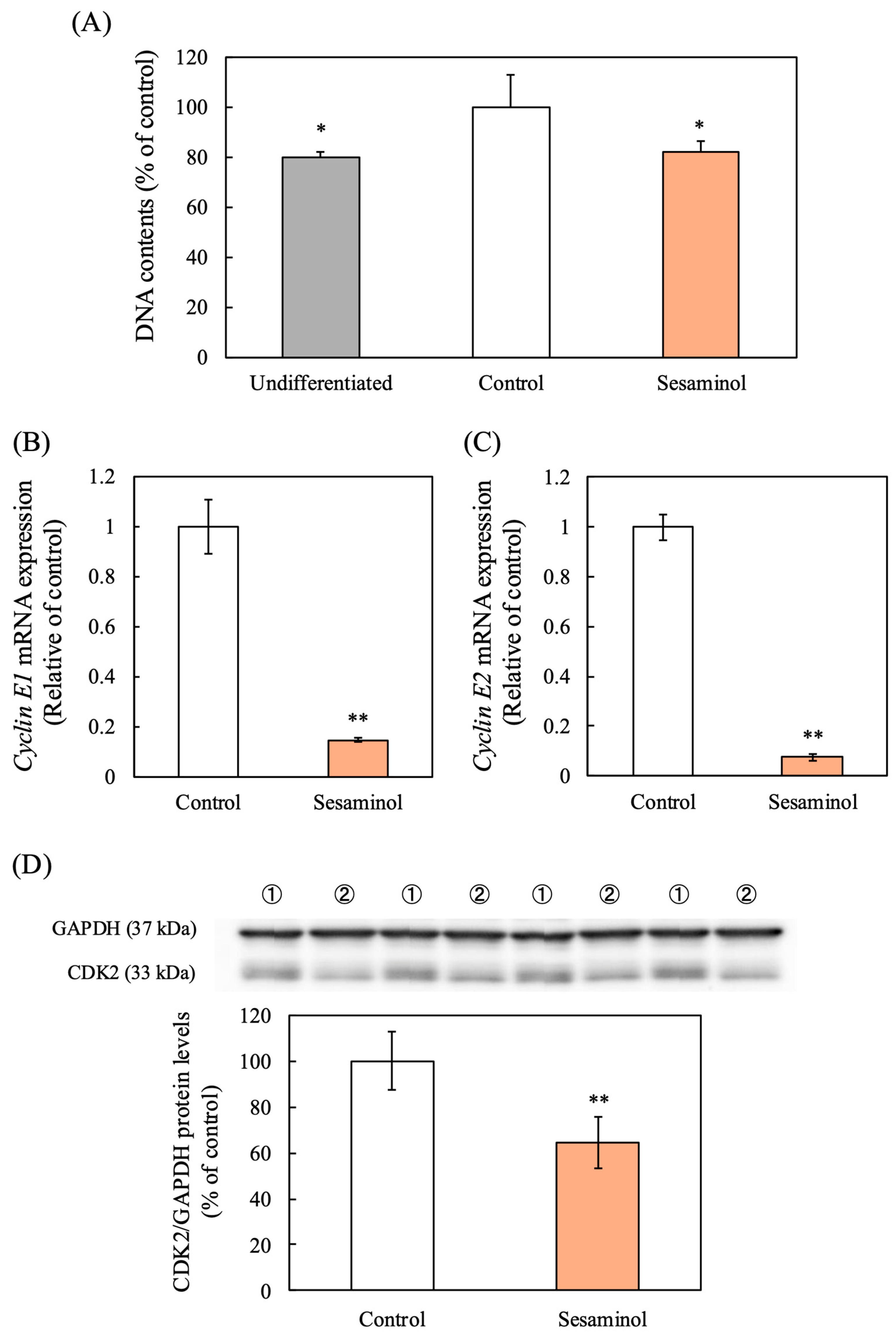
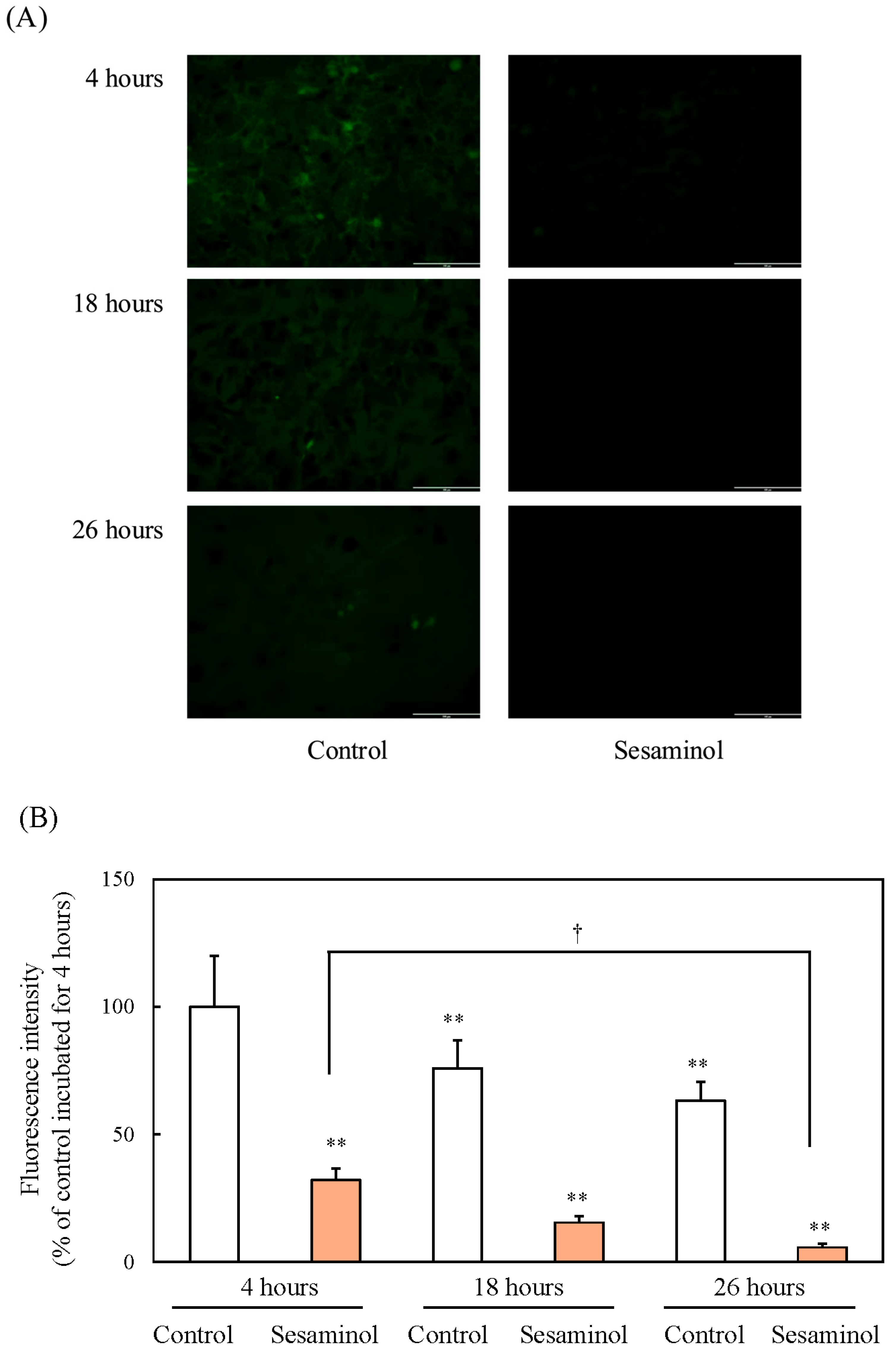
3.5. Effect of Sesaminol on DNA Synthesis in 3T3-L1 Adipocytes
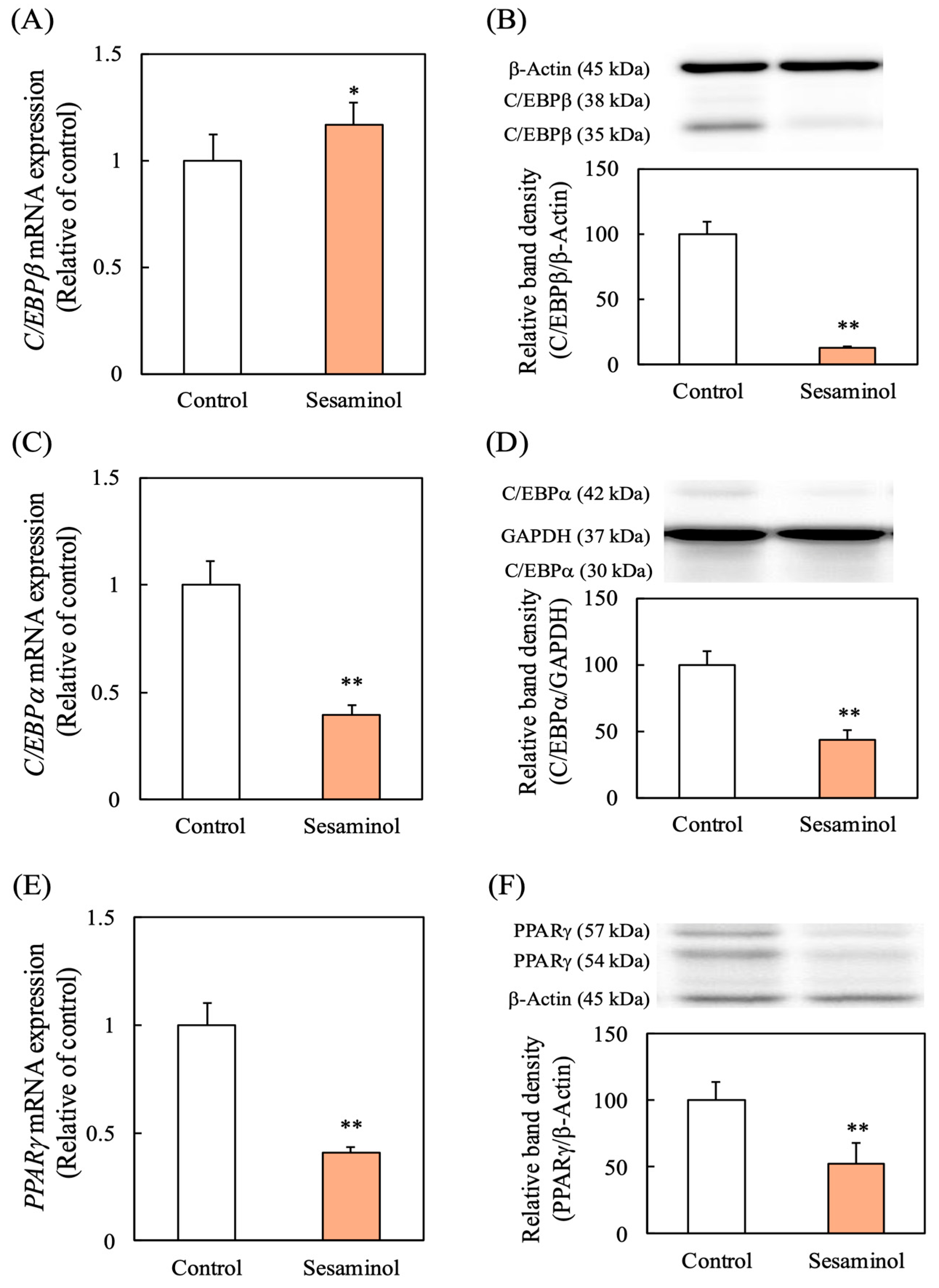
3.6. Effect of Sesaminol on Key Transcription Factors in Adipocyte Differentiation
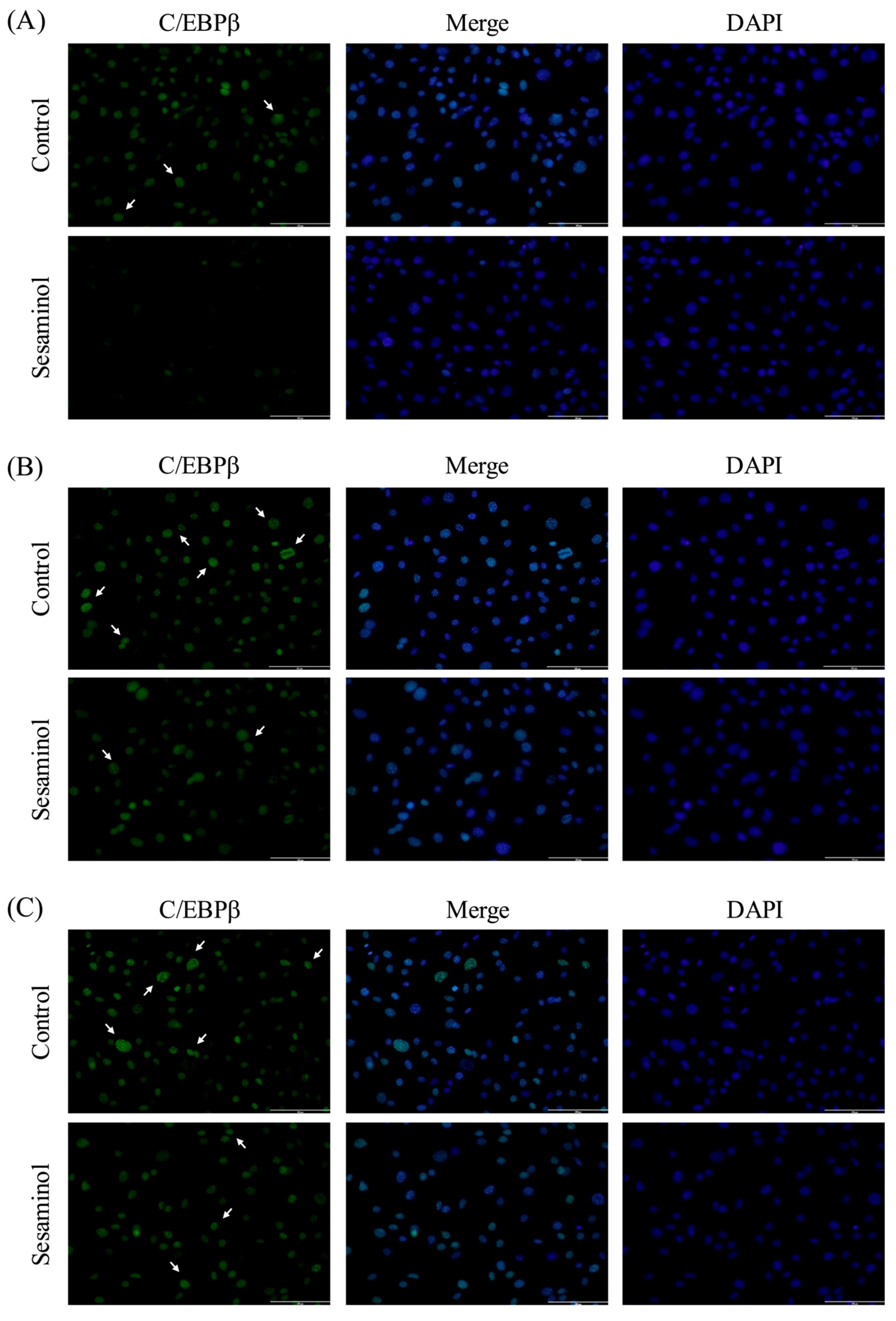
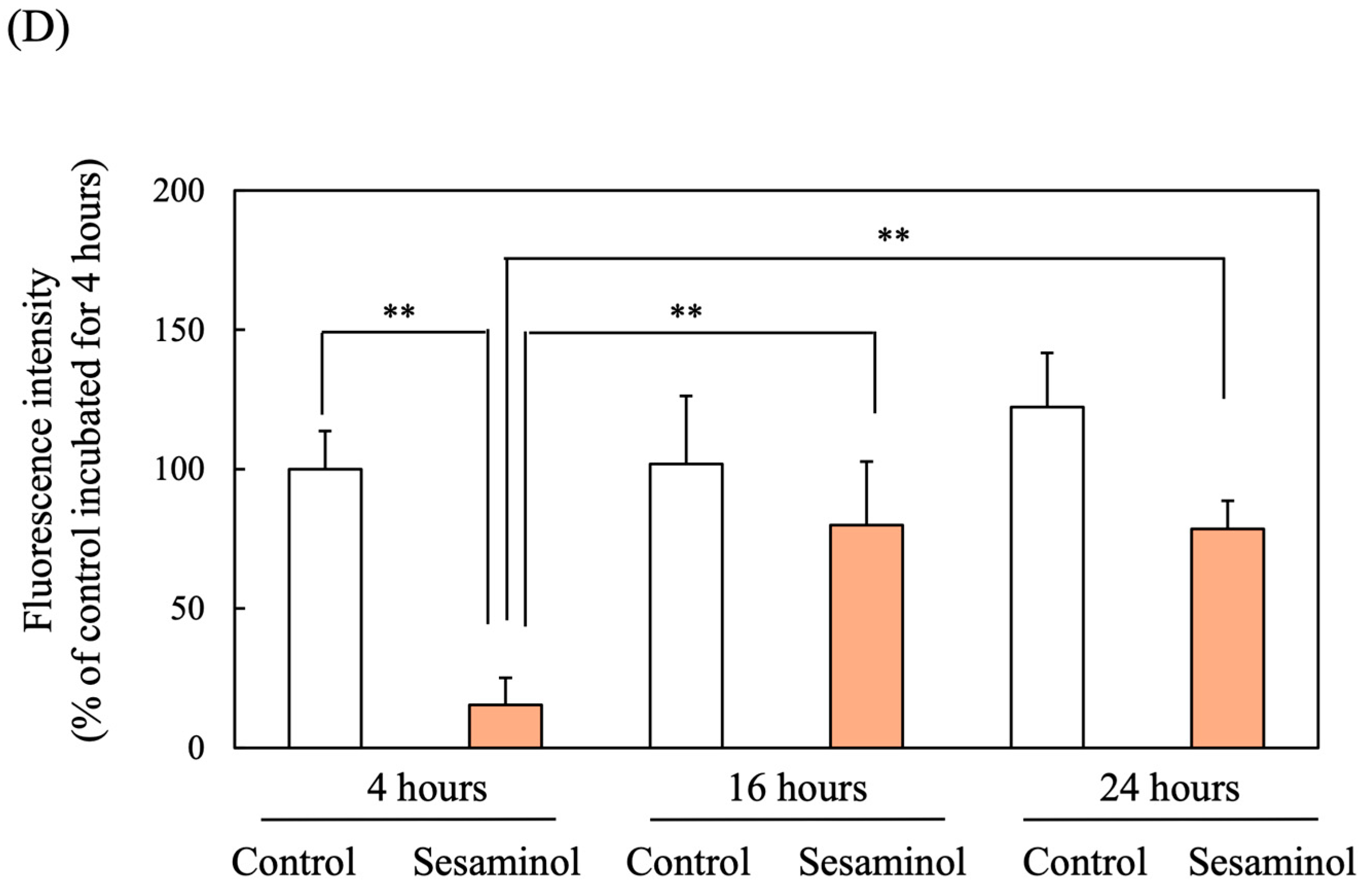
3.7. Effect of Sesaminol on C/EBPβ Localization to the Centromere
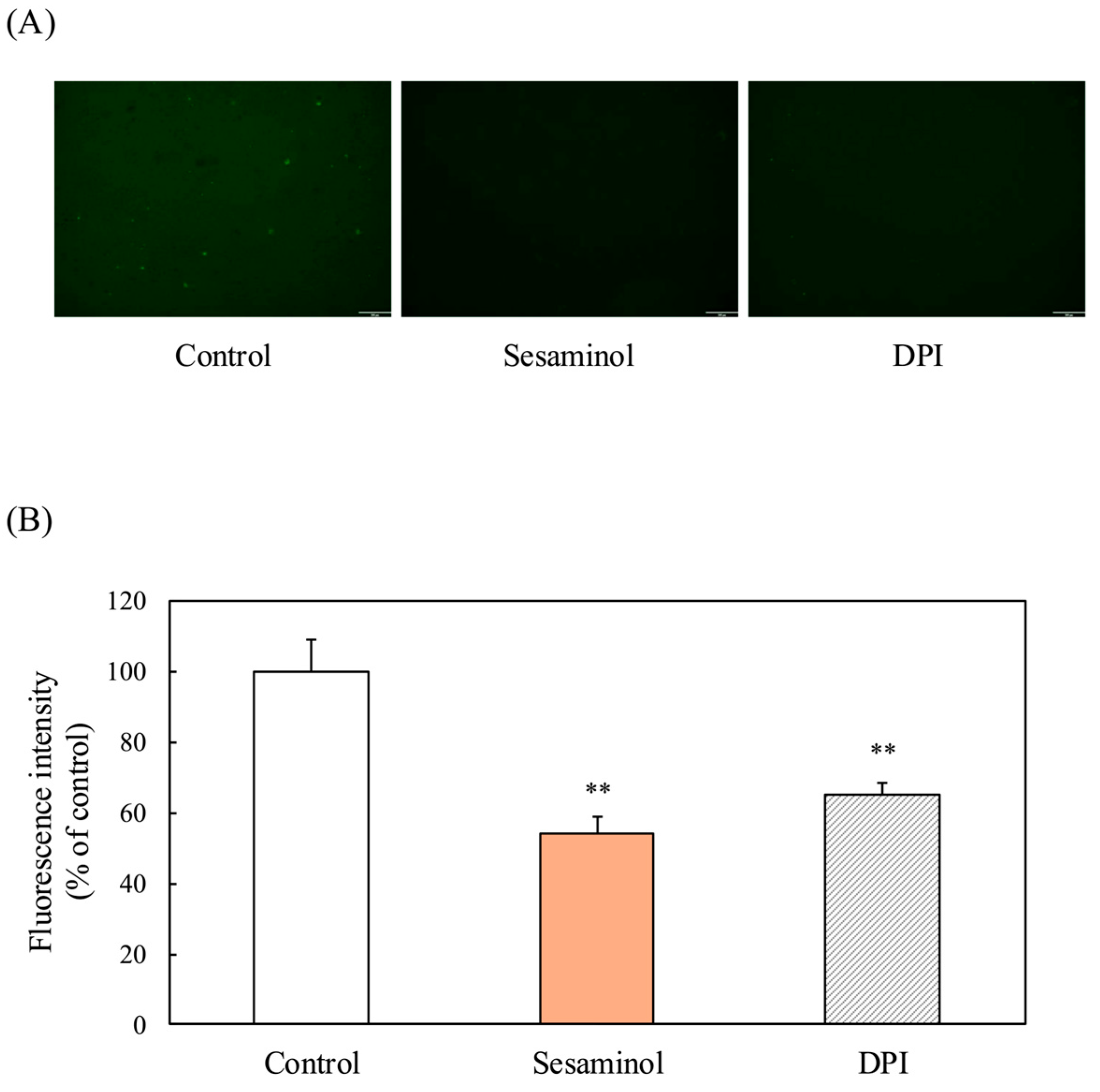
3.8. Effect of Sesaminol on Intracellular ROS Production in 3T3-L1 Preadipocytes
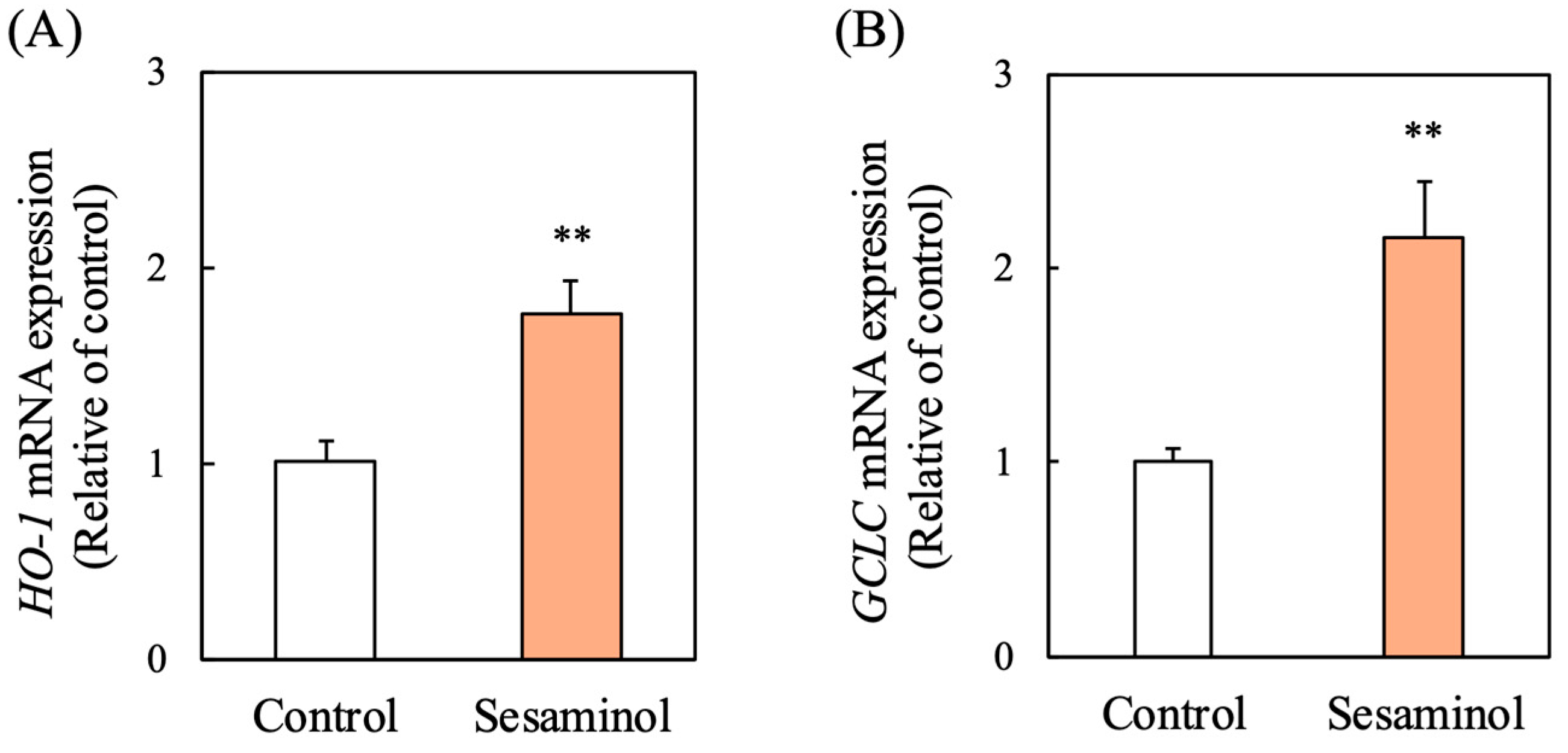
3.9. Effect of Sesaminol on Antioxidant Gene Expression
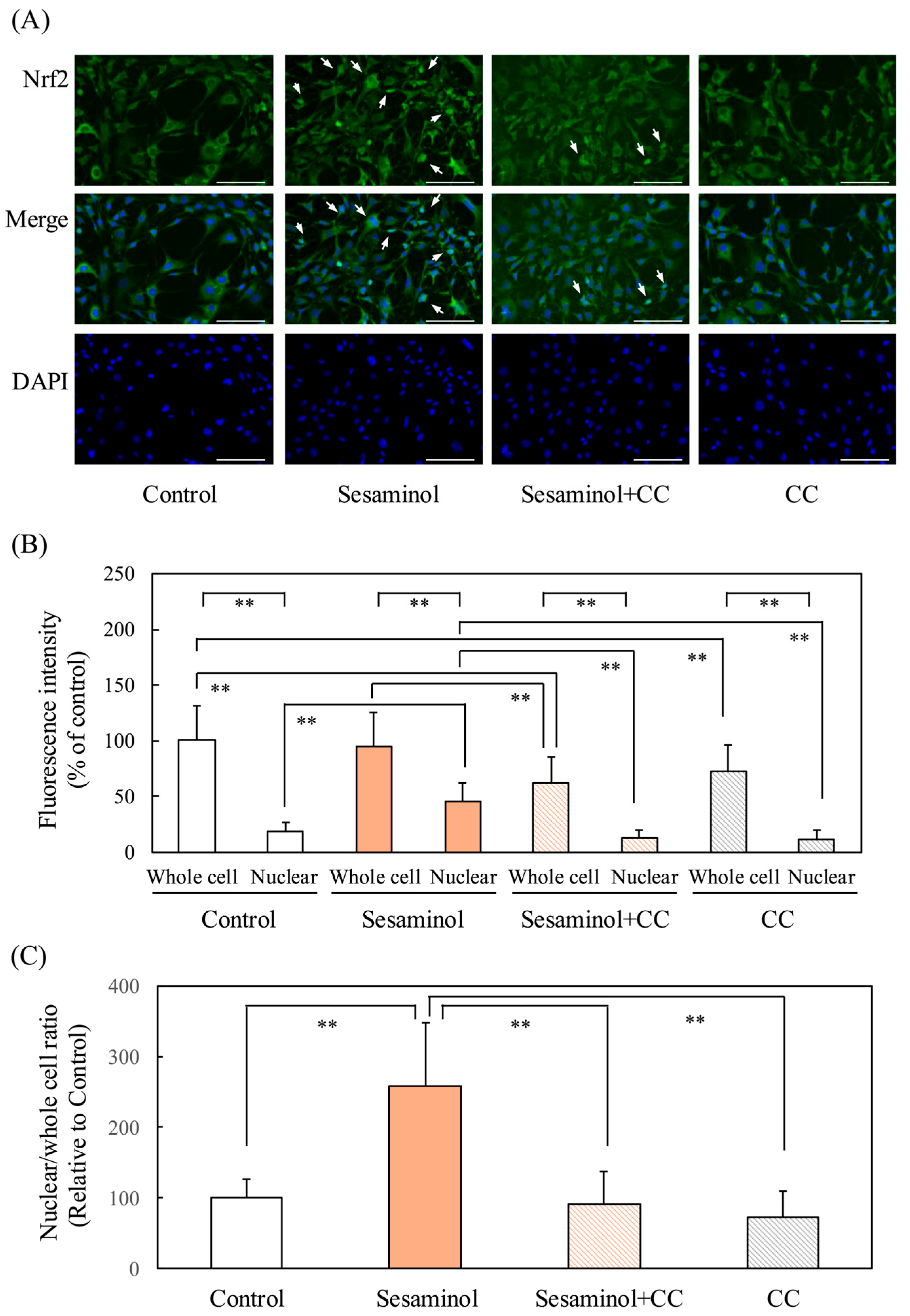
3.10. Effect of Sesaminol on Nrf2 Nuclear Translocation
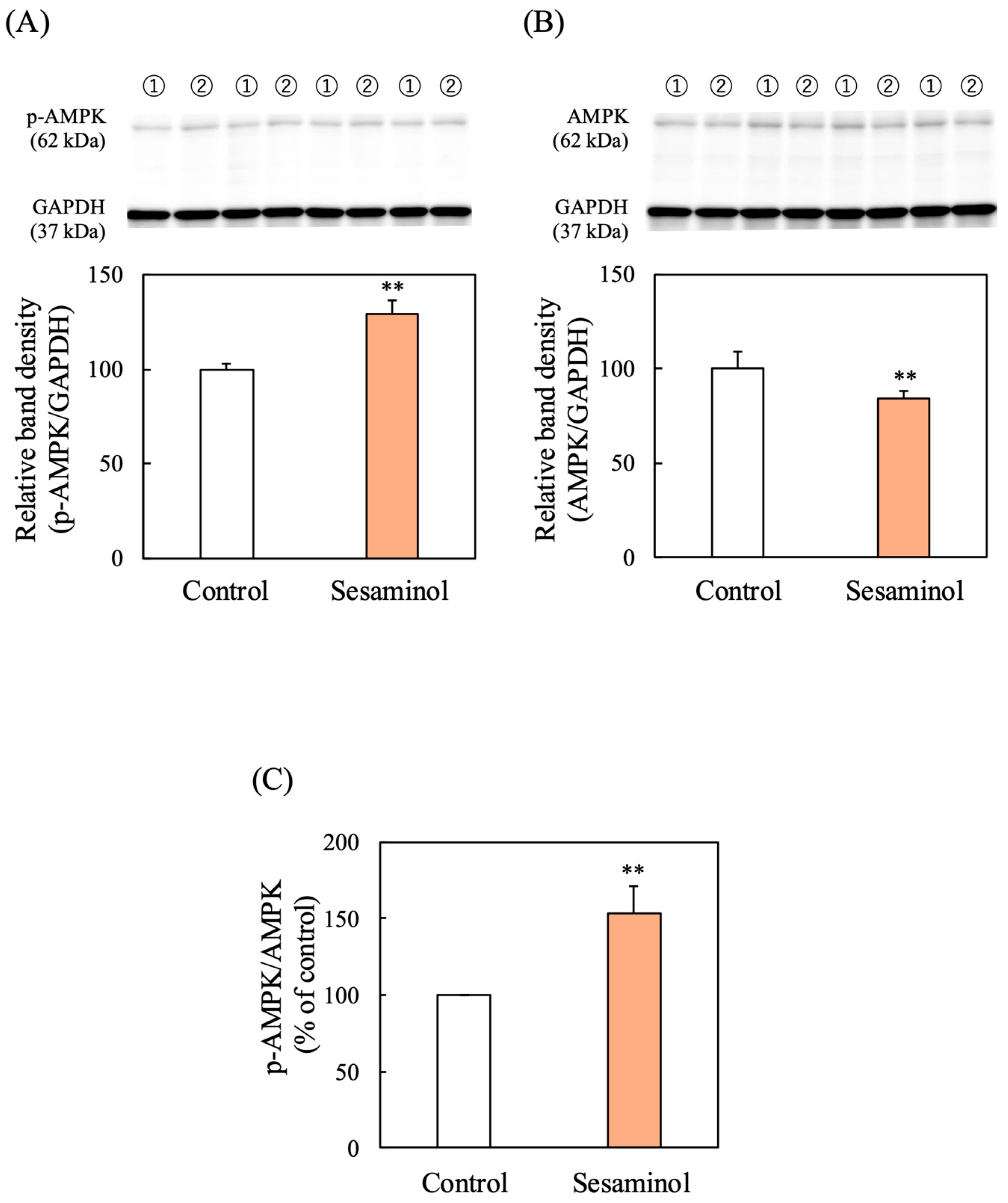
3.11. Effect of Sesaminol on AMPK Phosphorylation
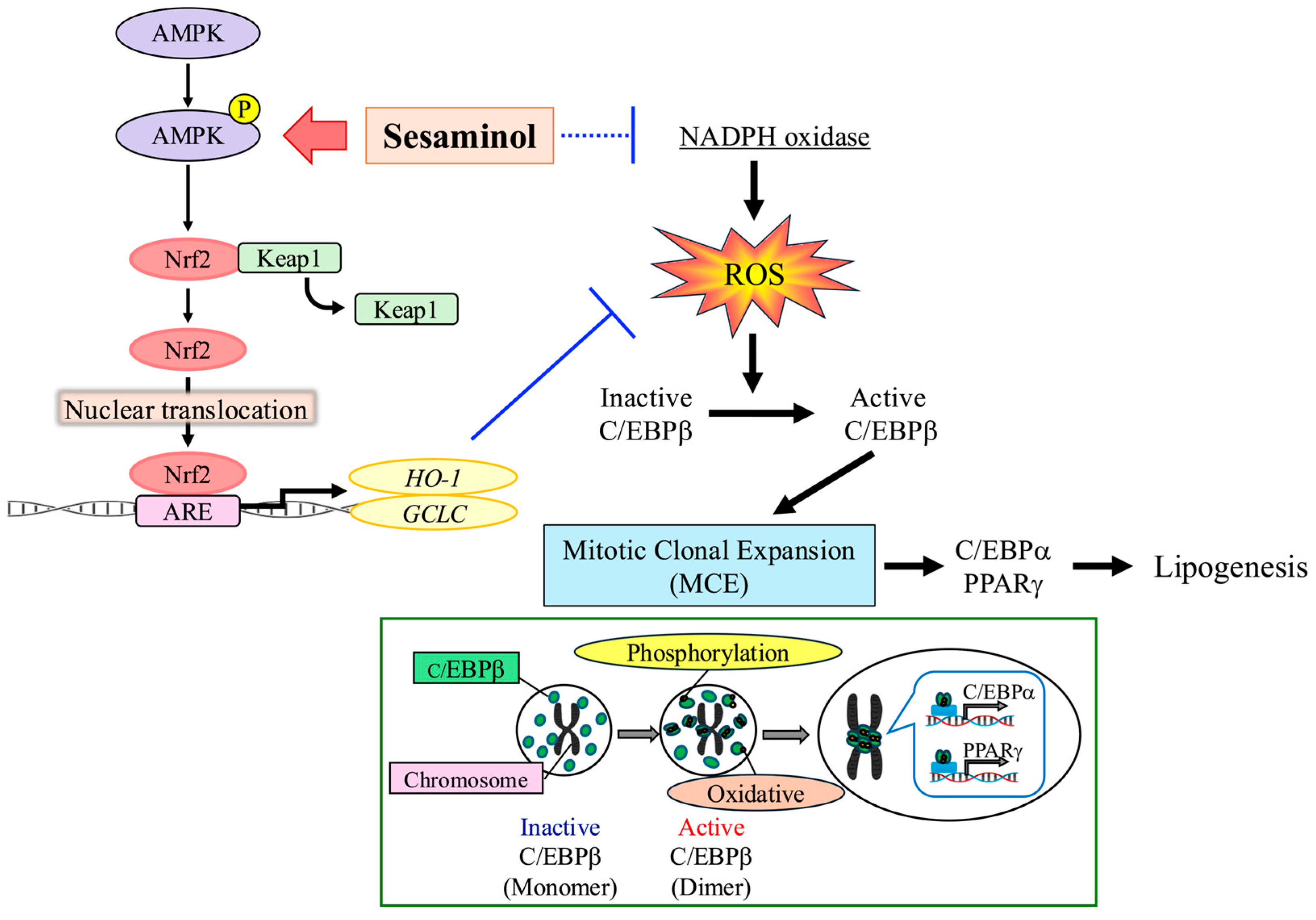
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MCE | mitotic clonal expansion |
| C/EBPβ | CCAAT/enhancer-binding protein beta |
| ROS | reactive oxygen species |
| DMSO | dimethyl sulfoxide |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMI | 0.25 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine and 0.2 μM insulin |
| TG | triacylglycerol |
| PBS (-) | Ca++ and Mg++-free phosphate-bufferes saline |
| GPDH | glycerol-3-phosphate dehydrogenase |
| qRT-PCR | quantitative reverse transcription-polymerase chain reaction |
| PVDF | polyvinylidene difluoride |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| DAPI | 46-diamino-2-phenylindole dihydrochloride |
| CDK2 | cyclin-dependent kinase 2 |
| PPARγ | peroxisome proliferator-activated receptor γ |
| DPI | diphenyleneiodonium chloride, |
| GCLC | glutamate–cysteine ligase catalytic subunit |
| HO-1 | heme oxygenase 1 |
| CC | compound C |
References
- Grgoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Han, J.H.; Jang, K.-W.; Park, M.-H.; Myung, C.-S. Garcinia cambogia suppresses adipogenesis in 3T3-L1 cells by inhibiting p90RSK and Stat3 activation during mitotic clonal expansion. J. Cell. Physiol. 2021, 236, 1922–1939. [Google Scholar] [CrossRef]
- Tang, Q.-Q.; Otto, T.C.; Lane, D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef]
- Tang, Q.-Q.; Gronborg, M.; Huang, H.; Kim, J.-W.; Otto, T.C.; Pande, A.; Lane, M.D. Sequential phosphorylation of CCAAT enhancer-binding protein β by MAPK and glycogen synthase kinase 3β is required for adipogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 9766–9775. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.-Q.; Otto, T.C.; Lane, M.D. CCAAT enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar] [CrossRef]
- Duarte, A.R.; Chenet, A.L.; de Almeida, F.J.S.; Andrada, C.M.B.; de Oliveira, M.R. The inhibition of heme oxygenase-1 (OH-1) abolishes the mitochondria protection induced by sesaminol in LPS-treated RAW 264.7 cells. Chem. Biol. Interact. 2018, 296, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Liou, C.I.; Li, Z.Y.; Lai, X.Y.; Fang, L.W.; Huang, W.C. Sesaminol suppresses the inflammatory response by inhibiting NF-kappaB/MAPK activation and upregulating AMP kinase signaling in RAW 264.7 macrophages. Inflamm. Res. 2015, 64, 577–588. [Google Scholar] [CrossRef]
- Kaji, H.; Matsui-Yuasa, I.; Matsumoto, K.; Omura, A.; Kiyomoto, K.; Kojima-Yuasa, A. Sesaminol prevents Parkinson’s disease by activating the Nrf2-ARE signaling pathway. Heliyon 2020, 6, e05342. [Google Scholar] [CrossRef]
- Nair, A.; Kuwahara, A.; Nagase, A.; Yamaguchi, A.H.; Yamazaki, T.; Hosoya, M.; Omura, A.; Kiyomoto, K.; Yamaguchi, M.-A.; Shimoyama, T.; et al. Purification, gene cloning, and biochemical characterization of β-glucosidase, capable of hydrolyzing sesaminol triglucoside from Paenibacillus sp. KB0599. PLoS ONE 2013, 8, e60538. [Google Scholar] [CrossRef] [PubMed]
- Riddell, R.J.; Clothier, R.H.; Balls, M. An evaluation of three in vitro cytotoxicity assays. Food Chem. Toxicol. 1986, 24, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Zacarias, J.L.; Castro-Muñozledo, F.; Kuri-Harcuch, M. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochem 1992, 97, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Weis, L.S.; Green, H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J. Biol. Chem. 1979, 254, 273–275. [Google Scholar] [CrossRef]
- Burton, K. Determination of DNA concentration with diphenyl amine. In Method in Enzymology; Grossman, L., Meidave, L., Eds.; Academic Press: New York, NY, USA, 1968; pp. 163–166. [Google Scholar]
- Tang, Q.Q.; Lane, M.D. Activatin and centromeric localization of CCAAT/enhancer-binding protein during the mitotic clonal expansion of adipocyte differentiation. Genes. Dev. 1999, 13, 2231–2241. [Google Scholar] [CrossRef]
- Furusawa, S.T.; Fujita, T.M.; Shimabukuro, M.; Iwaki, Y.; Yamada, Y.; Nakajima, M.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2003, 114, 1752–1761. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Li, J.; Chen, X.; Zhang, L.; Liu, Q.; Sun, Y.; Zhao, R. Post-translational regulation of C/EBPβ stability determines early adipogenic commitment in preadipocytes. iScience 2024, 27, 10765. [Google Scholar]
- Cao, Z.; Umek, R.M.; McKnight, L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Li, X.; Qian, S.W.; Guo, L.; Huang, H.Y.; He, Q.; Liu, Y.; Ma, C.-G.; Tang, Q.-Q. Transcriptional activation of histone H4 by C/EBPβ during the mitotic clonal expansion of 3T3-L1 adipocyte differentiation. Mol. Biol. Cell 2011, 22, 2165–2174. [Google Scholar] [CrossRef]
- Guo, L.; Chen, D.; Xu, J.; Wang, J.; Zhang, H.; Li, P.; Liu, Y.; Yang, X. CCAT/enhancer-binding protein β is required for mitotic clonal expansion during 3T3-L1 adipogenesis. J. Cell Physiol. 2022, 237, 2943–2956. [Google Scholar]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhao, M.; Huang, T.; Liu, B.; Chen, K.; Li, S.; Wang, Y.; Zhou, D. C/EBPβ regulates adipocyte differentiation via modulation of chromatin accessibility at PPARγ and C/EBPα loci. Front. Endcrinol. 2023, 14, 1123456. [Google Scholar]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.A.E.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, J.; Lee, S.; Choi, Y.; Kang, J.; Han, K.; Lim, D.; Cho, H. Centromeric localization of C/EBPβ is indispensable for transcriptional activation during adipogenesis. Mol. Cell Biol. 2022, 42, e00123-22. [Google Scholar]
- Tang, Q.-Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef]
- Guo, L.; Li, X.; Tang, Q.-Q. Transcriptional Regulation of Adipocyte Differentiation: A Central Role for CCAAT/Enhancer-binding Protein (C/EBP) β. J. Biol. Chem. 2015, 290, 755–761. [Google Scholar] [CrossRef]
- Johnson, P.F. Molecular stop signs: Regulation of cell-cycle arrest by C/EBP transcription factors. J. Cell Sci. 2005, 118, 2545–2555. [Google Scholar] [CrossRef]
- Pulido-Salgado, M.; Vidal-Taboada, J.M.; Saura, J. C/EBPβ and C/EBPδ transcription factors: Basic biology and roles in the CNS. Prog. Neurobiol. 2015, 132, 1–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, X.; Ruan, X.; Wei, Q.; Zhang, L.; Wo, L.; Huang, D.; Lin, L.; Wang, D.; Xia, L.; et al. SIRT2-mediated deacetylation and deubiquitination of C/EBPβ prevents ethanol-induced liver injury. Cell Discov. 2021, 7, 93. [Google Scholar] [CrossRef]
- Kowenz-Leutz, E.; Pless, O.; Dittmar, G.; Knoblich, M.; Leutz, A. Crosstalk between C/EBP B phosphorylation, arginine methylation, and SWI/SNF/Mediator implies an indexing transcription factor code. EMBO J. 2010, 29, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-L.; Lee, Y.-C.; Yang, W.-M.; Chang, W.-C.; Wang, J.-M. Sumoylation of LAP1 is involved in the HDAC4-mediated repression of COX-2 transcription. Nucleic Acids Res. 2008, 36, 6066–6079. [Google Scholar] [CrossRef]
- Seo, Y.J.; Kimm, K.J.; Koh, E.J.; Choi, J.; Lee, B.Y. Anti-adipogenesis mechanism of pterostilbene through the activation of heme oxygenase-1 in 3T3-L1 cells. Phytomedicine 2017, 33, 7–13. [Google Scholar] [CrossRef]
- Wagner, G.; Lindroos-Christensen, J.; Einwallner, E.; Husa, J.; Zapf, T.C.; Lipp, K.; Rauscher, S.; Gröger, M.; Spittler, A.; Loewe, R.; et al. HO-1 inhibits preadipocyte proliferation and differentiation at the onset of obesity via ROS dependent activation of Akt2. Sci. Rep. 2017, 7, 40881. [Google Scholar] [CrossRef]
- Zang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Lie, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Yang, J.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Inhibitory Effects of Butein on adipogenesis through Upregulation of the Nrf2/HO-1 Pathway in 3T3-L1 Adipocytes. Prev. Nutr. Food Sci. 2017, 22, 306–311. [Google Scholar] [CrossRef]
- Joo, M.S.; Kim, W.D.; Lee, K.Y.; Kim, J.H.; Koo, J.H.; Kim, S.G. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol. Cell. Biol. 2016, 36, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, Z.; Zhou, C.; Chen, K.; Li, X.; Wang, Z.; Ma, J.; Ma, Q.; Duan, W. Activation of Nrf2 by sulforaphane inhibits high glucose-induced progression of pancreatic cancer via AMPK dependent signaling. Cell. Physiol. Biochem. 2018, 50, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Oh, S.; Ko, H.-S.; Park, S.; Jeon, Y.-J.; Kim, J.; Yang, D.-K.; Park, K.W. Sesamolin suppresses adipocyte differentiation through Keap1-dependent Nrf2 activation in adipocytes. Nutr. Res. 2024, 128, 14–23. [Google Scholar] [CrossRef]
- Rajendran, R.; Suman, S.; Divakaran, S.J.; Swatikrishna, S.; Tripathi, P.; Jain, R.; Sagar, K.; Rajakumari, S. Sesaminol alters phospholipid metabolism and alleviates obesity-induced NAFLD. FASEB J. 2024, 38, e23835. [Google Scholar] [CrossRef]
- Gina, N.N.T.; Kuo, J.-L.; Wu, M.-L.; Chuang, S.-M. Sesamin and sesamolin potentially inhibit adipogenesis through downregulating the peroxisome proliferator-activated receptor γ protein expression and activity in 3T3-L1 cells. Nutr. Res. 2024, 123, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, T.; Xiao, H. The Implication of Oxidative Stress and AMPK-Nrf2 Antioxidative Signaling in Pneumonia Pathogenesis. Front. Endocrinol. 2020, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhou, Y.; Cheng, X.; Chen, J.; Cao, H.; Guo, X.; Zhang, C.; Zhuang, Y.; Hu, G. Baicalin Attenuates H2O2-Induced Oxidative Stress by Regulating the AMPK/Nrf2 Signaling Pathway in IPEC-J2 Cells. Int. J. Mol. Sci. 2023, 24, 9435. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.J.; Vasantha Rupasinghe, H.P. Elucidating the Mechanistic Interplay of AMPK and Nrf2 in MASLD: A Focus on Dietary Phytochemical Modulation of Lipid and Redox Homeostasis. Biochem. Biophys. Res. Commun. 2025, 777, 152300. [Google Scholar] [CrossRef] [PubMed]

| Genome | Base Sequence |
|---|---|
| C/EBPβ | Forward: AATCACTTAAAGATGTTCCTGCGG Reverse: ATGCTCGAAACGGAAAAGGTTC |
| C/EBPα | Forward: TTGAAGCACAATCGATCCATCC Reverse: GCACACTGCCATTGCACAAG |
| PPARγ | Forward: GGAGCCTAAGTTTGAGTTTGCTGTG Reverse: TGCAGCAGGTTGTCTTGGATG |
| HO-1 | Forward: GAGGCTAAGACCGCCTTCCT Reverse: ACGAAGTGACGCCATCTGTG |
| GCLC | Forward: ATCAAAGGCTTCTCAGCCAGAC Reverse: TATGCTGCAGGCTTGGAATGT |
| Cyclin E1 | Forward: GGTGGCTCCGACCTTTCAGT Reverse: ATCCCAGGGCTGACTGCTATC |
| Cyclin E2 | Forward: CAGACTCTCCGCAAGAAACCC Reverse: CAAGGGCTGATTCCTCCAGAC |
| Actin-β | Forward: CATCCGTAAAGACCTCTATGCCAAC Reverse: ATGGAGCCACCGATCCACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamatsu, S.; Nakata, M.; Norikura, T.; Sasaki, Y.; Matsui-Yuasa, I.; Omura, A.; Kiyomoto, K.; Kojima-Yuasa, A. Sesaminol Inhibits Adipogenesis by Suppressing Mitotic Clonal Expansion and Activating the Nrf2-ARE Pathway. Nutrients 2025, 17, 3242. https://doi.org/10.3390/nu17203242
Nakamatsu S, Nakata M, Norikura T, Sasaki Y, Matsui-Yuasa I, Omura A, Kiyomoto K, Kojima-Yuasa A. Sesaminol Inhibits Adipogenesis by Suppressing Mitotic Clonal Expansion and Activating the Nrf2-ARE Pathway. Nutrients. 2025; 17(20):3242. https://doi.org/10.3390/nu17203242
Chicago/Turabian StyleNakamatsu, Saki, Miki Nakata, Toshio Norikura, Yutaro Sasaki, Isao Matsui-Yuasa, Ayano Omura, Kunio Kiyomoto, and Akiko Kojima-Yuasa. 2025. "Sesaminol Inhibits Adipogenesis by Suppressing Mitotic Clonal Expansion and Activating the Nrf2-ARE Pathway" Nutrients 17, no. 20: 3242. https://doi.org/10.3390/nu17203242
APA StyleNakamatsu, S., Nakata, M., Norikura, T., Sasaki, Y., Matsui-Yuasa, I., Omura, A., Kiyomoto, K., & Kojima-Yuasa, A. (2025). Sesaminol Inhibits Adipogenesis by Suppressing Mitotic Clonal Expansion and Activating the Nrf2-ARE Pathway. Nutrients, 17(20), 3242. https://doi.org/10.3390/nu17203242







