Savoring Satiety: An Exploratory Analysis of the Neural Correlates of Sensory-Specific Satiety
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Test Group
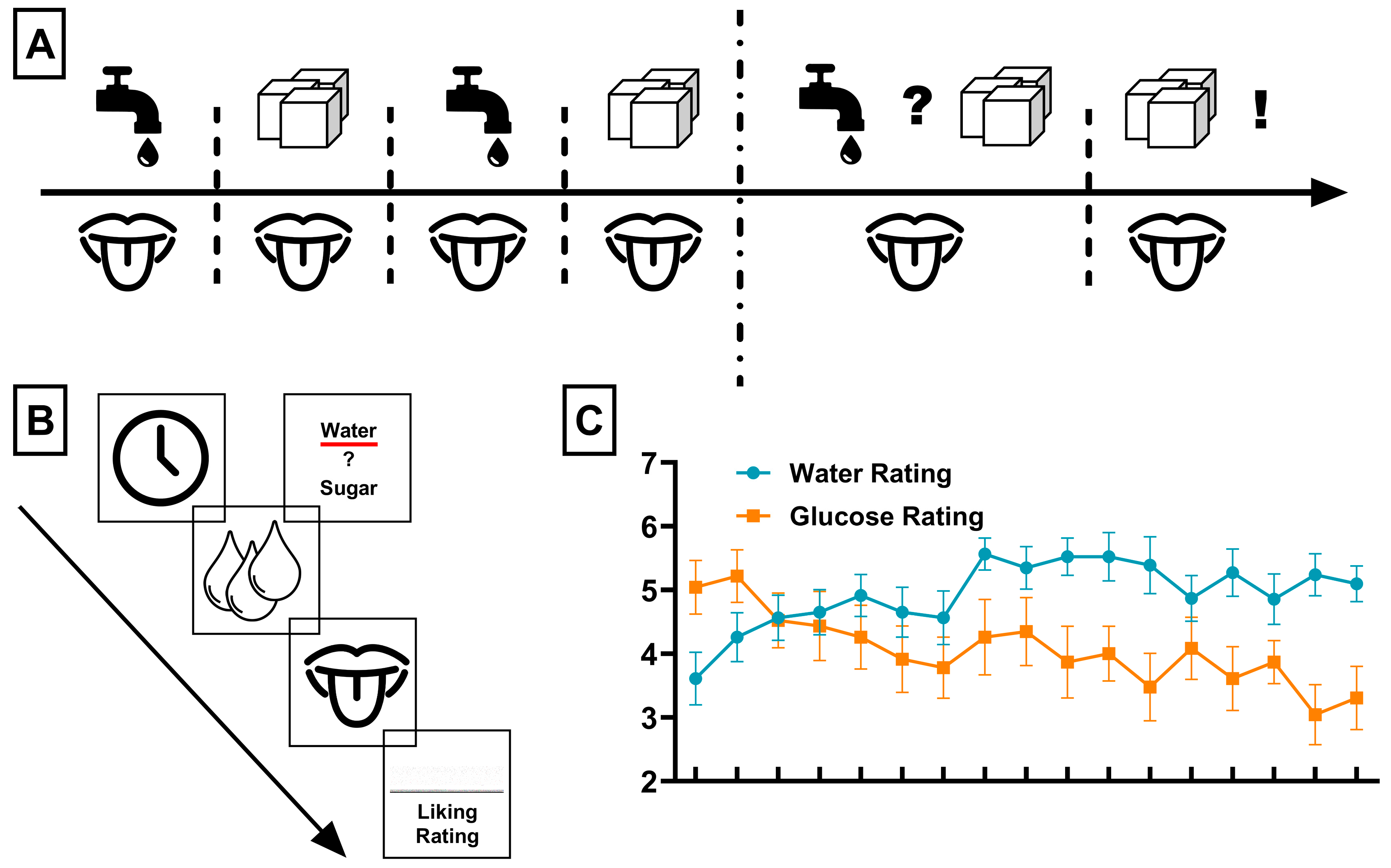
2.2. Procedure
2.3. fMRI Acquisition
2.4. fMRI Analysis
- (a)
- Choice of water during SSS compared to passive receipt of water before SSS (=influence of sensory-specific satiety on water perception).
- (b)
- Choice of water during SSS compared to passive receipt of glucose before SSS (=influence of increased hedonic perception of water during SSS when compared to an inherently hedonic stimulus).
- (c)
- Choice of water during SSS compared to passive receipt of glucose during SSS (=influence of increased hedonic perception of water during SSS when compared to SSS reduced hedonic perception of glucose).
- (d)
- Passive receipt of glucose before SSS compared to passive receipt of water before SSS (=comparison of glucose processing before SSS with a neutral stimulus).
- (e)
- Passive receipt of glucose during SSS compared to passive receipt of water before SSS (=comparison of SSS reduced hedonic perception of glucose to a neutral stimulus).
2.5. Behavioral Analysis
3. Results
3.1. Behavioral Results: Liking Ratings Associated with Sensory-Specific Satiety
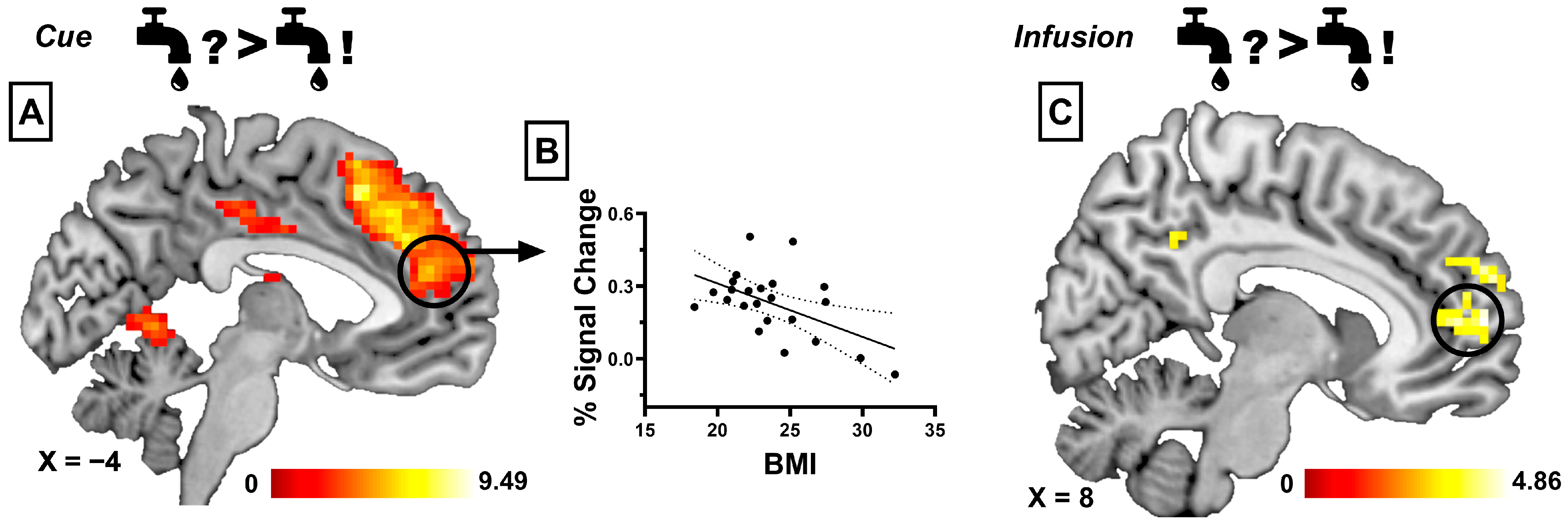
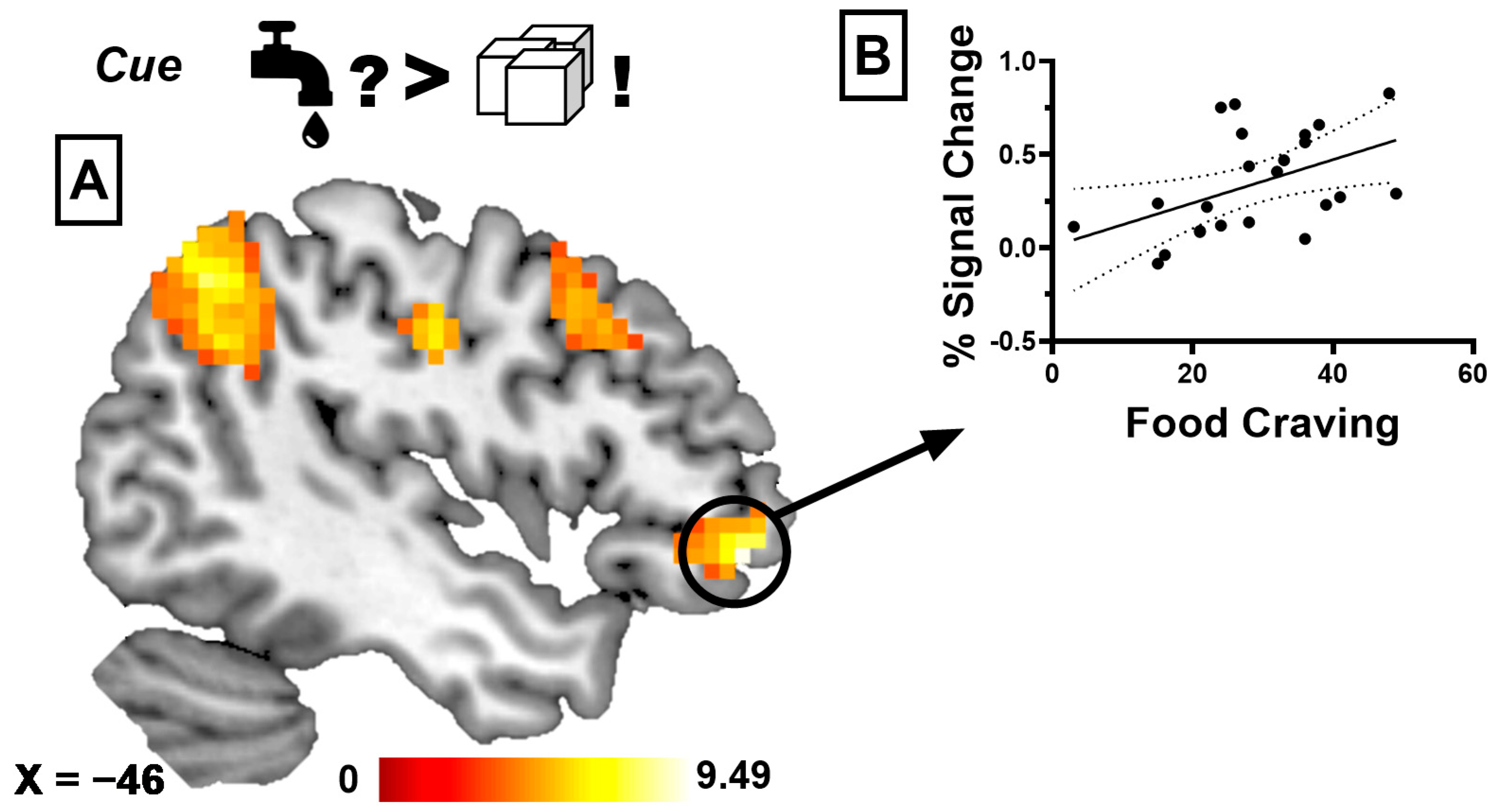
3.2. BOLD Responses Associated with Sensory-Specific Satiety
3.2.1. Taste Anticipation Phase
3.2.2. Infusion Phase
3.2.3. Correlational Analyses
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hendriks, A.E.M.; Nederkoorn, C.; van Lier, I.M.J.; van Belkom, B.; Bast, A.; Havermans, R.C. Sensory-specific satiety, the variety effect and physical context: Does change of context during a meal enhance food intake? Appetite 2021, 163, 105179. [Google Scholar] [CrossRef]
- Rolls, E.T.; Rolls, B.J.; Rowe, E.A. Sensory-specific and motivation-specific satiety for the sight and taste of food and water in man. Physiol. Behav. 1983, 30, 185–192. [Google Scholar] [CrossRef]
- Bell, E.A.; Roe, L.S.; Rolls, B.J. Sensory-specific satiety is affected more by volume than by energy content of a liquid food. Physiol. Behav. 2003, 78, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Kringelbach, M.L.; O’Doherty, J.P.; Rolls, E.T.; Andrews, C. Activation of the Human Orbitofrontal Cortex to a Liquid Food Stimulus Is Correlated With Its Subjective Pleasantness. Cereb. Cortex 2003, 13, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, L.B.; Møller, P.; Flint, A.; Martens, M.; Raben, A. Effect of Sensory Perception of Foods on Appetite and Food Intake: A Review of Studies on Humans. Int. J. Obes. 2003, 27, 1152–1166. [Google Scholar] [CrossRef]
- Yeomans, M.R. Olfactory Influences on Appetite and Satiety in Humans. Physiol. Behav. 2006, 87, 800–804. [Google Scholar] [CrossRef]
- Embling, R.; Pink, A.E.; Gatzemeier, J.; Price, M.; Lee, M.D.; Wilkinson, L.L. Effect of food variety on intake of a meal: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2021, 113, 716–741. [Google Scholar] [CrossRef]
- Raynor, H.A.; Niemeier, H.M.; Wing, R.R. Effect of limiting snack food variety on long-term sensory-specific satiety and monotony during obesity treatment. Eat. Behav. 2006, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Raynor, H.A.; Steeves, E.A.; Hecht, J.; Fava, J.L.; Wing, R.R. Limiting variety in non-nutrient-dense, energy-dense foods during a lifestyle intervention: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1305–1314. [Google Scholar] [CrossRef]
- Tey, S.L.; Brown, R.C.; Gray, A.R.; Chisholm, A.W.; Delahunty, C.M. Long-term consumption of high energy-dense snack foods on sensory-specific satiety and intake. Am. J. Clin. Nutr. 2012, 95, 1038–1047. [Google Scholar] [CrossRef]
- Myers, K.P. Sensory-specific satiety is intact in rats made obese on a high-fat high-sugar choice diet. Appetite 2017, 112, 196–200. [Google Scholar] [CrossRef]
- Havermans, R.C.; Roefs, A.; Nederkoorn, C.; Jansen, A. No rapid recovery of sensory-specific satiety in obese women. Flavour 2012, 1, 5. [Google Scholar] [CrossRef]
- Brondel, L.; Romer, M.; Wymelbeke, V.v.; Walla, P.; Jiang, T.; Deecke, L.; Rigaud, D. Sensory-Specific Satiety With Simple Foods in Humans: No Influence of BMI? Int. J. Obes. 2006, 31, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Snoek, H.M.; Huntjens, L.; Van Gemert, L.J.; De Graaf, C.; Weenen, H. Sensory-specific satiety in obese and normal-weight women. Am. J. Clin. Nutr. 2004, 80, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Mennella, J.A. Habituation to the pleasure elicited by sweetness in lean and obese women. Appetite 2012, 58, 800–805. [Google Scholar] [CrossRef]
- Inceu, G.; Nechifor, R.E.; Rusu, A.; Ciobanu, D.M.; Draghici, N.C.; Pop, R.M.; Craciun, A.E.; Porojan, M.; Negrut, M.; Roman, G.; et al. Post-COVID-19 Changes in Appetite—An Exploratory Study. Nutrients 2024, 16, 2349. [Google Scholar] [CrossRef]
- Masala, C.; Porcu, M.; Orofino, G.; Defazio, G.; Pinna, I.; Solla, P.; Ercoli, T.; Suri, J.S.; Spinato, G.; Saba, L. Neuroimaging evaluations of olfactory, gustatory, and neurological deficits in patients with long-term sequelae of COVID-19. Brain Imaging Behav. 2024, 18, 1480–1490. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Q.; Jiang, Z.; Mei, H.; Zeng, N.; Su, S.; Wu, S.; Ge, Y.; Li, P.; Lin, X.; et al. Brain abnormalities in survivors of COVID-19 after 2-year recovery: A functional MRI study. Lancet Reg. Health West. Pac. 2024, 47, 101086. [Google Scholar] [CrossRef]
- Nasir, S.M.; Yahya, N.; Manan, H.A. Functional brain alterations in COVID-19 patients using resting-state fMRI: A systematic review. Brain Imaging Behav. 2024, 18, 1582–1601. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Goto, T.K.; Leung, W.K. Basic taste processing recruits bilateral anteroventral and middle dorsal insulae: An activation likelihood estimation meta-analysis of fMRI studies. Brain Behav. 2017, 7, e00655. [Google Scholar] [CrossRef] [PubMed]
- Veldhuizen, M.G.; Albrecht, J.; Zelano, C.; Boesveldt, S.; Breslin, P.; Lundström, J.N. Identification of human gustatory cortex by activation likelihood estimation. Hum. Brain Mapp. 2011, 32, 2256–2266. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.A.; Giesbrecht, T.; Fallon, N.; Thomas, A.; Mela, D.J.; Kirkham, T.C. A Systematic Review and Activation Likelihood Estimation Meta-Analysis of fMRI Studies on Sweet Taste in Humans. J. Nutr. 2020, 150, 1619–1630. [Google Scholar] [CrossRef]
- Lasschuijt, M.; Graaf, K.d.; Mars, M. Effects of Oro-Sensory Exposure on Satiation and Underlying Neurophysiological Mechanisms—What Do We Know So Far? Nutrients 2021, 13, 1391. [Google Scholar] [CrossRef] [PubMed]
- O’doherty, J.; Rolls, E.T.; Francis, S.; Bowtell, R.; McGlone, F.; Kobal, G.; Renner, B.; Ahne, G. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport 2000, 11, 893–897. [Google Scholar]
- Zoon, H.F.A.; Ohla, K.; de Graaf, C.; Boesveldt, S. Modulation of event-related potentials to food cues upon sensory-specific satiety. Physiol. Behav. 2018, 196, 126–134. [Google Scholar] [CrossRef]
- Rolls, E.T. Sensory processing in the brain related to the control of food intake. Proc. Nutr. Soc. 2007, 66, 96–112. [Google Scholar] [CrossRef]
- Rolls, E.T. Taste, olfactory, and food reward value processing in the brain. Prog. Neurobiol. 2015, 127–128, 64–90. [Google Scholar] [CrossRef]
- Rolls, E.T. Chemosensory learning in the cortex. Front. Syst. Neurosci. 2011, 5, 78. [Google Scholar] [CrossRef]
- Rolls, E.T. The orbitofrontal cortex, food reward, body weight and obesity. Soc. Cogn. Affect. Neurosci. 2023, 18, nsab044. [Google Scholar] [CrossRef]
- Rolls, E.T. The Orbitofrontal Cortex and Reward. Cereb. Cortex 2000, 10, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. Taste, olfactory and food texture reward processing in the brain and the control of appetite. Proc. Nutr. Soc. 2012, 71, 488–501. [Google Scholar] [CrossRef]
- Sadler, J.R.; Shearrer, G.E.; Papantoni, A.; Yokum, S.T.; Stice, E.; Burger, K.S. Correlates of neural adaptation to food cues and taste: The role of obesity risk factors. Soc. Cogn. Affect. Neurosci. 2023, 18, nsab018. [Google Scholar] [CrossRef]
- Wittchen, H.; Zaudig, M.; Fydrich, T. Structured Clinical Interview for DSM-IV—German Version; Hoegrefe: Göttingen, Germany, 1997. [Google Scholar]
- Nijs, I.M.; Franken, I.H.; Muris, P. The modified Trait and State Food-Cravings Questionnaires: Development and validation of a general index of food craving. Appetite 2007, 49, 38–46. [Google Scholar] [CrossRef] [PubMed]
- van Strien, T.; Frijters, J.E.R.; Bergers, G.P.A.; Defares, P.B. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 1986, 5, 295–315. [Google Scholar] [CrossRef]
- Nagl, M.; Hilbert, A.; de Zwaan, M.; Braehler, E.; Kersting, A. The German Version of the Dutch Eating Behavior Questionnaire: Psychometric Properties, Measurement Invariance, and Population-Based Norms. PLoS ONE 2016, 11, e0162510. [Google Scholar] [CrossRef]
- Davidenko, O.; Bonny, J.M.; Morrot, G.; Jean, B.; Claise, B.; Benmoussa, A.; Fromentin, G.; Tome, D.; Nadkarni, N.; Darcel, N. Differences in BOLD responses in brain reward network reflect the tendency to assimilate a surprising flavor stimulus to an expected stimulus. Neuroimage 2018, 183, 37–46. [Google Scholar] [CrossRef]
- Veldhuizen, M.G.; Bender, G.; Constable, R.T.; Small, D.M. Trying to Detect Taste in a Tasteless Solution: Modulation of Early Gustatory Cortex by Attention to Taste. Chem. Senses 2007, 32, 569–581. [Google Scholar] [CrossRef]
- Brett, M.; Anton, J.-L.; Valabregue, R.; Poline, J.-B. Region of interest analysis using an SPM toolbox. In Proceedings of the 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan, 2–6 June 2002; Volume 16, p. 497. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Raynor, H.A.; Epstein, L.H. Dietary variety, energy regulation, and obesity. Psychol. Bull. 2001, 127, 325. [Google Scholar] [CrossRef]
- Costa, D.G.; Almeida, C.; Cavadas, C.; Carmo-Silva, S. A look on food intake and satiety: From humans to rodent models. Nutr. Rev. 2022, 80, 1942–1957. [Google Scholar] [CrossRef]
- Dalton, M.; Hollingworth, S.; Blundell, J.; Finlayson, G. Weak Satiety Responsiveness Is a Reliable Trait Associated with Hedonic Risk Factors for Overeating among Women. Nutrients 2015, 7, 7421–7436. [Google Scholar] [CrossRef]
- Small, D.M. Flavor is in the brain. Physiol. Behav. 2012, 107, 540–552. [Google Scholar] [CrossRef]
- Small, D.M.; Gregory, M.D.; Mak, Y.E.; Gitelman, D.; Mesulam, M.M.; Parrish, T. Dissociation of Neural Representation of Intensity and Affective Valuation in Human Gustation. Neuron 2003, 39, 701–711. [Google Scholar] [CrossRef]
- Duif, I.; Wegman, J.; Mars, M.M.; de Graaf, C.; Smeets, P.A.M.; Aarts, E. Effects of distraction on taste-related neural processing: A cross-sectional fMRI study. Am. J. Clin. Nutr. 2020, 111, 950–961. [Google Scholar] [CrossRef]
- Rushworth, M.F.; Kolling, N.; Sallet, J.; Mars, R.B. Valuation and decision-making in frontal cortex: One or many serial or parallel systems? Curr. Opin. Neurobiol. 2012, 22, 946–955. [Google Scholar] [CrossRef]
- Stice, E.; Burger, K. Neural vulnerability factors for obesity. Clin. Psychol. Rev. 2019, 68, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Yokum, S.; Stice, E. Weight gain is associated with changes in neural response to palatable food tastes varying in sugar and fat and palatable food images: A repeated-measures fMRI study. Am. J. Clin. Nutr. 2019, 110, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Turel, O.; Xiao, Z.; He, J.; He, Q. Impulsivity and neural mechanisms that mediate preference for immediate food rewards in people with vs without excess weight. Appetite 2022, 169, 105798. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, S.; Weber, S.C.; Graf, G.; Hinz, D.; Asarian, L.; Geary, N.; Leeners, B.; Hare, T.A.; Tobler, P.N. Reduced Neural Satiety Responses in Women Affected by Obesity. Neuroscience 2020, 447, 94–112. [Google Scholar] [CrossRef]
- Simon, J.J.; Becker, A.; Sinno, M.H.; Skunde, M.; Bendszus, M.; Preissl, H.; Enck, P.; Herzog, W.; Friederich, H.C. Neural Food Reward Processing in Successful and Unsuccessful Weight Maintenance. Obesity 2018, 26, 895–902. [Google Scholar] [CrossRef]
- Ciria, L.F.; Watson, P.; Vadillo, M.A.; Luque, D. Is the habit system altered in individuals with obesity? A systematic review. Neurosci. Biobehav. Rev. 2021, 128, 621–632. [Google Scholar] [CrossRef]
- Ziauddeen, H.; Farooqi, I.S.; Fletcher, P.C. Obesity and the brain: How convincing is the addiction model? Nat. Rev. Neurosci. 2012, 13, 279–286. [Google Scholar] [CrossRef]
- Thomas, J.M.; Higgs, S.; Dourish, C.T.; Hansen, P.C.; Harmer, C.J.; McCabe, C. Satiation attenuates BOLD activity in brain regions involved in reward and increases activity in dorsolateral prefrontal cortex: An fMRI study in healthy volunteers. Am. J. Clin. Nutr. 2015, 101, 701–708. [Google Scholar] [CrossRef]
- Rudebeck, P.H.; Murray, E.A. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J. Neurosci. 2011, 31, 10569–10578. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, L.N.; de Ridder, D.T.; Viergever, M.A.; Smeets, P.A. The first taste is always with the eyes: A meta-analysis on the neural correlates of processing visual food cues. Neuroimage 2011, 55, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, D.; Todd, J.; Du, J.; Yang, Z.; Lu, H.; Chen, H. Neural correlates of restrained eaters’ high susceptibility to food cues: An fMRI study. Neurosci. Lett. 2016, 631, 56–62. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, L.N.; de Ridder, D.T.; Viergever, M.A.; Smeets, P.A. Activation in inhibitory brain regions during food choice correlates with temptation strength and self-regulatory success in weight-concerned women. Front. Neurosci. 2014, 8, 308. [Google Scholar] [CrossRef]
- Hollmann, M.; Hellrung, L.; Pleger, B.; Schlögl, H.; Kabisch, S.; Stumvoll, M.; Villringer, A.; Horstmann, A. Neural correlates of the volitional regulation of the desire for food. Int. J. Obes. 2012, 36, 648–655. [Google Scholar] [CrossRef]
- Avery, J.A.; Liu, A.G.; Ingeholm, J.E.; Riddell, C.D.; Gotts, S.J.; Martin, A. Taste Quality Representation in the Human Brain. J. Neurosci. 2020, 40, 1042–1052. [Google Scholar] [CrossRef]
- Ulrich, M.; Steigleder, L.; Gron, G. Neural signature of the Food Craving Questionnaire (FCQ)-Trait. Appetite 2016, 107, 303–310. [Google Scholar] [CrossRef]
- Gearhardt, A.N.; Yokum, S.; Orr, P.T.; Stice, E.; Corbin, W.R.; Brownell, K.D. Neural correlates of food addiction. Arch. Gen. Psychiatry 2011, 68, 808–816. [Google Scholar] [CrossRef]
- Griffioen-Roose, S.; Finlayson, G.; Mars, M.; Blundell, J.E.; de Graaf, C. Measuring food reward and the transfer effect of sensory specific satiety. Appetite 2010, 55, 648–655. [Google Scholar] [CrossRef]
- Rogers, P.J.; Drumgoole, F.D.; Quinlan, E.; Thompson, Y. An analysis of sensory-specific satiation: Food liking, food wanting, and the effects of distraction. Learn. Motiv. 2021, 73, 101688. [Google Scholar] [CrossRef]
- Havermans, R.C.; Janssen, T.; Giesen, J.C.; Roefs, A.; Jansen, A. Food liking, food wanting, and sensory-specific satiety. Appetite 2009, 52, 222–225. [Google Scholar] [CrossRef]
- Castro, D.C.; Berridge, K.C. Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proc. Natl. Acad. Sci. USA 2017, 114, E9125–E9134. [Google Scholar] [CrossRef] [PubMed]
- Schier, L.A.; Hashimoto, K.; Bales, M.B.; Blonde, G.D.; Spector, A.C. High-resolution lesion-mapping strategy links a hot spot in rat insular cortex with impaired expression of taste aversion learning. Proc. Natl. Acad. Sci. USA 2014, 111, 1162–1167. [Google Scholar] [CrossRef]
- Forget, B.; Pushparaj, A.; Le Foll, B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol. Psychiatry 2010, 68, 265–271. [Google Scholar] [CrossRef]
- Tuerke, K.J.; Limebeer, C.L.; Fletcher, P.J.; Parker, L.A. Double dissociation between regulation of conditioned disgust and taste avoidance by serotonin availability at the 5-HT3 receptor in the posterior and anterior insular cortex. J. Neurosci. 2012, 32, 13709–13717. [Google Scholar] [CrossRef]
- Contreras, M.; Ceric, F.; Torrealba, F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science 2007, 318, 655–658. [Google Scholar] [CrossRef]
- Mohammadi, S.; Ghaderi, S. Post-COVID-19 conditions: A systematic review on advanced magnetic resonance neuroimaging findings. Neurol. Sci. 2024, 45, 1815–1833. [Google Scholar] [CrossRef] [PubMed]
- Ferrulli, A.; Senesi, P.; Terruzzi, I.; Luzi, L. Eating Habits and Body Weight Changes Induced by Variation in Smell and Taste in Patients with Previous SARS-CoV-2 Infection. Nutrients 2022, 14, 5068. [Google Scholar] [CrossRef]
- Pettemeridou, E.; Loizidou, M.; Trajkovic, J.; Constantinou, M.; De Smet, S.; Baeken, C.; Sack, A.T.; Williams, S.C.R.; Constantinidou, F. Cognitive and Psychological Symptoms in Post-COVID-19 Condition: A Systematic Review of Structural and Functional Neuroimaging, Neurophysiology, and Intervention Studies. Arch. Rehabil. Res. Clin. Transl. 2025, 7, 100461. [Google Scholar] [CrossRef] [PubMed]
- Grady, C.L.; Rieck, J.R.; Nichol, D.; Rodrigue, K.M.; Kennedy, K.M. Influence of sample size and analytic approach on stability and interpretation of brain-behavior correlations in task-related fMRI data. Hum. Brain Mapp. 2021, 42, 204–219. [Google Scholar] [CrossRef]
- Arnoni-Bauer, Y.; Bick, A.; Raz, N.; Imbar, T.; Amos, S.; Agmon, O.; Marko, L.; Levin, N.; Weiss, R. Is It Me or My Hormones? Neuroendocrine Activation Profiles to Visual Food Stimuli Across the Menstrual Cycle. J. Clin. Endocrinol. Metab. 2017, 102, 3406–3414. [Google Scholar] [CrossRef]
- Clarke, G.S.; Page, A.J.; Eldeghaidy, S. The gut-brain axis in appetite, satiety, food intake, and eating behavior: Insights from animal models and human studies. Pharmacol. Res. Perspect. 2024, 12, e70027. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Keast, R.S.J.; Roura, E. Salivary leptin and TAS1R2/TAS1R3 polymorphisms are related to sweet taste sensitivity and carbohydrate intake from a buffet meal in healthy young adults. Br. J. Nutr. 2017, 118, 763–770. [Google Scholar] [CrossRef] [PubMed]
| Mean ± SD | |
|---|---|
| Female/Male | 11/12 (n = 23) |
| Age | 28.6 ± 10.1 |
| BMI kg/m2 | 23.8 ± 3.3 (range 18.4–32.2) |
| FCQ-S | 28.6 ± 11.1 |
| FCQ-T | 52.6 ± 19.2 |
| DEBQ—Restraint Eating | 1.9 ± 0.7 |
| DEBQ—Emotional Eating | 1.8 ± 0.6 |
| DEBQ—External Eating | 2.6 ± 0.7 |
| MWT-B | 30.1 ± 4 |
| Water During SSS > Water Before SSS. | ||||||
|---|---|---|---|---|---|---|
| Brain Regions | k | t-Value | p-Value | x | y | z |
| Anterior Cingulate Cortex/Medial Frontal Cortex | 1741 | 9.48 | <0.001 | 6 | 44 | 20 |
| Right Angular Gyrus/Inferior Parietal Lobule | 417 | 6.41 | <0.001 | 51 | −46 | 44 |
| Left Angular Gyrus/Inferior Parietal Lobule | 607 | 8.16 | <0.001 | −45 | −58 | 47 |
| Right Anterior Insula/Orbitofronal Cortex | 129 | 7.62 | <0.001 | 48 | 23 | −7 |
| Left Anterior Insula/Orbitofronal Cortex | 236 | 6.37 | <0.001 | −45 | 20 | −13 |
| Right Middle Temporal Gyrus | 169 | 7.35 | <0.001 | 66 | −31 | −1 |
| Left Middle Temporal Gyrus | 186 | 7.14 | <0.001 | −57 | −40 | −4 |
| Right Posterior Cerebelum (Crus 1) | 188 | 5.79 | <0.001 | 36 | −73 | −22 |
| Left Posterior Cerebelum (Crus 1) | 183 | 6.13 | <0.001 | −27 | −73 | −28 |
| Anterior Vermis | 74 | 5.81 | 0.002 | 0 | −55 | −1 |
| Medial Cingulate Cortex | 72 | 5.45 | 0.002 | 3 | −19 | 38 |
| Caudate Nucleus | 39 | 5.22 | 0.042 | 15 | −1 | 20 |
| Water During SSS > Glucose Before SSS | ||||||
| Brain Regions | k | t-value | p-value | x | y | z |
| Right Angular Gyrus/Inferior Parietal Lobule | 72 | 5 | 0.003 | 48 | −46 | 44 |
| Left Angular Gyrus/Inferior Parietal Lobule | 240 | 7 | <0.001 | −48 | −49 | 44 |
| Middle Frontal Gyrus | 94 | 7.02 | 0.001 | −36 | 17 | 50 |
| Anterior Cingulate Cortex/Superior Frontal Cortex | 313 | 6.18 | <0.001 | −9 | 23 | 38 |
| Anterior Cingulate Cortex | 40 | 6.08 | 0.043 | 9 | 47 | 5 |
| Water During SSS > Glucose During SSS | ||||||
| Brain Regions | k | t-value | p-value | x | y | z |
| Middle Prefrontal Cortex | 155 | 7.39 | <0.001 | −36 | 14 | 53 |
| Left Orbitofrontal Cortex | 73 | 7.22 | 0.004 | −45 | 47 | −7 |
| Right Angular Gyrus/Inferior Parietal Lobule | 184 | 6.31 | <0.001 | 54 | −55 | 47 |
| Left Angular Gyrus/Inferior Parietal Lobule | 369 | 6.91 | <0.001 | −48 | −49 | 44 |
| Right Postcentral Gyrus | 88 | 6.89 | 0.001 | 60 | −7 | 32 |
| Left Postcentral Gyrus | 113 | 6.26 | <0.001 | −48 | −13 | 38 |
| Superior Frontal Cortex/Anterior Cingulate Cortex | 792 | 6.75 | <0.001 | −9 | 20 | 53 |
| Middle Frontal Gyrus | 85 | 5.32 | 0.002 | 39 | 26 | 35 |
| Posterior Cerebelum (Crus 1) | 45 | 5.02 | 0.035 | −27 | −70 | −25 |
| Glucose Before SSS > Water Before SSS | ||||||
| Brain Regions | k | t-value | p-value | x | y | z |
| Superior Temporal Gyrus | 144 | 6 | <0.001 | 60 | −52 | 23 |
| Middle Occipital Gyrus | 36 | 6 | 0.041 | −18 | −91 | −1 |
| Precuneus | 45 | 5 | 0.016 | 6 | −55 | 47 |
| Glucose During SSS > water Before SSS | ||||||
| Brain Regions | k | t-value | p-value | x | y | z |
| Superior Temporal Gyrus | 244 | 6.69 | <0.001 | 51 | −49 | 23 |
| Middle Occipital Gyrus | 58 | 4.88 | 0.011 | −30 | −85 | 29 |
| ParaHippocampal Gyrus | 47 | 4.87 | 0.028 | 30 | −28 | −19 |
| Precuneus | 70 | 4.54 | 0.005 | −12 | −49 | 44 |
| Water During SSS > Water Before SSS | ||||||
|---|---|---|---|---|---|---|
| Brain Regions | k | t-Value | p-Value | x | y | z |
| Medial Prefrontal Cortex | 169 | 4.85 | <0.001 | 9 | 59 | 8 |
| Water During SSS > Glucose Before SSS | ||||||
| Medial Prefrontal Cortex | 428 | 7 | <0.001 | 12 | 50 | 8 |
| Posterior Cingulate Cortex | 115 | 6 | 0.001 | 6 | −49 | 38 |
| Glucose During SSS > Water During SSS | ||||||
| Dorsal Medial Cingulate Cortex | 135 | 6.56 | 0.001 | 6 | 11 | 47 |
| Posterior Insula | 113 | 4.86 | 0.003 | 45 | −4 | 2 |
| Glucose During SSS > Water Before SSS | ||||||
| Medial Prefrontal Cortex | 84 | 6 | 0.005 | 3 | 50 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, J.J.; Müller, T.; Schöner, F.; Bendszus, M.; Friederich, H.-C. Savoring Satiety: An Exploratory Analysis of the Neural Correlates of Sensory-Specific Satiety. Nutrients 2025, 17, 3229. https://doi.org/10.3390/nu17203229
Simon JJ, Müller T, Schöner F, Bendszus M, Friederich H-C. Savoring Satiety: An Exploratory Analysis of the Neural Correlates of Sensory-Specific Satiety. Nutrients. 2025; 17(20):3229. https://doi.org/10.3390/nu17203229
Chicago/Turabian StyleSimon, Joe J., Tim Müller, Fabian Schöner, Martin Bendszus, and Hans-Christoph Friederich. 2025. "Savoring Satiety: An Exploratory Analysis of the Neural Correlates of Sensory-Specific Satiety" Nutrients 17, no. 20: 3229. https://doi.org/10.3390/nu17203229
APA StyleSimon, J. J., Müller, T., Schöner, F., Bendszus, M., & Friederich, H.-C. (2025). Savoring Satiety: An Exploratory Analysis of the Neural Correlates of Sensory-Specific Satiety. Nutrients, 17(20), 3229. https://doi.org/10.3390/nu17203229





