Co-Fermented Black Barley and Quinoa Alleviate Hepatic Inflammation via Regulating Metabolic Disorders and Gut Microbiota in Mice Fed with High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Preparation of FG with Lactobacillus
2.3. Animals and Treatments
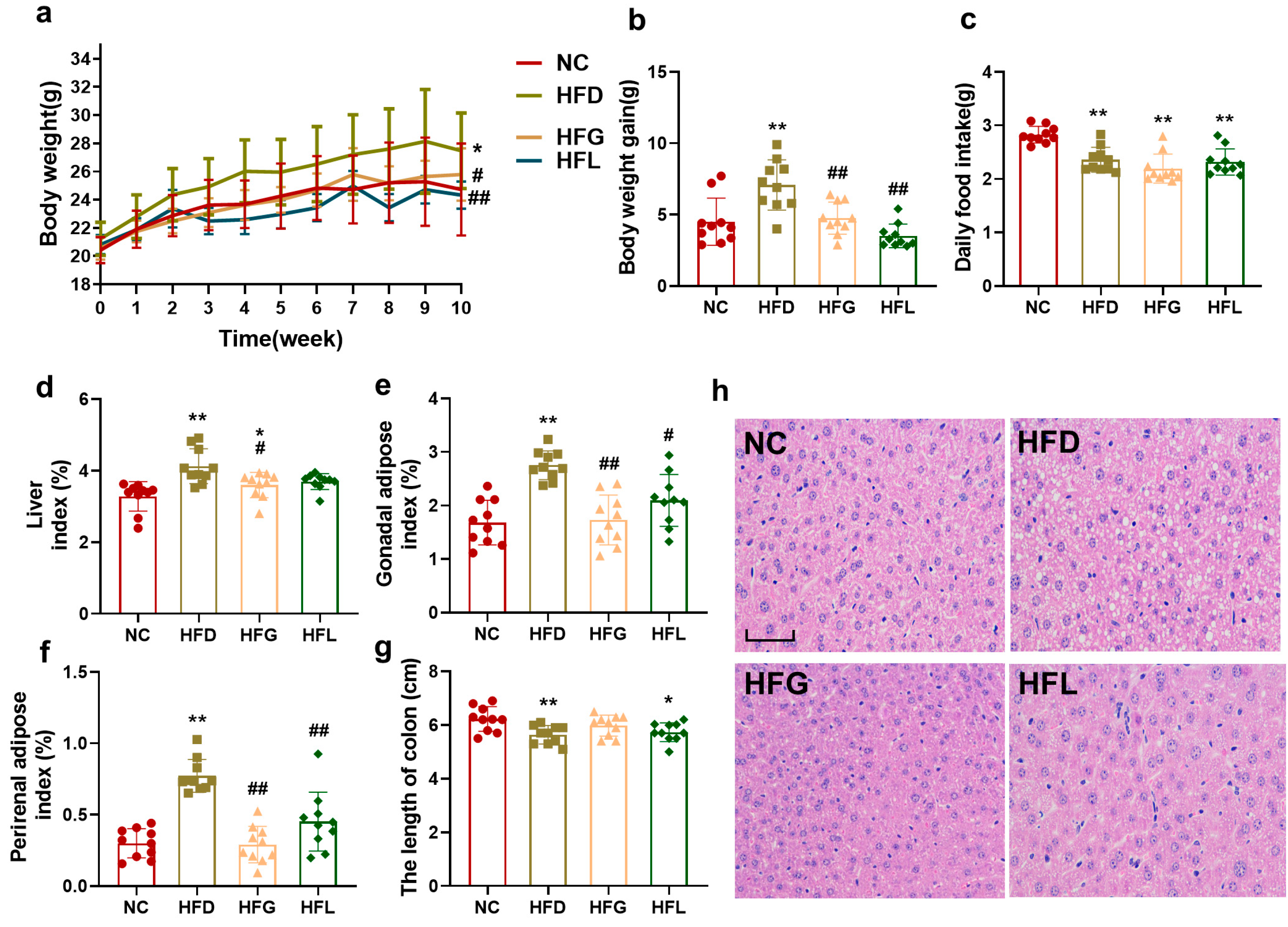
2.3.1. Analysis of Organs Index and Colon Length
2.3.2. Hepatic Hematoxylin–Eosin (HE) Staining Assay
2.3.3. Quantification of Inflammatory Cytokines Using Enzyme-Linked Immunosorbent Assay (ELISA)
2.3.4. RNA Extraction and Quantitative (q) Real-Time Polymerase Chain Reaction (qPCR)
2.3.5. Untargeted Metabolomic Analysis of Fecal and Hepatic Metabolites Using Ultra-Performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (UPLC-QTOF-MSE)
2.3.6. Metagenomic Analysis
2.4. Statistical Analysis
3. Results
3.1. Co-Fermented Quinoa and Black Barley (FG) Prevented High-Fat Diet (HFD)-Induced Obesity in Mice
3.2. FG Reduced Hepatic Inflammation in HFD-Fed Mice
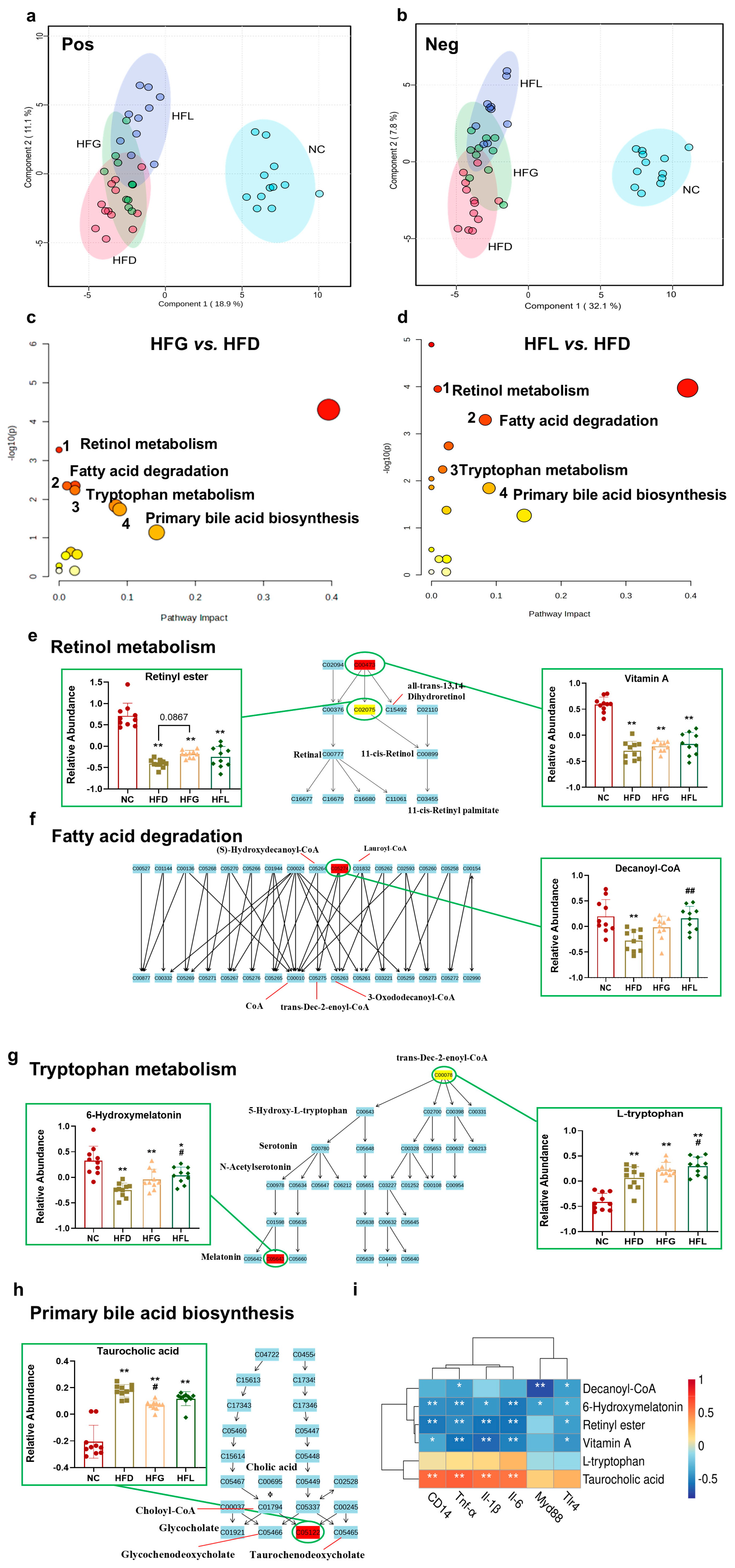
3.3. FG Reversed HFD-Induced Hepatic Metabolites Dysbiosis
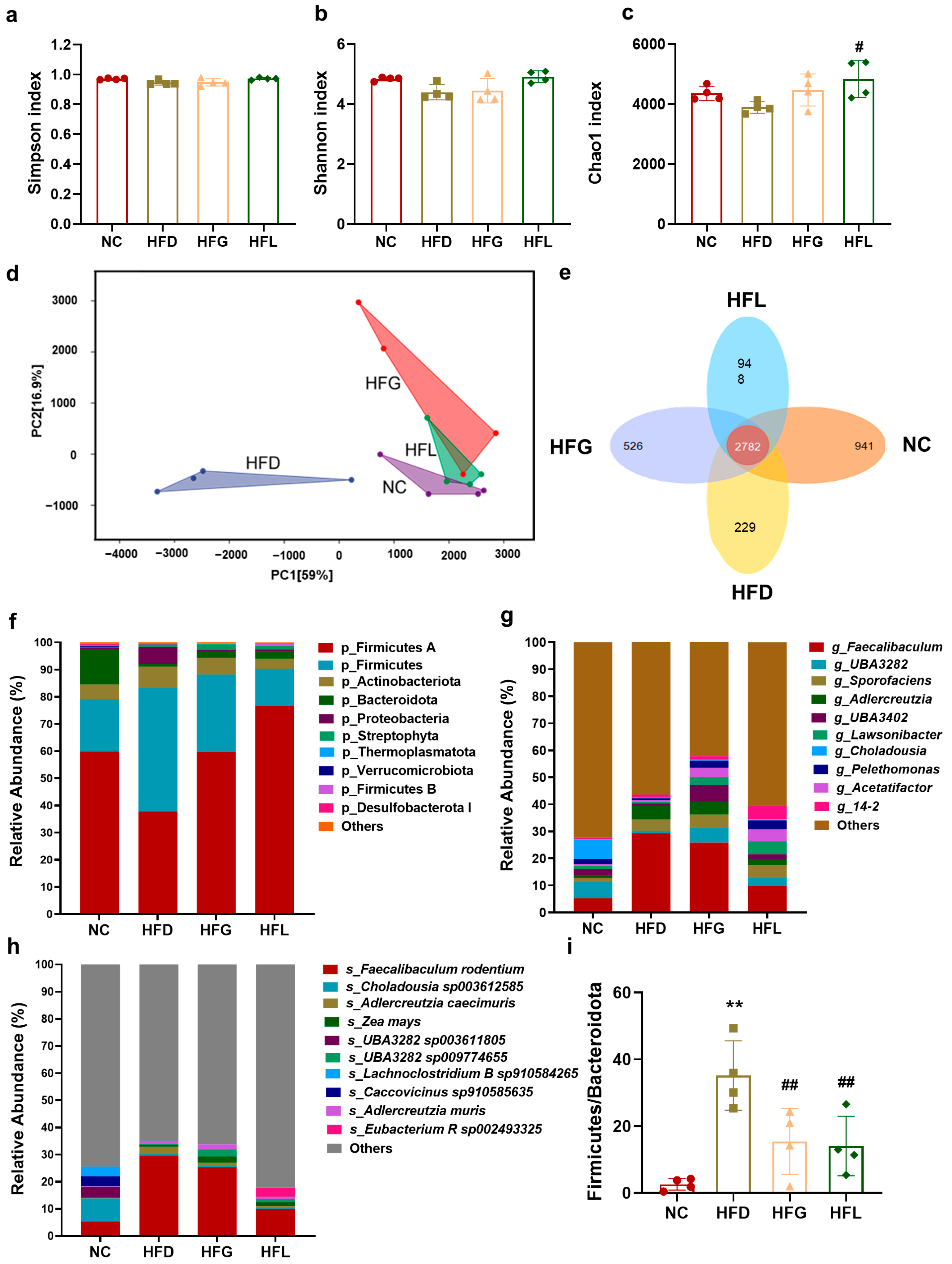
3.4. FG Rescued the Gut Microbiome Dysbiosis Caused by HFD
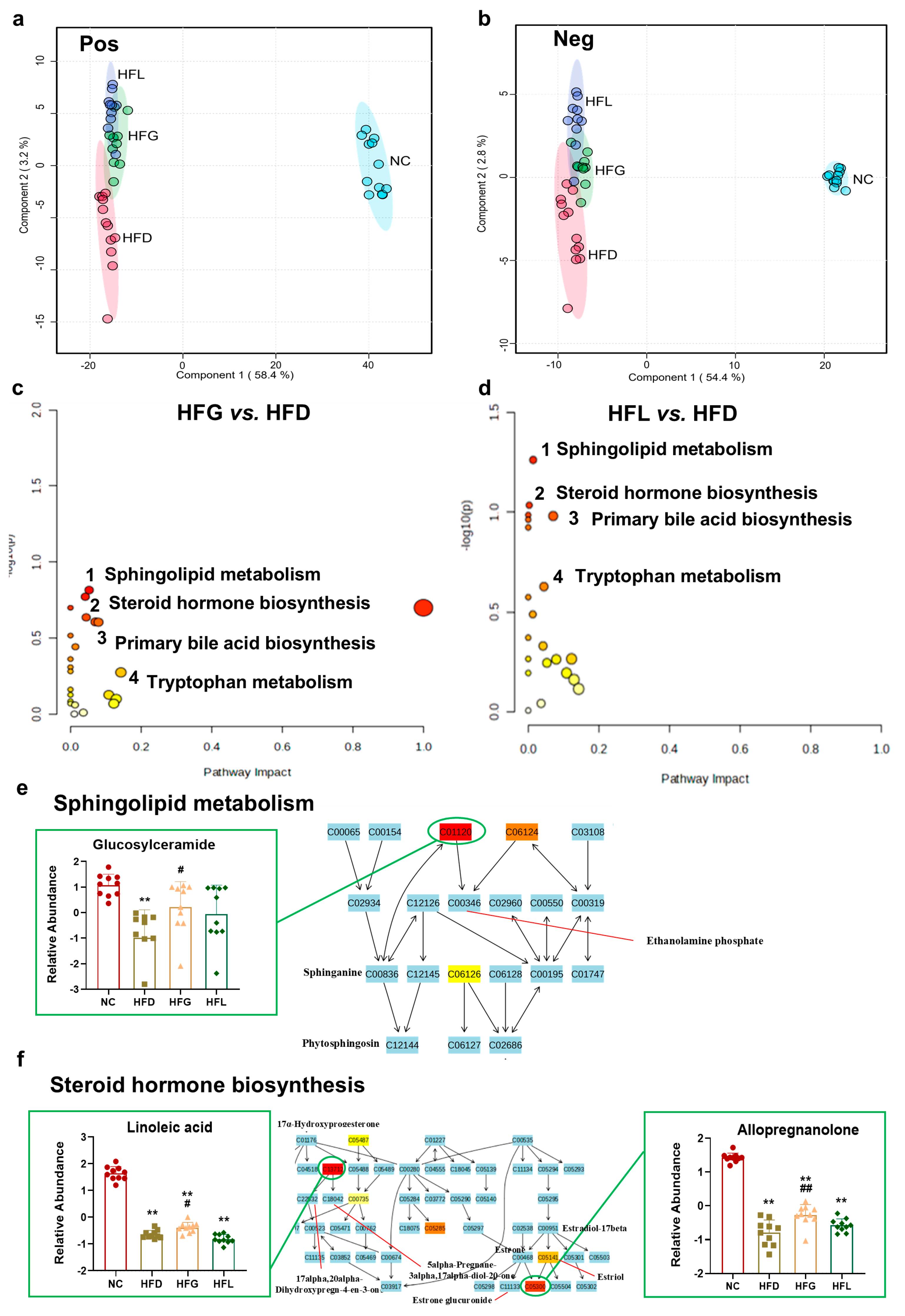
3.5. FG Partially Reversed HFD-Induced Fecal Metabolites Disorder
4. Discussion
4.1. FG Ameliorated Hepatic Chronic Inflammation Induced by Long-Term HFD
4.2. FG Ameliorated Hepatic Chronic Inflammation via Regulating Hepatic Metabolites and Tlr4/Myd88/CD14 Signalling Pathway
4.3. FG Alleviated Hepatic Inflammation via Regulating Gut Microbiota
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HFD | high-fat diet |
| FG | co-fermented whole grains black barley and quinoa |
| TNF-α | tumor necrosis factor-α |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| TLR4 | toll-like receptor 4 |
| Myd88 | myeloid differentiation primary response gene 88 |
| CD14 | cluster of differentiation 14 |
| BA | bile acid |
References
- Nutter, S.; Eggerichs, L.A.; Nagpal, T.S.; Ramos Salas, X.; Chin Chea, C.; Saiful, S.; Ralston, J.; Barata-Cavalcanti, O.; Batz, C.; Baur, L.A.; et al. Changing the global obesity narrative to recognize and reduce weight stigma: A position statement from the World Obesity Federation. Obes. Rev. 2023, 25, e13642. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 127, 1477–1500. [Google Scholar] [CrossRef]
- Taru, V.; Szabo, G.; Mehal, W.; Reiberger, T. Inflammasomes in chronic liver disease: Hepatic injury, fibrosis progression and systemic inflammation. J. Hepatol. 2024, 81, 895–910. [Google Scholar] [CrossRef]
- Gaggini, M.; Carli, F.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; Gambino, R.; Cassader, M.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.L.; Lau, H.C.H.; Wang, Y.X.; Chan, A.W.H.; Wei, H.; Yang, X.Y.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.W.; Rimal, B.; Jiang, C.T.; Chiang, J.Y.L.; Patterson, A.D. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2020, 19, 55–71. [Google Scholar] [CrossRef]
- Sun, J.Y.; Fan, J.M.; Li, T.T.; Yan, X.X.; Jiang, Y.H. Nuciferine Protects Against High-Fat Diet-Induced Hepatic Steatosis via Modulation of Gut Microbiota and Bile Acid Metabolism in Rats. J. Agric. Food Chem. 2022, 70, 12014–12028. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Z.; Wang, X.; Li, M.; Zhou, B.; Zhang, X. Solanum nigrum L. berries extract ameliorated the alcoholic liver injury by regulating gut microbiota, lipid metabolism, inflammation, and oxidative stress. Food Res. Int. 2024, 188, 114489. [Google Scholar] [CrossRef]
- Habermaass, V.; Bartoli, F.; Gori, E.; Dini, R.; Cogozzo, A.; Puccinelli, C.; Pierini, A.; Marchetti, V. Fecal Bile Acids in Canine Chronic Liver Disease: Results from 46 Dogs. Animals 2024, 14, 3051. [Google Scholar] [CrossRef]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef]
- Castro-Alba, V.; Lazarte, C.E.; Perez-Rea, D.; Carlsson, N.G.; Almgren, A.; Bergenståhl, B.; Granfeldt, Y. Fermentation of pseudocereals quinoa, canihua, and amaranth to improve mineral accessibility through degradation of phytate. J. Sci. Food Agric. 2019, 99, 5239–5248. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Michaelidou, A.-M.; Biliaderis, C.G. Fermented Cereal-based Products: Nutritional Aspects, Possible Impact on Gut Microbiota and Health Implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Ding, X.-W.; Zhong, L.-Y.; Zhu, C.; Nie, P.; Song, L.-H. Beneficial effects of Lactobacillus-fermented black barley on high fat diet-induced fatty liver in rats. Food Funct. 2021, 12, 6526–6539. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-H.; Zhong, L.-Y.; Jiang, H.-B.; Zhu, C.; Wei, F.-F.; Wu, Y.; Song, L.-H. Elucidation of the beneficial role of co-fermented whole grain quinoa and black barley with Lactobacillus on rats fed a western-style diet via a multi-omics approach. Food Res. Int. 2024, 187, 114345. [Google Scholar] [CrossRef]
- Zhong, L.Y.; Qin, L.A.; Ding, X.W.; Ma, L.; Wang, Y.; Liu, M.H.; Chen, H.; Yan, H.L.; Song, L.H. The regulatory effect of fermented black barley on the gut microbiota and metabolic dysbiosis in mice exposed to cigarette smoke. Food Res. Int. 2022, 157, 111465. [Google Scholar] [CrossRef]
- Jiang, H.B.; Nie, P.; Lin, Z.H.; Zhu, C.; Zhong, L.Y.; Wei, F.F.; Wu, Y.; Song, L.H. The polyphenols profile of co-fermented quinoa and black barley with Lactobacillus kisonensis and its in vitro bioactivities. Food Biosci. 2024, 58, 103712. [Google Scholar] [CrossRef]
- Dicken, S.J.; Batterham, R.L. The Role of Diet Quality in Mediating the Association between Ultra-Processed Food Intake, Obesity and Health-Related Outcomes: A Review of Prospective Cohort Studies. Nutrients 2021, 14, 23. [Google Scholar] [CrossRef]
- Mei, Z.; Hong, Y.; Yang, H.; Cai, S.; Hu, Y.; Chen, Q.; Yuan, Z.; Liu, X. Ferulic acid alleviates high fat diet-induced cognitive impairment by inhibiting oxidative stress and apoptosis. Eur. J. Pharmacol. 2023, 946, 175642. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.-x.; Guo, S.-d.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Wang, K.; Xue, Y.; Hu, S.; Jin, Y.; Zhu, G.; Shi, Q.; Rui, Y. Irisin alleviates obesity-induced bone loss by inhibiting interleukin 6 expression via TLR4/MyD88/NF-κB axis in adipocytes. J. Adv. Res. 2024, 69, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, Y.; Dai, X.; Liu, T.; Zhang, Y.; Wang, S.; Shi, H.; Yin, J.; Xu, T.; Zhu, R.; et al. Lycopene ameliorates islet function and down-regulates the TLR4/MyD88/NF-κB pathway in diabetic mice and Min6 cells. Food Funct. 2023, 14, 5090–5104. [Google Scholar] [CrossRef]
- Wei, Z.; Xue, Y.; Xue, Y.; Cheng, J.; Lv, G.; Chu, L.; Ma, Z.; Guan, S. Ferulic acid attenuates non-alcoholic steatohepatitis by reducing oxidative stress and inflammation through inhibition of the ROCK/NF-κB signaling pathways. J. Pharmacol. Sci. 2021, 147, 72–80. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Lv, L.X.; Jiang, H.Y.; Wang, K.C.; Yan, R.; Li, Y.T.; Ye, J.Z.; Wu, J.J.; Wang, Q.; Bian, X.Y.; et al. R0052 alleviates liver injury by modulating gut microbiome and metabolome in d-galactosamine-treated rats. Appl. Microbiol. Biot. 2019, 103, 9673–9686. [Google Scholar] [CrossRef]
- Amimo, J.O.; Michael, H.; Chepngeno, J.; Raev, S.A.; Saif, L.J.; Vlasova, A.N. Immune Impairment Associated with Vitamin A Deficiency: Insights from Clinical Studies and Animal Model Research. Nutrients 2022, 14, 5038. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Basafa Roodi, P.; Abaj, F.; Shab-Bidar, S.; Saedisomeolia, A.; Asbaghi, O.; Lak, M. Influence of Vitamin A supplementation on inflammatory biomarkers in adults: A systematic review and meta-analysis of randomized clinical trials. Sci. Rep. 2022, 12, 21384. [Google Scholar] [CrossRef]
- Melnikov, N.; Kamari, Y.; Kandel-Kfir, M.; Barshack, I.; Ben-Amotz, A.; Harats, D.; Shaish, A.; Harari, A. β-Carotene from the Alga Dunaliella bardawil Decreases Gene Expression of Adipose Tissue Macrophage Recruitment Markers and Plasma Lipid Concentrations in Mice Fed a High-Fat Diet. Mar. Drugs 2022, 20, 433. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.; Murray, P.J. Tryptophan and indole metabolism in immune regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 2019, 11, 2062. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tang, X.; Liang, Z.; Chen, M.; Sun, L. Taurocholic acid promotes hepatic stellate cell activation via S1PR2/p38 MAPK/YAP signaling under cholestatic conditions. Clin. Mol. Hepatol. 2023, 29, 465–481. [Google Scholar] [CrossRef]
- Sakamoto, M.; Iino, T.; Yuki, M.; Ohkuma, M. Lawsonibacter asaccharolyticus gen. nov., sp. nov., a butyrate-producing bacterium isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2018, 68, 2074–2081. [Google Scholar] [CrossRef]
- Fang, W.; Xue, H.; Chen, X.; Chen, K.; Ling, W. Supplementation with Sodium Butyrate Modulates the Composition of the Gut Microbiota and Ameliorates High-Fat Diet-Induced Obesity in Mice. J. Nutr. 2019, 149, 747–754. [Google Scholar] [CrossRef]
- Le Sayec, M.; Xu, Y.; Laiola, M.; Gallego, F.A.; Katsikioti, D.; Durbidge, C.; Kivisild, U.; Armes, S.; Lecomte, M.; Fança-Berthon, P.; et al. The effects of Aronia berry (poly)phenol supplementation on arterial function and the gut microbiome in middle aged men and women: Results from a randomized controlled trial. Clin. Nutr. 2022, 41, 2549–2561. [Google Scholar] [CrossRef]
- Prykhodko, O.; Burleigh, S.; Campanello, M.; Iresjö, B.-M.; Zilling, T.; Ljungh, Å.; Smedh, U.; Hållenius, F.F. Long-Term Changes to the Microbiome, Blood Lipid Profiles and IL-6 in Female and Male Swedish Patients in Response to Bariatric Roux-en-Y Gastric Bypass. Nutrients 2024, 16, 498. [Google Scholar] [CrossRef]
- Min, B.H.; Devi, S.; Kwon, G.H.; Gupta, H.; Jeong, J.J.; Sharma, S.P.; Won, S.M.; Oh, K.K.; Yoon, S.J.; Park, H.J.; et al. Gut microbiota-derived indole compounds attenuate metabolic dysfunction-associated steatotic liver disease by improving fat metabolism and inflammation. Gut Microbes 2024, 16, 2307568. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Garcia, K.; Alonso, C.; Iruarrizaga-Lejarreta, M.; D’Amato, M.; Crespo, A.; Iglesias, A.; Cubiella, J.; Bujanda, L.; Falcón-Pérez, J.M. Integrative Analysis of Fecal Metagenomics and Metabolomics in Colorectal Cancer. Cancers 2020, 12, 1142. [Google Scholar] [CrossRef]
- Xu, X.; Li, G.; Zhang, D.; Zhu, H.; Liu, G.h.; Zhang, Z. Gut Microbiota is Associated with Aging-Related Processes of a Small Mammal Species under High-Density Crowding Stress. Adv. Sci. 2023, 10, 2205346. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.F.; Jiang, H.B.; Zhu, C.; Zhong, L.Y.; Lin, Z.H.; Wu, Y.; Song, L.H. The co-fermentation of whole-grain black barley and quinoa improves murine cognitive impairment induced by a high-fat diet via altering gut microbial ecology and suppressing neuroinflammation. Food Funct. 2024, 15, 11667–11685. [Google Scholar] [CrossRef]
- Rovelli, V.; Longo, N. Phenylketonuria and the brain. Mol. Genet. Metab. 2023, 139, 107583. [Google Scholar] [CrossRef]
- Su, X.; Gao, Y.; Yang, R. Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells 2022, 11, 2296. [Google Scholar] [CrossRef]
- Gao, H.; Sun, M.; Li, A.; Gu, Q.; Kang, D.; Feng, Z.; Li, X.; Wang, X.; Chen, L.; Yang, H.; et al. Microbiota-derived IPA alleviates intestinal mucosal inflammation through upregulating Th1/Th17 cell apoptosis in inflammatory bowel disease. Gut Microbes 2025, 17, 2467235. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, S.; Li, S.; Zhang, Q.; Cai, Y.; Li, P.; Li, H.; Shen, B.; Liao, Q.; Hong, Y.; et al. Indoleacrylic acid produced by Parabacteroides distasonis alleviates type 2 diabetes via activation of AhR to repair intestinal barrier. BMC Biol. 2023, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Polonio, C.M.; McHale, K.A.; Sherr, D.H.; Rubenstein, D.; Quintana, F.J. The aryl hydrocarbon receptor: A rehabilitated target for therapeutic immune modulation. Nat. Rev. Drug Discov. 2025, 24, 610–630. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Fan, X.; Wu, Y. Pretreatment with Indole-3-Propionic Acid Attenuates Lipopolysaccharide-Induced Cardiac Dysfunction and Inflammation Through the AhR/NF-κB/NLRP3 Pathway. J. Inflamm. Res. 2024, 17, 5293–5309. [Google Scholar] [CrossRef]
- Zeng, J.; Fan, J.A.; Zhou, H.P. Bile acid-mediated signaling in cholestatic liver diseases. Cell Biosci. 2023, 13, 77. [Google Scholar] [CrossRef]
- Kiriyama, Y.; Nochi, H. The Biosynthesis, Signaling, and Neurological Functions of Bile Acids. Biomolecules 2019, 9, 232. [Google Scholar] [CrossRef]



| Gene Name | Forward Sequence (5’ to 3’) | Reverse Sequence (5’ to 3’) |
|---|---|---|
| Actb | TTCGCGGGCGACGAT | CATCTTTTCACGGTTGGCCT |
| Il-6 | GAGACTTCCATCCAGTTGCCT | TCTCCTCTCCGGACTTGTGA |
| Tnf-a | GCACCACCATCAAGGACTCA | GAGGCAACCTGACCACTCTC |
| Il-1b | GAGCACAAGCCTGTCTTCCT | TCTTGGCCGAGGACTAAGGA |
| Tlr4 | GCACTGTTCTTCTCCTGCCT | AGAGGTGGTGTAAGCCATGC |
| CD14 | AAGCAGATCTGGGGCAGTTC | CGCAGGGCTCCGAATAGAAT |
| Myd88 | AAGCAGCAGAACCAGGAGTC | CGAAAAGTTCCGGCGTTTGT |
| Substance | MS (m/z) | Retention Time (min) | Mass Error (ppm) | Formula | Adducts | VIP | p Value | NC/HFD | HFG/HFD | HFL/HFD |
|---|---|---|---|---|---|---|---|---|---|---|
| Retinyl ester | 303.23 | 9.60 | −0.81 | C20H30O2 | M + H − H2O | 2.11 | 5.7 × 10−14 | ↑ | ↑ | ↑ |
| Vitamin A | 269.23 | 9.60 | −1.50 | C20H30O | M + H − H2O | 2.00 | 2.38 × 10−13 | ↑ | ↑ | ↑ |
| Decanoyl-CoA | 922.26 | 0.93 | −1.35 | C31H54N7O17P3S | M + H | 1.09 | 1.36 × 10−4 | ↑ | ↑ | ↑ |
| 6-Hydroxymelatonin | 271.10 | 0.62 | −2.03 | C13H16N2O3 | M + Na | 1.03 | 4.97 × 10−4 | ↑ | ↑ | ↑ |
| L-tryptophan | 227.08 | 3.51 | −1.62 | C11H12N2O2 | M + Na | 1.01 | 2.03 × 10−8 | ↑ | ↑ | ↑ |
| Taurocholic acid | 498.29 | 6.09 | −0.82 | C26H45NO7S | M + H − H2O | 2.82 | 1.32 × 10−15 | ↓ | ↓ | ↓ |
| Cytidine monophosphate | 423.27 | 6.32 | −1.75 | C24H40O6 | M − H | 1.81 | 9.92 × 10−10 | ↑ | ↑ | ↑ |
| 1b-Hydroxycholic acid | 447.27 | 6.55 | −0.28 | C24H40O6 | M + Na | 1.85 | 6.59 × 10−27 | ↑ | ↑ | ↑ |
| Wharangin | 362.09 | 2.50 | −3.89 | C17H12O8 | M + NH4 | 1.61 | 1.62 × 10−35 | ↑ | ↑ | ↑ |
| L-a-Lysophosphatidylcholine | 490.29 | 7.41 | −0.68 | C22H46NO7P | M + H | 2.07 | 3.59 × 10−22 | ↑ | ↑ | ↑ |
| 7-Methylguanine | 166.07 | 0.93 | −2.31 | C6H7N5O | M − H | 1.60 | 5.49 × 10−29 | ↑ | ↑ | ↑ |
| N2-Succinyl-L-ornithine | 215.10 | 7.63 | −2.04 | C9H16N2O5 | M + H − H2O | 1.76 | 1.03 × 10−12 | ↑ | ↑ | ↑ |
| Luteolin 3’-glucuronide | 485.07 | 0.64 | 3.81 | C21H18O12 | M + Na | 2.16 | 4.09 × 10−32 | ↑ | ↑ | ↑ |
| Sulfolithocholylglycine | 514.28 | 5.75 | −1.67 | C26H43NO7S | M − H | 1.96 | 5.72 × 10−26 | ↓ | ↓ | ↓ |
| PE (15:0/20:2(11Z,14Z)) | 752.52 | 9.60 | 0.64 | C40H76NO8P | M + Na | 1.19 | 1.59 × 10−4 | ↑ | ↑ | ↑ |
| N-Acetylglutamine | 211.07 | 0.91 | −2.92 | C7H12N2O4 | M + Na | 1.70 | 3.57 × 10−29 | ↑ | ↑ | ↑ |
| PC (22:5(7Z,10Z,13Z,16Z,19Z)/14:0) | 802.54 | 7.18 | 1.16 | C44H78NO8P | M + Na | 1.52 | 2.81 × 10−31 | ↓ | ↓ | ↓ |
| Substance | MS (m/z) | Retention Time (min) | Mass Error (ppm) | Formula | Adducts | VIP | p Value | NC/HFD | HFG/HFD | HFL/HFD |
|---|---|---|---|---|---|---|---|---|---|---|
| Glucosylceramide | 682.46 | 6.98 | −2.78 | C36H69NO8 | M + K | 1.41 | 7.12 × 10−32 | ↑ | ↑ | ↑ |
| Linoleic acid | 303.23 | 7.38 | 0.72 | C18H32O2 | M + Na | 1.80 | 2.29 × 10−30 | ↑ | ↑ | ↑ |
| Allopregnanolone | 363.25 | 6.24 | −0.33 | C21H34O2 | M + FA − H | 1.99 | 7.87 × 10−8 | ↑ | ↑ | ↑ |
| Indoleacetaldehyde | 160.08 | 2.66 | −1.16 | C10H9NO | M + H | 2.38 | 2.18 × 10−4 | ↑ | ↑ | ↑ |
| 2-Indolecarboxylic acid | 160.04 | 3.55 | −2.63 | C9H7NO2 | M − H | 1.69 | 3.57 × 10−4 | ↑ | ↑ | ↑ |
| Chenodeoxycholic acid sulfate | 945.51 | 5.37 | −1.17 | C24H40O7S | 2M + H | 1.85 | 9.78 × 10−4 | ↓ | ↓ | ↓ |
| 3,7-Dihydroxy-12-oxocholanoic acid | 389.27 | 5.64 | −1.70 | C24H38O5 | M + H − H2O | 1.59 | 9.22 × 10−21 | ↑ | ↑ | ↑ |
| Trigonelline | 140.07 | 3.57 | 0.72 | C7H9NO2 | M + H | 1.52 | 1.51 × 10−10 | ↑ | ↑ | ↑ |
| Cinnamaldehyde | 177.06 | 6.24 | −2.07 | C9H8O | M + FA − H | 1.62 | 2.02 × 10−24 | ↑ | ↑ | ↑ |
| Tolnaftate | 632.24 | 0.77 | −0.39 | C19H17NOS | 2M + NH4 | 1.53 | 3 × 10−12 | ↑ | ↑ | ↑ |
| 11-Ketoetiocholanolone | 349.20 | 6.04 | −1.83 | C19H28O3 | M + FA − H | 2.43 | 4.27 × 10−8 | ↑ | ↑ | ↑ |
| Disialyllactose | 463.16 | 5.37 | −2.51 | C34H56N2O27 | M + 2H | 2.18 | 3.44 × 10−21 | ↑ | ↑ | ↑ |
| 11-Methyl-7-oxatetracyclo [6.3.1.01,6.04,11] dodecane | 178.14 | 8.37 | −0.49 | C12H18O | M + H − H2O, M + H | 2.26 | 9.06 × 10−17 | ↑ | ↑ | ↑ |
| (3β,17α,23R)-17,23-Epoxy-3,29-dihydroxy-27-norlanost-8-ene-15,24-dione | 473.33 | 6.59 | −0.91 | C29H44O5 | M + H | 1.87 | 3.97 × 10−26 | ↑ | ↑ | ↑ |
| (Z)-α-Bergamotenoic acid | 235.17 | 7.72 | −1.18 | C21H34O2 | M + H | 1.51 | 9.87 × 10−29 | ↑ | ↑ | ↑ |
| Sebacic acid | 183.10 | 5.57 | −2.44 | C10H18O4 | M-H2O − H | 1.62 | 1.04 × 10−15 | ↑ | ↑ | ↑ |
| Amylose | 160.04 | 3.55 | −2.63 | C9H7NO2 | M − H | 1.69 | 3.57 × 10−4 | ↑ | ↑ | ↑ |
| O-Desmethylangolensin | 257.08 | 5.66 | −1.17 | C15H14O4 | M−H | 1.64 | 2.32 × 10−12 | ↑ | ↑ | ↑ |
| Tetracosatetraenoic acid n-6 | 395.27 | 6.03 | −1.16 | C24H40O2 | M + Cl | 1.79 | 3.59 × 10−24 | ↑ | ↑ | ↑ |
| PC (18:3(6Z,9Z,12Z)/20:2(11Z,14Z)) | 842.55 | 6.83 | 0.24 | C46H82NO8P | M + Cl | 1.52 | 1.14 × 10−10 | ↓ | ↓ | ↓ |
| LysoPC (22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | 568.34 | 7.97 | 2.15 | C30H50NO7P | M + H | 1.76 | 1.03 × 10−12 | ↓ | ↓ | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, F.; Jiang, H.; Zhu, C.; Zhong, L.; Lin, Z.; Wu, Y.; Song, L. Co-Fermented Black Barley and Quinoa Alleviate Hepatic Inflammation via Regulating Metabolic Disorders and Gut Microbiota in Mice Fed with High-Fat Diet. Nutrients 2025, 17, 3228. https://doi.org/10.3390/nu17203228
Wei F, Jiang H, Zhu C, Zhong L, Lin Z, Wu Y, Song L. Co-Fermented Black Barley and Quinoa Alleviate Hepatic Inflammation via Regulating Metabolic Disorders and Gut Microbiota in Mice Fed with High-Fat Diet. Nutrients. 2025; 17(20):3228. https://doi.org/10.3390/nu17203228
Chicago/Turabian StyleWei, Fenfen, Huibin Jiang, Chuang Zhu, Lingyue Zhong, Zihan Lin, Yan Wu, and Lihua Song. 2025. "Co-Fermented Black Barley and Quinoa Alleviate Hepatic Inflammation via Regulating Metabolic Disorders and Gut Microbiota in Mice Fed with High-Fat Diet" Nutrients 17, no. 20: 3228. https://doi.org/10.3390/nu17203228
APA StyleWei, F., Jiang, H., Zhu, C., Zhong, L., Lin, Z., Wu, Y., & Song, L. (2025). Co-Fermented Black Barley and Quinoa Alleviate Hepatic Inflammation via Regulating Metabolic Disorders and Gut Microbiota in Mice Fed with High-Fat Diet. Nutrients, 17(20), 3228. https://doi.org/10.3390/nu17203228








