Clinical Applications of Probiotics in Pediatric Dentistry and Orthodontics—A Systematic Review
Abstract
1. Introduction
- -
- Orthodontic treatment: Patients with fixed appliances are prone to plaque accumulation and the growth of cariogenic microorganisms, which increase the risk of white spot lesions, caries, and gingivitis.
- -
- Pediatric dentistry: Early modulation of the oral microbiome through probiotics may reduce caries prevalence and improve periodontal health in children and adolescents.
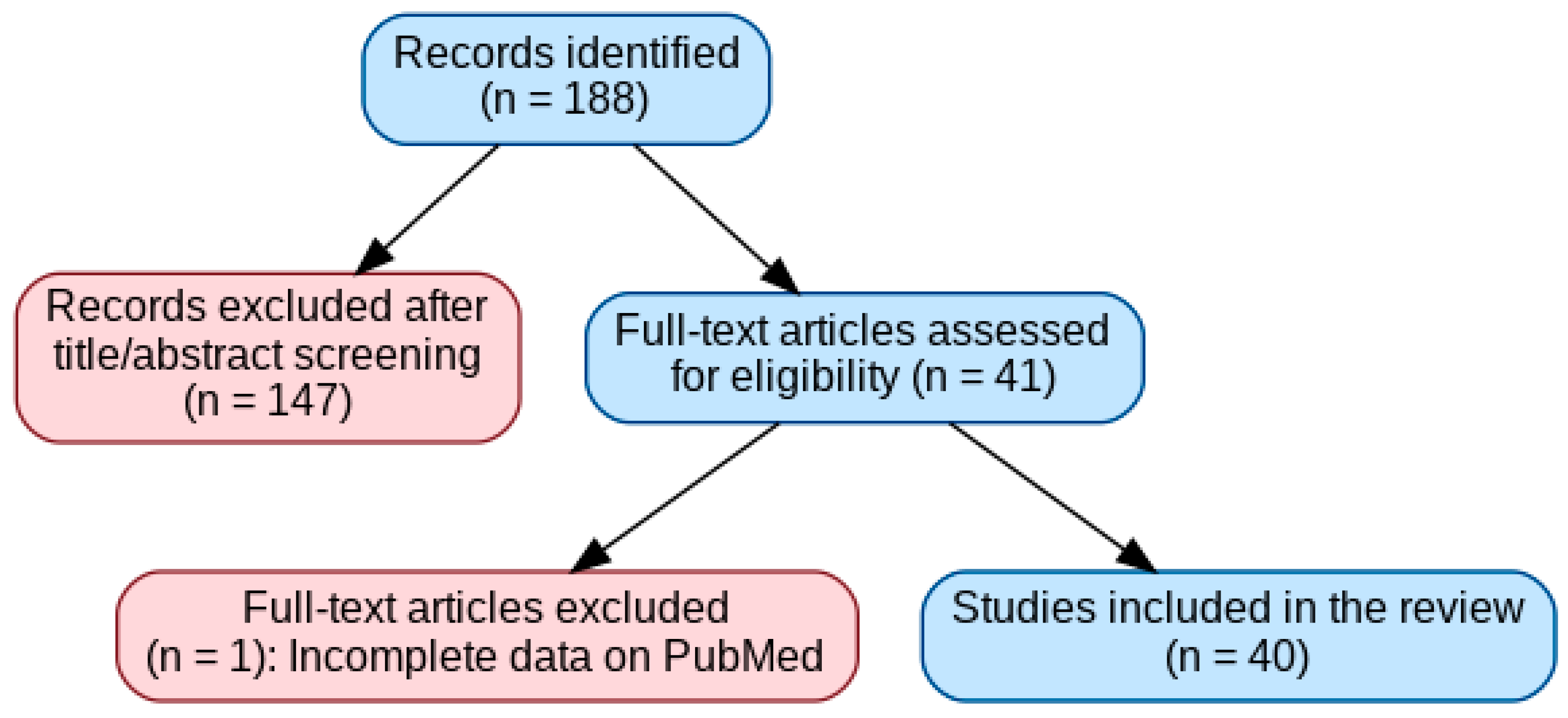
2. Materials and Methods
2.1. Protocol Employed
2.2. Risk of Bias
2.3. Review Hypotheses
2.4. Study Selection
- -
- 71 have periodontology as their topic;
- -
- 15 have caries as a topic;
- -
- 29 oral health in general;
- -
- 17 implantology;
- -
- 20 orthodontics;
- -
- 6 prosthetics;
- -
- 19 pedodontics;
- -
- 1 endodontics;
- -
- 4 on surgery;
- -
- 1 on COVID;
- -
- 1 smoking;
- -
- 1 pregnancy;
- -
- 2 dental materials;
- -
- 1 article was not relevant to the research.
2.5. Inclusion Criteria
2.6. Exclusion Criteria
2.7. Search Strategy
2.8. Data Selection and Coding
2.9. Statistical Analysis
3. Results
- Types of Included Studies
- -
- Randomized controlled trials (RCTs): 11 studies. These represent the highest level of evidence among the included studies and investigated the efficacy of probiotics under controlled clinical conditions.
- -
- Systematic reviews: 6 studies; These provided a comprehensive synthesis of the available evidence, assessing the overall effect of probiotics on oral health.
- -
- Meta-analyses: 2 studies. These studies combined data from multiple randomized clinical trials to provide an overall quantitative estimate of the effects of probiotics on oral health.
- -
- Clinical trials (non-RCTs): 9 studies. These evaluated probiotics without complete randomization.
- -
- Pilot studies: 4 studies. Conducted primarily to evaluate feasibility, these studies provided preliminary data on clinical benefits and tolerability.
- -
- In vitro studies: 4 studies. These evaluated the antimicrobial effects of probiotics and their interaction with orthodontic materials.
- -
- Preclinical/animal studies: 3 studies. Conducted in animal models, these explored mechanistic aspects, such as the impact on bone remodeling during orthodontic treatment.
- -
- Narrative review: 1 study. A narrative review addressed the potential use of Streptococcus spp. for the prevention of caries in patients with special needs.
- Setting of Included studies
- Area of the Mouth Assessed
- Comparisons Used in Included Studies
- Results of Included Studies
- -
- Microbiological outcomes: Some studies evaluated changes in the counts of Streptococcus mutans, Lactobacillus, and other bacterial strains in saliva or plaque.
- -
- Clinical outcomes: Some studies evaluated the plaque index, gingival bleeding index, and the development of white spot lesions.
- -
- Patient-focused outcomes: Other studies evaluated the potential reduction in halitosis, potential improvement of traumatic lesions in orthodontic patients, and changes in salivary pH.
- -
- Material-related outcomes: In vitro studies evaluated the effect of probiotics on the surface roughness and hardness of orthodontic alloys.
- -
- Bone-related outcomes: Animal studies evaluated the speed of orthodontic tooth movement and osteoclast activity.
- Methodological Quality of Included Studies
- Probiotics and orthodontics
- 2.
- Probiotics and Pediatric Dentistry
4. Discussion
Publication Bias and Sponsorship Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozen, M.; Dinleyici, E.C. The History of Probiotics: The Untold Story. Benef. Microbes 2015, 6, 159–165. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics History. J. Clin. Gastroenterol. 2016, 50, S116–S119. [Google Scholar] [CrossRef] [PubMed]
- Seminario-Amez, M.; López-López, J.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Jané-Salas, E. Probiotics and Oral Health: A Systematic Review. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e282–e288. [Google Scholar] [CrossRef]
- Santacroce, L.; Passarelli, P.C.; Azzolino, D.; Bottalico, L.; Charitos, I.A.; Cazzolla, A.P.; Colella, M.; Topi, S.; Godoy, F.G.; D’Addona, A. Oral Microbiota in Human Health and Disease: A Perspective. Exp. Biol. Med. 2023, 248, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.; Matsubara, V.H. Normal Oral Flora and the Oral Ecosystem. Dent. Clin. N. Am. 2017, 61, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Takeshita, T. The Oral Microbiome and Human Health. J. Oral Sci. 2017, 59, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.H.; Pourjafar, H.; Mirzakhani, E. A Comprehensive Review of the Application of Probiotics and Postbiotics in Oral Health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef]
- Ritthagol, W.; Saetang, C.; Teanpaisan, R. Effect of Probiotics Containing Lactobacillus paracasei SD1 on Salivary Mutans Streptococci and Lactobacilli in Orthodontic Cleft Patients: A Double-Blinded, Randomized, Placebo-Controlled Study. Cleft Palate-Craniofacial J. 2014, 51, 257–263. [Google Scholar] [CrossRef]
- Benic, G.Z.; Farella, M.; Morgan, X.C.; Viswam, J.; Heng, N.C.; Cannon, R.D.; Mei, L. Oral Probiotics Reduce Halitosis in Patients Wearing Orthodontic Braces: A Randomized, Triple-Blind, Placebo-Controlled Trial. J. Breath. Res. 2019, 13, 036010. [Google Scholar] [CrossRef]
- Gizani, S.; Petsi, G.; Twetman, S.; Caroni, C.; Makou, M.; Papagianoulis, L. Effect of the Probiotic Bacterium Lactobacillus reuteri on White Spot Lesion Development in Orthodontic Patients. Eur. J. Orthod. 2016, 38, 85–89. [Google Scholar] [CrossRef]
- Silva, N.L.N.V.; Della Bona, A.; Cardoso, M.; Callegari-Jacques, S.M.; Fornari, F. Lactobacillus Brevis CD2 Attenuates Traumatic Oral Lesions Induced by Fixed Orthodontic Appliance: A Randomized Phase 2 Trial. Orthod. Craniofac. Res. 2021, 24, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Tahir, K.; Barakaat, A.A.; Shoukat Ali, U.; Fida, M.; Sukhia, R.H. Effect of Probiotic Toothpaste and Regular Toothpaste on Gingival Health and Plaque Levels of Adult Orthodontic Patients—An Open Label Randomized Controlled Trial. Int. Orthod. 2025, 23, 100938. [Google Scholar] [CrossRef]
- Hedayati-Hajikand, T.; Lundberg, U.; Eldh, C.; Twetman, S. Effect of Probiotic Chewing Tablets on Early Childhood Caries—A Randomized Controlled Trial. BMC Oral Health 2015, 15, 112. [Google Scholar] [CrossRef]
- Alamoudi, N.M.; Almabadi, E.S.; El Ashiry, E.A.; El Derwi, D.A. Effect of Probiotic Lactobacillus reuteri on Salivary Cariogenic Bacterial Counts among Groups of Preschool Children in Jeddah, Saudi Arabia: A Randomized Clinical Trial. J. Clin. Pediatr. Dent. 2018, 42, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Stensson, M.; Koch, G.; Coric, S.; Abrahamsson, T.R.; Jenmalm, M.C.; Birkhed, D.; Wendt, L.K. Oral Administration of Lactobacillus reuteri During the First Year of Life Reduces Caries Prevalence in the Primary Dentition at 9 Years of Age. Caries Res. 2014, 48, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Kaklamanos, E.G.; Nassar, R.; Kalfas, S.; Al Halabi, M.; Kowash, M.; Hannawi, H.; Hussein, I.; Salami, A.; Hassan, A.; Senok, A.C. A Single-Centre Investigator-Blinded Randomised Parallel Group Clinical Trial to Investigate the Effect of Probiotic Strains Streptococcus Salivarius M18 and Lactobacillus acidophilus on Gingival Health of Pediatric Patients Undergoing Treatment with Fixed Orthodontic Appliances: Study Protocol. BMJ Open 2019, 9, e030638. [Google Scholar]
- Stecksén-Blicks, C.; Sjöström, I.; Twetman, S. Effect of Long-Term Consumption of Milk Supplemented with Probiotic Lactobacilli and Fluoride on Dental Caries and General Health in Preschool Children: A Cluster-Randomized Study. Caries Res. 2009, 43, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Nase, L.; Hatakka, K.; Savilahti, E.; Saxelin, M.; Pönkä, A.; Poussa, T.; Korpela, R.; Meurman, J.H. Effect of Long-Term Consumption of a Probiotic Bacterium, Lactobacillus rhamnosus GG, in Milk on Dental Caries and Caries Risk in Children. Caries Res. 2001, 35, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Robin, V.; Wim, T.; Maria, C.d.L.; Isabelle, L. Probiotics for Maintaining Oral Health during Fixed Orthodontic Treatment: A Systematic Review and Meta-Analysis. Int. J. Dent. Hyg. 2025, 23, 100–113. [Google Scholar] [CrossRef]
- Chen, W.; Ren, J.; Li, J.; Peng, S.; Zhang, C.; Lin, Y. Effects of Probiotics on the Oral Health of Patients Undergoing Orthodontic Treatment: A Systematic Review and Meta-Analysis. Eur. J. Orthod. 2023, 45, 599–611. [Google Scholar] [CrossRef]
- Pavlic, A.; Perissinotto, F.; Turco, G.; Contardo, L.; Stjepan, S. Do Chlorhexidine and Probiotics Solutions Provoke Corrosion of Orthodontic Mini-Implants? An In Vitro Study. Int. J. Oral Maxillofac. Implants 2019, 34, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Alp, S.; Baka, Z.M. Effects of Probiotics on Salivary Streptococcus mutans and Lactobacillus Levels in Orthodontic Patients. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Hadj-Hamou, R.; Senok, A.C.; Athanasiou, A.E.; Kaklamanos, E.G. Do Probiotics Promote Oral Health during Orthodontic Treatment with Fixed Appliances? A Systematic Review. BMC Oral Health 2020, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Pietri, F.K.; Rossouw, P.E.; Javed, F.; Michelogiannakis, D. Role of Probiotics in Oral Health Maintenance Among Patients Undergoing Fixed Orthodontic Therapy: A Systematic Review of Randomized Controlled Clinical Trials. Probiotics Antimicrob. Proteins 2020, 12, 1349–1359. [Google Scholar] [CrossRef]
- Lemos, I.d.S.; Jassé, F.F.d.A.; Suzuki, S.S.; Alencar, C.d.M.; Fujii, D.N.; Zaniboni, J.F.; Suzuki, H.; Segundo, A.S.G. Antimicrobial Activity of Probiotics against Oral Pathogens around Orthodontic Mini-Implants: An In Vitro Study. Dental Press. J. Orthod. 2021, 26, e2119350. [Google Scholar] [CrossRef] [PubMed]
- Duffles, L.F.; Menino, A.P.; Taira, T.M.; de Oliveira, S.; Salvador, S.L.; Messora, M.R.; Vinolo, M.A.R.; Fukada, S.Y. Probiotic Bifidobacterium animalis subsp. Lactis Consumption Slows down Orthodontic Tooth Movement in Mice. Arch. Oral Biol. 2022, 134, 105324. [Google Scholar] [CrossRef]
- Pazzini, C.A.; Pereira, L.J.; da Silva, T.A.; Montalvany-Antonucci, C.C.; Macari, S.; Marques, L.S.; de Paiva, S.M. Probiotic Consumption Decreases the Number of Osteoclasts during Orthodontic Movement in Mice. Arch. Oral Biol. 2017, 79, 30–34. [Google Scholar] [CrossRef]
- Kolip, D.; Yılmaz, N.; Gökkaya, B.; Kulan, P.; Kargul, B.; MacDonald, K.W.; Cadieux, P.A.; Burton, J.P.; James, K.M. Efficacy of Dentaq® Oral and ENT Health Probiotic Complex on Clinical Parameters of Gingivitis in Patients Undergoing Fixed Orthodontic Treatment: A Pilot Study. J. Clin. Dent. 2016, 27, 66–70. [Google Scholar]
- Jose, J.E.; Padmanabhan, S.; Chitharanjan, A.B. Systemic Consumption of Probiotic Curd and Use of Probiotic Toothpaste to Reduce Streptococcus mutans in Plaque around Orthodontic Brackets. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Gokce, G.; Savas, S.; Kucukyilmaz, E.; Veli, I. Effekte Verschiedener Zahnpasten Auf Bracketnahe White-Spot-Läsionen, Ermittelt Mit Quantitativer Lichtinduziertern Fluoreszenz (QLF): Eine In-vitro-Studie. J. Orofac. Orthop. 2017, 78, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Voronkova, A.V.; Smaglyuk, L.V. Changes in Biochemical Parameters of Oral Fluid in Patients during the Orthodontic Treatment with a Bracket System under the Action of a Developed Mucosal Gel with Probiotic. Wiad. Lek. 2018, 71, 496–500. [Google Scholar] [PubMed]
- Cildir, S.K.; Germec, D.; Sandalli, N.; Ozdemir, F.I.; Arun, T.; Twetman, S.; Caglar, E. Reduction of Salivary Mutans Streptococci in Orthodontic Patients during Daily Consumption of Yoghurt Containing Probiotic Bacteria. Eur. J. Orthod. 2009, 31, 407–411. [Google Scholar] [CrossRef]
- Sujlana, A.; Goyal, R.; Pannu, P.; Opal, S.; Bansal, P. Visual Pedagogy and Probiotics for Hearing Impaired Children: A Pilot Study. J. Indian Soc. Pedod. Prev. Dent. 2017, 35, 353–358. [Google Scholar] [CrossRef]
- Mato, E.G.; Montaño-Barrientos, B.J.; Rivas-Mundiña, B.; Aneiros, I.V.; López, L.S.; Posse, J.L.; Lamas, L.M. Anti-Caries Streptococcus spp.: A Potential Preventive Tool for Special Needs Patients. Spec. Care Dent. 2024, 44, 813–822. [Google Scholar] [CrossRef]
- Cortés-Dorantes, N.; Ruiz-Rodríguez, M.S.; Karakowsky-Kleiman, L.; Garrocho-Rangel, J.A.; Sánchez-Vargas, L.O.; Pozos-Guillén, A.J. Probiotics and Their Effect on Oral Bacteria Count in Children: A Pilot Study. Eur. J. Paediatr. Dent. 2015, 16, 56–60. [Google Scholar] [PubMed]
- Mayta-Tovalino, F.; Maguiña-Quispe, J.; Barja-Ore, J.; Hernandez, A.V. Efficacy of Probiotic Consumption on Oral Outcomes in Children and/or Adolescents: A Meta-Analysis. Int. Dent. J. 2024, 74, 1205–1219. [Google Scholar] [CrossRef]
- Kavitha, M.; Prathima, G.S.; Anusha, D.; Kengadaran, S.; Gayathri, K.; Vinothini, V. Evaluation of the Efficacy of Plaque Reduction and Gingival Health among 6-12 Years Old School Children before and after a Short Term Daily Intake of Probiotic Lozenge—A Comparative Study. Indian J. Dent. Res. 2022, 33, 184–187. [Google Scholar] [CrossRef]
- Borrell García, C.; Ribelles Llop, M.; García Esparza, M.Á.; Flichy-Fernández, A.J.; Marqués Martínez, L.; Izquierdo Fort, R. The Use of Lactobacillus reuteri DSM 17938 and ATCC PTA 5289 on Oral Health Indexes in a School Population: A Pilot Randomized Clinical Trial. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211031107. [Google Scholar] [CrossRef]
- Jindal, G.; Pandey, R.K.; Agarwal, J.; Singh, M. A Comparative Evaluation of Probiotics on Salivary Mutans Streptococci Counts in Indian Children. Eur. Arch. Pediatr. Dent. 2011, 12, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.; Trent, B.; Vorachek, A.; Kramer, S.; Esterly, R. Effectiveness of CRT at Measuring the Salivary Level of Bacteria in Caries Prone Children with Probiotic Therapy. J. Clin. Pediatr. Dent. 2013, 38, 55–60. [Google Scholar] [CrossRef]
- Lexner MO, B.S.D.G.T.S. Microbiological Profiles in Saliva and Supragingival Plaque from Caries-Active Adolescents before and after a Short-Term Daily Intake of Milk Supplemented with Probiotic Bacteria—A Pilot Study. Oral Health Prev. Dent. 2010, 8, 383–388. [Google Scholar]
- Taipale, T.; Pienihäkkinen, K.; Salminen, S.; Jokela, J.; Söderling, E. Bifidobacterium animalis subsp. Lactis BB-12 Administration in Early Childhood: A Randomized Clinical Trial of Effects on Oral Colonization by Mutans Streptococci and the Probiotic. Caries Res. 2012, 46, 69–77. [Google Scholar] [CrossRef]
- Taipale, T.; Pienihäkkinen, K.; Alanen, P.; Jokela, J.; Söderling, E. Administration of Bifidobacterium animalis subsp. Lactis Bb-12 in Early Childhood: A Post-Trial Effect on Caries Occurrence at Four Years of Age. Caries Res. 2013, 47, 364–372. [Google Scholar] [CrossRef]
- Maspero, C.; Galbiati, G.; Giannini, L.; Guenza, G.; Farronato, M. Class II division 1 malocclusions: Comparisons between one- and two-step treatment. Eur. J. Paediatr. Dent. 2018, 19, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Alanzi, A.; Honkala, S.; Honkala, E.; Varghese, A.; Tolvanen, M.; Söderling, E. Effect of Lactobacillus rhamnosus and Bifidobacterium lactis on Gingival Health, Dental Plaque, and Periodontopathogens in Adolescents: A Randomised Placebocontrolled Clinical Trial. Benef. Microbes 2018, 9, 593–602. [Google Scholar] [CrossRef] [PubMed]
| Topic | Authors (Year) | Study Design | Random Sequence Generation | Allocation Concealment | Blinding (Participants/Personnel) | Blinding (Outcome Assessment) | Incomplete Outcome Data | Selective Reporting | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|
| Orthodontics | Ritthagol W. et al. (2014) [9] | Double-blind RCT (cleft patients) | Low | Low | Low | Low | Low | Low | Low |
| Orthodontics | Benic G.Z. et al. (2019) [10] | Triple-blind RCT | Low | Low | Low | Low | Low | Low | Low |
| Orthodontics | Gizani S. et al. (2016) [11] | Double-blind RCT | Low | Low | Low | Low | Low | Low | Low |
| Orthodontics | Silva N.L.N.V. et al. (2021) [12] | Phase 2 RCT | Unclear | Low | Low | Low | Low | Low | Some concerns |
| Orthodontics | Tahir K. et al. (2025) [13] | Open-label RCT | Low | Low | High | High | Low | Low | High |
| Pediatric Dentistry | Hedayati-Hajikand T. et al. (2015) [14] | RCT | Low | Low | Low | Low | Low | Low | Low |
| Pediatric Dentistry | Alamoudi N.M. et al. (2018) [15] | RCT | Low | Low | Low | Low | Low | Low | Low |
| Pediatric Dentistry | Stensson M. et al. (2014) [16] | Single-blind, placebo-controlled study | Low | Low | Low | Low | Some concerns (attrition) | Low | Some concerns |
| Pediatric Dentistry/Orthodontics | Kaklamanos E.G. et al. (2019) [17] | RCT protocol | Low | Low | Low | Low | Not applicable | Not applicable | Low |
| Pediatric Dentistry | Stecksén-Blicks C. et al. (2009) [18] | Cluster RCT | Low | Low | High | High | Some concerns | Low | Some concerns |
| Pediatric Dentistry | Näse L. et al. (2001) [19] | RCT | Low | Low | Low | Low | Some concerns (attrition) | Low | Some concerns |
| Authors | Title | Year | Study Design | Participants (Number, Age, Gender) | Outcomes |
|---|---|---|---|---|---|
| Robin V, Wim T, Maria CL, Isabelle L. | Probiotics for maintaining oral health during fixed orthodontic treatment: A systematic review and meta-analysis [20]. | 2025 | Systematic review and meta-analysis | At least 10 patients/group undergoing fixed orthodontic therapy | Reduction in Streptococcus mutans counts, no significant effect on lactobacilli and white spot lesions. Interesting results on halitosis and oral traumatic lesions. Equivocal effects on plaque. |
| Chen W, Ren J, Li J, Peng S, Zhang C, Lin Y. | Effects of probiotics on the oral health of patients undergoing orthodontic treatment: a systematic review and meta-analysis [21]. | 2023 | Systematic review and meta-analysis | 405 Patients undergoing orthodontic treatment | Controversial results on clinical outcomes (plaque, gingival index). Significant reduction in Streptococcus mutans. |
| Pavlic A, Perissinotto F, Turco G, Contardo L, Spalj S. | Do Chlorhexidine and Probiotics Solutions Provoke Corrosion of Orthodontic Mini-implants? An In Vitro Study [22]. | 2019 | In vitro study | 0 | Probiotics increase the roughness of titanium implants, while chlorhexidine increases the roughness of steel implants. Chlorhexidine combined with probiotics reduces the microhardness of steel implants. |
| Alp S, Baka ZM. | Effects of probiotics on salivary Streptecoccus mutans and Lactobacillus levels in orthodontic patients [23]. | 2018 | Clinical study | 45 orthodontic patients: (27 F, 18 M) (3 groups of 15: 15 patients in the kefir group (7 girls, 8 boys; mean age, 14.3 ± 1.7 years), 15 patients in the dentifrice group (10 girls, 5 boys; mean age, 14.9 ± 2.0 years), and 15 patients in the control group (10 girls, 5 boys; mean age, 14.1 ± 2.1 years). | Significant reduction in Streptococcus mutans and Lactobacillus with the use of probiotics (kefir and toothpaste). Significant increase in buffering capacity with the use of probiotic-containing toothpaste. |
| Hadj-Hamou R, Senok AC, Athanasiou AE, Kaklamanos EG. | Do probiotics promote oral health during orthodontic treatment with fixed appliances? A systematic review [24]. | 2020 | systematic review | 0 | No significant effect of probiotics on gingival inflammation and enamel demineralization. |
| Pietri FK, Rossouw PE, Javed F, Michelogiannakis D. | Role of Probiotics in Oral Health Maintenance Among Patients Undergoing Fixed Orthodontic Therapy: a Systematic Review of Randomized Controlled Clinical Trials [25]. | 2020 | Systematic Review of Randomized Controlled Clinical Trials | The total number of participants across all included studies ranged between 24 and 85. In 8 studies, the patients’ ages ranged from 8 to 35 years. Six studies included both male and female participants. | Improvement of oral health and reduction in pathogenic bacteria with probiotics. Conflicting results on plaque and gingivitis. No effect on white spot lesions formation. |
| Ritthagol W, Saetang C, Teanpaisan R. | Effect of Probiotics Containing Lactobacillus paracasei SD1 on Salivary Mutans Streptococci and Lactobacilli in Orthodontic Cleft Patients: A Double-Blinded, Randomized, Placebo-Controlled Study [9]. | 2014 | Double-blinded, randomized, placebo-controlled study | 30 orthodontic patients with cleft lip and palate (mean age 19.22 ± 3.66 years) | Significant reduction in mutans streptococci and increase in lactobacilli with L. paracasei SD1. |
| Lemos IDS, Jassé FFA, Suzuki SS, Alencar CM, Fujii DN, Zaniboni JF, Suzuki H, Garcez Segundo AS. | Antimicrobial activity of probiotics against oral pathogens around orthodontic mini-implants: an in vitro study [26]. | 2021 | In vitro study | 120 mini-screw | L. casei, L. brevis, L. rhamnosus and Lactobacillus from fermented milk Batavito® showed antimicrobial activity against S. aureus. |
| Duffles LF, Menino AP, Taira TM, de Oliveira S, Salvador SL, Messora MR, Vinolo MAR, Fukada SY. | Probiotic Bifidobacterium animalis subsp. lactis consumption slows down orthodontic tooth movement in mice [27]. | 2022 | Clinical study | 30 male C57BL6/J mice | In mice, Bifidobacterium animalis subsp. lactis slowed orthodontic tooth movement. |
| Benic GZ, Farella M, Morgan XC, Viswam J, Heng NC, Cannon RD, Mei L. | Oral probiotics reduce halitosis in patients wearing orthodontic braces: a randomized, triple-blind, placebo-controlled trial [10]. | 2019 | Randomized, triple-blind, placebo-controlled trial | 64 patients with fixed orthodontic appliances (32 in the probiotic group, 32 in the placebo group) | Reduction in halitosis with Streptococcus salivarius M18. Mild effects on plaque and gingivitis. |
| Silva NLNV, Della Bona A, Cardoso M, Callegari-Jacques SM, Fornari F. | Lactobacillus brevis CD2 attenuates traumatic oral lesions induced by fixed orthodontic appliance: A randomized phase 2 trial [12]. | 2021 | randomized phase 2 trial | 20 orthodontic patients (14–57 years, 70% women in the probiotic group, 60% women in the placebo group) | The use of Lactobacillus brevis CD2 reduced the duration of traumatic oral lesions and pain. |
| Tahir K, Barakaat AA, Shoukat Ali U, Fida M, Sukhia RH. | Effect of probiotic toothpaste and regular toothpaste on gingival health and plaque levels of adult orthodontic patients—An open label randomized controlled trial [13]. | 2025 | Open label randomized controlled trial | 44 adult orthodontic patients (18–50 years) | Probiotic toothpaste improved the gingival bleeding index, but had no effect in the plaque index. |
| Gizani S, Petsi G, Twetman S, Caroni C, Makou M, Papagianoulis L. | Effect of the probiotic bacterium Lactobacillus reuteri on white spot lesion development in orthodontic patients [11]. | 2016 | Randomized double-blind placebo-controlled study | 85 patients (average age15.9 years) | Probiotic intake did not influence the development of white spot lesions. |
| Pazzini CA, Pereira LJ, da Silva TA, Montalvany-Antonucci CC, Macari S, Marques LS, de Paiva SM. | Probiotic consumption decreases the number of osteoclasts during orthodontic movement in mice [28]. | 2017 | Mice C57BL/6 | In mice, Bacillus Sub-ti-is was associated with a reduction in osteoclasts during orthodontic movement. | |
| Kolip D, Yılmaz N, Gökkaya B, Kulan P, Kargul B, MacDonald KW, Cadieux PA, Burton JP, James KM. | Efficacy of Dentaq® Oral and ENT Health Probiotic Complex on Clinical Parameters of Gingivitis in Patients Undergoing Fixed Orthodontic Treatment: A Pilot Study [29]. | 2016 | Pilot study | 15 patients (11–18 years) | Probiotics reduced plaque and gingival inflammation. |
| Jose JE, Padmanabhan S, Chitharanjan AB. | Systemic consumption of probiotic curd and use of probiotic toothpaste to reduce Streptococcus mutans in plaque around orthodontic brackets [30]. | 2013 | Clinical study | 60 orthodontic patients divided into 3 groups of 20: Group 1: control. Group 2: probiotic yogurt, Group 3: were asked to brush their teeth twice a day with a probiotic toothpaste (GD toothpaste). | Probiotic curd and probiotic toothpaste reduced Streptococcus mutans in plaque. |
| Gokce G, Savas S, Kucukyilmaz E, Veli I. | Effects of toothpastes on white spot lesions around orthodontic brackets using quantitative light-induced fluorescence (QLF): An in vitro study [31]. | 2017 | In vitro study | 45 extracted mandibular first molars in which caries were artificially recreated. | Significant differences between the initial and post-treatment QLF measurements of the demineralized enamel samples treated with the various agents (p < 0.05). In all experimental groups, a significant increase in fluorescence radiance and a decrease in lesion area were found (p = 0.000). |
| Voronkova AV, Smaglyuk LV. | Changes in biochemical parameters of oral fluid in patients during the orthodontic treatment with a bracket system under the action of a developed mucosal gel with probiotic [32]. | 2018 | Clinical study | 45 patients (18–24 years) | The gel with probiotics improved the biochemical parameters of the oral fluid. |
| Cildir SK, Germec D, Sandalli N, Ozdemir FI, Arun T, Twetman S, Caglar E. | Reduction of salivary mutans streptococci in orthodontic patients during daily consumption of yoghurt containing probiotic bacteria [33]. | 2009 | Double-blind, randomized crossover study | 24 adolescents (12–16 years) | Yogurt with Bifidobacterium animalis subsp. lactis DN-173010 reduced mutans streptococci. |
| Authors | Title | Year | Study Design | Participants (Number, Age, Gender) | Outcomes |
|---|---|---|---|---|---|
| Sujlana A, Goyal R, Pannu P, Opal S, Bansal P. | Visual pedagogy and probiotics for hearing impaired children: A pilot study [34]. | 2017 | Pilot study | 20 children with hearing problems and 20 healthy children | Probiotic mouthwash reduced GI and PI and increased salivary pH. |
| Mato EG, Montaño-Barrientos BJ, Rivas-Mundiña B, Aneiros IV, López LS, Posse JL, Lamas LM. | Anti-caries Streptococcus spp.: A potential preventive tool for special needs patients [35]. | 2024 | Narrative review | The text does not report data relating to a specific sample (number of patients), nor information on the sex or age of the participants. | Review of Streptococcus spp. with anti-Streptococcus mutans activity. |
| Cortés-Dorantes N, Ruiz-Rodríguez MS, Karakowsky-Kleiman L, Garrocho-Rangel JA, Sánchez-Vargas LO, Pozos-Guillén AJ. | Probiotics and their effect on oral bacteria count in children: a pilot study [36]. | 2015 | Pilot study | 40 patients (4–6 years) at high risk of caries | Probiotics reduced microbial count. |
| Mayta-Tovalino F, Maguiña-Quispe J, Barja-Ore J, Hernandez AV. | Efficacy of Probiotic Consumption on Oral Outcomes in Children and/or Adolescents: A Meta-Analysis [37]. | 2024 | Meta-analysis | 2622 between children and adolescents | Probiotics had no effect on caries reduction or Lactobacillus count. Positive effect on Streptococcus mutans count. |
| Kavitha M, Prathima GS, Anusha D, Kengadaran S, Gayathri K, Vinothini V. | Evaluation of the efficacy of plaque reduction and gingival health among 6–12 years old school children before and after a short term daily intake of probiotic lozenge—A comparative study [38]. | 2022 | Comparative study | 6–12 years | The group taking probiotics showed a statistically significant reduction in plaque indices compared to the placebo group, and a significant improvement in gingival health was also observed. |
| Borrell García C, Ribelles Llop M, García Esparza MÁ, Flichy-Fernández AJ, Marqués Martínez L, Izquierdo Fort R. | The use of Lactobacillus reuteri DSM 17938 and ATCC PTA 5289 on oral health indexes in a school population: A pilot randomized clinical trial [39]. | 2021 | pilot randomized clinical trial. | 27 teenagers, 12–18 years old | Probiotics reduced Streptococcus mutans in saliva and plaque. |
| Hedayati-Hajikand T, Lundberg U, Eldh C, Twetman S. | Effect of probiotic chewing tablets on early childhood caries—a randomized controlled trial. BMC Oral Health [14]. | 2015 | RCT | 138 children aged 2–3 years | The early development of childhood caries could be reduced through the administration of probiotic chewing tablets as an adjunct to the daily use of fluoride toothpaste in preschool children. |
| Jindal et al. | A comparative evaluation of probiotics on salivary mutans streptococci counts in Indian children [40] | 2011 | Clinical study | 150 healthy children aged 7–14 years | Statistically significant reduction in salivary Streptococcus mutans counts in groups taking probiotics |
| Alamoudi NM et al. | Effect of probiotic Lactobacillus reuteri on Salivary Cariogenic Bacterial Counts among Grounp of Preschool Children in Jeddah, Saudi Arabia: A Randomized Clinical Trial [15] | 2018 | RCT | 178 healthy children (3–6 years) | Probiotics containing L. reuteri significantly reduce cario-associated bacterial counts. |
| Stensson M et al. | Oral administration of Lactobacillus reuteri during the first year of life reduces caries prevalence in the primary dentition at 9 years of age [16]. | 2014 | Single-center, single-blind, placebo-controlled study | Children (1 year of age followed up to 9 years) N = 113 (60 Probiotic, 53 Placebo) | Reduced caries prevalence, lower prevalence of approximal carious lesions, fewer sites with gingivitis in the probiotic group. |
| Cannon M, et al. | Effectiveness of CRT at measuring the salivary level of bacteria in caries prone children with probiotic therapy [41]. | 2013 | Clinical study | 60 children 6–12 years old | Statistically significant difference in CRT results between pre- and post-use of probiotics. |
| Kaklamanos EG et al. | A single-centre investigator-blinded randomised parallel group clinical trial to investigate the effect of probiotic strains Streptococcus salivarius M18 and Lactobacillus acidophilus on gingival health of pediatric patients undergoing treatment with fixed orthodontic appliances: study protocol [17]. | 2019 | RCT | Pediatric patients undergoing orthodontic treatment N = 50 | Reduction in gingival bleeding. Reduction in plaque and gingival indices, change in oral microbiome composition. |
| Lexner MO et al. | Microbiological profiles in saliva and supragingival plaque from caries-active adolescents before and after a short-term daily intake of milk supplemented with probiotic bacteria—a pilot study [42]. | 2010 | Randomized, double-blind, placebo-controlled pilot study | Adolescents (with active caries) N = 18 | No statistically significant difference in microbial profiles or levels of caries-associated bacteria. |
| Stecksén-Blicks C et al. | Effect of long-term consumption of milk supplemented with probiotic lactobacilli and fluoride on dental caries and general health in preschool children: a cluster-randomized study [18]. | 2009 | Cluster randomized study | Preschool children (1–5 years) N = 248 | Reduced caries increment, fewer days of antibiotic therapy. |
| Näse L et al. | Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children [19]. | 2001 | Randomized, double-blind, placebo-controlled intervention study | Children (1–6 years) N = 594 | Less dental caries, lower Streptococcus mutans count, lower caries risk. |
| Taipale T et al. | Bifidobacterium animalis subsp. lactis BB-12 administration in early childhood: a randomized clinical trial of effects on oral colonization by mutans streptococci and the probiotic [43]. | 2012 | Double-blind, placebo-controlled study | Newborns (1–2 months at baseline, followed up to 2 years) N = 106 | Low MS colonization in children at 2 years, BB-12 has not permanently colonized the oral cavity. |
| Taipale T et al. | Administration of Bifidobacterium animalis subsp. lactis BB-12 in early childhood: a post-trial effect on caries occurrence at four years of age [44]. | 2013 | Post-trial analysis of a randomized, double-blind, placebo-controlled study | Children (1–2 months at baseline, followed up to 4 years) N = 106 | No difference in the occurrence of caries at 4 years. |
| Mayta-Tovalino F et al. | Efficacy of Probiotic Consumption on Oral Outcomes in Children and/or Adolescents: A Meta-Analysis [37]. | 2024 | Meta-analysis of randomized controlled trials (RCTs) | Children and adolescents (various ages) N = 2622 (in 19 RCTs) | Probiotics likely reduce Streptococcus mutans counts, no significant effect on dental caries or other outcomes. |
| Kavitha M et al. | Evaluation of the efficacy of plaque reduction and gingival health among 6–12 years old school children before and after a short term daily intake of probiotic lozenge—A comparative study [38]. | 2022 | Comparative study | Children (6–12 anni) N = 60 | Significantly reduced plaque scores and improved gum health with probiotic tablets. |
| Alanzi A et al. | Effect of Lactobacillus rhamnosus and Bifidobacterium lactis on gingival health, dental plaque, and periodontopathogens in adolescents: a randomised placebo-controlled clinical trial [45]. | 2018 | Randomized placebo-controlled clinical trial | Adolescents (13–15 years) N = 108 | Significant reduction in gingival index, reduction in A. actinomycetemcomitans, F. nucleatum and P. gingivalis. |
| Dixit A et al. | Comparative evaluation of antimicrobial efficacy of various intracanal medicament in young permanent teeth: An in vivo study [20]. | 2024 | In vivo study | Children (12–17 years) N = 30 | Probiotics showed antimicrobial efficacy comparable to triantibiotic paste against E. faecalis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannini, L.; Stella, G.; Cattaneo, G.; Dipalma, G.; Maspero, C. Clinical Applications of Probiotics in Pediatric Dentistry and Orthodontics—A Systematic Review. Nutrients 2025, 17, 3153. https://doi.org/10.3390/nu17193153
Giannini L, Stella G, Cattaneo G, Dipalma G, Maspero C. Clinical Applications of Probiotics in Pediatric Dentistry and Orthodontics—A Systematic Review. Nutrients. 2025; 17(19):3153. https://doi.org/10.3390/nu17193153
Chicago/Turabian StyleGiannini, Lucia, Giovanna Stella, Giovanni Cattaneo, Gianna Dipalma, and Cinzia Maspero. 2025. "Clinical Applications of Probiotics in Pediatric Dentistry and Orthodontics—A Systematic Review" Nutrients 17, no. 19: 3153. https://doi.org/10.3390/nu17193153
APA StyleGiannini, L., Stella, G., Cattaneo, G., Dipalma, G., & Maspero, C. (2025). Clinical Applications of Probiotics in Pediatric Dentistry and Orthodontics—A Systematic Review. Nutrients, 17(19), 3153. https://doi.org/10.3390/nu17193153










