Management of Post-Colonoscopy Syndrome with a Nutraceutical Intervention Based on Hericium erinaceus: A Retrospective Two-Arm Multicentre Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Investigated Nutraceutical Combination Compound
2.4. Collected Variables and Data Collection Timeline
2.5. Study Outcomes
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Sample
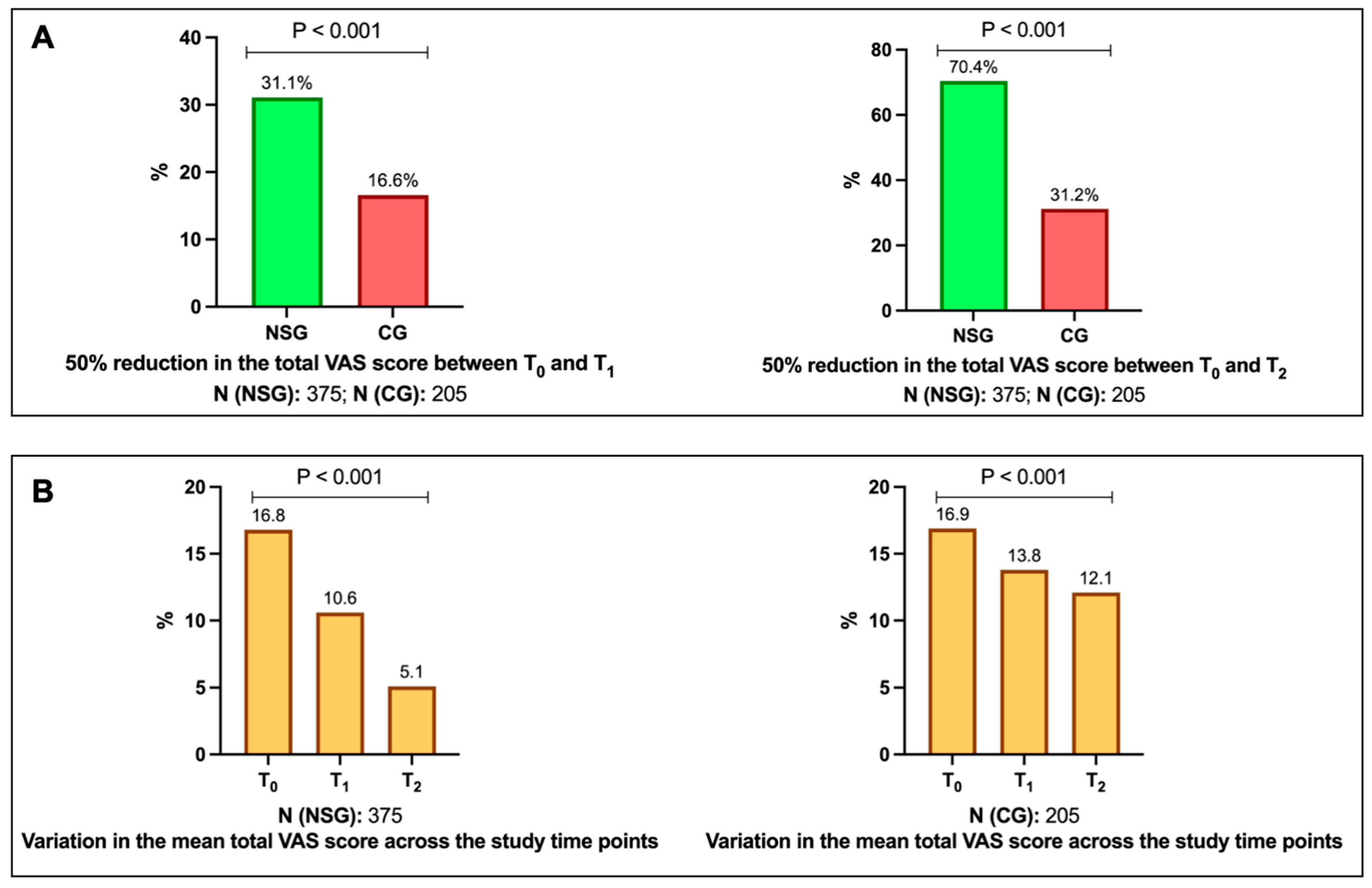
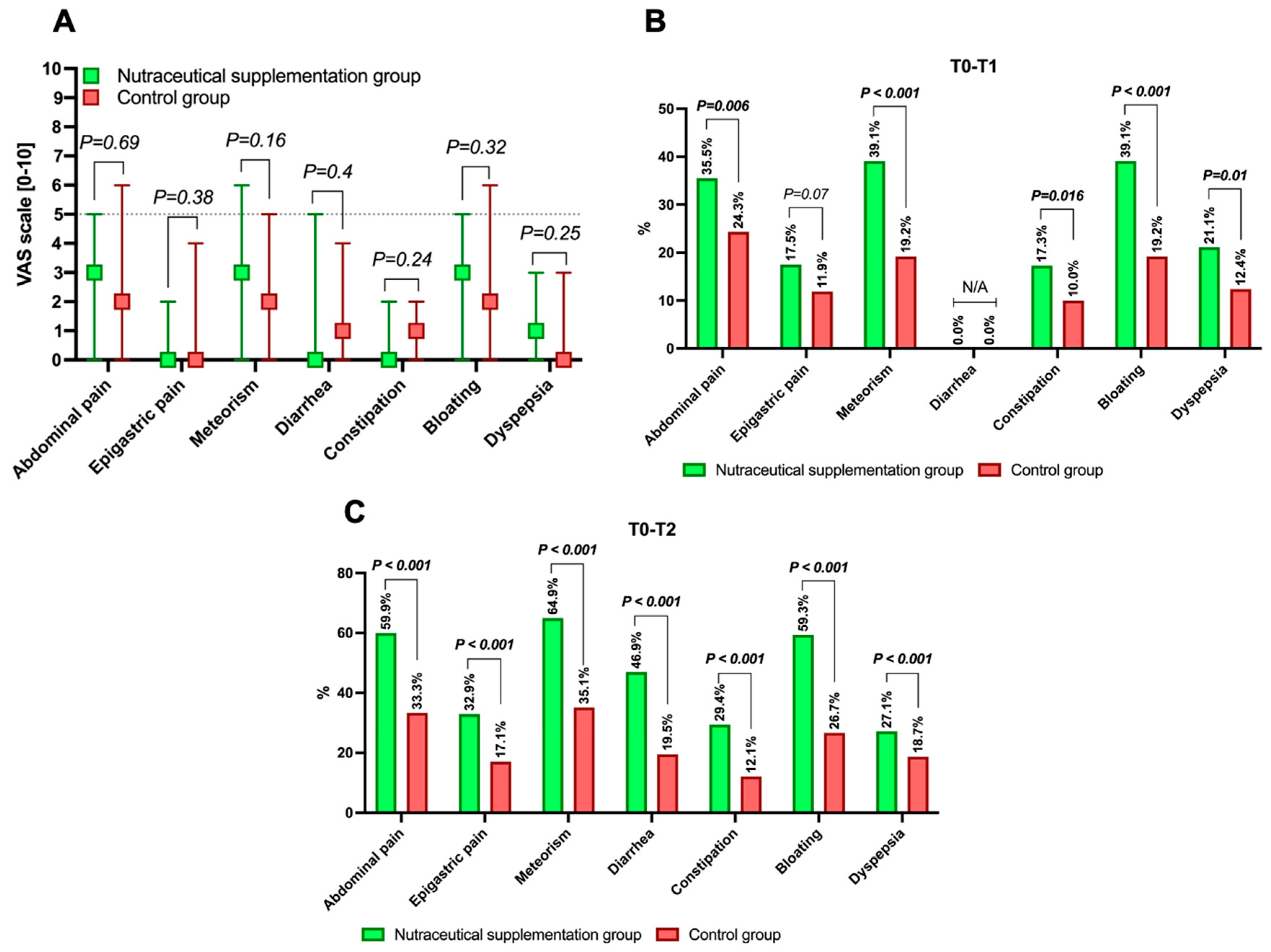
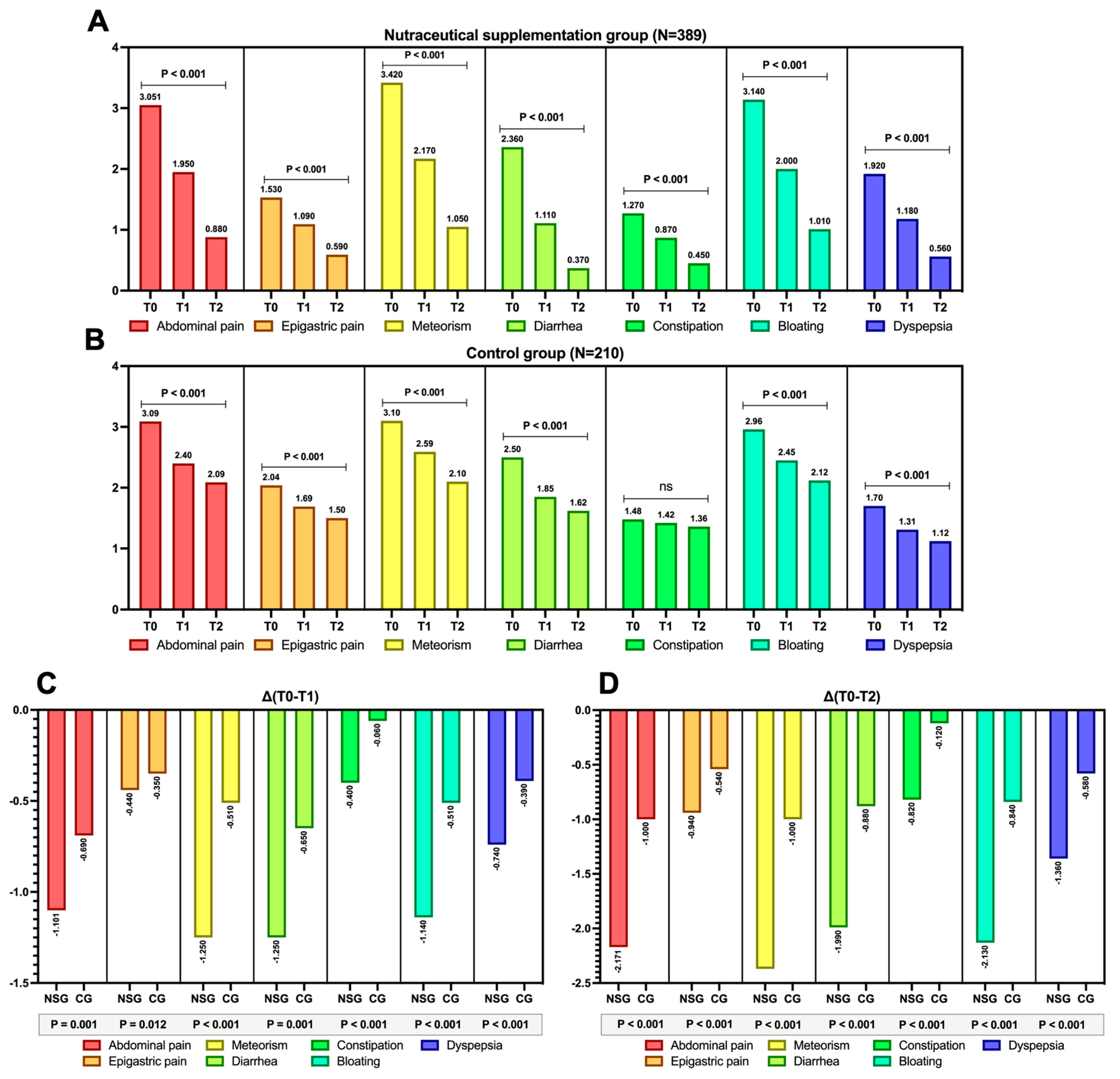
3.2. Study Outcomes: Results
3.3. VAS Scores Variations over Study Times
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Name and Surname | Affiliation |
|---|---|
| Luciana Avallone | Dipartimento di Medicina, Endoscopia Digestiva e Malattie Apparato Digerente, AUSL Ferrara, Ospedale del Delta, Ferrara, Italy |
| Stefania Baroni | Private practice, Milano, Italy |
| Barbara Battista | UOC di Gastroenterologia ed Endoscopia Digestiva Ospedale Santa Scolastica di Cassino, Roma, Italy |
| Antonio Bordonaro | UOS Gastroenterologia ed Endoscopia Digestiva, Ospedale Umberto I, Enna, Italy |
| Filippo Bova | Gastroenterologia, Ospedale Metropolitano di Reggio Calabria, Italy |
| Antongiulio Bucci | UOC di Gastroenterologia ed Endoscopia Digestiva, Ospedale San Paolo, Bari, Italy |
| Monica Carta | Gastroenterologia, Ospedale SS Annunziata, Sassari, Italy |
| Carlo Cellini | UOC di Gastroenterologia ed Endoscopia Digestiva, Ospedale Mazzini, Teramo, Italy |
| Andrea Cocco | UOC Transmurale Territoriale Gastroenterologia ed Endoscopia Digestiva Ospedale Sandro Pertini, Roma, Italy |
| Berardino D’Ascoli | UOSD Gastroenterologia, Ospedale Madonna delle Grazie, Matera, Italy |
| Francesco Decembrino | UOC Gastroenterologia ed Endoscopia Digestiva, E.E. Ospedale Regionale F. Miulli, Acquaviva delle Fonti, Bari, Italy |
| Massimo Devani | Reparto Endoscopia Digestiva Ospedale Rho, Milano, Italy |
| Vincenzo Di Martino | UOSD Endoscopia Digestiva-Ospedale dei Colli CTO, Napoli, Italy |
| Domenico Dominjanni | Ambulatorio Territoriale di Gastroenterologia ASL RM3, Roma, Italy |
| Enrica Evangelista | Ambulatorio Territoriale Gastroenterologia ASL Provincia di Frosinone, Frosinone, Italy |
| Lucia Falconi | UOC Gastroenterologia ed Endoscopia Digestiva, Ospedale Misericordia, Grosseto, Italy |
| Emanuele Ferrante | UO di Endoscopia Digestiva, ASL Napoli II Nord, PO San Giuliano, Giugliano in Campania, Italy |
| Katia Feole | Servizio di Gastroenterologia ed Endoscopia Digestiva Policlinico Casilino, Roma, Italy |
| Roberto Faggiani | UOC Gastroenterologia ed Endoscopia Digestiva Azienda Ospedale San Camillo Forlanini, Roma, Italy |
| Maria Domenica Franzese | UOC Gastroenterologia ed Endoscopia Digestiva, Ospedale Santa Maria delle Grazie, Pozzuoli, Italy |
| Maurizio Giovannone | UOC Gastroenterologia ed Endoscopia Digestiva Azienda Ospedale San Camillo Forlanini Roma, Italy |
| Donato Iannuzziello | Unità di Gastroenterologia, Mater Dei Hospital, Bari, Italy |
| Nicola Ierfone | UOC Chirurgia e Diagnostica Endoscopica-P.O. S.S. Filippo e Nicola, Avezzano, Italy |
| Giampiero Indennitate | Servizio di Endoscopia Digestiva, Casa di Cura Ulivella e Glicini, Firenze, Italy |
| Luca Isola | Gastroenterologia, Ospedale Evangelico Internazionale Genova Voltri, Genova, Italy |
| Roberto Lamanda | UOC Gastroenterologia ed Endoscopia Digestiva, Ospedale Santa Maria delle Grazie, Pozzuoli, Italy |
| Maria Antonia Lai | Endoscopia, Ospedale Businco, Azienda Ospedaliera Brotzu, Cagliari, Italy |
| Giovanni Lombardi | UOC Gastroenterologia ed Endoscopia Digestiva, Ospedale Cardarelli, Napoli, Italy |
| Tammaro Maisto | Servizio di Endoscopia Digestiva, Ospedale Santa Maria della Pietà, Casoria, Italy |
| Attilio Mancino | Private practice, Marsala, Italy |
| Giampiero Manes | Reparto Endoscopia Digestiva Ospedale Rho-Garbagnate, Garbagnate, Italy |
| Francesca Menasci | Servizio di Gastroenterologia ed Endoscopia Digestiva Policlinico Casilino, Roma, Italy |
| Giammarco Mocci | Gastroenterologia, Ospedale Brotzu, Cagliari, Italy |
| Antonino Morabito | Endoscopia Digestiva, Presidio Ospedaliero di Tropea, ASP Vibo Valentia, Italy |
| Caterina Mucherino | UOC Gastroenterologia AORN Sant’Anna e San Sebastiano, Caserta, Italy |
| Domenico Napoletano | Servizio di Endoscopia Digestiva, Clinica San Paolo, Aversa, Italy |
| Nicoletta Orzes | Private practice, Gorizia, Udine, Italy |
| Giuseppe Parisi | Private practice, Sant’Agata di Militello (ME), Italy |
| Marta Patturelli | UOC Gastroenterologia ed Endoscopia Digestiva, Ospedale Cardarelli, Napoli, Italy |
| Giuseppe Pianese | UOS Endoscopia Digestiva UOC Gastroenterologia Universitaria Ospedale Santa Maria Goretti, Latina, Italy |
| Giacomo Rando | UOC Gastroenterologia ed Endoscopia Digestiva-Ospedale Spirito Santo, Pescara, Italy |
| Rocco Ranaldo | UOC Medicina Interna, Ospedale Mazzolani Vandini, AUSL Ferrara, Italy |
| Raffaella Reati | Reparto Endoscopia Digestiva Ospedale Garbagnate, Milano, Italy |
| Antonio Romano | Private practice, Pisa, Italy |
| Stefano Rodinò | Azienda Ospedaliero Universitaria Renato Dulbecco, Catanzaro, Italy |
| Antonella Scarcelli | UOC Gastroenterologia ed Endoscopia Digestiva-AST Pesaro e Urbino, Pesaro/Fano, Italy |
| Silvia Sedda | UOC Gastroenterologia ed Endoscopia Digestiva, Ospedale del Mare, Napoli, Italy |
| Luca Sediari | Gastroenterologia, Ospedale Santa Maria della Misericordia, Perugia, Italy |
| Corrado Selvaggio | Gastroenterologia, Ospedale Maggiore di Modica, Ragusa, Italy |
| Nicola Sinnona | Servizio Gastroenterologia ed Endoscopia Digestiva Clinica San Marco, Latina, Italy |
| Francesco Tedone | UOC Gastroenterologia ed Endoscopia Digestiva, Ospedale Misericordia, Grosseto, Italy |
| Giuseppe Tarantino | AUO delle Marche-Clinica di Gastroenterologia, Epatologia ed Endoscopia Digestiva, Ancona, Italy |
| Lorenza Tifi | Gastroenterologia, USL Umbria 1, Perugia, Italy |
| Elisa Tiratterra | Gastroenterologo, Villa Erbosa-Gruppo San Donato, Bologna, Italy |
| Giuliana Vespere | UOC Gastroenterologia ed Endoscopia Digestiva, Ospedale del Mare, Napoli, Italy |
| Gabriele Vigliotti | UOSD Endoscopia Digestiva, Ospedale dei Colli CTO, Napoli, Italy |
| Paolo Usai Satta | Gastroenterologia, Azienda Ospedaliera Brotzu, Cagliari, Italy |
| Rita Ortenzi | USL Umbria 1, Perugia, Italy |
Appendix B
References
- Ladabaum, U.; Dominitz, J.A.; Kahi, C.; Schoen, R.E. Strategies for Colorectal Cancer Screening. Gastroenterology 2020, 158, 418–432. [Google Scholar] [CrossRef]
- Hassan, C.; East, J.; Radaelli, F.; Spada, C.; Benamouzig, R.; Bisschops, R.; Bretthauer, M.; Dekker, E.; Dinis-Ribeiro, M.; Ferlitsch, M.; et al. Bowel Preparation for Colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2019. Endoscopy 2019, 51, 775–794. [Google Scholar] [CrossRef]
- Gravina, A.G.; Pellegrino, R.; Romeo, M.; Palladino, G.; Cipullo, M.; Iadanza, G.; Olivieri, S.; Zagaria, G.; De Gennaro, N.; Santonastaso, A.; et al. Quality of Bowel Preparation in Patients with Inflammatory Bowel Disease Undergoing Colonoscopy: What Factors to Consider? World J. Gastrointest. Endosc. 2023, 15, 133–145. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Boffetta, P.; Radaelli, F.; Cadoni, S.; Hassan, C.; Frazzoni, L.; Anderloni, A.; Laterza, L.; La Marca, M.; Rogai, F.; et al. Incidence, Risk and Protective Factors of Symptoms after Colonoscopy. Dig. Liver Dis. 2022, 54, 1698–1705. [Google Scholar] [CrossRef]
- Steffenssen, M.W.; Al-Najami, I.; Baatrup, G. Patient-Reported Minor Adverse Events after Colonoscopy: A Systematic Review. Acta Oncol. 2019, 58, S22–S28. [Google Scholar] [CrossRef]
- Liu, X.; Gao, J. Examination of Core Clinical Symptoms of Postoperative Fatigue Syndrome Following Colonoscopy With Sedation. J. Perianesth. Nurs. 2025, 40, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Reumkens, A.; Rondagh, E.J.A.; Bakker, C.M.; Winkens, B.; Masclee, A.A.M.; Sanduleanu, S. Post-Colonoscopy Complications: A Systematic Review, Time Trends, and Meta-Analysis of Population-Based Studies. Am. J. Gastroenterol. 2016, 111, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Makker, J.; Shaikh, D.; Patel, H.; Hanumanthu, S.; Sun, H.; Zaidi, B.; Ravi, M.; Balar, B. Characteristics of Patients with Post-Colonoscopy Unplanned Hospital Visit: A Retrospective Single-Center Observational Study. Clin. Exp. Gastroenterol. 2021, 14, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Meroni, G.; Pace, F.; Grossi, E.; Casini, V.; Drago, L. Bacterial Network Community in Fecal and Endoluminal Microbiota after Colonoscopy. New Microbiol. 2020, 43, 22–27. [Google Scholar] [PubMed]
- Drago, L.; Toscano, M.; De Grandi, R.; Casini, V.; Pace, F. Persisting Changes of Intestinal Microbiota after Bowel Lavage and Colonoscopy. Eur. J. Gastroenterol. Hepatol. 2016, 28, 532–537. [Google Scholar] [CrossRef]
- Vajravelu, R.K.; Shapiro, J.M.; Ni, J.; Thanawala, S.U.; Lewis, J.D.; El-Serag, H.B. Risk for Post-Colonoscopy Irritable Bowel Syndrome in Patients With and Without Antibiotic Exposure: A Retrospective Cohort Study. Clin. Gastroenterol. Hepatol. 2022, 20, e1305–e1322. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Wang, H.-P.; Chiu, H.-M.; Lin, C.-P.; Huang, S.-P.; Lai, Y.-P.; Wu, M.-S.; Chen, M.-F.; Lin, J.-T. Factors Determining Post-Colonoscopy Abdominal Pain: Prospective Study of Screening Colonoscopy in 1000 Subjects. J. Gastroenterol. Hepatol. 2006, 21, 1575–1580. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Muzellina, V.N.; Alvianto, S.; Widjanarko, N.D. Utilization of Probiotics in Relieving Post-Colonoscopy Gastrointestinal Symptoms: A Systematic Review and Meta-Analysis. Rom. J. Intern. Med. 2024, 62, 387–403. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Kalra, E.K. Nutraceutical—Definition and Introduction. AAPS PharmSci 2003, 5, 25. [Google Scholar] [CrossRef]
- Gravina, A.G.; Pellegrino, R.; Auletta, S.; Palladino, G.; Brandimarte, G.; D’Onofrio, R.; Arboretto, G.; Imperio, G.; Ventura, A.; Cipullo, M.; et al. Hericium erinaceus, a Medicinal Fungus with a Centuries-Old History: Evidence in Gastrointestinal Diseases. World J. Gastroenterol. 2023, 29, 3048–3065. [Google Scholar] [CrossRef]
- Brandimarte, G.; Frajese, G.V.; Bargiggia, S.; Castellani, D.; Cocco, A.; Colucci, R.; Evangelista, E.; Gravina, A.G.; Napoletano, D.; Nardi, E.; et al. Performance of a Multicompounds Nutraceutical Formulation in Patients with Symptomatic Uncomplicated Diverticular Disease. Minerva Gastroenterol. 2022, 68, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; D’Avino, A.; Brandimarte, G.; Mocci, G.; Pellegrino, R.; Savarino, E.V.; Gravina, A.G.; The Hericium-Uc Study Group. null Enhancing Oral 5-ASA Effectiveness in Mild-to-Moderate Ulcerative Colitis through an H. Erinaceus-Based Nutraceutical Add-on Multi-Compound: The “HERICIUM-UC” Two-Arm Multicentre Retrospective Study. Pharmaceutics 2024, 16, 1133. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Pellegrino, R.; Palladino, G.; Coppola, A.; Brandimarte, G.; Tuccillo, C.; Ciardiello, F.; Romano, M.; Federico, A. Hericium erinaceus, in Combination with Natural Flavonoid/Alkaloid and B3/B8 Vitamins, Can Improve Inflammatory Burden in Inflammatory Bowel Diseases Tissue: An Ex Vivo Study. Front. Immunol. 2023, 14, 1215329. [Google Scholar] [CrossRef] [PubMed]
- Goodyear, M.D.E.; Krleza-Jeric, K.; Lemmens, T. The Declaration of Helsinki. BMJ 2007, 335, 624–625. [Google Scholar] [CrossRef]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global Prevalence of Irritable Bowel Syndrome According to Rome III or IV Criteria: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.J.; Calderwood, A.H.; Doros, G.; Fix, O.K.; Jacobson, B.C. The Boston Bowel Preparation Scale: A Valid and Reliable Instrument for Colonoscopy-Oriented Research. Gastrointest. Endosc. 2009, 69, 620–625. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; di Mario, F.; Nardone, G.; Scarpignato, C.; Picchio, M.; Elisei, W.; DICA Italian Group. The “DICA” Endoscopic Classification for Diverticular Disease of the Colon Shows a Significant Interobserver Agreement among Community Endoscopists. J. Gastrointestin. Liver Dis. 2019, 28, 23–27. [Google Scholar] [CrossRef]
- Heller, G.Z.; Manuguerra, M.; Chow, R. How to Analyze the Visual Analogue Scale: Myths, Truths and Clinical Relevance. Scand. J. Pain. 2016, 13, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, H.-S.; Park, H.J. Adverse Events Related to Colonoscopy: Global Trends and Future Challenges. World J. Gastroenterol. 2019, 25, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.; Liu, T.; Yi, C.; Wong, M.; Lei, W.; Chen, C. Influence of Probiotics on Gastrointestinal Symptoms in Patients Undergoing Colonoscopy. Adv. Dig. Med. 2021, 8, 27–32. [Google Scholar] [CrossRef]
- Uchiyama, K.; Ando, T.; Kishimoto, E.; Nishimura, T.; Imamoto, E.; Takagi, T.; Ishikawa, T.; Naito, Y.; Itoh, Y. Correlation of Gastrointestinal Symptom Rating Scale and Frequency Scale for the Symptoms of Gastroesophageal Reflux Disease with Endoscopic Findings. Scand. J. Gastroenterol. 2024, 59, 1220–1228. [Google Scholar] [CrossRef]
- Bonavina, L.; Ariani, A.; Ficano, L.; Iannuzziello, D.; Pasquale, L.; Aragona, S.E.; Drago, L.; Ciprandi, G.; On Digestive Disorders, I.S.G. Lactobacillus plantarum LP01, Lactobacillus Lactis Subspecies Cremoris LLC02, and Lactobacillus delbrueckii LDD01 in Patients Undergoing Bowel Preparation. Acta Biomed. 2019, 90, 13–17. [Google Scholar] [CrossRef]
- Nordström, E.A.; Teixeira, C.; Montelius, C.; Jeppsson, B.; Larsson, N. Lactiplantibacillus plantarum 299v (LP299V®): Three Decades of Research. Benef. Microbes 2021, 12, 441–465. [Google Scholar] [CrossRef]
- Liu, R.-H.; Sun, A.-Q.; Liao, Y.; Tang, Z.-X.; Zhang, S.-H.; Shan, X.; Hu, J.-T. Lactiplantibacillus plantarum Regulated Intestinal Microbial Community and Cytokines to Inhibit Salmonella yyphimurium Infection. Probiotics Antimicrob. Proteins 2023, 15, 1355–1370. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Knøchel, S. Bioprotection Potential of Lacticaseibacillus rhamnosus LRH01 and Lactiplantibacillus plantarum LP01 against Spoilage-Associated Penicillium Strains in Yoghurt. Molecules 2023, 28, 7397. [Google Scholar] [CrossRef] [PubMed]
- Bonavina, L.; Arini, A.; Ficano, L.; Iannuzziello, D.; Pasquale, L.; Aragona, S.E.; Ciprandi, G.; On Digestive Disorders, I.S.G. Post-Surgical Intestinal Dysbiosis: Use of an Innovative Mixture (Lactobacillus plantarum LP01, Lactobacillus lactis Subspecies cremoris LLC02, Lactobacillus delbrueckii LDD01). Acta Biomed. 2019, 90, 18–23. [Google Scholar] [CrossRef]
- Bonavina, L.; Arini, A.; Ficano, L.; Iannuzziello, D.; Pasquale, L.; Aragona, S.E.; Ciprandi, G.; On Digestive Disorders, I.S.G. Abincol® (Lactobacillus plantarum LP01, Lactobacillus lactis Subspecies cremoris LLC02, Lactobacillus delbrueckii LDD01), an Oral Nutraceutical, Pragmatic Use in Patients with Chronic Intestinal Disorders. Acta Biomed. 2019, 90, 8–12. [Google Scholar] [CrossRef]
- de Jesus, L.C.L.; Freitas, A.D.S.; Dutra, J.d.C.F.; Campos, G.M.; Américo, M.F.; Laguna, J.G.; Dornelas, E.G.; Carvalho, R.D.d.O.; Vital, K.D.; Fernandes, S.O.A.; et al. Lactobacillus Delbrueckii CIDCA 133 Fermented Milk Modulates Inflammation and Gut Microbiota to Alleviate Acute Colitis. Food Res. Int. 2024, 186, 114322. [Google Scholar] [CrossRef]
- Del Piano, M.; Anderloni, A.; Balzarini, M.; Ballarè, M.; Carmagnola, S.; Montino, F.; Orsello, M.; Pagliarulo, M.; Tari, R.; Soattini, L.; et al. The Innovative Potential of Lactobacillus rhamnosus LR06, Lactobacillus pentosus LPS01, Lactobacillus plantarum LP01, and Lactobacillus delbrueckii Subsp. delbrueckii LDD01 to Restore the “Gastric Barrier Effect” in Patients Chronically Treated with PPI: A Pilot Study. J. Clin. Gastroenterol. 2012, 46, S18–S26. [Google Scholar] [CrossRef]
- Baek, M.G.; Kim, K.W.; Yi, H. Subspecies-Level Genome Comparison of Lactobacillus Delbrueckii. Sci. Rep. 2023, 13, 3171. [Google Scholar] [CrossRef]
- Mogna, L.; Deidda, F.; Nicola, S.; Amoruso, A.; Del Piano, M.; Mogna, G. In Vitro Inhibition of Klebsiella pneumoniae by Lactobacillus delbrueckii Subsp. delbrueckii LDD01 (DSM 22106): An Innovative Strategy to Possibly Counteract Such Infections in Humans? J. Clin. Gastroenterol. 2016, 50, S136–S139. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and Its Probiotic and Food Potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Laitila, A.; Ouwehand, A.C. Bifidobacterium animalis Subsp. lactis HN019 Effects on Gut Health: A Review. Front. Nutr. 2021, 8, 790561. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, X.; Chen, X.; Hai, D.; Wei, C.; Zhang, L.; Li, P. The Functional Roles of Lactobacillus acidophilus in Different Physiological and Pathological Processes. J. Microbiol. Biotechnol. 2022, 32, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-Q.; Geng, Y.; Guan, Q.; Ren, Y.; Guo, L.; Lv, Q.; Lu, Z.-M.; Shi, J.-S.; Xu, Z.-H. Influence of Short-Term Consumption of Hericium erinaceus on Serum Biochemical Markers and the Changes of the Gut Microbiota: A Pilot Study. Nutrients 2021, 13, 1008. [Google Scholar] [CrossRef]
- Karunaratne, T.B.; Okereke, C.; Seamon, M.; Purohit, S.; Wakade, C.; Sharma, A. Niacin and Butyrate: Nutraceuticals Targeting Dysbiosis and Intestinal Permeability in Parkinson’s Disease. Nutrients 2020, 13, 28. [Google Scholar] [CrossRef]
- Habtemariam, S. Berberine Pharmacology and the Gut Microbiota: A Hidden Therapeutic Link. Pharmacol. Res. 2020, 155, 104722. [Google Scholar] [CrossRef]
- Feng, J.; Li, Z.; Ma, H.; Yue, Y.; Hao, K.; Li, J.; Xiang, Y.; Min, Y. Quercetin Alleviates Intestinal Inflammation and Improves Intestinal Functions via Modulating Gut Microbiota Composition in LPS-Challenged Laying Hens. Poult. Sci. 2023, 102, 102433. [Google Scholar] [CrossRef]
- Saturni, S.; Bellini, F.; Braido, F.; Paggiaro, P.; Sanduzzi, A.; Scichilone, N.; Santus, P.A.; Morandi, L.; Papi, A. Randomized Controlled Trials and Real Life Studies. Approaches and Methodologies: A Clinical Point of View. Pulm. Pharmacol. Ther. 2014, 27, 129–138. [Google Scholar] [CrossRef]
- Liu, F.; Panagiotakos, D. Real-World Data: A Brief Review of the Methods, Applications, Challenges and Opportunities. BMC Med. Res. Methodol. 2022, 22, 287. [Google Scholar] [CrossRef]
- Dang, A. Real-World Evidence: A Primer. Pharmaceut. Med. 2023, 37, 25–36. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Papez, V.; Chang, W.H.; Mueller, S.H.; Denaxas, S.; Lai, A.G. Comparing Clinical Trial Population Representativeness to Real-World Populations: An External Validity Analysis Encompassing 43 895 Trials and 5 685 738 Individuals across 989 Unique Drugs and 286 Conditions in England. Lancet Healthy Longev. 2022, 3, e674–e689. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.E.; Booth, C.M. Real-World Data: Bridging the Gap between Clinical Trials and Practice. eClinicalMedicine 2024, 78, 102915. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Kunisaki, R.; Kato, J.; Murakami, M.; Nishio, M.; Ogashiwa, T.; Yoshida, T.; Kimura, H.; Kitano, M. Patient Self-Reported Symptoms Using Visual Analog Scales Are Useful to Estimate Endoscopic Activity in Ulcerative Colitis. Intest. Res. 2018, 16, 579–587. [Google Scholar] [CrossRef]
- Bengtsson, M.; Ohlsson, B. The Brief Visual Analogue Scale for Irritable Bowel Syndrome Questionnaire Can Be Used to Evaluate Psychological Well-Being in Patients with Irritable Bowel Syndrome. Eur. J. Intern. Med. 2013, 24, e82–e83. [Google Scholar] [CrossRef]
- Bengtsson, M.; Persson, J.; Sjölund, K.; Ohlsson, B. Further Validation of the Visual Analogue Scale for Irritable Bowel Syndrome after Use in Clinical Practice. Gastroenterol. Nurs. 2013, 36, 188–198. [Google Scholar] [CrossRef]
- Chan, W.W.; Schroeder, M.; Richardson, A.; Shah, N.; Muftah, M.; Muftah, S.; Siboni, S.; Sozzi, M.; Wong, M.-W.; Chen, C.-L.; et al. Validation of Esophageal Global Symptom Severity as a Patient-Reported Outcome for Evaluation of Reflux Symptoms. Am. J. Gastroenterol. 2025, 120, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Scarpignato, C. Pharmacologic Treatment of GERD in Adolescents: Is Esophageal Mucosal Protection an Option? Ther. Adv. Gastroenterol. 2022, 15, 17562848221115319. [Google Scholar] [CrossRef] [PubMed]

| Mean Values | In One Capsule | %RNV 1 | In Two Capsules | %RNV 1 |
|---|---|---|---|---|
| Hericium plv. tit. 5% polysaccharides | 262.5 mg | - | 525 mg | - |
| Hericium e.s. tit. 30% polysaccharides | 112.5 μg | - | 225 mg | - |
| Quercetin 98% | 37.5 mg | - | 75 mg | - |
| Biotin | 112.5 μg | 225 | 225 μg | 450 |
| Niacin | 13.5 mg | 84.3 | 27 mg | 168.6 |
| Berberis e.s. tit. 97% berberine | 37.5 mg | - | 75 mg | - |
| Sodium butyrate | 150 mg | - | 300 mg | - |
| Lactobacillus acidophilus SGL11 | 2.5 bn CFU 2 | - | 5 bn CFU 2 | - |
| Bifidobacterium animalis subsp. lactis SGB06 | 1 bn CFU 2 | - | 2 bn CFU 2 | - |
| Lactiplantibacillus plantarum SGL07 | 0.5 bn CFU 2 | - | 1 bn CFU 2 | - |
| Parameter | Nutraceutical Supplementation Group (N = 389) | Control Group (N = 210) | p-Value 1 |
|---|---|---|---|
| Age | 57.3 (46.8–64.5) | 57.7 (47.8–66) | 0.34 |
| Sex | |||
| Males | 191 (49.1%) | 99 (47.1%) | 0.66 |
| Active smoking | 137 (35.2%) | 72 (34.3%) | 0.85 |
| Alcohol consumers | 122 (31.4%) | 60 (28.6%) | 0.4 |
| Appendectomy | 113 (29%) | 60 (28.6%) | 0.92 |
| Comorbidity | |||
| Cardiovascular | 138 (35.4%) | 68 (32.3%) | 0.45 |
| Respiratory | 47 (12.1%) | 26 (12.4%) | 0.89 |
| Metabolic | 128 (32.9%) | 49 (23.3%) | 0.015 |
| Musculoskeletal | 50 (12.9%) | 23 (11%) | 0.6 |
| Neurological | 26 (6.7%) | 9 (4.3%) | 0.27 |
| Other | 48 (12.33%) | 32 (15.2%) | 0.37 |
| Patient’s standard diet | |||
| Mediterranean | 323 (83%) | 186 (88.6%) | 0.23 |
| Vegan/Vegetarian | 10 (2.6%) | 6 (2.9%) | 0.89 |
| Predominantly meat-based | 31 (8%) | 9 (4.3%) | 0.09 |
| Predominantly fish-based | 11 (2.8%) | 5 (2.4%) | 0.68 |
| Gluten-free Low | 4 (1%) | 3 (1.4%) | 0.55 |
| FODMAP | 10 (2.6%) | 1 (0.5%) | 0.12 |
| Colonoscopy indication | |||
| Cancer screening | 147 (37.8%) | 87 (41.4%) | 0.56 |
| Cancer familiar history | 101 (26%) | 56 (26.7%) | 0.68 |
| Lower GI bleeding | 77 (19.8%) | 40 (19.1%) | 0.84 |
| Other | 64 (16.4%) | 27 (12.8%) | 0.34 |
| Bowel preparation | |||
| 2 L PEG plus citrate/simethicone 2 | 119 (30.6%) | 53 (25.2%) | 0.66 |
| 2 L PEG plus ascorbate 3 | 25 (6.4%) | 9 (4.3%) | 0.12 |
| 2 L PEG plus ascorbate 4 | 124 (31.9%) | 64 (30.5%) | 0.85 |
| Magnesium citrate-based 5 | 2 (0.5%) | 2 (1%) | 0.4 |
| 4 L PEG 6 | 119 (30.6%) | 82 (39%) | 0.21 |
| DICA score | 158 (40.6%) | 88 (41.9%) | 0.1 |
| 1 | 98 (25.2%) | 66 (31.4%) | 0.55 |
| 2 | 41 (10.5%) | 18 (8.5%) | 0.66 |
| 3 | 19 (4.8%) | 4 (1.9%) | 0.08 |
| BBPS | 7 (6–9) | 7 (6–8) | <0.001 |
| BBPS < 6 (Suboptimal) | 33 (8.4%) | 32 (15.2%) | <0.001 |
| Diet before bowel preparation (days) | 3 (3–4) | 3 (3–3) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tursi, A.; D’Avino, A.; Brandimarte, G.; Mocci, G.; Pellegrino, R.; Federico, A.; Savarino, E.V.; Gravina, A.G.; the HERICIUM-COLON Study Group. Management of Post-Colonoscopy Syndrome with a Nutraceutical Intervention Based on Hericium erinaceus: A Retrospective Two-Arm Multicentre Analysis. Nutrients 2025, 17, 3152. https://doi.org/10.3390/nu17193152
Tursi A, D’Avino A, Brandimarte G, Mocci G, Pellegrino R, Federico A, Savarino EV, Gravina AG, the HERICIUM-COLON Study Group. Management of Post-Colonoscopy Syndrome with a Nutraceutical Intervention Based on Hericium erinaceus: A Retrospective Two-Arm Multicentre Analysis. Nutrients. 2025; 17(19):3152. https://doi.org/10.3390/nu17193152
Chicago/Turabian StyleTursi, Antonio, Alessandro D’Avino, Giovanni Brandimarte, Giammarco Mocci, Raffaele Pellegrino, Alessandro Federico, Edoardo Vincenzo Savarino, Antonietta Gerarda Gravina, and the HERICIUM-COLON Study Group. 2025. "Management of Post-Colonoscopy Syndrome with a Nutraceutical Intervention Based on Hericium erinaceus: A Retrospective Two-Arm Multicentre Analysis" Nutrients 17, no. 19: 3152. https://doi.org/10.3390/nu17193152
APA StyleTursi, A., D’Avino, A., Brandimarte, G., Mocci, G., Pellegrino, R., Federico, A., Savarino, E. V., Gravina, A. G., & the HERICIUM-COLON Study Group. (2025). Management of Post-Colonoscopy Syndrome with a Nutraceutical Intervention Based on Hericium erinaceus: A Retrospective Two-Arm Multicentre Analysis. Nutrients, 17(19), 3152. https://doi.org/10.3390/nu17193152









