Hydration Status in Geriatric Patients—Subjective Impression or Objective Parameter? The Hydr-Age-Study
Abstract
1. Introduction
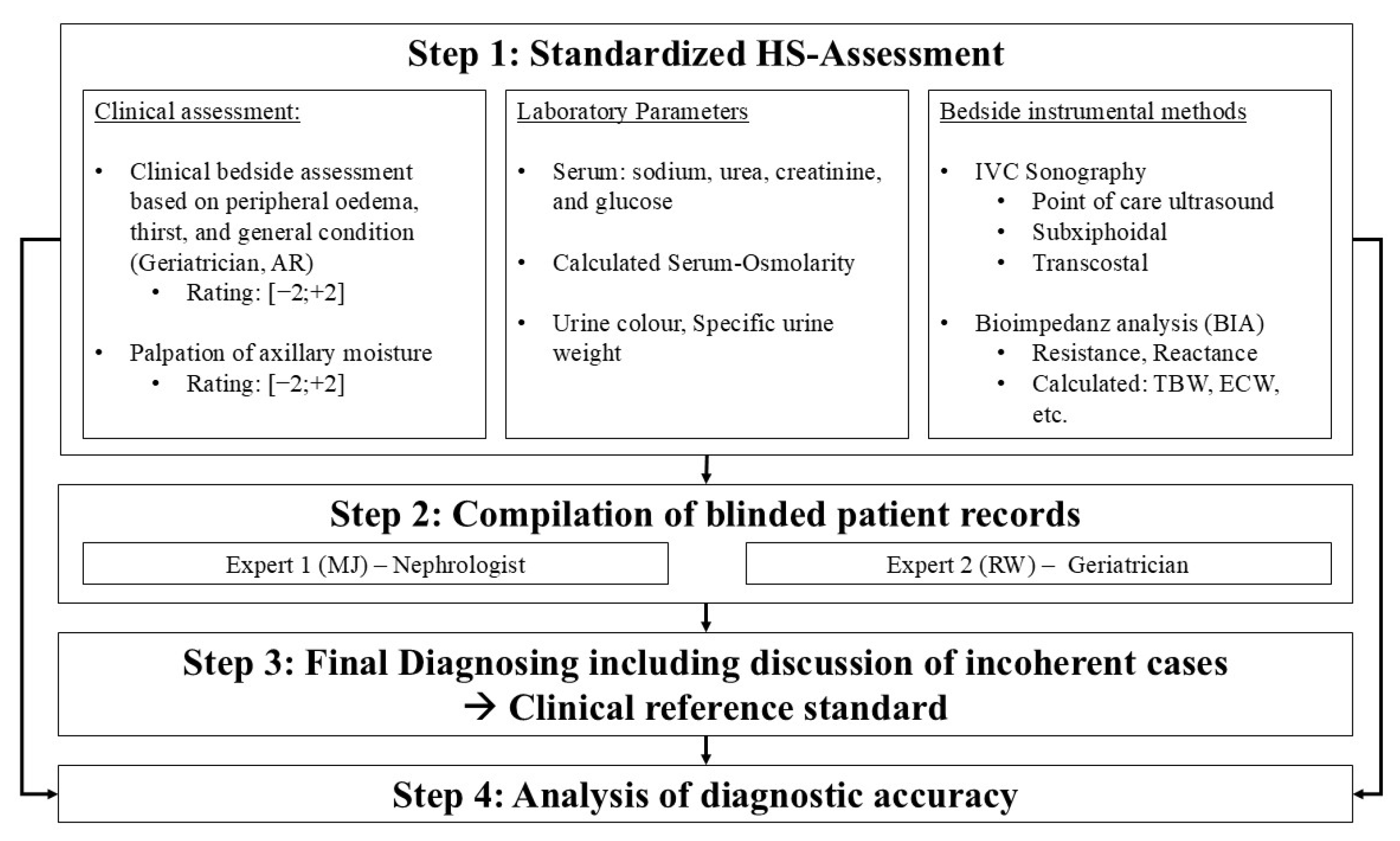
2. Materials and Methods
2.1. The Missing Gold Standard
2.2. Study Design and Patients
2.3. Baseline Procedures
2.4. Clinical Reference Standard
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics and Group Differences
3.2. Laboratory Measurements
3.3. Inter-Rater Reliability
3.4. ROC Analysis and Diagnostic Accuracy in Assessing the HS
3.4.1. Detecting Hypohydration
- Axillary Dryness
- Inferior vena cava sonography
3.4.2. Detecting Hyperhydration
- Inferior vena cava sonography
4. Discussion
4.1. Hypohydration
4.1.1. Laboratory Parameters
4.1.2. Axillary Dryness
4.1.3. Inferior Vena Cava Ultrasound
4.2. Hyperhydration
4.2.1. Inferior Vena Cava Ultrasound
4.2.2. Body Weight and BMI
4.3. Strengths and Limitations
5. Conclusions
- Integrating the palpation of the axillary skin as part of the routine physical examination not only helps to train the investigators in identifying the underreported clinical sign of a “dry axilla” but also helps to evaluate the overall HS in older patients.
- Performing IVC sonography can be challenging. It is therefore crucial to guide young physicians in this procedure, which would be the first step for improving the assessment of the overall hydration status.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HS | Hydration Status |
| IVC | Inferior vena Cava |
| BUN:Cr | Blood Urea Nitrogen to Creatinine Ratio |
| BIA | Bioimpedance Analysis |
| ECW | Extracellular Water |
| TBW | Total Body Water |
| ECW/TBW | Extracellular Water to Total Body Water Ratio |
| MMSE | Mini-Mental State Examination |
| SD | Standard Deviation |
| IQR | Interquartile Range |
| ROC | Receiver Operating Characteristic |
| AUC | Area Under the Curve |
| PPV | Positive Predictive Value |
| NPV | Negative Predictive Value |
| LR+ | Positive Likelihood Ratio |
| LR− | Negative Likelihood Ratio |
| CI | Confidence Interval |
| BMI | Body Mass Index |
| BIVA | Bioelectrical Impedance Vector Analysis |
| FFM | Fat Free Mass |
References
- Zhang, X.; Li, X.-Y. Prevalence of hyponatremia among older inpatients in a general hospital. Eur. Geriatr. Med. 2020, 11, 685–692. [Google Scholar] [CrossRef]
- Parkinson, E.; Hooper, L.; Fynn, J.; Wilsher, S.H.; Oladosu, T.; Poland, F.; Roberts, S.; van Hout, E.; Bunn, D. Low-intake dehydration prevalence in non-hospitalised older adults: Systematic review and meta-analysis. Clin. Nutr. 2023, 42, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Monnerat, S.; Christ-Crain, M.; Refardt, J. Hyponatraemia in ageing. Nat. Rev. Endocrinol. 2025, 21, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Helderman, J.H.; Vestal, R.E.; Rowe, J.W.; Tobin, J.D.; Andres, R.; Robertson, G.L. The response of arginine vasopressin to intravenous ethanol and hypertonic saline in man: The impact of aging. J. Gerontol. 1978, 33, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.A.; Fulop, T. Clinical aspects of changes in water and sodium homeostasis in the elderly. Rev. Endocr. Metab. Disord. 2017, 18, 49–66. [Google Scholar] [CrossRef]
- Schrier, R.W. Interactions between angiotensin II and arginine vasopressin in water homeostasis. Kidney Int. 2009, 76, 137–139. [Google Scholar] [CrossRef]
- Deißler, L.; Wirth, R.; Frilling, B.; Janneck, M.; Rösler, A. Hydration Status Assessment in Older Patients. Dtsch. Arztebl. Int. 2023, 120, 663–669. [Google Scholar] [CrossRef]
- Andreucci, V.E.; Russo, D.; Cianciaruso, B.; Andreucci, M. Some sodium, potassium and water changes in the elderly and their treatment. Nephrol. Dial. Transpl. 1996, 11 (Suppl. S9), 9–17. [Google Scholar] [CrossRef][Green Version]
- Hooper, L.; Abdelhamid, A.; Attreed, N.J.; Campbell, W.W.; Channell, A.M.; Chassagne, P.; Culp, K.R.; Fletcher, S.J.; Fortes, M.B.; Fuller, N.; et al. Clinical symptoms, signs and tests for identification of impending and current water-loss dehydration in older people. Cochrane Database Syst. Rev. 2015, 2015, CD009647. [Google Scholar] [CrossRef]
- Bunn, D.K.; Hooper, L. Signs and Symptoms of Low-Intake Dehydration Do Not Work in Older Care Home Residents-DRIE Diagnostic Accuracy Study. J. Am. Med. Dir. Assoc. 2019, 20, 963–970. [Google Scholar] [CrossRef]
- Siervo, M.; Bunn, D.; Prado, C.M.; Hooper, L. Accuracy of prediction equations for serum osmolarity in frail older people with and without diabetes. Am. J. Clin. Nutr. 2014, 100, 867–876. [Google Scholar] [CrossRef]
- Diederich, H.; Burkhardt, H. Diagnostic efficacy of bedside ultrasound to detect dehydration in older patients attending an emergency care unit. Z. Gerontol. Geriatr. 2020, 54, 130–135. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J.; Corbett, J.; Forni, L.; Hooper, L.; Hughes, F.; Minto, G.; Moss, C.; Price, S.; Whyte, G.; Woodcock, T.; et al. A multidisciplinary consensus on dehydration: Definitions, diagnostic methods and clinical implications. Ann. Med. 2019, 51, 232–251. [Google Scholar] [CrossRef] [PubMed]
- Orso, D.; Guglielmo, N.; Federici, N.; Cugini, F.; Ban, A.; Mearelli, F.; Copetti, R. Accuracy of the caval index and the expiratory diameter of the inferior vena cava for the diagnosis of dehydration in elderly. J. Ultrasound 2016, 19, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E. Assessing hydration status: The elusive gold standard. J. Am. Coll. Nutr. 2007, 26, 575S–584S. [Google Scholar] [CrossRef]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]
- Khajuria, A.; Krahn, J. Osmolality revisited—Deriving and validating the best formula for calculated osmolality. Clin. Biochem. 2005, 38, 514–519. [Google Scholar] [CrossRef]
- Armstrong, L.E. Performing in Extreme Environments; Human Kinetics: Champaign, IL, USA; Leeds, UK, 2000; ISBN 0880118377. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
- Altman, D.G.; Machin, D.; Bryant, T.N.; Gardner, M.J. (Eds.) Statistic with Confidence: Confidence Intervals and Statistical Guidelines, 2nd ed.; BMJ Books: London, UK, 2000; ISBN 0-7279-1375-1. [Google Scholar]
- Rösler, A.; Lehmann, F.; Krause, T.; Wirth, R.; von Renteln-Kruse, W. Nutritional and hydration status in elderly subjects: Clinical rating versus bioimpedance analysis. Arch. Gerontol. Geriatr. 2010, 50, e81–e85. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, A.; Harvey, K.; Ash, S.; Battistutta, D. Clinical assessment of dehydration in older people admitted to hospital: What are the strongest indicators? Arch. Gerontol. Geriatr. 2008, 47, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Paulis, S.J.C.; Everink, I.H.J.; Halfens, R.J.G.; Lohrmann, C.; Schols, J.M.G.A. Prevalence and Risk Factors of Dehydration Among Nursing Home Residents: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 19, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, X.; Poulia, K.A.; Chourdakis, M. What’s new about hydration in dementia? Curr. Opin. Clin. Nutr. Metab. Care 2025, 28, 20–24. [Google Scholar] [CrossRef]
- Hoen, L.; Pfeffer, D.; Zapf, R.; Raabe, A.; Hildebrand, J.; Kraft, J.; Kalkhof, S. Association of Drug Application and Hydration Status in Elderly Patients. Nutrients 2021, 13, 1929. [Google Scholar] [CrossRef]
- Lauriola, M.; Mangiacotti, A.; D’Onofrio, G.; Cascavilla, L.; Paris, F.; Paroni, G.; Seripa, D.; Greco, A.; Sancarlo, D. Neurocognitive Disorders and Dehydration in Older Patients: Clinical Experience Supports the Hydromolecular Hypothesis of Dementia. Nutrients 2018, 10, 562. [Google Scholar] [CrossRef]
- Edmonds, C.J.; Foglia, E.; Booth, P.; Fu, C.H.Y.; Gardner, M. Dehydration in older people: A systematic review of the effects of dehydration on health outcomes, healthcare costs and cognitive performance. Arch. Gerontol. Geriatr. 2021, 95, 104380. [Google Scholar] [CrossRef]
- Shimizu, M.; Kinoshita, K.; Hattori, K.; Ota, Y.; Kanai, T.; Kobayashi, H.; Tokuda, Y. Physical signs of dehydration in the elderly. Intern. Med. 2012, 51, 1207–1210. [Google Scholar] [CrossRef]
- Kinoshita, K.; Hattori, K.; Ota, Y.; Kanai, T.; Shimizu, M.; Kobayashi, H.; Tokuda, Y. The measurement of axillary moisture for the assessment of dehydration among older patients: A pilot study. Exp. Gerontol. 2013, 48, 255–258. [Google Scholar] [CrossRef]
- Paulus, M.C.; Melchers, M.; van Es, A.; Kouw, I.W.K.; van Zanten, A.R.H. The urea-to-creatinine ratio as an emerging biomarker in critical care: A scoping review and meta-analysis. Crit. Care 2025, 29, 175. [Google Scholar] [CrossRef]
- Manoeuvrier, G.; Bach-Ngohou, K.; Batard, E.; Masson, D.; Trewick, D. Diagnostic performance of serum blood urea nitrogen to creatinine ratio for distinguishing prerenal from intrinsic acute kidney injury in the emergency department. BMC Nephrol. 2017, 18, 173. [Google Scholar] [CrossRef] [PubMed]
- Porter, T.R.; Shillcutt, S.K.; Adams, M.S.; Desjardins, G.; Glas, K.E.; Olson, J.J.; Troughton, R.W. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: A report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2015, 28, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Masugata, H.; Senda, S.; Okuyama, H.; Murao, K.; Inukai, M.; Hosomi, N.; Iwado, Y.; Noma, T.; Kohno, M.; Himoto, T.; et al. Age-related decrease in inferior vena cava diameter measured with echocardiography. Tohoku J. Exp. Med. 2010, 222, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Sliwa-Hahnle, K.; Jing, Z.-C.; Gibbs, J.S.R. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef]
- Neuendorff, N.R.; Wirth, R.; Stoev, K.; Schnepper, M.; Levermann, I.; Wang, B.; Giehl, C.; Trampisch, U.S.; Funk, L.; Pourhassan, M. Dehydration and Malnutrition-Similar Yet Different: Data from a Prospective Observational Study in Older Hospitalized Patients. Nutrients 2025, 17, 1004. [Google Scholar] [CrossRef]
- Hoyle, G.E.; Chua, M.; Soiza, R.L. Volaemic assessment of the elderly hyponatraemic patient: Reliability of clinical assessment and validation of bioelectrical impedance analysis. QJM Int. J. Med. 2011, 104, 35–39. [Google Scholar] [CrossRef]
- Park, K.H.; Shin, J.-H.; Hwang, J.H.; Kim, S.H. Utility of Volume Assessment Using Bioelectrical Impedance Analysis in Critically Ill Patients Receiving Continuous Renal Replacement Therapy: A Prospective Observational Study. Korean J. Crit. Care Med. 2017, 32, 256–264. [Google Scholar] [CrossRef]
- Chung, Y.J.; Lee, G.R.; Kim, H.S.; Kim, E.Y. Effect of rigorous fluid management using monitoring of ECW ratio by bioelectrical impedance analysis in critically ill postoperative patients: A prospective, single-blind, randomized controlled study. Clin. Nutr. 2024, 43, 2164–2176. [Google Scholar] [CrossRef]
| Characteristics n (%) | Total 101 (100) | Hypohydrated 12 (11.9) | Euhydrated 69 (68.3) | Hyperhydrated 20 (19.8) | p |
|---|---|---|---|---|---|
| Age [years] * | 80.1 (±6.97) | 81.1 (±6.20) | 80.3 (±6.95) | 78.8 (±7.62) | 0.594 † |
| Gender [female, n] *** | 59.0 (58.40) | 10.0 (83.30) | 40.0 (58.00) | 9.0 (45.00) | 0.103 ‡ |
| Height [cm] * | 167.1 (±8.84) | 161.4 (±5.28) | 167.4 (±8.33) | 169.9 (±10.88) | 0.011 † |
| Weight [kg] * | 76.9 (±19.91) | 61.7 (±13.68) | 74.6 (±17.00) | 94.1 (±21.62) | <0.001 † |
| BMI [kg/m2] * | 27.4 (±5.88) | 23.6 (±4.92) | 26.5 (±5.12) | 32.5 (±5.91) | <0.001 † |
| MMSE [Points] ** | 27.0 (±3.0) | 26.5 (±5.25) | 27.0 (±3.0) | 27.0 (±2.75) | 0.594 † |
| Characteristics n (%) | Total 101 (100) | Hypohydrated 12 (11.9) | Euhydrated 69 (68.3) | Hyperhydrated 20 (19.8) | p |
|---|---|---|---|---|---|
| Serum Parameter | |||||
| S-Osmolarity [mOsmol/L] * | 291.4 (±13.37) | 294.8 (±13.71) | 291.3 (±11.42) | 289.7 (±18.95) | 0.773 † |
| S-Sodium [mmol/L] * | 136.3 (±5.87) | 137.6 (±7.87) | 136.62 (±4.69) | 134.15 (±7.79) | 0.629 † |
| S-Creatinine [mg/dL] * | 1.22 (±0.87) | 1.43 (±1.237) | 1.108 (±0.728) | 1.455 (±1.014) | 0.290 † |
| S-Urea [mg/dL] * | 51.3 (±39.99) | 54.9 (±51.35) | 46.4 (±32.51) | 66.5 (±53.09) | 0.539 † |
| BUN:Cr * | 20.3 (±10.11) | 19.2 (±7.62) | 20.4 (±10.47) | 20.7 (±10.56) | 0.990 † |
| S-Potassium [mmol/L] * | 4.08 (±0.52) | 3.94 (±0.322) | 4.08 (±0.503) | 4.14 (±0.662) | 0.521 † |
| S-Glucose [mg/dL] * | 124.2 (±59.86) | 130.8 (±87.16) | 122.4 (±55.91) | 126.5 (±56.62) | 0.600 † |
| Urine Parameters | |||||
| Urine color ** [0;6 (±IQR)] | 4.00 (3.00) | 4.0 (±3.25) | 4.0 (±3.75) | 5.0 (±3.50) | 0.387 † |
| Specific urine gravity [g/mL(±SD)] * | 1.0169 (±0.01314) | 1.0164 (±0.00888) | 1.0180 (±0.0149) | 1.0133 (±0.0066) | 0.238 † |
| Diagnostic Method | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR− (95% CI) |
|---|---|---|---|---|---|---|
| Palpation of axillary moisture | ||||||
| Dry axilla *,# | 83.3 (55.2; 95.3) | 82.8 (73.5; 89.3) | 40.0 (23.4; 59.3) | 97.3 (90.7; 99.3) | 4.83 (2.86; 8.17) | 0.20 (0.06; 0.72) |
| Maximum IVC diameter, subxiphoidal angle | ||||||
| cut-off < 1.55 cm ** | 63.64 (35.38; 84.83) | 81.01 (71.01; 88.14) | 31.82 (16.36; 52.68) | 94.12 (85.83; 97.69) | 3.35 (1.77; 6.34) | 0.45 (0.20; 0.99) |
| cut-off ≤ 1.95 cm ** | 90.9 (62.3; 98.4) | 50.6 (39.8; 61.4) | 20.4 (11.5; 33.6) | 97.6 (87.4; 99.6) | 1.84 (1.38; 2.46) | 0.18 (0.03; 1.18) |
| cut-off < 2.15 cm ** | 100.00 (74.12; 100.00) | 36.71 (26.93; 47.72) | 18.03 (10.38; 29.47) | 100.00 (88.30; 100.00) | 1.58 (1.34; 1.87) | 0.00 (0.00; 0.00) |
| Diagnostic Method | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR− (95% CI) |
|---|---|---|---|---|---|---|
| Maximum IVC diameter, subxiphoidal angle | ||||||
| cut-off ≥ 2.50 cm ** | 47.37 (27.33; 68.29) | 90.14 (81.02; 95.14) | 56.25 (33.18; 76.90) | 86.49 (76.88; 92.49) | 4.80 (2.06; 11.22) | 0.58 (0.38; 0.90) |
| cut-off ≥ 2.15 cm ** | 73.7 (51.2; 88.2) | 78.9 (68.0; 86.8) | 48.3 (31.4; 65.6) | 91.8 (82.2; 96.5) | 3.49 (2.07; 5.89) | 0.33 (0.16; 0.71) |
| cut-off ≥ 1.55 cm ** | 94.74 (75.36; 99.06) | 28.58 (19.32; 40.05) | 26.47 (17.45; 38.01) | 95.24 (77.33; 99.15) | 1.33 (1.11; 1.59) | 0.18 (0.02; 1.29) |
| Maximum IVC diameter, transcostal angle | ||||||
| cut-off ≥ 2.50 cm *** | 55.56 (26.66; 81.12) | 91.43 (77.62; 97.04) | 62.50 (30.57; 86.32) | 88.89 (74.68; 95.59) | 6.48 (1.90; 22.17) | 0.49 (0.23; 1.02) |
| cut-off ≥ 2.15 cm *** | 88.9 (56.5; 98.0) | 71.4 (54.9; 83.7) | 44.4 (24.6; 66.3) | 96.2 (81.1; 99.3) | 3.11 (1.76; 5.52) | 0.16 (0.02; 1.00) |
| cut-off ≥ 1.55 cm *** | 100.00 (70.08; 100.00) | 25.71 (14.16; 42.07) | 25.71 (14.16; 42.07) | 100.00 (70.08; 100.00) | 1.35 (1.11; 1.64) | 0.00 (0.00; 0.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deissler, L.; Janneck, M.; Wirth, R.; Fierenz, A.; Thiem, U.; Rösler, A. Hydration Status in Geriatric Patients—Subjective Impression or Objective Parameter? The Hydr-Age-Study. Nutrients 2025, 17, 3129. https://doi.org/10.3390/nu17193129
Deissler L, Janneck M, Wirth R, Fierenz A, Thiem U, Rösler A. Hydration Status in Geriatric Patients—Subjective Impression or Objective Parameter? The Hydr-Age-Study. Nutrients. 2025; 17(19):3129. https://doi.org/10.3390/nu17193129
Chicago/Turabian StyleDeissler, Linda, Matthias Janneck, Rainer Wirth, Alexander Fierenz, Ulrich Thiem, and Alexander Rösler. 2025. "Hydration Status in Geriatric Patients—Subjective Impression or Objective Parameter? The Hydr-Age-Study" Nutrients 17, no. 19: 3129. https://doi.org/10.3390/nu17193129
APA StyleDeissler, L., Janneck, M., Wirth, R., Fierenz, A., Thiem, U., & Rösler, A. (2025). Hydration Status in Geriatric Patients—Subjective Impression or Objective Parameter? The Hydr-Age-Study. Nutrients, 17(19), 3129. https://doi.org/10.3390/nu17193129






