Effects of Infant Formula Type on Early Childhood Growth Outcomes: A Retrospective Cohort Study †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Population
2.3. Exposures
2.4. Outcomes
2.5. Data Collection
2.6. Statistical Analysis
3. Results
Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| CDC | Center for Disease Control and Prevention |
| CMF | Cow Milk-based Formula |
| BMI | Body Mass Index |
References
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.C.; Davy, O.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P.; et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files-Development of Files and Prevalence Estimates for Selected Health Outcomes; National Health Statistics Reports; CDC Stacks: Atlanta, Georgia, 2021. [Google Scholar] [CrossRef]
- Gracner, T.; Boone, C.; Gertler, P.J. Exposure to sugar rationing in the first 1000 days of life protected against chronic disease. Science 2024, 386, 1043–1048. [Google Scholar] [CrossRef]
- DiGirolamo, A.M.; Ochaeta, L.; Flores, R.M.M. Early Childhood Nutrition and Cognitive Functioning in Childhood and Adolescence. Food Nutr. Bull. 2020, 41, S31–S40. [Google Scholar] [CrossRef]
- Kaar, J.L.; Crume, T.; Brinton, J.T.; Bischoff, K.J.; McDuffie, R.; Dabelea, D. Maternal obesity, gestational weight gain, and offspring adiposity: The exploring perinatal outcomes among children study. J. Pediatr. 2014, 165, 509–515. [Google Scholar] [CrossRef]
- Robertson, R.C.; Edens, T.J.; Carr, L.; Mutasa, K.; Gough, E.K.; Evans, C.; Geum, H.M.; Baharmand, I.; Gill, S.K.; Ntozini, R.; et al. The gut microbiome and early-life growth in a population with high prevalence of stunting. Nat. Commun. 2023, 14, 654. [Google Scholar] [CrossRef]
- Meek, J.Y.; Noble, L.; Breastfeeding, S.O. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Breastfeeding Report Card United States. Available online: https://www.cdc.gov/breastfeeding-data/media/pdfs/2024/05/2020-breastfeeding-report-card-h.pdf (accessed on 1 March 2025).
- U.S. Department of Agriculture. Dietary Guidelines for Americans, 2020–2025, 9th ed.; USDA: Washington, DC, USA, 2020. [Google Scholar]
- Strzalkowski, A.J.; Jarvinen, K.M.; Schmidt, B.; Young, B.E. Protein and carbohydrate content of infant formula purchased in the United States. Clin. Exp. Allergy 2022, 52, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Infant Formula. Available online: https://www.fda.gov/food/resources-you-food/infant-formula (accessed on 8 May 2025).
- Anderson, C.E.; Whaley, S.E.; Goran, M.I. Lactose-reduced infant formula with corn syrup solids and obesity risk among participants in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). Am. J. Clin. Nutr. 2022, 116, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Dedon, L.R.; Ozcan, E.; Rani, A.; Sela, D.A. Bifidobacterium infantis Metabolizes 2’Fucosyllactose-Derived and Free Fucose Through a Common Catabolic Pathway Resulting in 1,2-Propanediol Secretion. Front. Nutr. 2020, 7, 583397. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Rips-Goodwin, A.R.; Jun, D.; Griebel-Thompson, A.; Kong, K.L.; Fazzino, T.L. US infant formulas contain primarily added sugars: An analysis of infant formulas on the US market. J. Food Compos. Anal. 2025, 141, 107369. [Google Scholar] [CrossRef]
- Sullivan, P.B.; Juszczak, E.; Bachlet, A.M.; Lambert, B.; Vernon-Roberts, A.; Grant, H.W.; Eltumi, M.; McLean, L.; Alder, N.; Thomas, A.G. Gastrostomy tube feeding in children with cerebral palsy: A prospective, longitudinal study. Dev. Med. Child. Neurol. 2005, 47, 77–85. [Google Scholar] [CrossRef]
- Roberts, K.H.; Barks, J.D.E.; Glass, H.C.; Soul, J.S.; Chang, T.; Wusthoff, C.J.; Chu, C.J.; Massey, S.L.; Abend, N.S.; Lemmon, M.E.; et al. Feeding and developmental outcomes after neonatal seizures-A prospective observational study. Ann. Child Neurol. Soc. 2023, 1, 209–217. [Google Scholar] [CrossRef]
- Holst, L.M.; Serrano, F.; Shekerdemian, L.; Ravn, H.B.; Guffey, D.; Ghanayem, N.S.; Monteiro, S. Impact of feeding mode on neurodevelopmental outcome in infants and children with congenital heart disease. Congenit. Heart Dis. 2019, 14, 1207–1213. [Google Scholar] [CrossRef]
- Banerjee, I.; Forsythe, L.; Skae, M.; Avatapalle, H.B.; Rigby, L.; Bowden, L.E.; Craigie, R.; Padidela, R.; Ehtisham, S.; Patel, L.; et al. Feeding Problems Are Persistent in Children with Severe Congenital Hyperinsulinism. Front. Endocrinol. 2016, 7, 8. [Google Scholar] [CrossRef]
- WHO. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar] [CrossRef]
- Ogden, C.L.; Flegal, K.M. Changes in terminology for childhood overweight and obesity. Natl. Health Stat. Rep. 2010, 25, 1–5. [Google Scholar]
- American Academy of Pediatrics. Bright Futures Infancy Tools. Available online: https://www.aap.org/en/practice-management/bright-futures/bright-futures-materials-and-tools/bright-futures-tool-and-resource-kit/bright-futures-infancy-tools/?utm_source (accessed on 2 March 2025).
- Santillan, D.A.; Santillan, M.K.; Davis, H.A.; Crooks, M.; Flanagan, P.J.; Ortman, C.E.; Faro, E.Z.; Hunter, S.K.; Knosp, B.K. Implementation of a Maternal Child Knowledgebase. AMIA Jt. Summits Transl. Sci. Proc. 2022, 2022, 432–438. [Google Scholar]
- Langley-Evans, S.C.; Moran, V.H. Childhood obesity: Risk factors, prevention and management. Matern. Child Nutr. 2014, 10, 453–455. [Google Scholar] [CrossRef]
- Gamboa-Gamboa, T.; Fantin, R.; Cordoba, J.; Caravaca, I.; Gomez-Duarte, I. Relationship between childhood obesity and socio-economic status among primary school children in Costa Rica. Public Health Nutr. 2021, 24, 3825–3833. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Ainiwan, D.; Alifu, X.; Cheng, H.; Qiu, Y.; Zhou, H.; Liu, H.; Yu, Y. Breastfeeding, Gestational Diabetes Mellitus, Size at Birth and Overweight/Obesity in Early Childhood. Nutrients 2024, 16, 1351. [Google Scholar] [CrossRef] [PubMed]
- Voerman, E.; Santos, S.; Patro Golab, B.; Amiano, P.; Ballester, F.; Barros, H.; Bergstrom, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef] [PubMed]
- Rani, U.; Story, W.T.; Ten Eyck, P.; Imdad, A. The Association of Infant Formula Type with Growth Outcomes: Does Formula Choice Matter? In Proceedings of the Pediatric Academic Societies (PAS) Meeting, Honolulu, HI, USA, 27 April 2025. [Google Scholar]
- Young, B. Variation in Infant Formula Macronutrient Ingredients Is Associated with Infant Anthropometrics. Nutrients 2020, 12, 3465. [Google Scholar] [CrossRef]
- Merritt, R.J. Should we be concerned about the use of lactose-free infant formulas? J. Pediatr. Gastroenterol. Nutr. 2024, 79, 929–933. [Google Scholar] [CrossRef]
- Romero-Velarde, E.; Delgado-Franco, D.; García-Gutiérrez, M.; Gurrola-Díaz, C.; Larrosa-Haro, A.; Montijo-Barrios, E.; Muskiet, F.A.J.; Vargas-Guerrero, B.; Geurts, J. The Importance of Lactose in the Human Diet: Outcomes of a Mexican Consensus Meeting. Nutrients 2019, 11, 2737. [Google Scholar] [CrossRef]
- Mennella, J.A.; Ventura, A.K.; Beauchamp, G.K. Differential growth patterns among healthy infants fed protein hydrolysate or cow-milk formulas. Pediatrics 2011, 127, 110–118. [Google Scholar] [CrossRef]
- Imdad, A.; Sherwani, R.; Wall, K. Pediatric Formulas: An Update. Pediatr. Rev. 2024, 45, 394–405. [Google Scholar] [CrossRef]
- Kong, K.L.; Burgess, B.; Morris, K.S.; Re, T.; Hull, H.R.; Sullivan, D.K.; Paluch, R.A. Association Between Added Sugars from Infant Formulas and Rapid Weight Gain in US Infants and Toddlers. J. Nutr. 2021, 151, 1572–1580. [Google Scholar] [CrossRef]
- Ogbuanu, C.; Glover, S.; Probst, J.; Liu, J.; Hussey, J. The effect of maternity leave length and time of return to work on breastfeeding. Pediatrics 2011, 127, e1414–e1427. [Google Scholar] [CrossRef]
- Aziz-Bose, R.; Paul, M.A.; Flamand, Y.; Kelly, C.A.; Duhaney, L.; Umaretiya, P.J.; Lokko, L.; Griffin, M.; Hawkins, A.; Cole, P.D.; et al. Public Insurance as a Proxy Measure of Household Poverty-Exposures Among Children with Hematologic Malignancies. Blood 2024, 144, 3750. [Google Scholar] [CrossRef]
- Monuteaux, M.C.; Du, M.; Neuman, M.I. Evaluation of Insurance Type as a Proxy for Socioeconomic Status in the Pediatric Emergency Department: A Pilot Study. Ann. Emerg. Med. 2024, 83, 562–567. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. National Academies of Sciences, Engineering, and Medicine. National Academies of Sciences, Engineering, and Medicine. Chapter 3: Infant formula regulatory framework before the 2022 shortage. In Challenges in Supply, Market Competition, and Regulation of Infant Formula in the United States; The National Academies Press: Washington, DC, USA, 2024. [Google Scholar]
- Abrams, S.A.; Du, N. Perspective: Operation stork speed: Strategies for reviewing and advising on infant formula. Am. J. Clin. Nutr. 2025, 121, 1220–1223. [Google Scholar] [CrossRef] [PubMed]

| Variable | Breastfeeding (N = 1922) | Standard Formula (N = 2301) | Non-Standard Formula (N = 1292) | p-Value |
|---|---|---|---|---|
| Sex, N (%) | 0.2763 | |||
| Female | 979 (50.9%) | 1143 (49.7%) | 621 (48%) | |
| Male | 944 (49.1%) | 1158 (50.3%) | 672 (52%) | |
| Insurance, N (%) | <0.0001 | |||
| Medicaid | 355 (18.8%) | 816 (35.8%) | 421 (32.9%) | |
| Other | 374 (19.9%) | 482 (21.2%) | 275 (21.5%) | |
| Private | 1155 (61.3%) | 981 (43.1%) | 584 (45.6%) | |
| Race, N (%) | <0.0001 | |||
| Black | 83 (5.1%) | 407 (20.3%) | 121 (11.4%) | |
| Other (Asian, American Indians, Pacific Islanders) | 237 (14.6%) | 482 (24%) | 196 (18.5%) | |
| White | 1309 (80.4%) | 1116 (55.7%) | 741 (70%) | |
| Gestational Diabetes, N (%) | 153 (9.4%) | 224 (11.2%) | 112 (10.6%) | 0.2134 |
| Maternal Obesity, N (%) | 217 (13.3%) | 409 (20.4%) | 265 (25.1%) | <0.0001 |
| Maternal Anemia, N (%) | 372 (22.8%) | 602 (30%) | 282 (26.7%) | <0.0001 |
| Mother’s Age (Years), Median (IQR) | 31 (28–34) | 30 (26–34) | 30 (26–33) | <0.0001 |
| Gestational Age (Days), Median (IQR) | 276 (272–281) | 275 (271–280) | 274 (270–279) | <0.0001 |
| Birth Weight (g), Median (IQR) | 3445 (3165–3748) | 3375 (3095–3685) | 3385 (3095–3680) | <0.0001 |
| Birth Length (cm), Median (IQR) | 51.0 (49.5–52.5) | 51 (49.5–52.1) | 50.8 (49.5–52.1) | 0.0035 |
| Cow’s milk at 1 year of age * | 422 (47.0%) | 850 (66.3%) | 490 (63.6%) | <0.0001 |
| Breast milk at 1 year of age * | 651 (72.5%) | 236 (18.4%) | 86 (11.2%) | <0.0001 |
| Formula at 1 year of age * | 52 (5.8%) | 524 (40.8%) | 347 (45.1%) | <0.0001 |
| Soy milk at 1 year of age * | 16 (1.8%) | 13 (1.0%) | 14 (1.8%) | 0.2103 |
| Feeding Group Comparison | Age (Months) | Z-Score | Unadjusted Mean Diff (95% CI) | Unadjusted p-Value | Adjusted Mean Difference (95% CI) | p-Value |
|---|---|---|---|---|---|---|
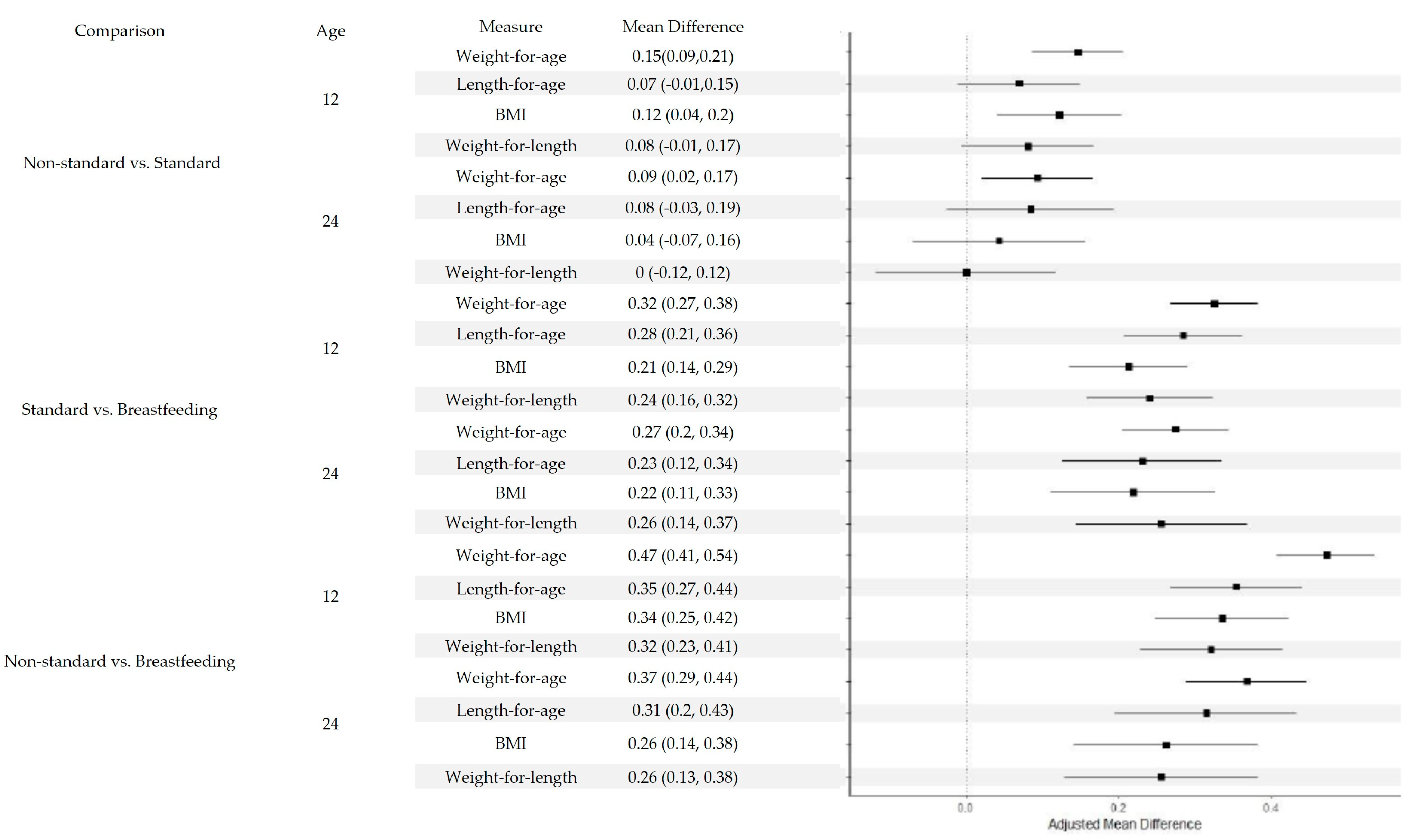
| Non-standard vs. Standard | 12 | Weight-for-age | 0.154 (0.094, 0.214) | <0.0001 | 0.146 (0.086, 0.206) | <0.0001 |
| 12 | Length-for-age | 0.034 (0.046, 0.114) | 0.4055 | 0.069 (−0.011, 0.149) | 0.0898 | |
| 12 | BMI | 0.146 (0.065, 0.228) | 0.0004 | 0.122 (0.041, 0.203) | 0.0032 | |
| 12 | Weight-for-length | 0.088 (0.001, 0.176) | 0.0487 | 0.081 (−0.006, 0.167) | 0.0681 | |
| 24 | Weight-for-age | 0.103 (0.031, 0.176) | 0.0052 | 0.093 (0.020, 0.166) | 0.012 | |
| 24 | Length-for-age | 0.045(−0.065, 0.156) | 0.4209 | 0.084 (−0.026, 0.194) | 0.1356 | |
| 24 | BMI | 0.068 (−0.045, 0.181) | 0.2368 | 0.043 (−0.070, 0.156) | 0.4552 | |
| 24 | Weight-for-length | 0.010 (−0.109, 0.129) | 0.8714 | 0.000 (−0.118, 0.118) | 0.9997 | |
| Standard vs. Breastfeeding | 12 | Weight-for-age | 0.339 (0.283, 0.395) | <0.0001 | 0.324 (0.267, 0.382) | <0.0001 |
| 12 | Length-for-age | 0.334 (0.258, 0.410) | <0.0001 | 0.284 (0.207, 0.361) | <0.0001 | |
| 12 | BMI | 0.207 (0.130, 0.284) | <0.0001 | 0.212 (0.135, 0.290) | <0.0001 | |
| 12 | Weight-for-length | 0.235 (0.153, 0.318) | <0.0001 | 0.240 (0.157, 0.323) | <0.0001 | |
| 24 | Weight-for-age | 0.295 (0.226, 0.363) | <0.0001 | 0.274 (0.204, 0.343) | <0.0001 | |
| 24 | Length-for-age | 0.284 (0.179, 0.388) | <0.0001 | 0.230 (0.125, 0.335) | <0.0001 | |
| 24 | BMI | 0.212 (0.106, 0.319) | <0.0001 | 0.218 (0.111, 0.326) | <0.0001 | |
| 24 | Weight-for-length | 0.249 (0.137, 0.361) | <0.0001 | 0.255 (0.144, 0.367) | <0.0001 | |
| Non-standard vs. Breastfeeding | 12 | Weight-for-age | 0.493 (0.429, 0.557) | <0.0001 | 0.471 (0.406, 0.535) | <0.0001 |
| 12 | Length-for-age | 0.368 (0.282, 0.454) | <0.0001 | 0.353 (0.267, 0.439) | <0.0001 | |
| 12 | BMI | 0.354 (0.266, 0.441) | <0.0001 | 0.335 (0.247, 0.422) | <0.0001 | |
| 12 | Weight-for-length | 0.324 (0.230, 0.418) | <0.0001 | 0.320 (0.227, 0.414) | <0.0001 | |
| 24 | Weight-for-age | 0.398 (0.320, 0.476) | <0.0001 | 0.367 (0.288, 0.445) | <0.0001 | |
| 24 | Length-for-age | 0.329 (0.211, 0.447) | <0.0001 | 0.314 (0.195, 0.432) | <0.0001 | |
| 24 | BMI | 0.280 (0.160, 0.401) | <0.0001 | 0.262 (0.141, 0.382) | <0.0001 | |
| 24 | Weight-for-length | 0.259 (0.132, 0.385) | <0.0001 | 0.255 (0.1290.382) | <0.0001 |
| Unadjusted | |||||||
|---|---|---|---|---|---|---|---|
| Subset | Age (Months) | Feeding | Reference | Mean Diff | 95% Lower | 95% Upper | p -Value |
| Male | 12 | Non-standard Formula | Standard Formula | 0.090 | −0.018 | 0.198 | 0.1036 |
| 12 | Standard Formula | Breastfeeding | 0.232 | 0.128 | 0.336 | <0.0001 | |
| 12 | Non-standard Formula | Breastfeeding | 0.322 | 0.206 | 0.438 | <0.0001 | |
| 24 | Non-standard Formula | Standard Formula | 0.066 | −0.086 | 0.217 | 0.396 | |
| 24 | Standard Formula | Breastfeeding | 0.204 | 0.061 | 0.346 | 0.0051 | |
| 24 | Non-standard Formula | Breastfeeding | 0.269 | 0.108 | 0.431 | 0.0011 | |
| Female | 12 | Non-standard Formula | Standard Formula | 0.229 | 0.126 | 0.332 | <0.0001 |
| 12 | Standard Formula | Breastfeeding | 0.194 | 0.098 | 0.291 | <0.0001 | |
| 12 | Non-standard Formula | Breastfeeding | 0.424 | 0.314 | 0.533 | <0.0001 | |
| 24 | Non-standard Formula | Standard Formula | 0.079 | −0.065 | 0.224 | 0.2824 | |
| 24 | Standard Formula | Breastfeeding | 0.255 | 0.116 | 0.394 | 0.0003 | |
| 24 | Non-standard Formula | Breastfeeding | 0.334 | 0.179 | 0.489 | <0.0001 | |
| Black | 12 | Non-standard Formula | Standard Formula | 0.035 | −0.216 | 0.287 | 0.7823 |
| 12 | Standard Formula | Breastfeeding | 0.202 | −0.117 | 0.521 | 0.2141 | |
| 12 | Non-standard Formula | Breastfeeding | 0.237 | −0.127 | 0.602 | 0.2022 | |
| 24 | Non-standard Formula | Standard Formula | −0.209 | −0.552 | 0.134 | 0.2319 | |
| 24 | Standard Formula | Breastfeeding | 0.429 | −0.048 | 0.906 | 0.0784 | |
| 24 | Non-standard Formula | Breastfeeding | 0.220 | −0.304 | 0.743 | 0.4108 | |
| White | 12 | Non-standard Formula | Standard Formula | 0.164 | 0.067 | 0.260 | 0.0009 |
| 12 | Standard Formula | Breastfeeding | 0.232 | 0.143 | 0.321 | <0.0001 | |
| 12 | Non-standard Formula | Breastfeeding | 0.396 | 0.299 | 0.493 | <0.0001 | |
| 24 | Non-standard Formula | Standard Formula | 0.101 | −0.035 | 0.236 | 0.1464 | |
| 24 | Standard Formula | Breastfeeding | 0.195 | 0.073 | 0.317 | 0.0018 | |
| 24 | Non-standard Formula | Breastfeeding | 0.295 | 0.160 | 0.431 | <0.0001 | |
| Other | 12 | Non-standard Formula | Standard Formula | −0.028 | −0.214 | 0.158 | 0.7687 |
| 12 | Standard Formula | Breastfeeding | 0.417 | 0.228 | 0.607 | <0.0001 | |
| 12 | Non-standard Formula | Breastfeeding | 0.389 | 0.168 | 0.611 | 0.0006 | |
| 24 | Non-standard Formula | Standard Formula | 0.051 | −0.197 | 0.299 | 0.6889 | |
| 24 | Standard Formula | Breastfeeding | 0.424 | 0.160 | 0.688 | 0.0017 | |
| 24 | Non-standard Formula | Breastfeeding | 0.474 | 0.174 | 0.775 | 0.002 | |
| Hispanic | 12 | Non-standard Formula | Standard Formula | −0.030 | −0.279 | 0.219 | 0.8134 |
| 12 | Standard Formula | Breastfeeding | 0.476 | 0.178 | 0.774 | 0.0018 | |
| 12 | Non-standard Formula | Breastfeeding | 0.446 | 0.117 | 0.776 | 0.008 | |
| 24 | Non-standard Formula | Standard Formula | 0.069 | −0.253 | 0.390 | 0.676 | |
| 24 | Standard Formula | Breastfeeding | 0.204 | −0.204 | 0.612 | 0.3272 | |
| 24 | Non-standard Formula | Breastfeeding | 0.273 | −0.162 | 0.707 | 0.2193 | |
| Non-Hispanic | 12 | Non-standard Formula | Standard Formula | 0.170 | 0.084 | 0.256 | 0.0001 |
| 12 | Standard Formula | Breastfeeding | 0.180 | 0.099 | 0.260 | <0.0001 | |
| 12 | Non-standard Formula | Breastfeeding | 0.349 | 0.258 | 0.440 | <0.0001 | |
| 24 | Non-standard Formula | Standard Formula | 0.080 | −0.041 | 0.202 | 0.1963 | |
| 24 | Standard Formula | Breastfeeding | 0.195 | 0.084 | 0.307 | 0.0006 | |
| 24 | Non-standard Formula | Breastfeeding | 0.276 | 0.148 | 0.403 | <0.0001 | |
| Medicaid | 12 | Non-standard Formula | Standard Formula | 0.179 | 0.040 | 0.318 | 0.0116 |
| 12 | Standard Formula | Breastfeeding | 0.315 | 0.150 | 0.481 | 0.0002 | |
| 12 | Non-standard Formula | Breastfeeding | 0.494 | 0.313 | 0.676 | <0.0001 | |
| 24 | Non-standard Formula | Standard Formula | 0.042 | −0.154 | 0.237 | 0.676 | |
| 24 | Standard Formula | Breastfeeding | 0.269 | 0.034 | 0.505 | 0.025 | |
| 24 | Non-standard Formula | Breastfeeding | 0.311 | 0.058 | 0.564 | 0.0162 | |
| Private | 12 | Non-standard Formula | Standard Formula | 0.203 | 0.099 | 0.307 | 0.0001 |
| 12 | Standard Formula | Breastfeeding | 0.173 | 0.082 | 0.265 | 0.0002 | |
| 12 | Non-standard Formula | Breastfeeding | 0.376 | 0.273 | 0.480 | <0.0001 | |
| 24 | Non-standard Formula | Standard Formula | 0.058 | −0.088 | 0.204 | 0.4348 | |
| 24 | Standard Formula | Breastfeeding | 0.213 | 0.087 | 0.339 | 0.0009 | |
| 24 | Non-standard Formula | Breastfeeding | 0.271 | 0.125 | 0.417 | 0.0003 | |
| Other insurance | 12 | Non-standard Formula | Standard Formula | 0.028 | −0.138 | 0.194 | 0.7426 |
| 12 | Standard Formula | Breastfeeding | 0.274 | 0.112 | 0.436 | 0.0009 | |
| 12 | Non-standard Formula | Breastfeeding | 0.302 | 0.124 | 0.480 | 0.0009 | |
| 24 | Non-standard Formula | Standard Formula | 0.156 | −0.077 | 0.388 | 0.1891 | |
| 24 | Standard Formula | Breastfeeding | 0.173 | −0.059 | 0.405 | 0.1434 | |
| 24 | Non-standard Formula | Breastfeeding | 0.329 | 0.074 | 0.584 | 0.0114 | |
| <4000 g | 12 | Non-standard Formula | Standard Formula | 0.140 | 0.055 | 0.224 | 0.0012 |
| 12 | Standard Formula | Breastfeeding | 0.189 | 0.109 | 0.270 | <0.0001 | |
| 12 | Non-standard Formula | Breastfeeding | 0.329 | 0.239 | 0.420 | <0.0001 | |
| 24 | Non-standard Formula | Standard Formula | 0.087 | −0.032 | 0.205 | 0.1518 | |
| 24 | Standard Formula | Breastfeeding | 0.178 | 0.066 | 0.290 | 0.0018 | |
| 24 | Non-standard Formula | Breastfeeding | 0.265 | 0.139 | 0.390 | <0.0001 | |
| >4000 g | 12 | Non-standard Formula | Standard Formula | 0.261 | −0.028 | 0.550 | 0.0765 |
| 12 | Standard Formula | Breastfeeding | 0.375 | 0.129 | 0.621 | 0.0028 | |
| 12 | Non-standard Formula | Breastfeeding | 0.636 | 0.331 | 0.941 | <0.0001 | |
| 24 | Non-standard Formula | Standard Formula | −0.034 | −0.412 | 0.344 | 0.8587 | |
| 24 | Standard Formula | Breastfeeding | 0.537 | 0.193 | 0.881 | 0.0023 | |
| 24 | Non-standard Formula | Breastfeeding | 0.503 | 0.087 | 0.918 | 0.0179 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, U.; Alwasila, R.; Story, W.T.; Ten Eyck, P.; Hoberg, A.; Santillan, D.A.; Imdad, A. Effects of Infant Formula Type on Early Childhood Growth Outcomes: A Retrospective Cohort Study. Nutrients 2025, 17, 3111. https://doi.org/10.3390/nu17193111
Rani U, Alwasila R, Story WT, Ten Eyck P, Hoberg A, Santillan DA, Imdad A. Effects of Infant Formula Type on Early Childhood Growth Outcomes: A Retrospective Cohort Study. Nutrients. 2025; 17(19):3111. https://doi.org/10.3390/nu17193111
Chicago/Turabian StyleRani, Uzma, Roba Alwasila, William T. Story, Patrick Ten Eyck, Asher Hoberg, Donna A. Santillan, and Aamer Imdad. 2025. "Effects of Infant Formula Type on Early Childhood Growth Outcomes: A Retrospective Cohort Study" Nutrients 17, no. 19: 3111. https://doi.org/10.3390/nu17193111
APA StyleRani, U., Alwasila, R., Story, W. T., Ten Eyck, P., Hoberg, A., Santillan, D. A., & Imdad, A. (2025). Effects of Infant Formula Type on Early Childhood Growth Outcomes: A Retrospective Cohort Study. Nutrients, 17(19), 3111. https://doi.org/10.3390/nu17193111






