Potential Therapeutic Effects of Oolong Tea Phytochemicals on NLRP3 Inflammasome Assembly and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Tea Material
2.3. Extraction
2.4. Determination of Total Phenolic Content
2.5. Determination of Total Flavonoid Content
2.6. Determination of Total Condensed Tannins Content
2.7. Determination of Proanthocyanins Content
2.8. HPLC Analysis of Phytochemicals
2.9. Specific Conditions
2.10. Effect of OLT Extract on NLRP3 Inflammasome Activation
2.11. Determination of ASC Oligomerization
2.12. Determination of ROS Levels
2.13. Statistical Analysis
3. Results
3.1. Analysis of Bioactive Phytochemicals from OLT Extract
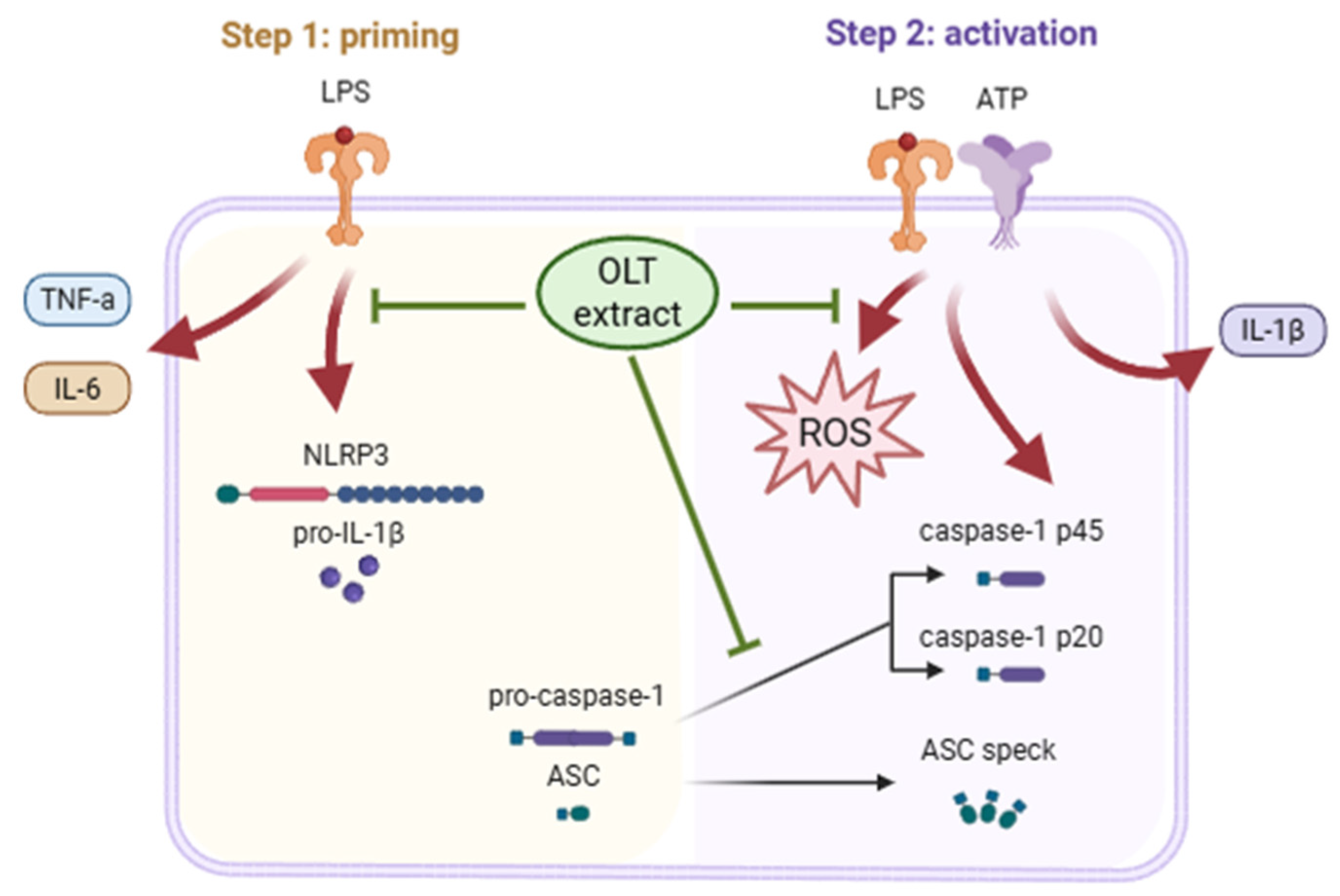
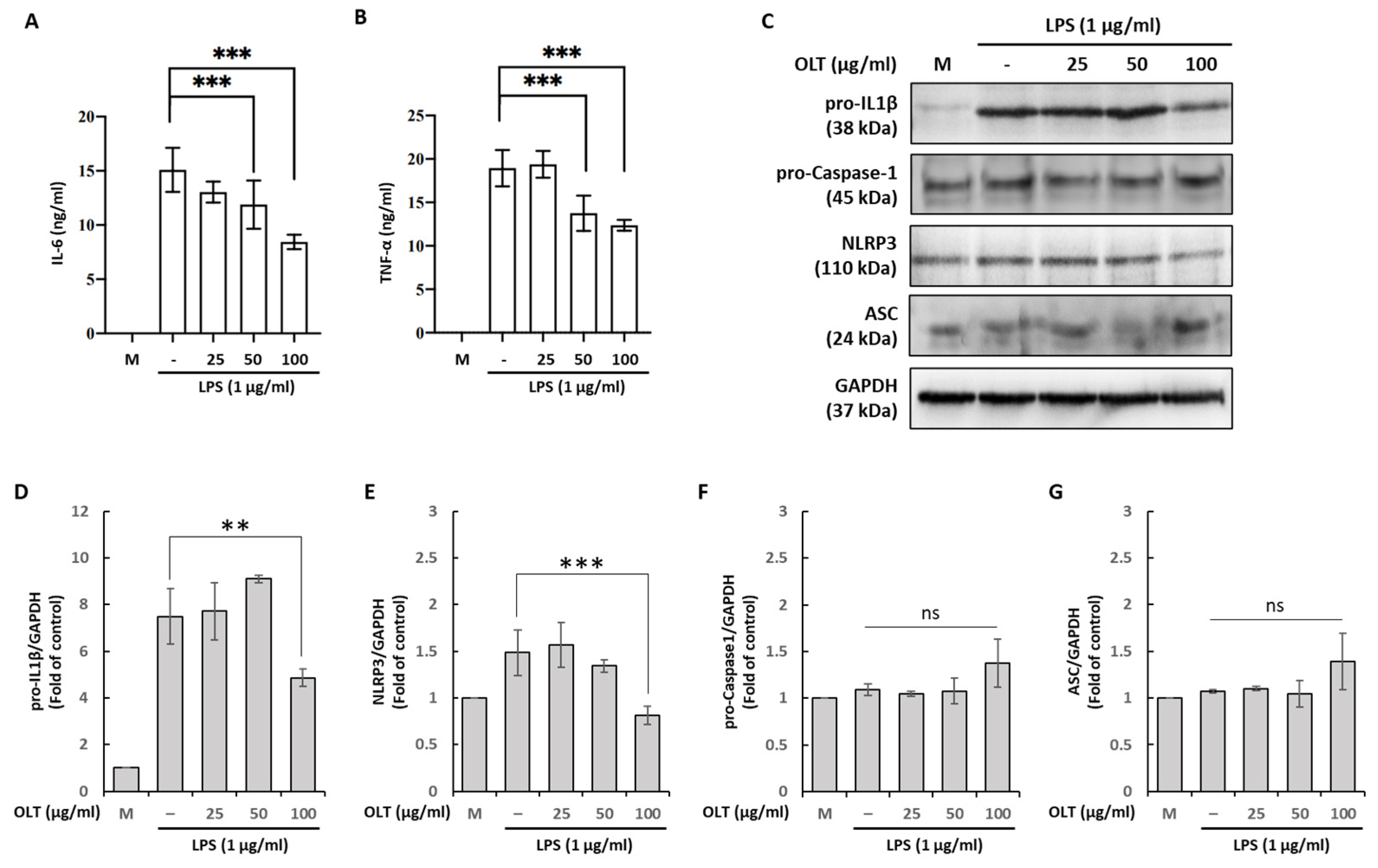
3.2. OLT Reduces LPS Priming Outputs (IL-6/TNF-α, pro-IL-1β, NLRP3) While ASC and Pro-Caspase-1 Remain Unchanged
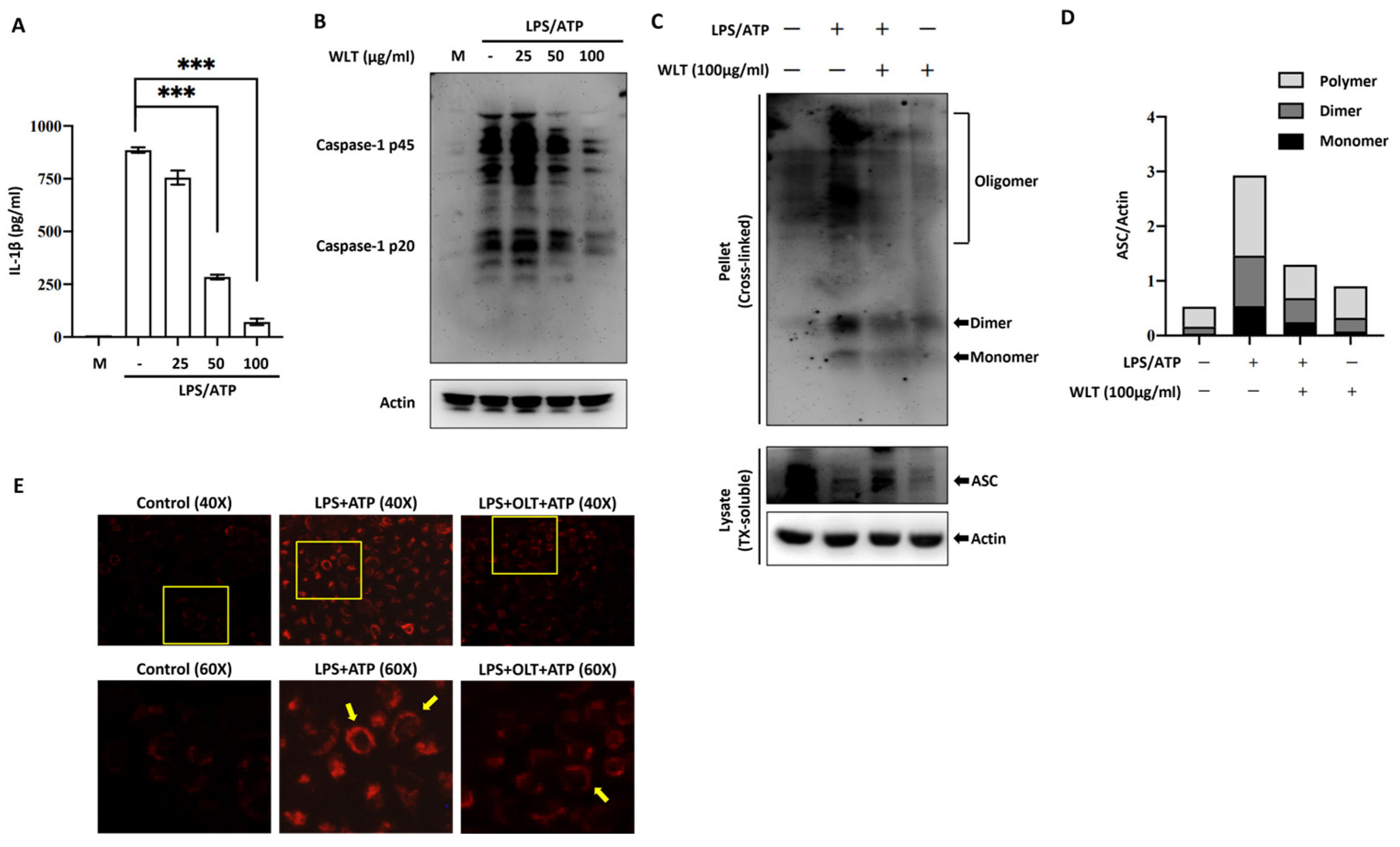
3.3. OLT Inhibits NLRP3 Inflammasome Activation by Suppressing IL-1β Secretion, Caspase-1 Activation, and ASC Oligomerization
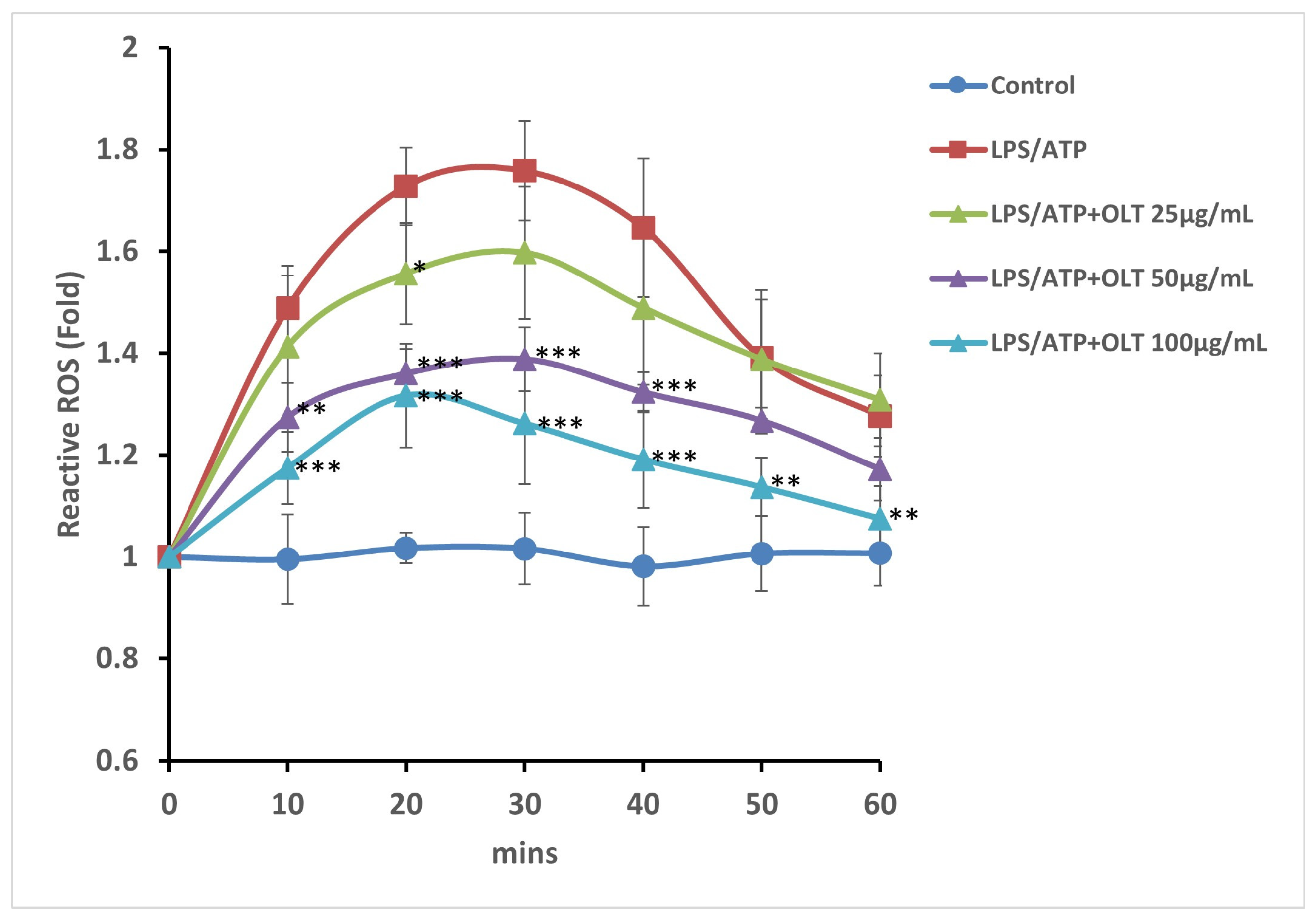
3.4. OLT Extracts Attenuate LPS/ATP-Induced ROS Generation in a Time- and Dose-Dependent Manner
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Knowledge Repository. Fao.org. 2025. Available online: https://openknowledge.fao.org/items/b25fb1f7-b819-4024-9344-d7b651fefdba (accessed on 19 September 2025).
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, T.; Zhao, S.; Zhu, Y.; Feng, C.; Zhan, J.; Li, S.; Ho, C.-T.; Gosslau, A. Multifunctional health-promoting effects of oolong tea and its products. Food Sci. Hum. Wellness 2022, 11, 512–523. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Tea flavonoids: Their functions, utilisation and analysis. Trends Food Sci. Technol. 2000, 11, 152–160. [Google Scholar] [CrossRef]
- Ho, C.T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Chen, Y.L.; Duan, J.; Jiang, Y.M.; Shi, J.; Peng, L.; Xue, S.; Kakuda, Y. Production, Quality, and Biological Effects of Oolong Tea (Camellia sinensis). Food Rev. Int. 2010, 27, 1–15. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lin, H.-H.; Chang, H.; Chuang, L.-T.; Hsieh, C.-Y.; Lu, S.-H.; Hung, C.-F.; Chang, J.-F. Prophylactic Effects of Purple Shoot Green Tea on Cytokine Immunomodulation through Scavenging Free Radicals and NO in LPS-Stimulated Macrophages. Curr. Issues Mol. Biol. 2022, 44, 3980–4000. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Chen, S.-J.; Huang, C.-C.; Liu, W.-C.; Lai, M.-T.; Kao, T.-Y.; Yang, W.-S.; Yang, C.-H.; Hsu, C.-P.; Chang, J.-F. Tocilizumab Exerts Anti-Tumor Effects on Colorectal Carcinoma Cell Xenografts Corresponding to Expression Levels of Interleukin-6 Receptor. Pharmaceuticals 2024, 17, 127. [Google Scholar] [CrossRef]
- Lin, C.-C.; Hsieh, C.-Y.; Chen, L.-F.; Chen, Y.-C.; Ho, T.-H.; Chang, S.-C.; Chang, J.-F. Versatile Effects of GABA Oolong Tea on Improvements in Diastolic Blood Pressure, Alpha Brain Waves, and Quality of Life. Foods 2023, 12, 4101. [Google Scholar] [CrossRef]
- Tang, G.-Y.; Meng, X.; Gan, R.-Y.; Zhao, C.-N.; Liu, Q.; Feng, Y.-B.; Li, S.; Wei, X.-L.; Atanasov, A.G.; Corke, H.; et al. Health Functions and Related Molecular Mechanisms of Tea Components: An Update Review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Comparison of Antimutagenic Effect of Various Tea Extracts (Green, Oolong, Pouchong, and Black Tea). J. Food Prot. 1994, 57, 54–58. [Google Scholar] [CrossRef]
- Hibasami, H.; Jin, Z.X.; Hasegawa, M.; Urakawa, K.; Nakagawa, M.; Ishii, Y.; Yoshioka, K. Oolong tea polyphenol extract induces apoptosis in human stomach cancer cells. Anticancer. Res. 2000, 20, 4403–4406. [Google Scholar]
- Nakazato, K.; Takeo, T. Anti-Inflammatory Effect of Oolong Tea Polyphenols. J. Agric. Chem. Soc. Jpn. 1998, 72, 51–54. [Google Scholar] [CrossRef][Green Version]
- Xiao, J.; Sarker, S.D.; Asakawa, Y. Handbook of Dietary Phytochemicals; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar][Green Version]
- Lee, Y.R.; Moon, G.H.; Shim, D.; Kim, J.C.; Lee, K.J.; Chung, K.H.; An, J.H. Neuroprotective effects of fermented tea in MPTP-induced Parkinson’s disease mouse model via MAPK signaling-mediated regulation of inflammation and antioxidant activity. Food Res. Int. 2023, 164, 112133. [Google Scholar] [CrossRef]
- Weerawatanakorn, M.; Hung, W.-L.; Pan, M.-H.; Li, S.; Li, D.; Wan, X.; Ho, C.-T. Chemistry and health beneficial effects of oolong tea and theasinensins. Food Sci. Hum. Wellness 2015, 4, 133–146. [Google Scholar] [CrossRef]
- Hisanaga, A.; Ishida, H.; Sakao, K.; Sogo, T.; Kumamoto, T.; Hashimoto, F.; Hou, D.-X. Anti-inflammatory activity and molecular mechanism of Oolong tea theasinensin. Food Funct. 2014, 5, 1891–1897. [Google Scholar] [CrossRef]
- Pan, M.H.; Yu Chih Liang Shoei-Yn Lin-Shiau Zhu, N.; Ho, C.T.; Lin, J. Induction of Apoptosis by the Oolong Tea Polyphenol Theasinensin A through Cytochrome c Release and Activation of Caspase-9 and Caspase-3 in Human U937 Cells. J. Agric. Food Chem. 2000, 48, 6337–6346. [Google Scholar] [CrossRef]
- Shirai, T.; Sato, A.; Chida, K.; Hayakawa, H.; Akiyama, J.; Iwata, M.; Taniguchi, M.; Reshad, K.; Hara, Y. Epigallocatechin Gallate-Induced Histamine Release in Patients with Green Tea-Induced Asthma. Ann. Allergy Asthma Immunol. 1997, 79, 65–69. [Google Scholar] [CrossRef]
- Shirai, T.; Hayakawa, H.; Akiyama, J.; Iwata, M.; Chida, K.; Nakamura, H.; Taniguchi, M.; Reshad, K. Food allergy to green tea. J. Allergy Clin. Immunol. 2003, 112, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.S.; Johnson, J.A.; Peppers, B.; Haig Tcheurekdjian Hostoffer, R. A case of green tea (Camellia sinensis) imbibement causing possible anaphylaxis. Ann. Allergy Asthma Immunol. 2017, 118, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.-H.S.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar]
- Truong, V.L.; Jeong, W.S. Cellular Defensive Mechanisms of Tea Polyphenols: Structure-Activity Relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Bajaj, P.R.; Singhal, R.S. Tea Polyphenols as Nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2008, 7, 229–254. [Google Scholar] [CrossRef]
- Khasnabis, J.; Rai, C.; Roy, A. Determination of Tannin Content by Titrimetric Method from Different Types of Tea. J. Chem. Pharm. Res. 2015, 7, 238–241. [Google Scholar]
- Zhang, H.; Qi, R.; Mine, Y. The impact of oolong and black tea polyphenols on human health. Food Biosci. 2019, 29, 55–61. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Que, X.; Zheng, S.; Song, Q.; Pei, H.; Zhang, P. Fantastic voyage: The journey of NLRP3 inflammasome activation. Genes Dis. 2024, 11, 819–829. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Ho, C.T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Gritsenko, A.; Green, J.P.; Brough, D.; Lopez-Castejon, G. Mechanisms of NLRP3 priming in inflammaging and age related diseases. Cytokine Growth Factor Rev. 2020, 55, 15–25. [Google Scholar] [CrossRef]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef]
- Lin, Y.S.; Tsai, Y.J.; Tsay, J.S.; Lin, J.K. Factors Affecting the Levels of Tea Polyphenols and Caffeine in Tea Leaves. J. Agric. Food Chem. 2003, 51, 1864–1873. [Google Scholar] [CrossRef]
- Luximon-Ramma, A.; Bahorun, T.; Soobrattee, M.A.; Aruoma, O.I. Antioxidant Activities of Phenolic, Proanthocyanidin, and Flavonoid Components in Extracts of Cassia fistula. J. Agric. Food Chem. 2002, 50, 5042–5047. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R.; Sorsa, S. Testing the Effects of Drying Methods on Willow Flavonoids, Tannins, and Salicylates. J. Chem. Ecol. 2001, 27, 779–789. [Google Scholar] [CrossRef]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef]
- Ling, Y.; Zhao, Y.; Li, Z.; Zhang, G.; Wu, Y. Determination of catechins and caffeine in tea and tea beverages by high-performance liquid chromatography. Wei Sheng Yan Jiu J. Hyg. Res. 2005, 34, 187–190. [Google Scholar]
- Lin, Y.; Luo, T.; Weng, A.; Huang, X.; Yao, Y.; Fu, Z.; Li, Y.; Liu, A.; Li, X.; Chen, D.; et al. Gallic Acid Alleviates Gouty Arthritis by Inhibiting NLRP3 Inflammasome Activation and Pyroptosis Through Enhancing Nrf2 Signaling. Front. Immunol. 2020, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-Y.; Feng, Y.-M.; Kong, W.-S.; Li, S.-N.; Sun, X.-J.; Zhou, G.; Xie, R.-F.; Zhou, X. Gallic acid ameliorates dextran sulfate sodium-induced ulcerative colitis in mice via inhibiting NLRP3 inflammasome. Front. Pharmacol. 2023, 25, 14. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-κB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Song, K.-Y.; Feng, J.-Z.; Huang, S.-Y.; Guo, X.-M.; Zhang, L.; Zhang, G.; Huo, Y.-C.; Zhang, R.-R.; Ma, Y.; et al. Caffeine Inhibits Activation of the NLRP3 Inflammasome via Autophagy to Attenuate Microglia-Mediated Neuroinflammation in Experimental Autoimmune Encephalomyelitis. J. Mol. Neurosci. 2021, 72, 97–112. [Google Scholar] [CrossRef]
- Tian, X.; Xue, Y.; Xie, G.; Zhou, Y.; Xiao, H.; Ding, F.; Zhang, M. (-)-Epicatechin ameliorates cigarette smoke-induced lung inflammation via inhibiting ROS/NLRP3 inflammasome pathway in rats with COPD. Toxicol. Appl. Pharmacol. 2021, 429, 115674. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, F.; Zhang, X.; Xu, W.; Wang, Y.; Yao, Y.; Han, Z.; Xia, D. (−)-Epicatechin Ameliorates Monosodium Urate-Induced Acute Gouty Arthritis Through Inhibiting NLRP3 Inflammasome and the NF-κB Signaling Pathway. Front. Pharmacol. 2022, 13, 799552. [Google Scholar] [CrossRef]
- Jhang, J.J.; Lu, C.C.; Yen, G.C. Epigallocatechin gallate inhibits urate crystals-induced peritoneal inflammation in C57BL/6 mice. Mol. Nutr. Food Res. 2016, 60, 2297–2303. [Google Scholar] [CrossRef]
- Di, M.; Zhang, Q.; Wang, J.; Xiao, X.; Huang, J.; Ma, Y.; Yang, H.; Li, M. Epigallocatechin-3-gallate (EGCG) attenuates inflammatory responses and oxidative stress in lipopolysaccharide (LPS)-induced endometritis via silent information regulator transcript-1 (SIRT1)/nucleotide oligomerization domain (NOD)-like receptor pyrin domain-containing 3 (NLRP3) pathway. J. Biochem. Mol. Toxicol. 2022, 36, e23203. [Google Scholar]
- Gao, Z.; Han, Y.; Hu, Y.; Wu, X.; Wang, Y.; Zhang, X.; Fu, J.; Zou, X.; Zhang, J.; Chen, X.; et al. Targeting HO-1 by Epigallocatechin-3-Gallate Reduces Contrast-Induced Renal Injury via Anti-Oxidative Stress and Anti-Inflammation Pathways. PLoS ONE 2016, 11, e0149032. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Yang, G.; Park, Y.B.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Epigallocatechin-3-Gallate Prevents Acute Gout by Suppressing NLRP3 Inflammasome Activation and Mitochondrial DNA Synthesis. Molecules 2019, 24, 2138. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Q.; Wang, T.; Kan, Z.; Li, X.; Hu, L.; Peng, C.-Y.; Qian, F.; Wang, Y.; Granato, D. Green tea polyphenols and epigallocatechin-3-gallate protect against perfluorodecanoic acid induced liver damage and inflammation in mice by inhibiting NLRP3 inflammasome activation. Food Res. Int. 2020, 127, 108628. [Google Scholar] [CrossRef]
- Lim, H.; Min, D.S.; Park, H.; Kim, H.P. Flavonoids interfere with NLRP3 inflammasome activation. Toxicol. Appl. Pharmacol. 2018, 355, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2023, 24, 340. [Google Scholar] [CrossRef]
- Samanta, S. Potential Bioactive Components and Health Promotional Benefits of Tea (Camellia sinensis). J. Am. Coll. Nutr. 2020, 41, 65–93. [Google Scholar] [CrossRef]
- Lagha, A.B.; Grenier, D. Tea polyphenols inhibit the activation of NF-κB and the secretion of cytokines and matrix metalloproteinases by macrophages stimulated with Fusobacterium nucleatum. Sci. Rep. 2016, 6, 34520. [Google Scholar] [CrossRef]
- Karunaweera, N.; Raju, R.; Gyengesi, E.; Münch, G. Plant polyphenols as inhibitors of NF-κB induced cytokine production—A potential anti-inflammatory treatment for Alzheimer’s disease? Front. Mol. Neurosci. 2015, 8, 24. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Xiao, Y.; Zhang, W.; Wu, S.; Qin, T.; Yue, Y.; Qian, W.; Li, L. NLRP3 Inflammasome and Inflammatory Diseases. Oxidative Med. Cell. Longev. 2020, 2020, e4063562. [Google Scholar] [CrossRef] [PubMed]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.L. Redox Regulation of NLRP3 Inflammasomes: ROS as Trigger or Effector? Antioxid. Redox Signal. 2015, 22, 1111–1129. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Ullevig, S.L.; Short, J.D.; Wang, L.; Ahn, Y.J.; Asmis, R. Ursolic Acid and Related Analogues: Triterpenoids with Broad Health Benefits. Antioxidants 2021, 10, 1161. [Google Scholar] [CrossRef]
- Farhan, M. Green Tea Catechins: Nature’s Way of Preventing and Treating Cancer. Int. J. Mol. Sci. 2022, 23, 10713. [Google Scholar] [CrossRef]
| Time (min) | Solvent A (%) | Solvent B (%) |
|---|---|---|
| 0 | 90 | 10 |
| 10 | 90 | 10 |
| 24 | 80 | 20 |
| 30 | 78 | 22 |
| 35 | 75 | 25 |
| 35.1 | 90 | 10 |
| 45 | 90 | 10 |
| Phytochemical Compounds | Content |
|---|---|
| Total phenolics (mg GAE/g DW) | 321.95 ± 10.58 |
| Total flavonoids (mg CTE/g DW) | 64.82 ± 0.83 |
| Condensed tannins (mg CE/g DW) | 233.67 ± 6.61 |
| Proanthocyanidins (mg CCE/g DW) | 10.88 ± 0.46 |
| Compounds | Structure | Molecular Formula | Molecular Weight (g/mol) | Extract Content (µg/mg) | mmol/g | NLRP3-Related Publications |
|---|---|---|---|---|---|---|
| Gallic acid (GA) | 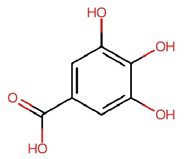 | C7H6O5 | 170.12 | 4.28 ± 0.02 | 0.025 | Lin et al., 2020 [42] Yu et al., 2023 [43] |
| Gallocatechin (GC) |  | C15H14O7 | 306.27 | 61.64 ± 0.30 | 0.201 | – |
| Epigallocatechin (EGC) | 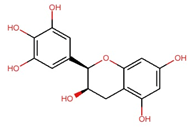 | C15H14O7 | 306.27 | 97.33 ± 0.51 | 0.318 | – |
| Catechin (C) | 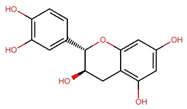 | C15H14O6 | 290.27 | 12.98 ± 0.03 | 0.045 | – |
| Caffeine |  | C8H10N4O2 | 194.19 | 32.14 ± 0.59 | 0.166 | Zhao et al., 2019 [44] Wang et al., 2022 [45] |
| Epicatechin (EC) | 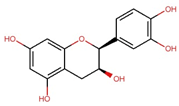 | C15H14O6 | 290.27 | 17.27 ± 0.09 | 0.059 | Tian et al., 2021 [46] Wu et al., 2022 [47] |
| Epigallocatechin gallate (EGCG) | 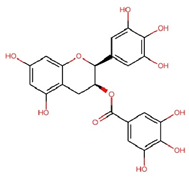 | C22H18O11 | 458.4 | 119.97 ± 2.40 | 0.262 | Abundant related research and articles can be found; here are a selected few: Jhang et al., 2016 [48] Di et al., 2022 [49] Gao et al., 2016 [50] Lee et al., 2019 [51] Wang et al., 2020 [52] |
| Gallocatechin gallate (GCG) | 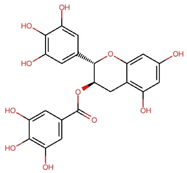 | C22H18O11 | 458.4 | 82.52 ± 1.64 | 0.180 | – |
| Epicatechin gallate (ECG) | 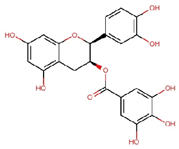 | C22H18O10 | 442.4 | 17.92 ± 0.38 | 0.041 | – |
| Catechin gallate (CG) | 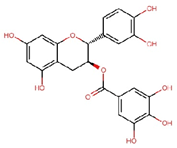 | C22H18O10 | 442.4 | 2.90 ± 0.18 | 0.007 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.-S.; Yang, S.-N.; Chang, Y.-P.; Wu, C.-S.; Yang, H.-C.; Chang, J.-F. Potential Therapeutic Effects of Oolong Tea Phytochemicals on NLRP3 Inflammasome Assembly and Oxidative Stress. Nutrients 2025, 17, 3106. https://doi.org/10.3390/nu17193106
Wang M-S, Yang S-N, Chang Y-P, Wu C-S, Yang H-C, Chang J-F. Potential Therapeutic Effects of Oolong Tea Phytochemicals on NLRP3 Inflammasome Assembly and Oxidative Stress. Nutrients. 2025; 17(19):3106. https://doi.org/10.3390/nu17193106
Chicago/Turabian StyleWang, Ming-Shyan, Szu-Nian Yang, Yi-Ping Chang, Chi-Sheng Wu, Hung-Chi Yang, and Jia-Feng Chang. 2025. "Potential Therapeutic Effects of Oolong Tea Phytochemicals on NLRP3 Inflammasome Assembly and Oxidative Stress" Nutrients 17, no. 19: 3106. https://doi.org/10.3390/nu17193106
APA StyleWang, M.-S., Yang, S.-N., Chang, Y.-P., Wu, C.-S., Yang, H.-C., & Chang, J.-F. (2025). Potential Therapeutic Effects of Oolong Tea Phytochemicals on NLRP3 Inflammasome Assembly and Oxidative Stress. Nutrients, 17(19), 3106. https://doi.org/10.3390/nu17193106







