Effects of 30-Day High-Dose Omega-3 Fatty Acid Supplementation on Plasma Oxidative Stress Enzyme Activities in Recreational and Trained Runners: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Gas Chromatography–Mass Spectrometry Analysis for Plasma AA, EPA, and DHA Levels
2.4. Enzyme Activity Assays
2.5. Statistical Analysis
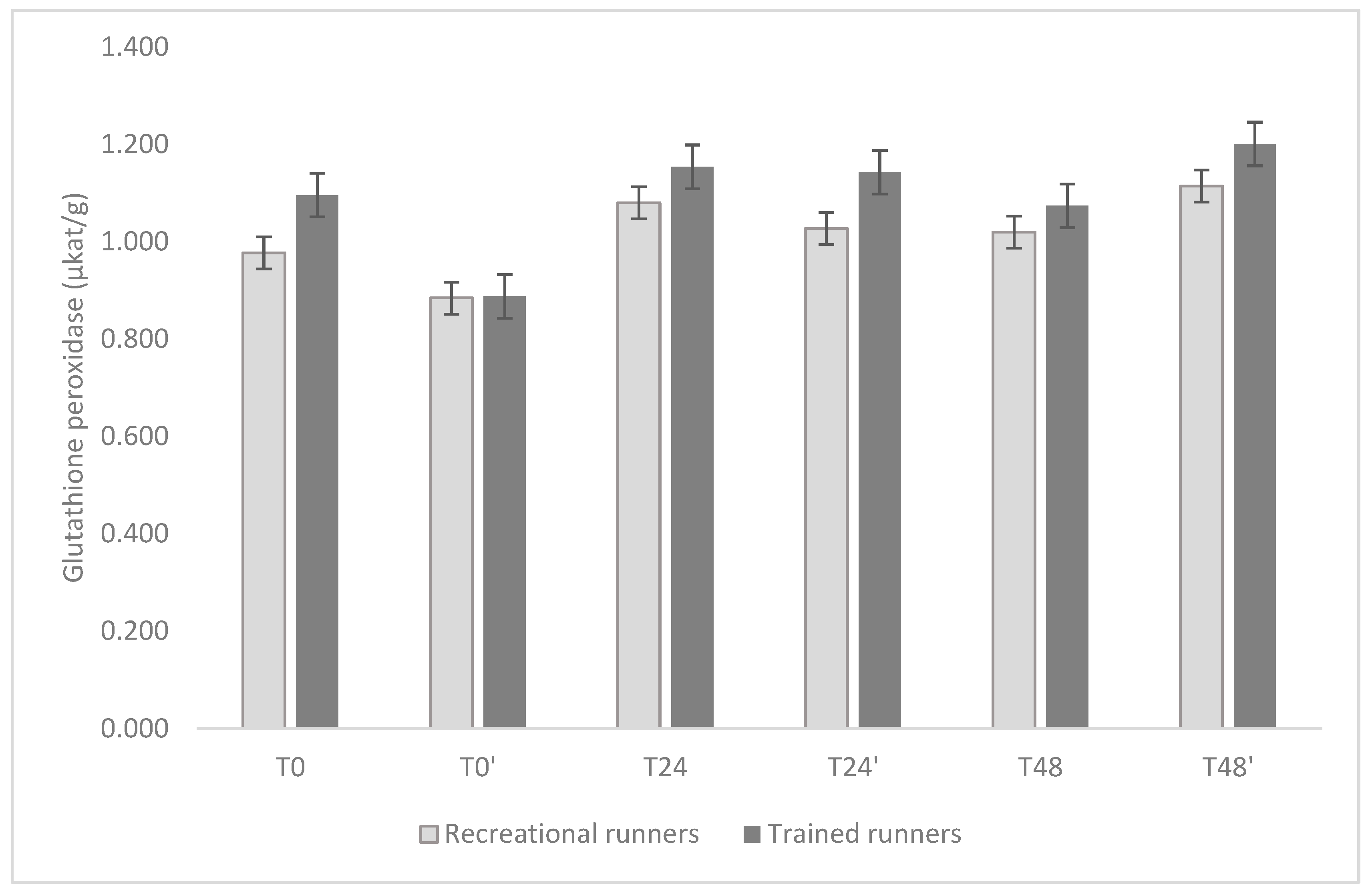
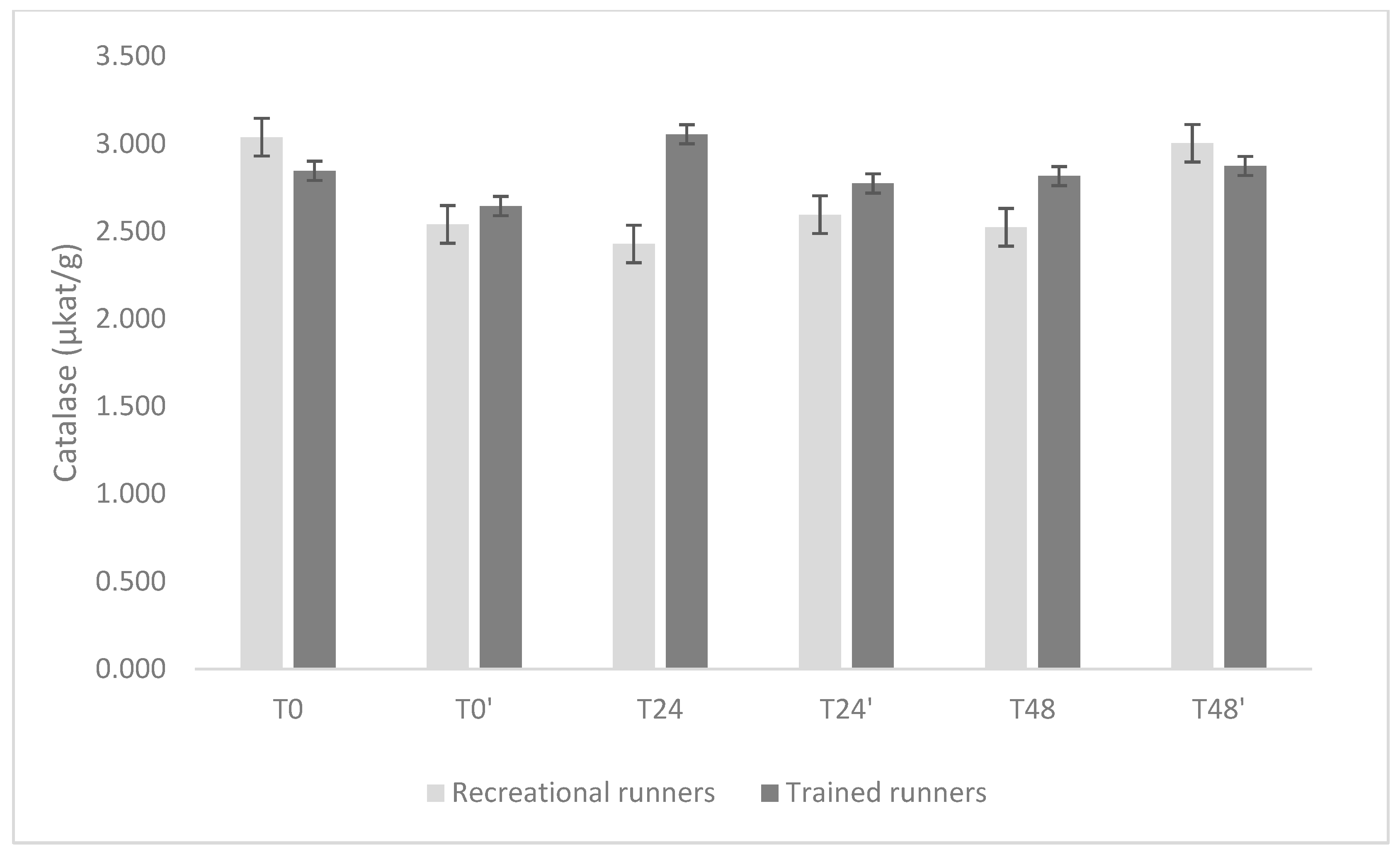
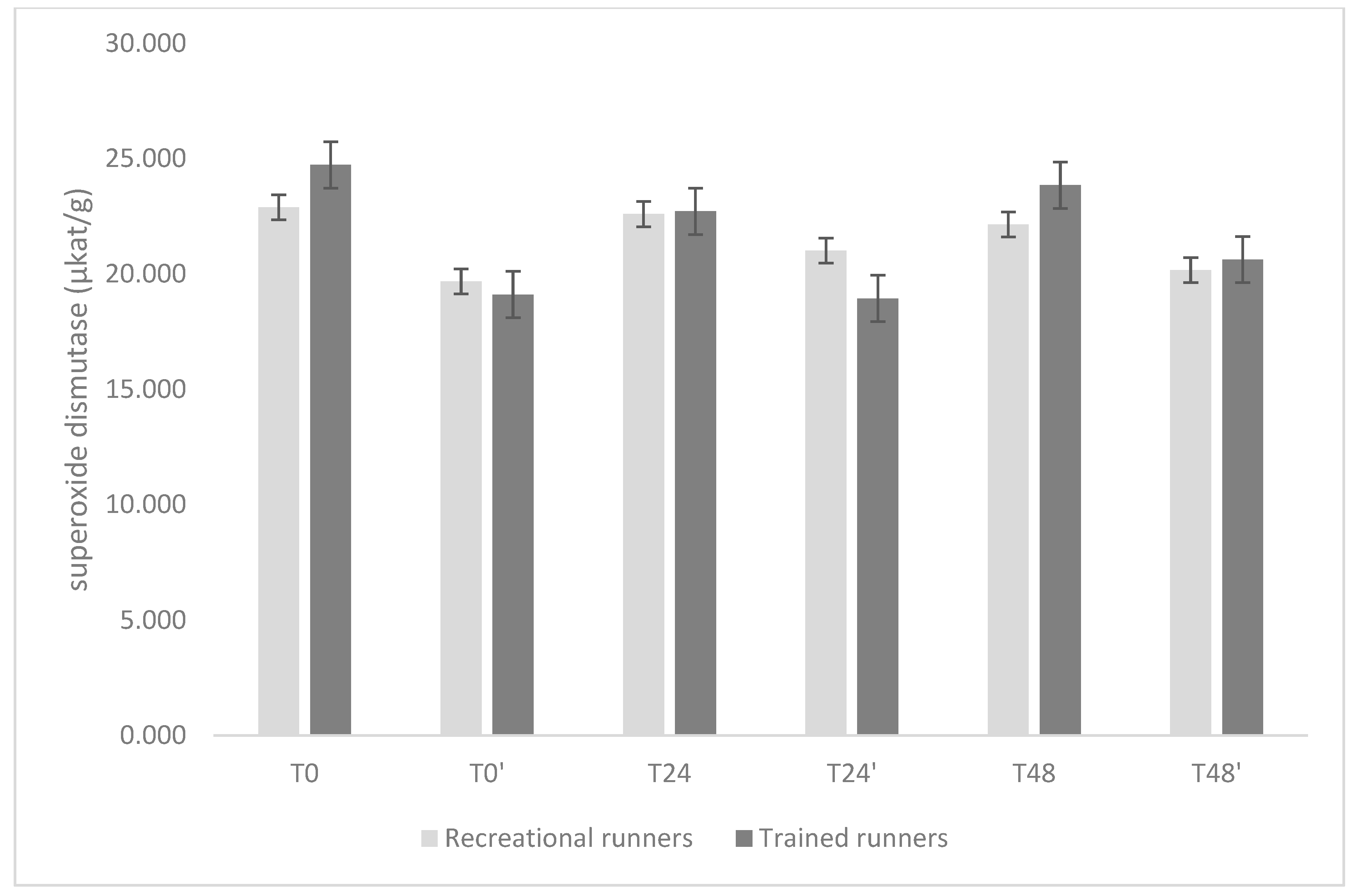
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rennemark, M.; Jogréus, C.; Elmståhl, S.; Welmer, A.; Wimo, A.; Sanmartin-Berglund, J. Relationships Between Frequency of Moderate Physical Activity and Longevity: An 11-Year Follow-up Study. Gerontol. Geriatr. Med. 2018, 4, 233372141878656. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J. Redox Regulation of Adaptive Responses in Skeletal Muscle to Contractile Activity. Free Radic. Biol. Med. 2009, 47, 1267–1275. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants Prevent Health-Promoting Effects of Physical Exercise in Humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]
- Silva, L.A.; Pinho, C.A.; Scarabelot, K.S.; Fraga, D.B.; Volpato, A.M.J.; Boeck, C.R.; Souza, C.T.; Streck, E.L.; Pinho, R.A. Physical Exercise Increases Mitochondrial Function and Reduces Oxidative Damage in Skeletal Muscle. Eur. J. Appl. Physiol. 2009, 105, 861–867. [Google Scholar] [CrossRef]
- Viña, J.; Gimeno, A.; Sastre, J.; Desco, C.; Asensi, M.; Pallardó, F.V.; Cuesta, A.; Ferrero, J.A.; Terada, L.S.; Repine, J.E. Mechanism of Free Radical Production in Exhaustive Exercise in Humans and Rats; Role of Xanthine Oxidase and Protection by Allopurinol. IUBMB Life 2000, 49, 539–544. [Google Scholar] [CrossRef]
- Silva, L.A.; Pinho, C.A.; Silveira, P.C.L.; Tuon, T.; De Souza, C.T.; Dal-Pizzol, F.; Pinho, R.A. Vitamin e Supplementation Decreases Muscular and Oxidative Damage but Not Inflammatory Response Induced by Eccentric Contraction. J. Physiol. Sci. 2010, 60, 51–57. [Google Scholar] [CrossRef]
- Palazzetti, S.; Richard, M.J.; Favier, A.; Margaritis, I. Overloaded Training Increases Exercise-Induced Oxidative Stress and Damage. Can. J. Appl. Physiol. 2003, 28, 588–604. [Google Scholar] [CrossRef] [PubMed]
- Panissa, V.L.G.; Fukuda, D.H.; Staibano, V.; Marques, M.; Franchini, E. Magnitude and Duration of Excess of Post-Exercise Oxygen Consumption between High-Intensity Interval and Moderate-Intensity Continuous Exercise: A Systematic Review. Obes. Rev. 2021, 22, e13099. [Google Scholar] [CrossRef]
- Tauler, P.; Aguiló, A.; Guix, P.; Jiménez, F.; Villa, G.; Tur, J.A.; Córdova, A.; Pons Biescas, A. Pre-Exercise Antioxidant Enzyme Activities Determine the Antioxidant Enzyme Erythrocyte Response to Exercise. J. Sports Sci. 2005, 23, 5–13. [Google Scholar] [CrossRef]
- SIES, H. Oxidative Stress: Introductory Remarks; Pergamon Press Inc.: Oxford, UK, 1985; ISBN 0126427607. [Google Scholar]
- Sies, H.; Cadenas, E. Oxidative Stress: Damage to Intact Cells and Organs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985, 311, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; McConkey, D.J.; Bellomo, G.; Nicotera, P. Role of Ca2+ in Toxic Cell Killing. Trends Pharmacol. Sci. 1989, 10, 281–285. [Google Scholar] [CrossRef]
- Jones, D.P. Redefining Oxidative Stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Le Moal, E.; Pialoux, V.; Juban, G.; Groussard, C.; Zouhal, H.; Chazaud, B.; Mounier, R. Redox Control of Skeletal Muscle Regeneration. Antioxid. Redox Signal. 2017, 27, 276–310. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond Oxidative Stress: An Immunologist’s Guide to Reactive Oxygen Species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef]
- Halliwell, B.; Cross, C.E. Oxygen-Derived Species: Their Relation to Human Disease and Environmental Stress. Environ. Health Perspect. 1994, 102, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Knap, B.; Prezelj, M.; Buturović-Ponikvar, J.; Ponikvar, R.; Bren, A.F. Antioxidant Enzymes Show Adaptation to Oxidative Stress in Athletes and Increased Stress in Hemodialysis Patients. Ther. Apher. Dial. 2009, 13, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Yashodhara, B.M.; Umakanth, S.; Pappachan, J.M.; Bhat, S.K.; Kamath, R.; Choo, B.H. Omega-3 Fatty Acids: A Comprehensive Review of Their Role in Health and Disease. Postgrad. Med. J. 2009, 85, 84–90. [Google Scholar] [CrossRef]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, Interconversion, and Dose Response of n-3 Fatty Acids in Humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef]
- Lane, K.E.; Wilson, M.; Hellon, T.G.; Davies, I.G. Bioavailability and Conversion of Plant Based Sources of Omega-3 Fatty Acids–a Scoping Review to Update Supplementation Options for Vegetarians and Vegans. Crit. Rev. Food Sci. Nutr. 2022, 62, 4982–4997. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramaniapillai, M.; Fan, B.; Lu, C.; Mclntyer, R.S. Efficacy of Omega-3 PUFAs in Depression: A Meta-Analysis. Transl. Psychiatry 2019, 9, 190. [Google Scholar] [CrossRef]
- Elagizi, A.; Lavie, C.J.; O’Keefe, E.; Marshall, K.; O’Keefe, J.H.; Milani, R. V An Update on Omega-3 Polyunsaturated Fatty Acids and Cardiovascular Health. Nutrients 2021, 13, 204. [Google Scholar] [CrossRef]
- Tatsumi, Y.; Kato, A.; Sango, K.; Himeno, T.; Kondo, M.; Kato, Y.; Kamiya, H.; Nakamura, J.; Kato, K. Omega-3 Polyunsaturated Fatty Acids Exert Anti-Oxidant Effects through the Nuclear Factor (Erythroid-Derived 2)-Related Factor 2 Pathway in Immortalized Mouse Schwann Cells. J. Diabetes Investig. 2019, 10, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Sakai, C.; Ishida, M.; Ohba, H.; Yamashita, H.; Uchida, H.; Yoshizumi, M.; Ishida, T. Fish Oil Omega-3 Polyunsaturated Fatty Acids Attenuate Oxidative Stress-Induced DNA Damage in Vascular Endothelial Cells. PLoS ONE 2017, 12, 1–13. [Google Scholar] [CrossRef]
- Bang, H.Y.; Park, S.A.; Saeidi, S.; Na, H.K.; Surh, Y.J. Docosahexaenoic Acid Induces Expression of Heme Oxygenase-1 and NAD(P)H: Quinone Oxidoreductase through Activation of Nrf2 in Human Mammary Epithelial Cells. Molecules 2017, 22, 969. [Google Scholar] [CrossRef]
- Mendelsohn, A.R.; Larrick, J.W. Trade-Offs between Anti-Aging Dietary Supplementation and Exercise. Rejuvenation Res. 2013, 16, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, S. Effects of Dietary Polyunsaturated Fatty Acids on Mitochondria. Curr. Pharm. Des. 2009, 15, 4103–4116. [Google Scholar] [CrossRef]
- Goldfarb, A.H.; McKenzie, M.J.; Bloomer, R.J. Gender Comparisons of Exercise-Induced Oxidative Stress: Influence of Antioxidant Supplementation. Appl. Physiol. Nutr. Metab. 2007, 32, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Filaire, E.; Massart, A.; Rouveix, M.; Portier, H.; Rosado, F.; Durand, D. Effects of 6 Weeks of N-3 Fatty Acids and Antioxidant Mixture on Lipid Peroxidation at Rest and Postexercise. Eur. J. Appl. Physiol. 2011, 111, 1829–1839. [Google Scholar] [CrossRef]
- Xin, G.; Eshaghi, H. Effect of Omega-3 Fatty Acids Supplementation on Indirect Blood Markers of Exercise-Induced Muscle Damage: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Food Sci. Nutr. 2021, 9, 6429–6442. [Google Scholar] [CrossRef]
- Żebrowska, A.; Hall, B.; Stolecka-Warzecha, A.; Stanula, A.; Sadowska-Krępa, E. The Effect of Omega-3 Fatty Acid Supplementation on Serum Adipocytokines, Lipid Profile and Biochemical Markers of Inflammation in Recreational Runners. Nutrients 2021, 13, 456. [Google Scholar] [CrossRef]
- Buonocore, D.; Verri, M.; Giolitto, A.; Doria, E.; Ghitti, M.; Dossena, M. Effect of 8-Week n-3 Fatty-Acid Supplementation on Oxidative Stress and Inflammation in Middle- and Long-Distance Running Athletes: A Pilot Study. J. Int. Soc. Sports Nutr. 2020, 17, 1–19. [Google Scholar] [CrossRef]
- Arnold, L.E.; Young, A.S.; Belury, M.A.; Cole, R.M.; Gracious, B.; Seidenfeld, A.M.; Wolfson, H.; Fristad, M.A. Omega-3 Fatty Acid Plasma Levels before and after Supplementation: Correlations with Mood and Clinical Outcomes in the Omega-3 and Therapy Studies. J. Child Adolesc. Psychopharmacol. 2017, 27, 223–233. [Google Scholar] [CrossRef]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative Stress. Sport. Med. 2006, 36, 327–358. [Google Scholar] [CrossRef]
- Tong, T.K.; Lin, H.; Lippi, G.; Nie, J.; Tian, Y. Serum Oxidant and Antioxidant Status in Adolescents Undergoing Professional Endurance Sports Training. Oxidative Med. Cell. Longev. 2012, 2012, 741239. [Google Scholar] [CrossRef] [PubMed]
- Brites, F.D.; Evelson, P.A.; Christiansen, M.G.; Nicol, M.F.; Basílico, M.J.; Wikinski, R.W.; Llesuy, S.F. Soccer Players under Regular Training Show Oxidative Stress but an Improved Plasma Antioxidant Status. Clin. Sci. 1999, 96, 381–385. [Google Scholar] [CrossRef]
- Evelson, P.; Gambino, G.; Travacio, M.; Jaita, G.; Verona, J.; Maroncelli, C.; Wikinski, R.; Llesuy, S.; Brites, F. Higher Antioxidant Defences in Plasma and Low Density Lipoproteins from Rugby Players. Eur. J. Clin. Investig. 2002, 32, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, D.Z.; Cubrilo, D.G.; Barudzic, N.S.; Vuletic, M.S.; Zivkovic, V.I.; Nesic, M.; Radovanovic, D.; Djuric, D.M.; Jakovljevic, V.L. Comparison of Blood pro/Antioxidant Levels before and after Acute Exercise in Athletes and Non-Athletes. Gen. Physiol. Biophys. 2012, 31, 211–219. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: The Dose-Response Revolution. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 175–197. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Goto, S. Exercise and Hormesis: Oxidative Stress-Related Adaptation for Successful Aging. Biogerontology 2005, 6, 71–75. [Google Scholar] [CrossRef]
- Ji, L.L.; Gomez-Cabrera, M.C.; Vina, J. Exercise and Hormesis: Activation of Cellular Antioxidant Signaling Pathway. Ann. N. Y. Acad. Sci. 2006, 1067, 425–435. [Google Scholar] [CrossRef]
- Miyazaki, H.; Oh-ishi, S.; Ookawara, T.; Kizaki, T.; Toshinai, K.; Ha, S.; Haga, S.; Ji, L.L.; Ohno, H. Strenuous Endurance Training in Humans Reduces Oxidative Stress Following Exhausting Exercise. Eur. J. Appl. Physiol. 2001, 84, 1–6. [Google Scholar] [CrossRef]
- Robertson, J.D.; Maughan, R.J.; Duthie, G.G.; Morrice, P.C. Increased Blood Antioxidant Systems of Runners in Response to Training Load. Clin. Sci. 1991, 80, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Groussard, C.; Rannou-Bekono, F.; Machefer, G.; Chevanne, M.; Vincent, S.; Sergent, O.; Cillard, J.; Gratas-Delamarche, A. Changes in Blood Lipid Peroxidation Markers and Antioxidants after a Single Sprint Anaerobic Exercise. Eur. J. Appl. Physiol. 2003, 89, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.; Schwartz, D.D.; Quindry, J.; Barberio, M.D.; Foster, E.B.; Jones, K.W.; Pascoe, D.D. Lymphocyte Enzymatic Antioxidant Responses to Oxidative Stress Following High-Intensity Interval Exercise. J. Appl. Physiol. 2011, 110, 730–737. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Do Antioxidant Supplements Interfere with Skeletal Muscle Adaptation to Exercise Training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and Exercise Performance: With a Focus on Vitamin e and c Supplementation. Int. J. Environ. Res. Public Health 2020, 17, 8452. [Google Scholar] [CrossRef]
- Holley, A.K.; Bakthavatchalu, V.; Velez-Roman, J.M.; St. Clair, D.K. Manganese Superoxide Dismutase: Guardian of the Powerhouse. Int. J. Mol. Sci. 2011, 12, 7114–7162. [Google Scholar] [CrossRef]
- Ji, L.L.; Katz, A.; Fu, R.; Griffiths, M.; Spencer, M. Blood Glutathione Status during Exercise: Effect of Carbohydrate Supplementation. J. Appl. Physiol. 1993, 74, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Fanò, G.; Mecocci, P.; Vecchiet, J.; Belia, S.; Fulle, S.; Polidori, M.C.; Felzani, G.; Senin, U.; Vecchiet, L.; Beal, M.F. Age and Sex Influence on Oxidative Damage and Functional Status in Human Skeletal Muscle. J. Muscle Res. Cell Motil. 2001, 22, 345–351. [Google Scholar] [CrossRef]
- Packer, L. Oxidants, Antioxidant Nutrients and the Athlete. J. Sports Sci. 1997, 15, 353–363. [Google Scholar] [CrossRef]
- Mena, P.; Maynar, M.; Gutierrez, J.M.; Maynar, J.; Timon, J.; Campillo, J.E. Erythrocyte Free Radical Scavenger Enzymes in Bicycle Professional Racers. Adaptation to Training. Int. J. Sports Med. 1991, 12, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, Y.; Jamurtas, A.Z.; Nikolaidis, M.G.; Fatouros, I.G.; Koutedakis, Y.; Papassotiriou, I.; Kouretas, D. Sampling Time Is Crucial for Measurement of Aerobic Exercise-Induced Oxidative Stress. Med. Sci. Sports Exerc. 2007, 39, 1107–1113. [Google Scholar] [CrossRef] [PubMed]

| Runner Group | Omega-3 Supplementation Period | Age (Years) | Height (cm) | Weight (kg) | Body Mass Index (kg/m2) |
|---|---|---|---|---|---|
| All (n = 21) | T0 | 25.29 ± 5.99 | 180.05 ± 5.91 | 71.03 ± 7.38 | 21.94 ± 2.26 |
| T0′ | nd | nd | 71.59 ± 7.90 | 22.10 ± 2.40 | |
| Recreational (n = 11) | T0 | 27.91 ± 4.70 | 178.55 ± 6.15 | 72.23 ± 7.67 | 22.65 ± 1.86 |
| T0′ | nd | nd | 72.86 ± 8.37 | 22.93 ± 2.10 | |
| Trained (n = 10) | T0 | 22.67 ± 5.73 | 181.92 ± 6.27 | 70.10 ± 6.71 | 21.23 ± 2.32 |
| T0′ | nd | nd | 70.27 ± 6.68 | 21.31 ± 2.33 |
| Runner Group | Omega-3 Supplementation Period | Statistic | AA (µg/mL) | r | p | EPA (µg/mL) | r | p | DHA (µg/mL) | r | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All participants (n = 21) | T0 | Mean ± SD | 158.60 ± 62.06 | −0.26 | 0.31 | 159.05 ± 80.60 | −0.68 | 0.001 | 32.22 ± 18.82 | −0.68 | < 0.001 |
| Median (Q1–Q3) | 137.45 (119.63–175.95) | 160.98 (108.48–186.74) | 26.93 (18.20–40.71) | ||||||||
| T0′ | Mean ± SD | 178.18 ± 66.08 | 244.14 ± 108.92 | 88.49 ± 32.50 | |||||||
| Median (Q1–Q3) | 153.64 (128.56–236.34) | 243.63 (158.09–296.31) | 83.07 (56.32–106.88) | ||||||||
| Recreational (n = 11) | T0 | Mean ± SD | 155.75 ± 54.74 | −0.55 | 0.12 | 152.89 ± 56.79 | −0.76 | 0.032 | 24.32 ± 8.03 | −0.76 | 0.03 |
| Median (Q1–Q3) | 129.51 (119.10–186.57) | 158.62 (126.78–174.19) | 24.78 (16.85–30.80) | ||||||||
| T0′ | Mean ± SD | 196.80 ± 71.71 | 258.33 ± 117.61 | 94.66 ± 33.99 | |||||||
| Median (Q1–Q3) | 158.72 (144.69–251.18) | 259.87 (160.95–289.86) | 87.94 (67.78–120.16) | ||||||||
| Trained (n = 10) | T0 | Mean ± SD | 161.74 ± 72.17 | 0.02 | 1.00 | 165.83 ± 103.71 | −0.64 | 0.073 | 40.92 ± 23.56 | −0.64 | 0.08 |
| Median (Q1–Q3) | 140.74 (132.07–165.05) | 166.27 (87.17–204.23) | 41.09 (20.86–59.00) | ||||||||
| T0′ | Mean ± SD | 157.69 ± 55.67 | 228.52 ± 102.37 | 81.69 ± 31.06 | |||||||
| Median (Q1–Q3) | 152.30 (104.30–199.47) | 220.85 (139.74–296.90) | 81.53 (53.55–102.22) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinšek, B.; Skitek, M.; Kosjek, T.; Bedrač, L.; Benedik, E. Effects of 30-Day High-Dose Omega-3 Fatty Acid Supplementation on Plasma Oxidative Stress Enzyme Activities in Recreational and Trained Runners: A Pilot Study. Nutrients 2025, 17, 2985. https://doi.org/10.3390/nu17182985
Martinšek B, Skitek M, Kosjek T, Bedrač L, Benedik E. Effects of 30-Day High-Dose Omega-3 Fatty Acid Supplementation on Plasma Oxidative Stress Enzyme Activities in Recreational and Trained Runners: A Pilot Study. Nutrients. 2025; 17(18):2985. https://doi.org/10.3390/nu17182985
Chicago/Turabian StyleMartinšek, Bojan, Milan Skitek, Tina Kosjek, Leon Bedrač, and Evgen Benedik. 2025. "Effects of 30-Day High-Dose Omega-3 Fatty Acid Supplementation on Plasma Oxidative Stress Enzyme Activities in Recreational and Trained Runners: A Pilot Study" Nutrients 17, no. 18: 2985. https://doi.org/10.3390/nu17182985
APA StyleMartinšek, B., Skitek, M., Kosjek, T., Bedrač, L., & Benedik, E. (2025). Effects of 30-Day High-Dose Omega-3 Fatty Acid Supplementation on Plasma Oxidative Stress Enzyme Activities in Recreational and Trained Runners: A Pilot Study. Nutrients, 17(18), 2985. https://doi.org/10.3390/nu17182985






