Analysis of the Stimulative Effect of Arginine on Translation Initiation of Protein Synthesis in Skeletal Muscle
Highlights
- Arginine stimulated the translation initiation step of protein synthesis in the skeletal muscle of food-deprived mice.
- The effect of arginine on mouse skeletal muscle required a marked elevation of circulating arginine levels.
- In mouse skeletal muscle, arginine enhanced the translation initiation independently of IGF-1.
- Arginine stimulated translation initiation in cultured myotubes via the GPRC6A/PI3K/AKT/mTORC1 signaling pathway.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Analysis of Serum IGF-1 Level
2.3. Serum Amino Acid Analysis
2.4. Cell Culture
2.5. Immunoblotting
2.6. Statistical Analyses
3. Results
3.1. Effects of Arg on Translation Initiation in Skeletal Muscle of Mice
3.2. Effect of Arg on Translation Initiation in C2C12 Myotubes
3.3. Comparison of the Effect of Arg and Leu on Translation Initiation in C2C12 Myotubes
3.4. Effect of Arg Metabolites on Translation Initiation in C2C12 Myotubes
3.5. Involvement of the PI3K/AKT and MAPK Pathways in Arg-Induced Stimulation of Translation Initiation in C2C12 Myotubes
3.6. Involvement of GPRC6A in Arg-Induced Stimulation of Translation Initiation
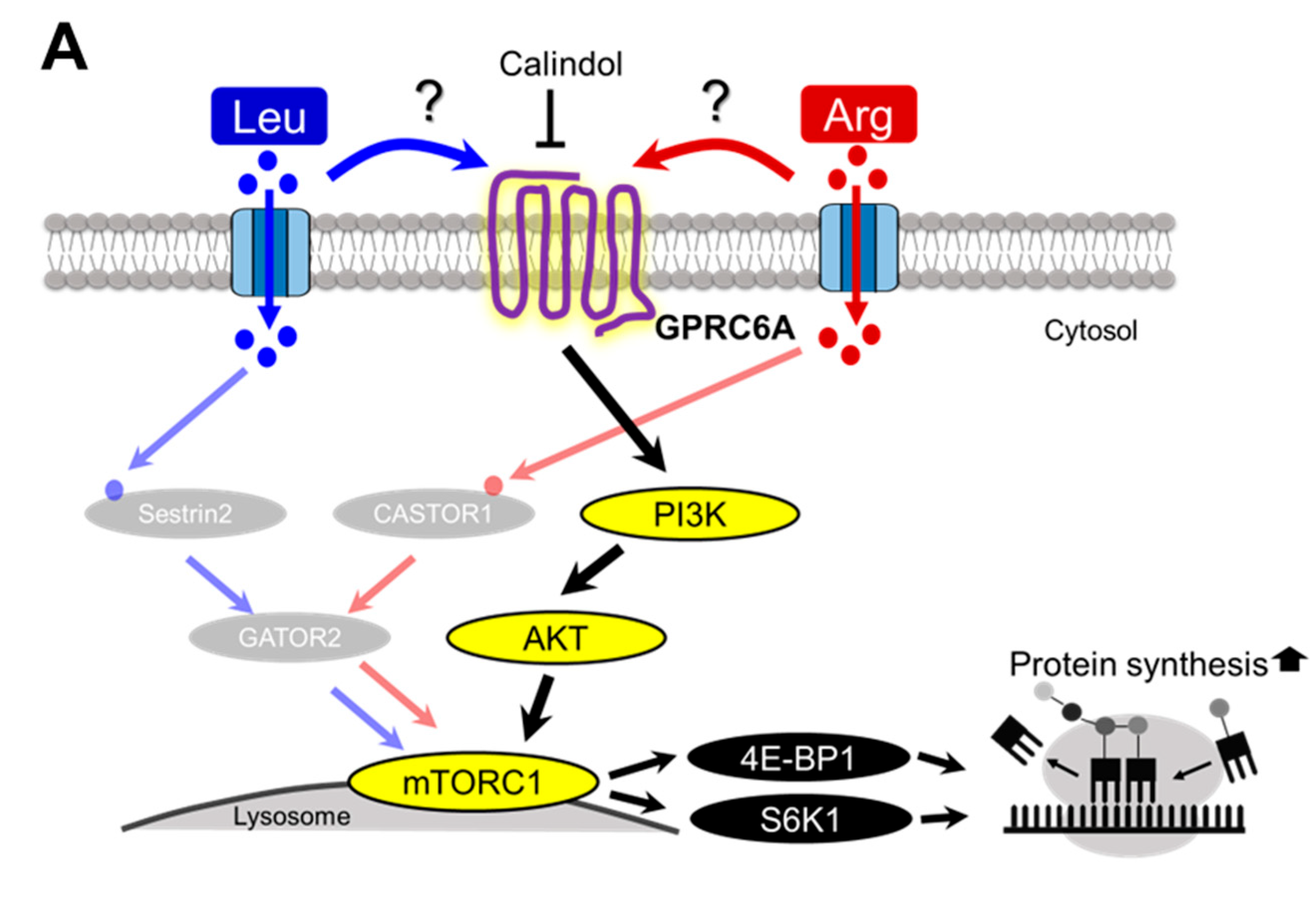
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bar-Peled, L.; Sabatini, D.M. Regulation of mTORC1 by Amino Acids. Trends Cell Biol. 2014, 24, 400–406. [Google Scholar] [CrossRef]
- Moro, T.; Ebert, S.M.; Adams, C.M.; Rasmussen, B.B. Amino Acid Sensing in Skeletal Muscle. Trends Endocrinol. Metab. 2016, 27, 796–806. [Google Scholar] [CrossRef]
- Rundqvist, H.C.; Esbjörnsson, M.; Rooyackers, O.; Österlund, T.; Moberg, M.; Apro, W.; Blomstrand, E.; Jansson, E. Influence of Nutrient Ingestion on Amino Acid Transporters and Protein Synthesis in Human Skeletal Muscle after Sprint Exercise. J. Appl. Physiol. 2017, 123, 1501–1515. [Google Scholar] [CrossRef]
- Drummond, M.J.; Glynn, E.L.; Fry, C.S.; Timmerman, K.L.; Volpi, E.; Rasmussen, B.B. An Increase in Essential Amino Acid Availability Upregulates Amino Acid Transporter Expression in Human Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1011–E1018. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine Stimulates Translation Initiation Skeletal Muscle of Postabsorptive Rats via a Rapamycin-Sensitive Pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, F. Regulation of Protein Synthesis by Branched-Chain Amino Acids in Vivo. Biochem. Biophys. Res. Commun. 2004, 313, 417–422. [Google Scholar] [CrossRef]
- Lian, J.; Yan, X.H.; Peng, J.; Jiang, S.W. The Mammalian Target of Rapamycin Pathway and Its Role in Molecular Nutrition Regulation. Mol. Nutr. Food Res. 2008, 52, 393–399. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, B.; Zhang, Y. 4E-BP1, a Multifactor Regulated Multifunctional Protein. Cell Cycle 2016, 15, 781–786. [Google Scholar] [CrossRef]
- Yoon, M.S. mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Mao, Z.; Zhang, W. Role of mTOR in Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2018, 19, 2043. [Google Scholar] [CrossRef] [PubMed]
- Ruvinsky, I.; Katz, M.; Dreazen, A.; Gielchinsky, Y.; Saada, A.; Freedman, N.; Mishani, E.; Zimmerman, G.; Kasir, J.; Meyuhas, O. Mice Deficient in Ribosomal Protein S6 Phosphorylation Suffer from Muscle Weakness That Reflects a Growth Defect and Energy Deficit. PLoS ONE 2009, 4, e5618. [Google Scholar] [CrossRef]
- Han, J.M.; Jeong, S.J.; Park, M.C.; Kim, G.; Kwon, N.H.; Kim, H.K.; Ha, S.H.; Ryu, S.H.; Kim, S. Leucyl-tRNA Synthetase Is an Intracellular Leucine Sensor for the mTORC1-Signaling Pathway. Cell 2012, 149, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. Sestrin2 Is a Leucine Sensor for the mTORC1 Pathway. Science 2016, 351, 43–48. [Google Scholar] [CrossRef]
- Ban, H.; Shigemitsu, K.; Yamatsuji, T.; Haisa, M.; Nakajo, T.; Takaoka, M.; Nobuhisa, T.; Gunduz, M.; Tanaka, N.; Naomoto, Y. Arginine and Leucine Regulate P70 S6 Kinase and 4E-BP1 in Intestinal Epithelial Cells. Int. J. Mol. Med. 2004, 13, 537–543. [Google Scholar] [CrossRef]
- Rebsamen, M.; Pochini, L.; Stasyk, T.; De Araújo, M.E.G.; Galluccio, M.; Kandasamy, R.K.; Snijder, B.; Fauster, A.; Rudashevskaya, E.L.; Bruckner, M.; et al. SLC38A9 Is a Component of the Lysosomal Amino Acid Sensing Machinery That Controls mTORC1. Nature 2015, 519, 477–481. [Google Scholar] [CrossRef]
- Wang, S.; Tsun, Z.Y.; Wolfson, R.L.; Shen, K.; Wyant, G.A.; Plovanich, M.E.; Yuan, E.D.; Jones, T.D.; Chantranupong, L.; Comb, W.; et al. Metabolism. Lysosomal Amino Acid Transporter SLC38A9 Signals Arginine Sufficiency to mTORC1. Science 2015, 347, 188–194. [Google Scholar] [CrossRef]
- Xia, J.; Wang, R.; Zhang, T.; Ding, J. Structural Insight into the Arginine-Binding Specificity of CASTOR1 in Amino Acid-Dependent mTORC1 Signaling. Cell Discov. 2016, 2, 16035. [Google Scholar] [CrossRef]
- Collier, S.R.; Collins, E.; Kanaley, J.A. Oral Arginine Attenuates the Growth Hormone Response to Resistance Exercise. J. Appl. Physiol. 2006, 101, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Umeda, M.; Hiramoto, M.; Watanabe, A.; Tsunoda, N.; Imai, T. Arginine-Induced Insulin Secretion in Endoplasmic Reticulum. Biochem. Biophys. Res. Commun. 2015, 466, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.N.; Holzenberger, M.; Shih, D.Q.; Ozcan, U.; Stoffel, M.; Magnuson, M.A.; Kahn, C.R. β-Cell-Specific Deletion of the Igf1 Receptor Leads to Hyperinsulinemia and Glucose Intolerance but Does Not Alter β-Cell Mass. Nat. Genet. 2002, 31, 111–115. [Google Scholar] [CrossRef]
- Aguiar, A.F.; Vechetti-Júnior, I.J.; Souza, R.W.; Piedade, W.P.; Pacagnelli, F.L.; Leopoldo, A.S.; Casonatto, J.; Dal-Pai-Silva, M. Nitric Oxide Synthase Inhibition Impairs Muscle Regrowth Following Immobilization. Nitric Oxide—Biol. Chem. 2017, 69, 22–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, G.; Morris, S.M. Arginine Metabolism: Nitric Oxide and Beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Wang, R.; Jiao, H.; Zhao, J.; Wang, X.; Lin, H. L-Arginine Enhances Protein Synthesis by Phosphorylating mTOR (Thr 2446) in a Nitric Oxide-Dependent Manner in C2C12 Cells. Oxid. Med. Cell. Longev. 2018, 2018, 7569127. [Google Scholar] [CrossRef]
- Noeh, F.M.; Wenzel, A.; Harris, N.; Milakofsky, L.; Hofford, J.M.; Pell, S.; Vogel, W.H. The Effects of Arginine Administration on the Levels of Arginine, Other Amino Acids and Related Amino Compounds in the Plasma, Heart, Aorta, Vena Cava, Bronchi and Pancreas of the Rat. Life Sci. 1996, 58, PL131–PL138. [Google Scholar] [CrossRef]
- Yoshizawa, F.; Mochizuki, S.; Sugahara, K. Differential Dose Response of mTOR Signaling to Oral Administration of Leucine in Skeletal Muscle and Liver of Rats. Biosci. Biotechnol. Biochem. 2013, 77, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.P.; Andrabi, K.; Kozlowski, M.T.; Grove, J.R.; Avruch, J. Multiple Independent Inputs Are Required for Activation of the P70 S6 Kinase. Mol. Cell. Biol. 1995, 15, 2333–2340. [Google Scholar] [CrossRef]
- Yoshizawa, F.; Sekizawa, H.; Hirayama, S.; Hatakeyama, A.; Nagasawa, T.; Sugahara, K. Time Course of Leucine-Induced 4E-BP1 and S6K1 Phosphorylation in the Liver and Skeletal Muscle of Rats. J. Nutr. Sci. Vitaminol. 2001, 47, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, F.; Hirayama, S.; Sekizawa, H.; Nagasawa, T.; Sugahara, K. Oral Administration of Leucine Stimulates Phosphorylation of 4E-BP1 and S6K1 in Skeletal Muscle but Not in Liver of Diabetic Rats. J. Nutr. Sci. Vitaminol. 2002, 48, 59–64. [Google Scholar] [CrossRef]
- Tsugawa, Y.; Handa, H.; Imai, T. Arginine Induces IGF-1 Secretion from the Endoplasmic Reticulum. Biochem. Biophys. Res. Commun. 2019, 514, 1128–1132. [Google Scholar] [CrossRef]
- Rommel, C.; Bodine, S.C.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-Induced Skeletal Myotube Hypertrophy by Pl(3)K/Alt/mTOR and Pl(3)K/Akt/GSK3 Pathways. Nat. Cell Biol. 2001, 3, 1009–1013. [Google Scholar] [CrossRef]
- Palmer, R.M.; Ashton, D.S.; Moncada, S. Vascular Endothelial Cells Synthesize Nitric Oxide from L-Arginine. Nature 1988, 333, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Stuehr, D.J. Enzymes of the L-Arginine to Nitric Oxide Pathway. J. Nutr. 2004, 134, 2748S–2751S. [Google Scholar] [CrossRef]
- Le Plénier, S.; Goron, A.; Sotiropoulo, A.; Archambault, E.; Guihenneuc, C.; Walrand, S.; Salles, J.; Jourdan, M.; Neveux, N.; Cynober, L.; et al. Citrulline Directly Modulates Muscle Protein Synthesis via the PI3K/MAPK/4E-BP1 Pathway in a Malnourished State: Evidence from in Vivo, Ex Vivo, and in Vitro Studies. Am. J. Physiol.—Endocrinol. Metab. 2017, 312, E27–E36. [Google Scholar] [CrossRef]
- Kokubo, T.; Maeda, S.; Tazumi, K.; Nozawa, H.; Miura, Y.; Kirisako, T. The Effect of L-Ornithine on the Phosphorylation of mTORC1 Downstream Targets in Rat Liver. Prev. Nutr. Food Sci. 2015, 20, 238–245. [Google Scholar] [CrossRef]
- Huang, R.; Dai, Q.; Yang, R.; Duan, Y.; Zhao, Q.; Haybaeck, J. A Review: PI3K/AKT/mTOR Signaling Pathway and Its Regulated Eukaryotic Translation Initiation Factors May Be a Potential Therapeutic Target in Esophageal Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 817916. [Google Scholar] [CrossRef] [PubMed]
- Shahbazian, D.; Roux, P.P.; Mieulet, V.; Cohen, M.S.; Raught, B.; Taunton, J.; Wb, J.; Blenis, J.; Pende, M.; Sonenberg, N. The mTOR/PI3K and MAPK Pathways Converge on eIF4B to Control Its Phosphorylation and Activity. EMBO J. 2006, 25, 2781–2791. [Google Scholar] [CrossRef]
- Duchêne, S.; Audouin, E.; Crochet, S.; Duclos, M.J.; Tesseraud, S. Involvement of the ERK1/2 MAPK Pathway in Insulin-Induced S6K1 Activation in Avian Cells. Domest. Anim. Endocrinol. 2008, 34, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.R.; Andjelkovic, M.; Caudwell, B.; Cron, P.; Morrice, N.; Cohen, P.; Hemmings, B.A. Mechanism of Activation of Protein Kinase B by Insulin and IGF-1. EMBO J. 1996, 15, 6541–6551. [Google Scholar] [CrossRef]
- Domma, A.J.; Henderson, L.A.; Goodrum, F.D.; Moorman, N.J.; Kamil, J.P. Human Cytomegalovirus Attenuates AKT Activity by Destabilizing Insulin Receptor Substrate Proteins. J. Virol. 2023, 97, e0056323. [Google Scholar] [CrossRef]
- Fujiwara, T.; Kanazawa, S.; Ichibori, R.; Tanigawa, T.; Magome, T.; Shingaki, K.; Miyata, S.; Tohyama, M.; Hosokawa, K. L-Arginine Stimulates Fibroblast Proliferation through the GPRC6A-ERK1/2 and PI3K/Akt Pathway. PLoS ONE 2014, 9, e92168. [Google Scholar] [CrossRef]
- Yao, K.; Yin, Y.L.; Chu, W.; Liu, Z.; Deng, D.; Li, T.; Huang, R.; Zhang, J.; Tan, B.; Wang, W.; et al. Dietary Arginine Supplementation Increases mTOR Signaling Activity in Skeletal Muscle of Neonatal Pigs. J. Nutr. 2008, 138, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Sun, L.; Liu, C.; Su, L.; Chen, X.; Yang, Z.; Hu, G.; Zhang, M.; Zhao, L.; Jin, Y. Effect of Dietary Arginine Supplementation on Protein Synthesis, Meat Quality and Flavor in Growing Lambs. Meat Sci. 2023, 204, 109291. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Castillo, L.; Chapman, T.E.; Yu, Y.M.; Ajami, A.; Burke, J.F.; Young, V.R. Dietary Arginine Uptake by the Splanchnic Region in Adult Humans. Am. J. Physiol.—Endocrinol. Metab. 1993, 265, E532–E539. [Google Scholar] [CrossRef]
- Morita, M.; Hayashi, T.; Ochiai, M.; Maeda, M.; Yamaguchi, T.; Ina, K.; Kuzuya, M. Oral supplementation with a combina-tion of L-citrulline and L-arginine rapidly increases plasma L-arginine concentration and enhances NO bioavailability. Biochem. Biophys. Res. Commun. 2014, 454, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Köhler, E.S.; Sankaranarayanan, S.; van Ginneken, C.J.; van Dijk, P.; Vermeulen, J.L.; Ruijter, J.M.; Lamers, W.H.; Bruder, E. The human neonatal small intestine has the potential for arginine synthesis; developmental changes in the expression of arginine-synthesizing and -catabolizing enzymes. BMC Dev. Biol. 2008, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am. J. Physiol. 1997, 272, G1382–G1390. [Google Scholar] [CrossRef]
- De Jonge, W.J.; Dingemanse, M.A.; de Boer, P.A.; Lamers, W.H.; Moorman, A.F. Arginine-metabolizing enzymes in the developing rat small intestine. Pediatr. Res. 1998, 43, 442–451. [Google Scholar] [CrossRef]
- Yeo, T.W.; Lampah, D.A.; Rooslamiati, I.; Gitawati, R.; Tjitra, E.; Kenangalem, E.; Price, R.N.; Duffull, S.B.; Anstey, N.M. A randomized pilot study of L-arginine infusion in severe falciparum malaria: Preliminary safety, efficacy and pharmacoki-netics. PLoS ONE 2013, 29, e69587. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Poeze, M.; Deutz, N.E. Arginine infusion in patients with septic shock increases nitric oxide production without haemodynamic instability. Clin. Sci. 2015, 128, 57–67. [Google Scholar] [CrossRef]
- Buel, G.R.; Dang, H.Q.; Asara, J.M.; Blenis, J.; Mutvei, A.P. Prolonged Deprivation of Arginine or Leucine Induces PI3K/Akt-Dependent Reactivation of mTORC1. J. Biol. Chem. 2022, 298, 102030. [Google Scholar] [CrossRef]
- Hay, N.; Sonenberg, N. Upstream and Downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef]
- Powis, K.; De Virgilio, C. Conserved Regulators of Rag GTPases Orchestrate Amino Acid-Dependent TORC1 Signaling. Cell Discov. 2016, 2, 15049. [Google Scholar] [CrossRef] [PubMed]
- Doxsey, D.D.; Shen, K. Purification and Biochemical Characterization of the Rag GTPase Heterodimer. Methods Enzymol. 2022, 675, 131–158. [Google Scholar] [CrossRef]
- Xu, D.; Shimkus, K.L.; Lacko, H.A.; Kutzler, L.; Jefferson, L.S.; Kimball, S.R. Evidence for a Role for Sestrin1 in Mediating Leucine-Induced Activation of mTORC1 in Skeletal Muscle. Am. J. Physiol.—Endocrinol. Metab. 2019, 316, E817–E828. [Google Scholar] [CrossRef]
- Floyd, J.C.; Fajans, S.S.; Pek, S.; Thiffault, C.A.; Knopf, R.F.; Conn, J.W. Synergistic Effect of Essential Amino Acids and Glucose upon Insulin Secretion in Man. Diabetes 1970, 19, 109–115. [Google Scholar] [CrossRef]
- Pek, S.; Santiago, J.C.; Tai, T.Y. L-Leucine-Induced Secretion of Glucagon and Insulin, and the “off-Response”to l-Leucine in Vitro. I. Characterization of the Dynamics of Secretion. Endocrinology 1978, 103, 1208–1218. [Google Scholar] [CrossRef]
- Scott, P.H.; Brunn, G.J.; Kohn, A.D.; Roth, R.A.; Lawrence, J.C. Evidence of Insulin-Stimulated Phosphorylation and Activation of the Mammalian Target of Rapamycin Mediated by a Protein Kinase B Signaling Pathway. Proc. Natl. Acad. Sci. USA 1998, 95, 7772–7777. [Google Scholar] [CrossRef] [PubMed]
- Taha, C.; Liu, Z.; Jin, J.; Al-Hasani, H.; Sonenberg, N.; Klip, A. Opposite Translational Control of GLUT1 and GLUT4 Glucose Transporter mRNAs in Response Insulin. Role of Mammalian Target of Rapamycin, Protein Kinase B, and Phosphatidylinositol 3-Kinase in GLUT1 mRNA Translation. J. Biol. Chem. 1999, 274, 33085–33091. [Google Scholar] [CrossRef] [PubMed]
- Peyrollier, K.; Hajduch, E.; Blair, A.S.; Hyde, R.; Hundal, H.S. L-Leucine Availability Regulates Phosphatidylinositol 3-Kinase, P70 S6 Kinase and Glycogen Synthase Kinase-3 Activity in L6 Muscle Cells: Evidence for the Involvement of the Mammalian Target of Rapamycin (mTOR) Pathway in the L-Leucine-Induced Up-Regulation of System A Amino Acid Transport. Biochem. J. 2000, 350 Pt 2, 361–368. [Google Scholar] [PubMed]
- Sato, T.; Ito, Y.; Nedachi, T.; Nagasawa, T. Lysine Suppresses Protein Degradation through Autophagic-Lysosomal System in C2C12 Myotubes. Mol. Cell. Biochem. 2014, 391, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.S.; Son, K.; Arauz, E.; Han, J.M.; Kim, S.; Chen, J. Leucyl-tRNA Synthetase Activates Vps34 in Amino Acid-Sensing mTORC1 Signaling. Cell Rep. 2016, 16, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, M.G.; Hamilton, D.L.; Murray, J.T.; Taylor, P.M.; Baar, K. mVps34 Is Activated Following High-Resistance Contractions. J. Physiol. 2009, 587, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.; Maetzel, D.; Maddocks, O.D.; Otten, G.; Ratcliff, M.; Smith, G.R.; Dunlop, E.A.; Passos, J.F.; Davies, O.R.; Jaenisch, R.; et al. Control of TSC2-Rheb Signaling Axis by Arginine Regulates mTORC1 Activity. Elife 2016, 5, e11058. [Google Scholar] [CrossRef]
- Clemmensen, C.; Smajilovic, S.; Wellendorph, P.; Bräuner-Osborne, H. The GPCR, Class C, Group 6, Subtype A (GPRC6A) Receptor: From Cloning to Physiological Function. Br. J. Pharmacol. 2014, 171, 1129–1141. [Google Scholar] [CrossRef]
- Ge, Y.; Li, F.; He, Y.; Cao, Y.; Guo, W.; Hu, G.; Liu, J.; Fu, S. L-Arginine Stimulates the Proliferation of Mouse Mammary Epithelial Cells and the Development of Mammary Gland in Pubertal Mice by Activating the GPRC6A/PI3K/AKT/mTOR Signalling Pathway. J. Anim. Physiol. Anim. Nutr. 2022, 106, 1383–1395. [Google Scholar] [CrossRef]
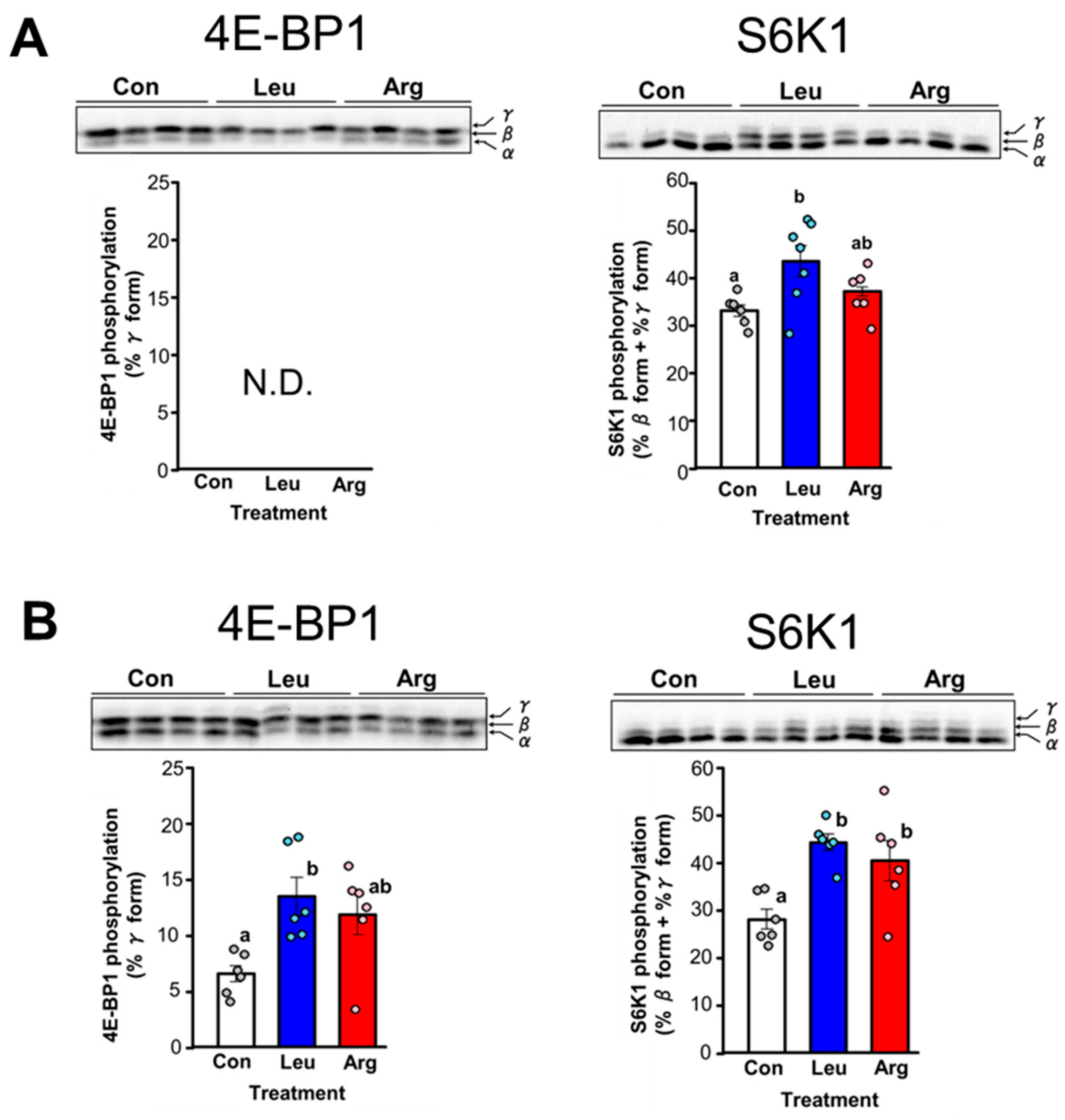

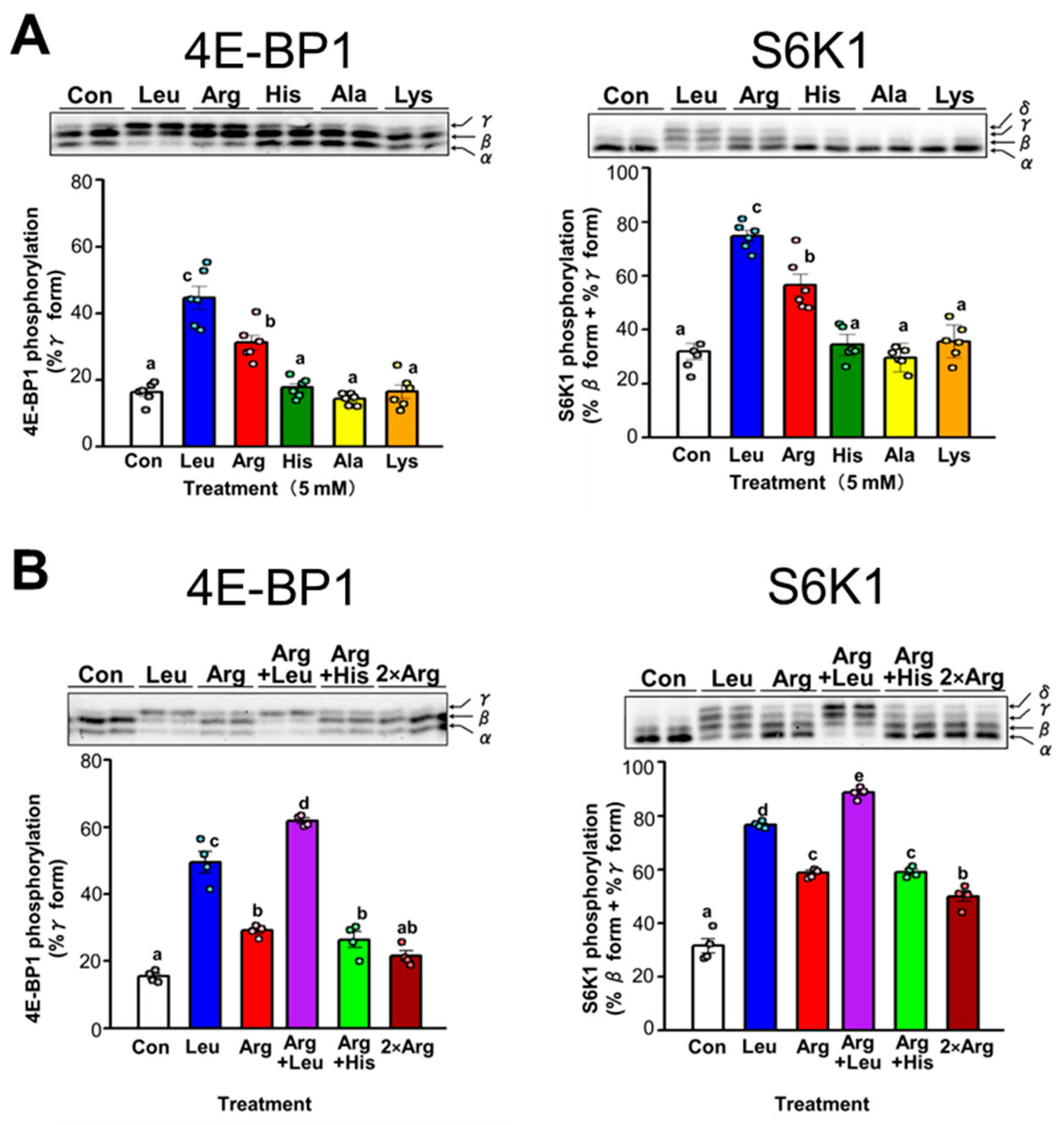
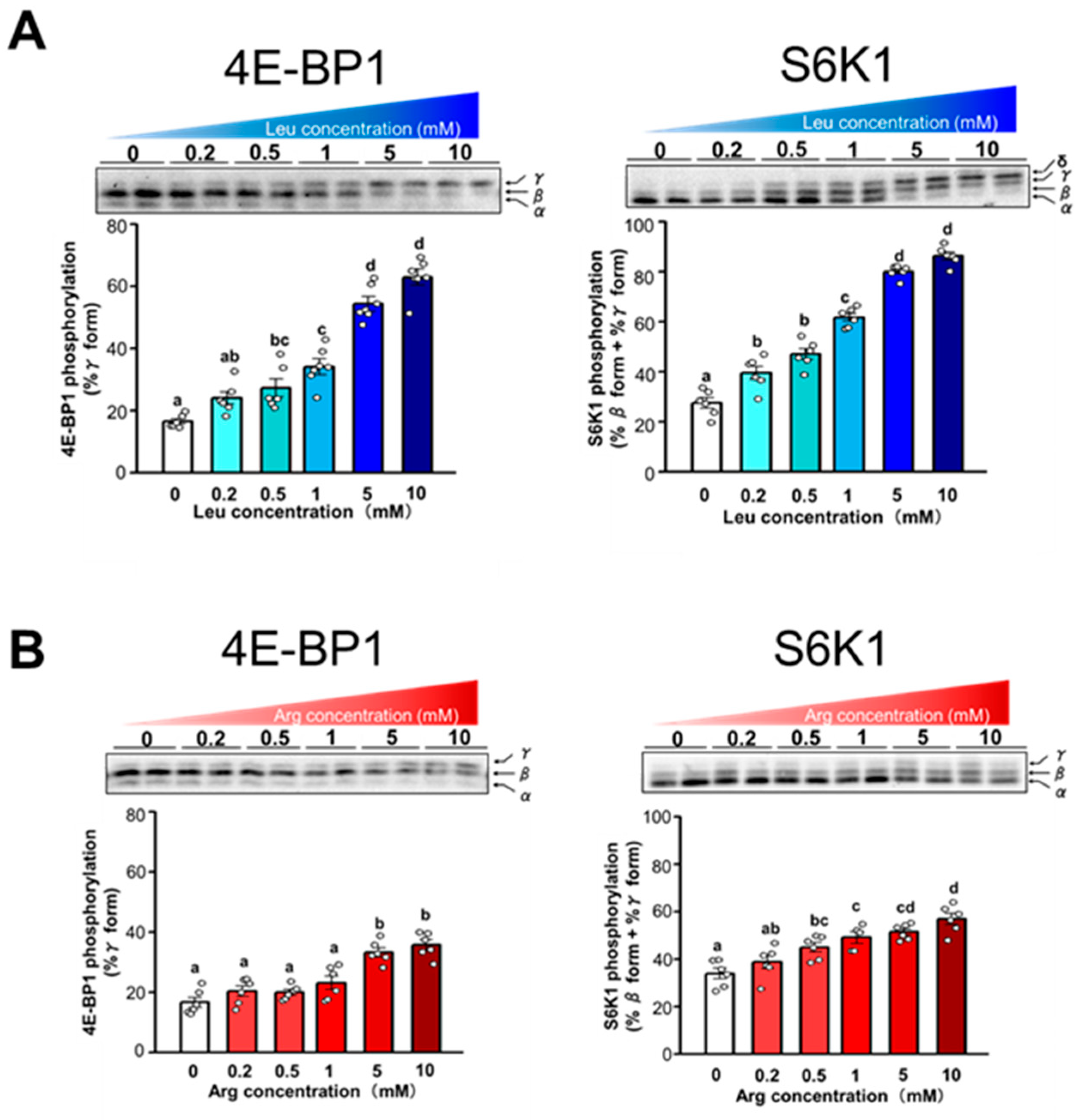
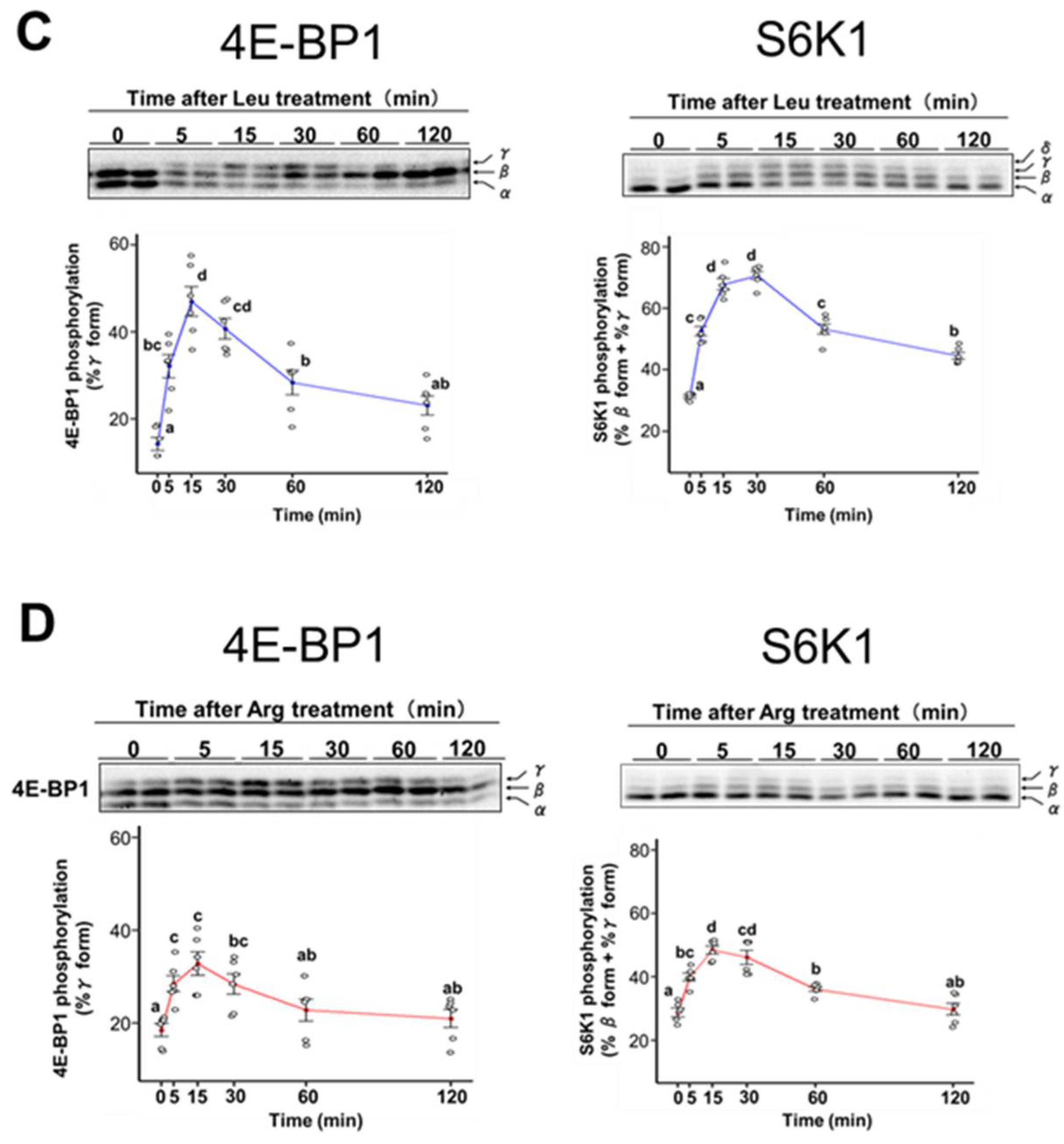
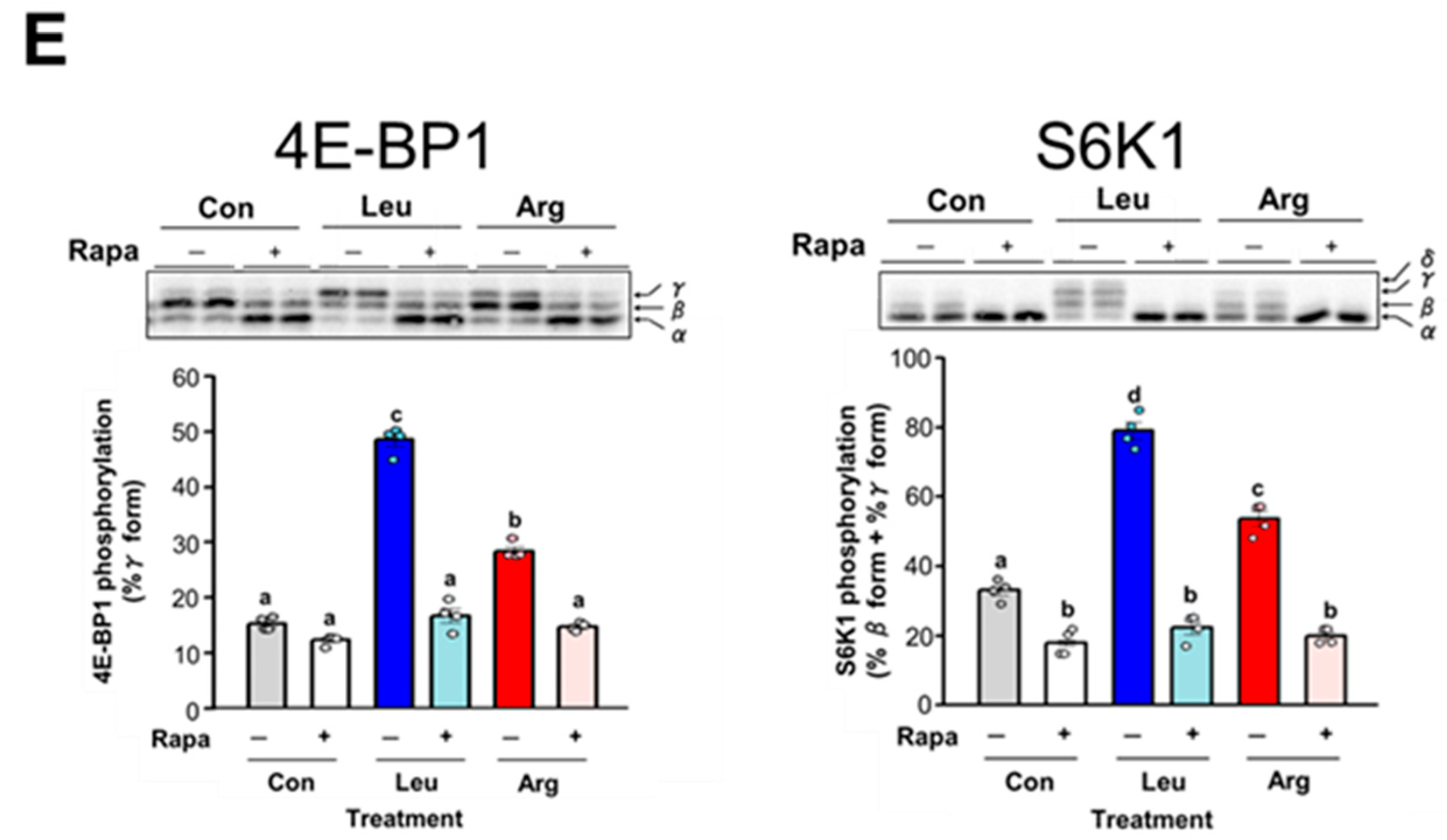
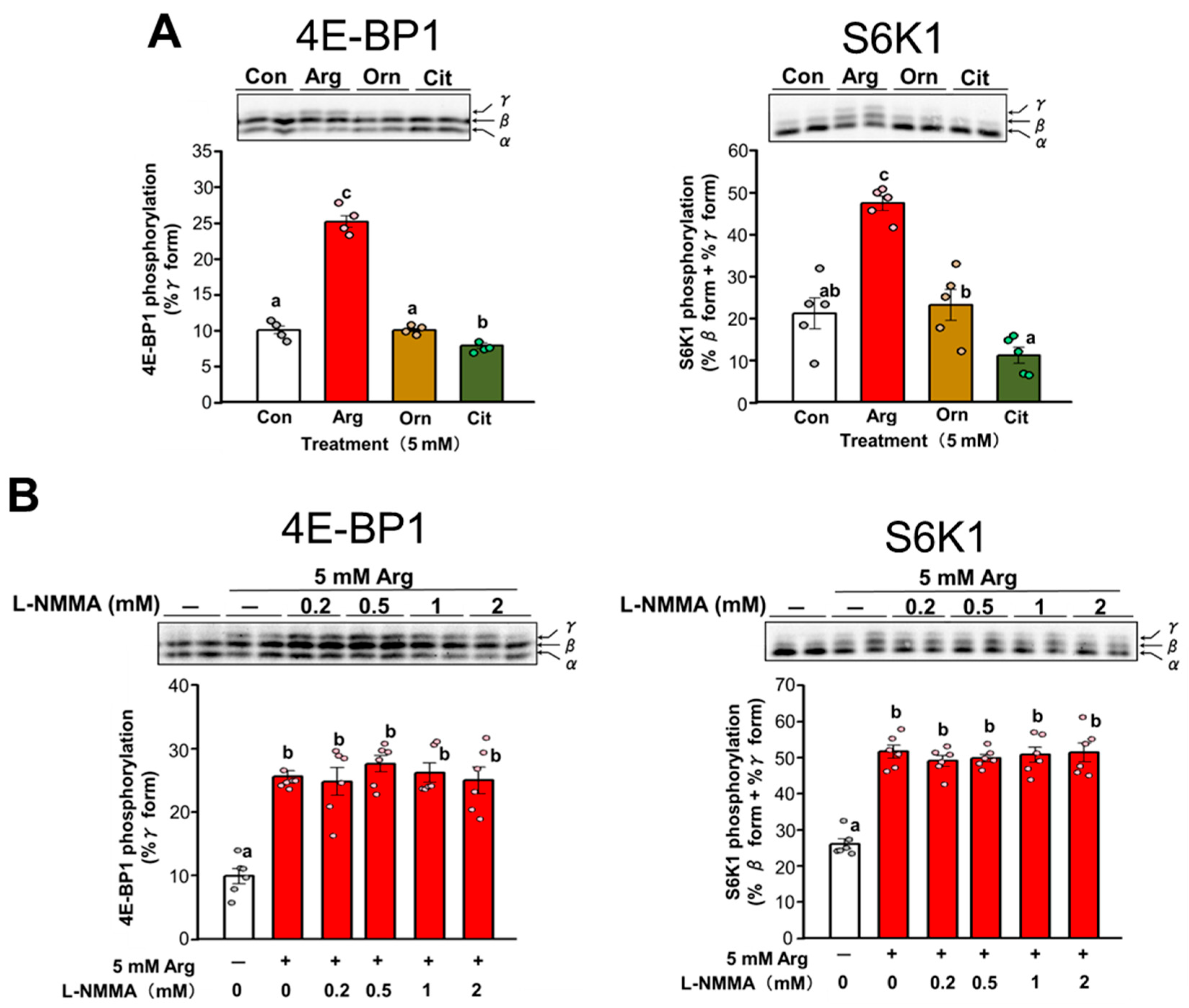
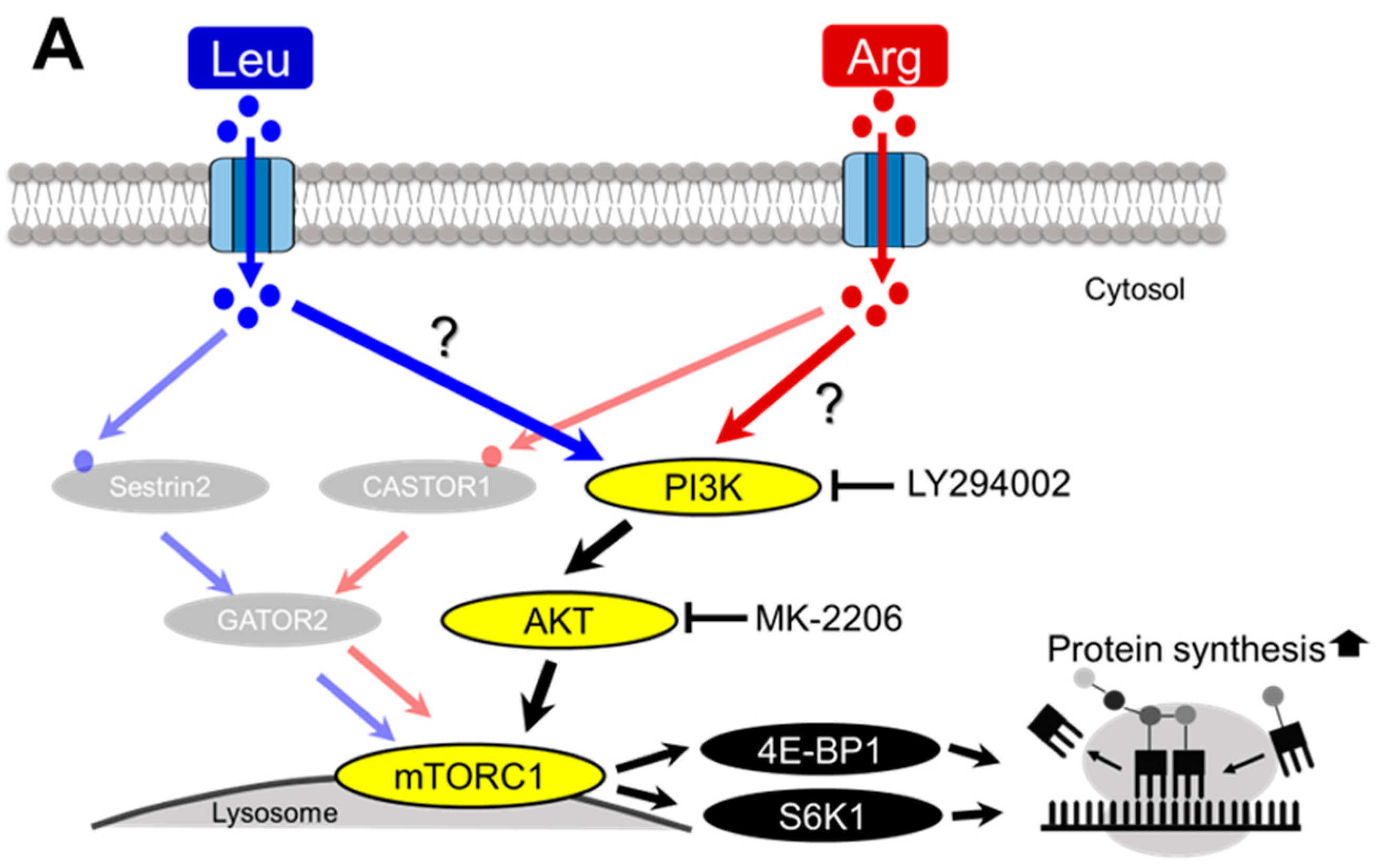

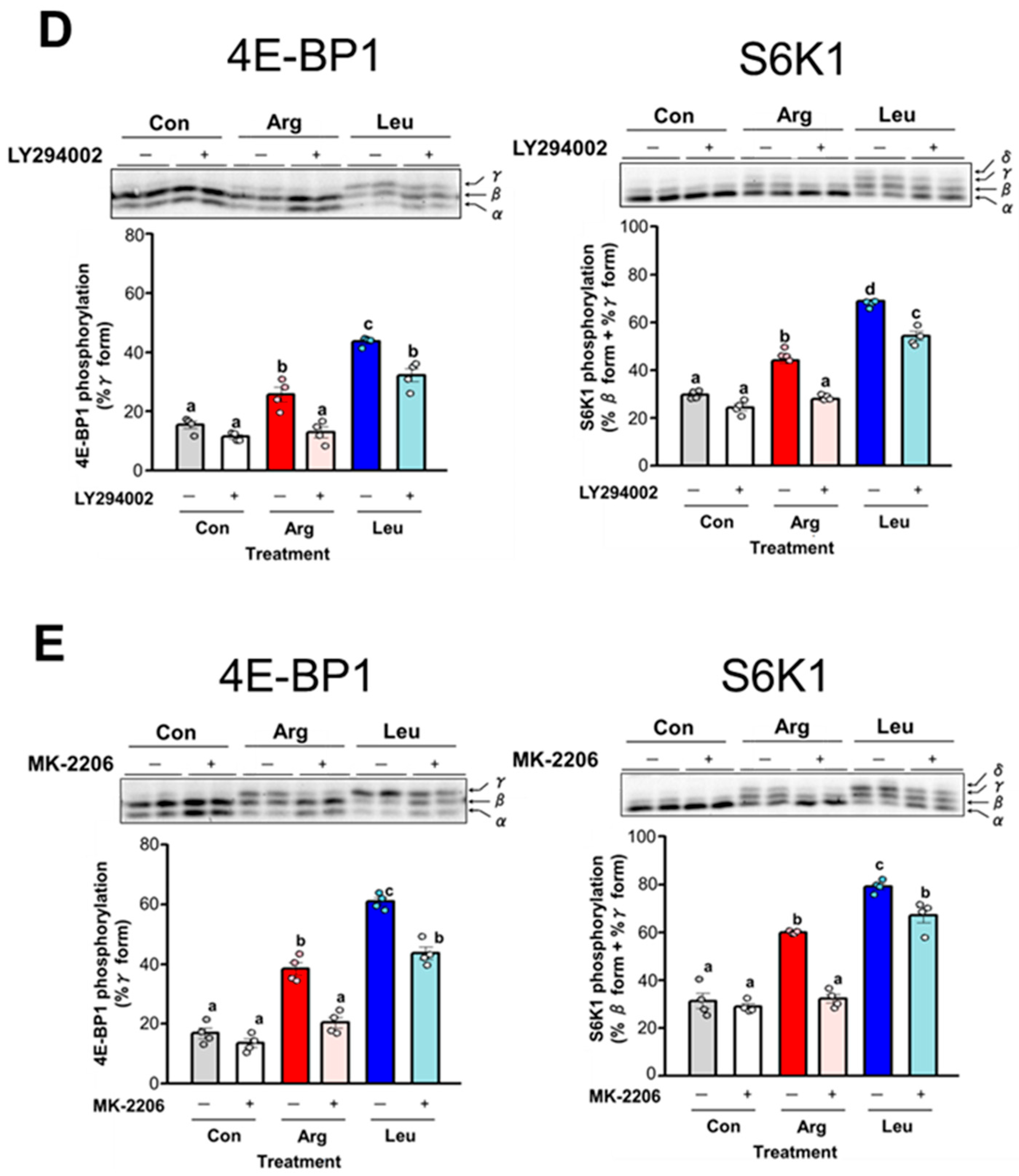

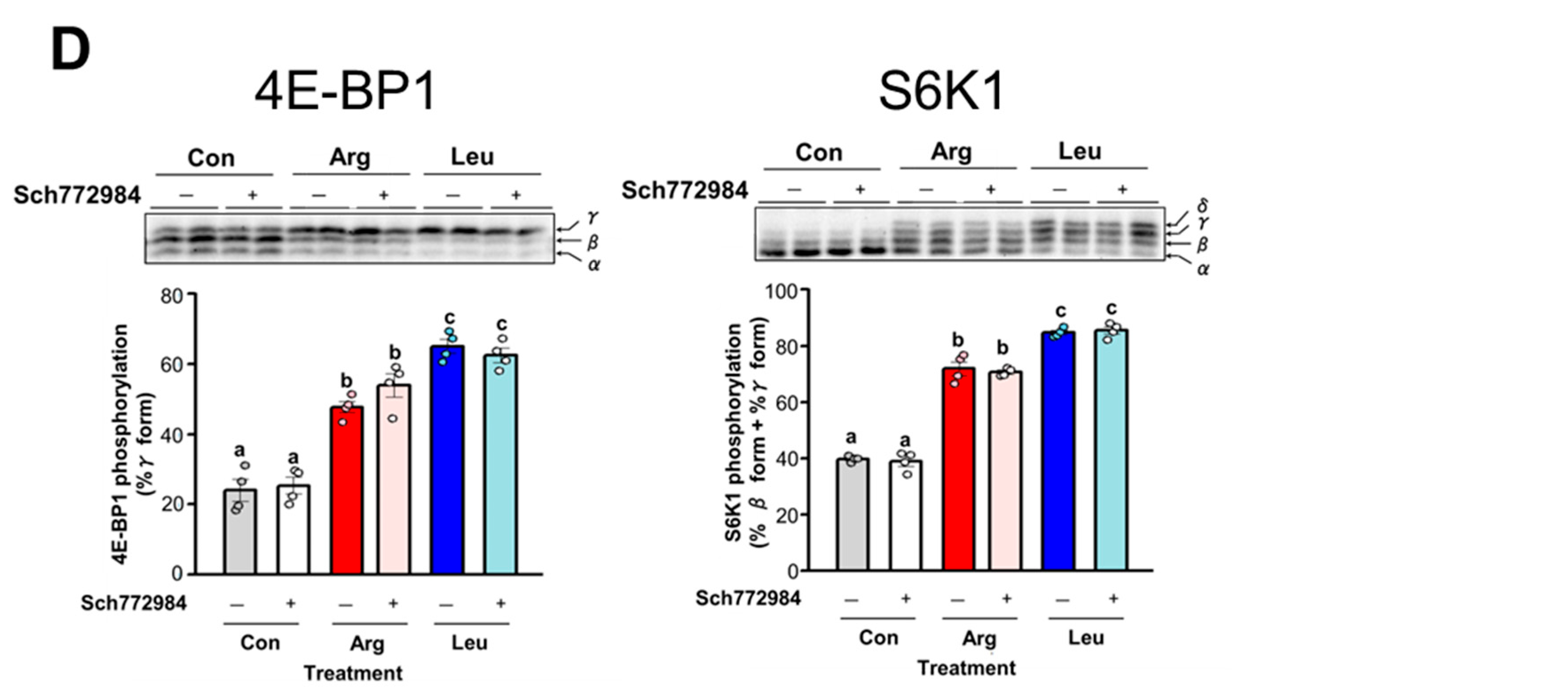

| Amino Acid Concentration in Serum (nmol/mL) | Oral Administration | Intraperitoneal Administration | ||||
|---|---|---|---|---|---|---|
| Con | Leu | Arg | Con | Leu | Arg | |
| Leu | 195.2 ± 9.1 a | 518.7 ± 40.7 b | 188.6 ± 18.4 a | 230.8 ± 26.8 a | 890.7 ± 43.7 b | 276.1 ± 21.5 a |
| Arg | 65.2 ± 4.8 a | 66.5 ± 8.2 a | 132.5 ± 15.8 b | 77.6 ± 4.5 a | 82.9 ± 11.8 a | 312.9 ± 62.7 b |
| Orn | 40.4 ± 4.5 a | 45.0 ± 5.8 a | 232.1 ± 27.2 b | 36.1 ± 2.9 a | 54.4 ± 4.5 a | 710.0 ± 65.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, D.; Takami, Y.; Sato, Y.; Toyoshima, Y.; Yoshizawa, F. Analysis of the Stimulative Effect of Arginine on Translation Initiation of Protein Synthesis in Skeletal Muscle. Nutrients 2025, 17, 2981. https://doi.org/10.3390/nu17182981
Suzuki D, Takami Y, Sato Y, Toyoshima Y, Yoshizawa F. Analysis of the Stimulative Effect of Arginine on Translation Initiation of Protein Synthesis in Skeletal Muscle. Nutrients. 2025; 17(18):2981. https://doi.org/10.3390/nu17182981
Chicago/Turabian StyleSuzuki, Daisuke, Yuki Takami, Yusuke Sato, Yuka Toyoshima, and Fumiaki Yoshizawa. 2025. "Analysis of the Stimulative Effect of Arginine on Translation Initiation of Protein Synthesis in Skeletal Muscle" Nutrients 17, no. 18: 2981. https://doi.org/10.3390/nu17182981
APA StyleSuzuki, D., Takami, Y., Sato, Y., Toyoshima, Y., & Yoshizawa, F. (2025). Analysis of the Stimulative Effect of Arginine on Translation Initiation of Protein Synthesis in Skeletal Muscle. Nutrients, 17(18), 2981. https://doi.org/10.3390/nu17182981







