A Standardised Exercise Protocol to Induce Oxidative Stress in Humans: Validation with a Dietary Polyphenol Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Size
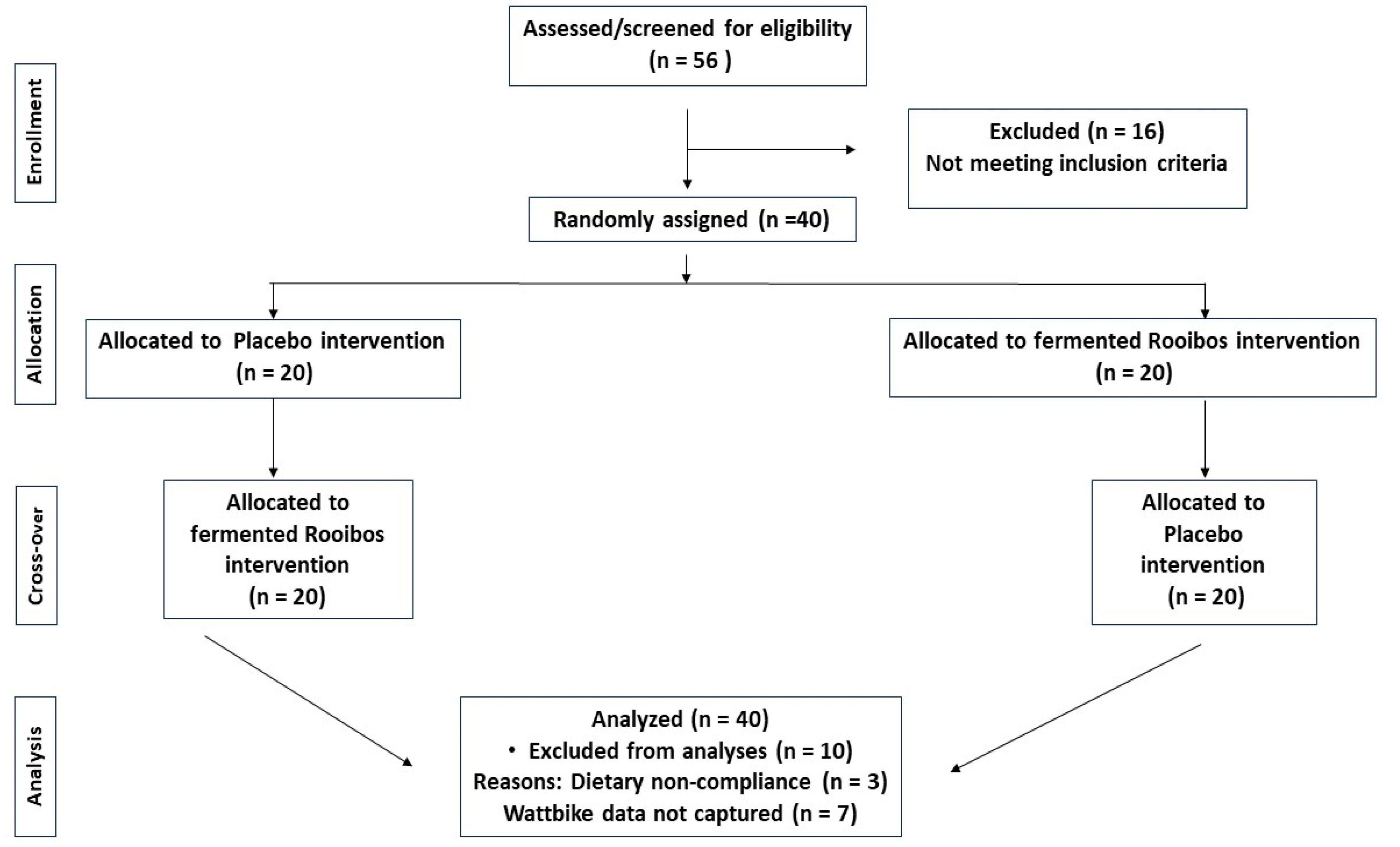
2.2. Participant Screening and Recruitment
2.3. Randomisation and Controls
2.4. Intervention Beverages
2.5. Trial Exercise Protocol
2.5.1. Warm-Up
- A 5 min warm-up, at 50–60 rpm on air resistance setting 3 on the Wattbike Pro
2.5.2. Exercise Test Protocol to Induce Oxidative Stress
- Pedal in a seated position for 1 min at the starting power of 100 watts (W), at a cadence rate of between 70 and 100 rpm.
- Increase the air resistance setting and/or cadence as necessary every 1 min to ensure a 15 W increase in power (W) output every 1 min; this allowed the body to adapt to the increasing workload and for a steady heart rate to be achieved (at that level).
- Keep increasing the power (W) output by 15 W every minute until the rider reaches the rate of perceived exertion (RPE) of somewhat hard—level 13 on the Borg scale rate of perceived exertion.
- The test would be terminated if the rider/participant experienced any adverse symptoms during the exercise test prior to achieving their sub-maximum exertion of 75–80% as per heart rate and/or the participant rating the exertion level, as per the Borg scale at 13 (somewhat hard).
- The test would also be terminated if the participant experienced an emergency (physical and/or mental) e.g., the participant felt unwell and/or became overly anxious.
- Once the participant reaches level 13 on the Borg scale, he stops cycling for 1 min and then is asked to complete sets of 10 s sprints (10 max or until the participant reaches the RPE of maximal exertion—level 20 on the Borg scale), separated by 15 s of passive recovery rest periods.
2.6. Blood Sampling and Processing
2.7. Oxidative Stress Markers
2.7.1. Reduced Glutathione
2.7.2. Oxidised Glutathione
2.7.3. The Redox Status of Glutathione
2.7.4. Conjugated Dienes
2.7.5. Thiobarbituric Acid Reactive Substances (TBARSs)
2.7.6. Protein Carbonylation
2.7.7. Unconjugated Bilirubin
2.7.8. Ferric Reducing Antioxidant Power (FRAP) Assay
2.7.9. Oxidative DNA Damage
2.8. Clinical Chemistry Markers
2.9. Statistical Analysis
3. Results
3.1. Anthropometric Measurements and Health Indicators
3.2. Oxidative Stress Parameters
3.2.1. Glutathione Redox Status
3.2.2. Oxidative Stress Status and Lipid and Protein Damage
3.2.3. Oxidative DNA Damage
3.3. Clinical Chemistry Markers
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAE | Ascorbic acid equivalents |
| ALB | Albumin |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BHT | Butylated hydroxytoluene |
| BMI | Body mass index |
| BSA | Bovine serum albumin |
| BUN | Blood urea nitrogen |
| CD | Conjugated dienes |
| CE | Catechin equivalents |
| CK | Creatine kinase |
| CREAT | Creatinine |
| DBIL | Direct bilirubin |
| DBP | Diastolic blood pressure |
| DDW | Double-distilled water |
| DNPH | Dinitrophenylhydrazine |
| DTNB | 5,5′-dithiobis-2-nitrobenzoic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| FPG | Formamidopyrimidine DNA glycosylase |
| FRAP | Ferric reducing antioxidant power |
| GAE | Gallic acid equivalents |
| GGT | Gamma glutamyl transferase |
| GLUC | Glucose |
| GR | Glutathione reductase |
| GSH | Reduced glutathione |
| GSSG | Oxidised glutathione |
| H2O2 | Hydrogen peroxide |
| Hb | Haemoglobin |
| HPLC | High-performance liquid chromatography |
| LAC | Lactate |
| LDH | Lactate dehydrogenase |
| M2VP | Methyl-2-vinylpyridinium |
| MDA | Malondialdehyde |
| MPA | Meta-phosphoric acid |
| NADPH | Nicotinamide adenine dinucleotide phosphate hydrogen |
| PC | Protein carbonyls |
| QE | Quercetin equivalents |
| RPE | Rate of perceived exertion |
| RT | Room temperature |
| SBP | Systolic blood pressure |
| SST | Serum separates |
| TBARS | Thiobarbituric acid reactive substances |
| TBIL | Total bilirubin |
| TCA | Trichloro acetic acid |
| TE | Trolox equivalents |
| TEAC | Trolox equivalent antioxidant capacity |
| tGSH | Total reduced glutathione |
| TP | Total protein |
| UA | Uric acid |
| UCB | Unconjugated bilirubin |
| W | Watts |
References
- Sies, H.; Cadenas, E. Oxidative stress: Damage to intact cells and organs. Philos. Trans. R. Soc. London. B Biol. Sci. 1985, 311, 617–631. [Google Scholar] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 552535. [Google Scholar] [CrossRef]
- Ji, L.L.; Yeo, D. Oxidative stress: An evolving definition. Fac. Rev. 2021, 10, 13. [Google Scholar] [CrossRef]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, N.; Eren, E. Oxidative Stress and Sterile Inflammation in Multiple Sclerosis; IOP Publishing: Bristol, UK, 2019; Chapter 7; pp. 1–32. [Google Scholar] [CrossRef]
- De, G.A.D.L.R.; Riva, L.; Trujillo, L.A.S.; González-Hernández, J.C. Assessment on Oxidative Stress in Animals: From Experimental Models to Animal Production. In Importance of Oxidative Stress and Antioxidant System in Health and Disease; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Jones, A.M. Measurement of the maximum oxygen uptake Vo2max: Vo2peak is no longer acceptable. J. Appl. Physiol. 2017, 122, 997–1002. [Google Scholar] [CrossRef]
- Copetti, C.L.K.; Orssatto, L.B.; Diefenthaeler, F.; Silveira, T.T.; da Silva, E.L.; de Liz, S.; Mendes, B.C.; Rieger, D.K.; Vieira, F.G.K.; de Fragas Hinnig, P.; et al. Acute effect of juçara juice (Euterpe edulis Martius) on oxidative stress biomarkers and fatigue in a high-intensity interval training session: A single-blind cross-over randomized study. J. Funct. Foods 2020, 67, 103835. [Google Scholar] [CrossRef]
- Durstenfeld, M.S.; Sun, K.; Tahir, P.; Peluso, M.J.; Deeks, S.G.; Aras, M.A.; Grandis, D.J.; Long, C.S.; Beatty, A.; Hsue, P.Y. Use of Cardiopulmonary Exercise Testing to Evaluate Long COVID-19 Symptoms in Adults: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, e2236057. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takase, B. Exercise stress testing as the significant clinical modality for management of hypertension. Hypertens. Res. 2012, 35, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Bramati, L.; Aquilano, F.; Pietta, P. Unfermented rooibos tea: Quantitative characterization of flavonoids by HPLC-UV and determination of the total antioxidant activity. J. Agric. Food Chem. 2003, 51, 7472–7474. [Google Scholar] [CrossRef] [PubMed]
- Asensi, M.; Sastre, J.; Pallardo, F.V.; Lloret, A.; Lehner, M.; Garcia-De-La Asuncion, J.; Viña, J. Ratio of reduced to oxidized glutathione as indicator of oxidative stress status and DNA damage. Methods Enzymol. 1999, 299, 267–276. [Google Scholar]
- Recknagel, R.O.; Glende, E.A. Detection of lipid conjugated dienes. Methods Enzymol. 1984, 105, 331–337. [Google Scholar]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation if thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free. Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Colombo, G.; Clerici, M.; Garavaglia, M.E.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. A step-by-step protocol for assaying protein carbonylation in biological samples. J. Chromatogr. B 2016, 1019, 178–190. [Google Scholar] [CrossRef]
- Augustyniak, E.; Adam, A.; Wojdyla, K.; Rogowska-Wrzesinska, A.; Willetts, R.; Korkmaz, A.; Atalay, M.; Weber, D.; Grune, T.; Borsa, C.; et al. Validation of protein carbonyl measurement: A multi-centre study. Redox Biol. 2015, 4, 149–157. [Google Scholar] [CrossRef]
- Wallner, M.; Blassnigg, S.M.; Marisch, K.; Pappenheim, M.T.; Müllner, E.; Mölzer, C.; Nersesyan, A.; Marculescu, R.; Doberer, D.; Knasmüller, S.; et al. Effects of unconjugated bilirubin on chromosomal damage in individuals with Gilbert’s syndrome measured with the micronucleus cytome assay. Mutagenesis 2012, 27, 731–735. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Draxler, A.; Franzke, B.; Cortolezis, J.T.; Gillies, N.A.; Unterberger, S.; Aschauer, R.; Zöhrer, P.A.; Bragagna, L.; Kodnar, J.; Strasser, E.M.; et al. The Effect of Elevated Protein Intake on DNA Damage in Older People: Comparative Secondary Analysis of Two Randomized Controlled Trials. Nutrients 2021, 13, 3479. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Pinho, R.A.; Ugbolue, U.C.; He, Y.; Meng, Y.; Gu, Y. Effect of Running Exercise on Oxidative Stress Biomarkers: A Systematic Review. Front. Physiol. 2021, 11, 610112. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Pecorari, M.; Serafini, M.; Crozier, A. Bioavailability of C-linked dihydrochalcone and flavanone glucosides in humans following ingestion of unfermented and fermented rooibos teas. J. Agric. Food Chem. 2009, 57, 7104–7111. [Google Scholar] [CrossRef] [PubMed]
- Marnewick, J.L.; Rautenbach, F.; Venter, I.; Neethling, H.; Blackhurst, D.M.; Wolmarans, P.; Macharia, M. Effects of rooibos (Aspalathus linearis) on oxidative stress and biochemical parameters in adults at risk for cardiovascular disease. J. Ethnopharmacol. 2011, 133, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Villano, D.; Pecorari, M.; Francesca, M.T.; Raguzzini, A.; Stalmach, A.; Crozier, A.; Tubili, C.; Serafini, M. Unfermented and fermented rooibos teas (Aspalathus linearis) increase plasma total antioxidant capacity in healthy humans. Food Chem. 2010, 123, 679–683. [Google Scholar] [CrossRef]
- Breiter, T.; Laue, C.; Kressel, G.; Gröll, S.; Engelhardt, U.H.; Hahn, A. Bioavailability and antioxidant potential of rooibos flavonoids in humans following the consumption of different rooibos formulations. Food Chem. 2011, 128, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Escobar, M.A.; Oliveira, M.W.; Behr, G.A.; Zanotto-Filho, A.L.; Ilha, L.U.; Cunha, G.D.; Oliveira, A.R.; Moreira, J.C. Oxidative stress in young football (soccer) players in intermittent high intensity exercise protocol. J. Exerc. Physiol. 2009, 12, 1–10. [Google Scholar]
- Vezzoli, A.; Dellanoce, C.; Mrakic-Sposta, S.; Montorsi, M.; Moretti, S.; Tonini, A.; Pratali, L.; Accinni, R. Oxidative Stress Assessment in Response to Ultra endurance Exercise: Thiols Redox Status and ROS Production according to Duration of a Competitive Race. Oxidative Med. Cell. Longev. 2016, 2016, 6439037. [Google Scholar] [CrossRef]
- Spanidis, Y.; Stagos, D.; Orfanou, M.; Goutzourelas, N.; Bar-Or, D.; Spandidos, D.; Kouretas, D. Variations in Oxidative Stress Levels in 3 Days Follow-up in Ultramarathon Mountain Race Athletes. J. Strength Cond. Res. 2017, 31, 582–594. [Google Scholar] [CrossRef]
- Jówko, E.; Długołęcka, B.; Makaruk, B.; Cieśliński, I. The effect of green tea extract supplementation on exercise-induced oxidative stress parameters in male sprinters. Eur. J. Nutr. 2015, 54, 783–791. [Google Scholar] [CrossRef]
- Ferreira, P.R.; Marins, J.C.B.; de Oliveira, L.L.; Bastos, D.S.S.; Júnior, D.T.S.; da Silva, C.D.; Fontes, E.A.F. Beverage based on whey permeate with phenolic extract of jabuticaba peel: A pilot study on effects on muscle and oxidative stress in trained individuals. J. Funct. Foods. 2020, 65, 103749. [Google Scholar] [CrossRef]
- Thirupathi, A.; Wang, M.; Lin, J.K.; Fekete, G.; István, B.; Baker, J.S.; Gu, Y. Effect of Different Exercise Modalities on Oxidative Stress: A Systematic Review. Biomed. Res. Int. 2021, 2021, 1947928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bojarczuk, A.; Dzitkowska-Zabielska, M. Polyphenol Supplementation and Antioxidant Status in Athletes: A Narrative Review. Nutrients 2023, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef]
- Jenkins, R.R. Exercise and oxidative stress methodology: A critique. Am. J. Clin. Nutr. 2000, 72, 670S674S. [Google Scholar] [CrossRef] [PubMed]
- Moslemi, E.; Dehghan, P.; Khani, M.; Sarbakhsh, P.; Sarmadi, B. The effects of date seed (Phoenix dactylifera) supplementation on exercise-induced oxidative stress and aerobic and anaerobic performance following high-intensity interval training sessions: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2023, 129, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hai, C. Novel insights into redox system and the mechanism of redox regulation. Mol. Biol. Rep. 2016, 43, 607–628. [Google Scholar] [CrossRef]
- Yavari, A.; Javadi, M.; Mirmiran, P.; Bahadoran, Z. Exercise-induced oxidative stress and dietary antioxidants. Asian J. Sports Med. 2015, 6, e24898. [Google Scholar] [CrossRef]
- Neubauer, O.; Reichhold, S.; Nersesyan, A.; König, D.; Wagner, K.H. Exercise-induced DNA damage: Is there a relationship with inflammatory responses? Exerc. Immunol. Rev. 2008, 14, 51–72. [Google Scholar]
- Tryfidou, D.V.; McClean, C.; Nikolaidis, M.G.; Davison, G.W. DNA Damage Following Acute Aerobic Exercise: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 103–127. [Google Scholar] [CrossRef]
- Richardson, R.S.; Noyszewski, E.A.; Kendrick, K.F.; Leigh, J.S.; Wagner, P.D. Myoglobin 02 Desaturation during Exercise. J. Clin. Investig. 1995, 96, 1916–1926. [Google Scholar] [CrossRef]
- Arbogast, S.; Reid, M.B. Oxidant activity in skeletal muscle fibers is influenced by temperature, CO2 level, and muscle-derived nitric oxide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R698–R705. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Reid, M.B. Muscle-derived ROS and thiol regulation in muscle fatigue. J. Appl. Physiol. 2008, 104, 853–860. [Google Scholar] [CrossRef]
- Furrer, R.; Hawley, J.A.; Handschin, C. Themolecular athlete: Exercise physiology from mechanisms to medals. Physiol. Rev. 2023, 103, 1693–1787. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.K.; Senefeld, J.W. Sex differences in human performance. J. Physiol. 2024, 602, 4129–4156. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; for the CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Fermented Rooibos Beverage (375 mL) | Placebo Beverage (375 mL) |
|---|---|---|
| Flavonols (mg QE) | 93.7 ± (1.67) | 11.27 ± 5.03 |
| Flavanols (mg CE) | 37.6 ± 3.67 | 0 |
| Total polyphenolic content (mg GAE) | 333 ± 1.72 | 0 |
| FRAP (µmol AAE) | 1701 ± 4.55 | 52.55 ± 2.86 |
| TEAC (µmol TE) | 2118 ± 19.3 | 0 |
| Aspalathin (mg) | 8.37 ± 0.04 | 0 |
| Orientin (mg) | 6.69 ± 0.03 | 0 |
| Isoorientin (mg) | 15.50 ± 0.17 | 0 |
| Isovitexin (mg) | 2.52 ± 0.05 | 0 |
| Vitexin (mg) | 1.95 ± 0.03 | 0 |
| Hyperroside (mg) | 7.26 ± 0.05 | 0 |
| Quercetin (mg) | 2.37 ± 0.02 | 0 |
| Luteolin (mg) | 0.57 ± 0.02 | 0 |
| Chrysoeriol (mg) | 0.11 ± 0.00 | 0 |
| Variable | Placebo Session | Rooibos Session | p Value |
|---|---|---|---|
| Age | 26.23 (6.53) | 26.23 (6.53) | 1.0 |
| Mass (kg) | 73.45 (14.31) | 73.45 (14.31) | 1.0 |
| Height (m) | 1.73 (0.08) | 1.73 (0.08) | 1.0 |
| Waist circumference (cm) | 102 (8.38) | 102 (8.38) | 1.0 |
| BMI (kg/m) | 24.45 (4.22) | 24.45 (4.22) | 1.0 |
| SBP (mm Hg) | 125.4 (11.57) | 124.5 (10.59) | 0.563 |
| DBP (mm Hg) | 73.33 (7.76) | 73.80 (8.23) | 0.573 |
| Hb (g/dL) | 15.28 (1.23) | 15.21 (1.08) | 0.676 |
| Glucose (mmol/L) | 4.91 (0.23) | 4.97 (0.33) | 0.359 |
| Total cholesterol (mmol/L) | 4.51 (0.10) | 4.56 (0.15) | 0.979 |
| Marker | Group | 0 h | 1.5 h | IAE | 1 h Rest | 24 h Rest | p Value | 0–1.5 h | 0 h-IAE | 0–1 h Rest | 0–24 h Rest | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Time | Time x Group | Group | %∆ | %∆ | %∆ | %∆ | ||
| tGSH [µmol/L] | Placebo | 1330 | 800 | 1370 | 878 | 1643 | 1033 | 1489 | 827 | 1327 | 800 | <0.001 | 0.055 | 0.617 | 3% | 24% | 12% | 0% |
| Rooibos | 1313 | 855 | 1603 | 1010 | 1708 | 1170 | 1446 | 893 | 1648 | 983 | 22% | 30% | 10% | 26% | ||||
| GSH [µmol/L] | Placebo | 1306 | 816 | 1344 | 895 | 1615 | 1049 | 1470 | 836 | 1314 | 806 | <0.001 | 0.063 | 0.604 | 3% | 24% | 13% | 1% |
| Rooibos | 1300 | 860 | 1580 | 1019 | 1686 | 1180 | 1431 | 899 | 1637 | 986 | 22% | 30% | 10% | 26% | ||||
| GSSG [µmol/L] | Placebo | 11.97 | 20.67 | 12.74 | 24.46 | 14.22 | 24.03 | 9.42 | 10.75 | 6.34 | 6.71 | 0.003 | 0.436 | 0.427 | 6% | 19% | −21% | −47% |
| Rooibos | 6.58 | 5.68 | 11.71 | 13.57 | 10.58 | 12.28 | 7.27 | 7.59 | 5.16 | 4.03 | 78% | 61% | 11% | −22% | ||||
| GSH/GSSG Ratio | Placebo | 309 | 264 | 343 | 352 | 383 | 491 | 393 | 407 | 424 | 380 | 0.003 | 0.429 | 0.816 | 11% | 24% | 27% | 37% |
| Rooibos | 380 | 476 | 327 | 349 | 396 | 416 | 377 | 359 | 484 | 477 | −14% | 4% | −1% | 27% | ||||
| Marker | Group | 0 h | 1.5 h | IAE | 1 h Rest | 24 h Rest | p Value | 0–1.5 h | 0 h-IAE | 0–1 h Rest | 0–24 h Rest | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Time | Time x Group | Group | %∆ | %∆ | %∆ | %∆ | ||
| CD [µmol/L] | Placebo | 15.41 | 3.44 | 15.01 | 3.41 | 15.91 | 3.18 | 15.71 | 3.77 | 15.44 | 3.67 | 0.051 | 0.374 | 0.962 | −3% | 3% | 2% | 0% |
| Rooibos | 15.33 | 3.81 | 15.25 | 3.41 | 15.63 | 3.65 | 15.30 | 3.38 | 15.77 | 3.39 | −1% | 2% | 0% | 3% | ||||
| TBARS [µmol/L] | Placebo | 10.56 | 1.28 | 10.28 | 1.32 | 10.17 | 1.27 | 10.37 | 1.29 | 10.70 | 1.11 | 0.004 | 0.876 | 0.689 | −3% | −4% | −2% | 1% |
| Rooibos | 10.89 | 1.33 | 10.25 | 1.31 | 10.20 | 1.09 | 10.36 | 1.30 | 10.81 | 1.14 | −6% | −6% | −5% | −1% | ||||
| PC [µmol/L] | Placebo | 2.30 | 0.60 | 2.26 | 0.77 | 2.33 | 0.70 | 2.40 | 0.67 | 2.48 | 1.00 | 0.728 | 0.144 | 0.760 | −2% | 1% | 4% | 8% |
| Rooibos | 2.36 | 0.64 | 2.61 | 0.59 | 2.43 | 0.72 | 2.24 | 0.58 | 2.35 | 0.83 | 11% | 3% | −5% | 0% | ||||
| UCB [µmol/L] | Placebo | 5.53 | 2.89 | 5.65 | 3.34 | 5.90 | 3.79 | 5.49 | 3.16 | 5.10 | 3.43 | 0.002 | 0.206 | 0.996 | 2% | 7% | −1% | −8% |
| Rooibos | 5.04 | 3.07 | 5.56 | 2.76 | 6.41 | 3.36 | 5.30 | 2.88 | 5.39 | 3.50 | 10% | 27% | 5% | 7% | ||||
| FRAP [µmol/L] | Placebo | 1020 | 161 | 1021 | 140 | 1055 | 172 | 1169 | 179 | 1006 | 132 | <0.001 | 0.463 | 0.881 | 0% | 3% | 15% | −1% |
| Rooibos | 1010 | 173 | 1021 | 227 | 1028 | 228 | 1207 | 201 | 1034 | 158 | 1% | 2% | 20% | 2% | ||||
| Marker | Group | 0 h | 1.5 h | IAE | 1 h Rest | 24 h Rest | p Value | 0–1.5 h | 0 h-IAE | 0–1 h Rest | 0–24 h Rest | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Time | Time x Group | Group | %∆ | %∆ | %∆ | %∆ | ||
| Lysis [% tail DNA] | Placebo | 23.92 | 8.28 | 26.60 | 7.75 | 27.15 | 7.41 | 26.27 | 7.38 | 26.39 | 7.23 | 0.011 | 0.228 | 0.314 | 11% | 13% | 10% | 10% |
| Rooibos | 26.63 | 8.42 | 27.79 | 7.46 | 28.34 | 7.53 | 29.71 | 7.27 | 26.45 | 7.17 | 4% | 6% | 12% | −1% | ||||
| H2O2 [% tail DNA] | Placebo | 26.79 | 8.29 | 30.46 | 8.62 | 31.80 | 7.88 | 32.00 | 7.06 | 31.65 | 8.10 | <0.001 | 0.519 | 0.176 | 14% | 19% | 19% | 18% |
| Rooibos | 31.00 | 11.53 | 32.26 | 8.39 | 33.52 | 8.63 | 35.55 | 9.76 | 33.78 | 9.04 | 4% | 8% | 15% | 9% | ||||
| FPG [% tail DNA] | Placebo | 5.42 | 4.51 | 7.30 | 3.79 | 6.93 | 3.52 | 8.29 | 4.67 | 9.07 | 4.90 | <0.001 | 0.956 | 0.667 | 35% | 28% | 53% | 67% |
| Rooibos | 5.50 | 4.37 | 8.09 | 4.83 | 6.90 | 5.24 | 8.80 | 4.61 | 9.46 | 4.45 | 47% | 25% | 60% | 72% | ||||
| Marker | Group | 0 h | 1.5 h | IAE | 1 h Rest | 24 H Rest | p Value | 0−1.5 h | 0 h-IAE | 0−1 h Rest | 0−24 h Rest | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Time | Time x Group | Group | %∆ | %∆ | %∆ | %∆ | ||
| AST [U/L] | Placebo | 31.16 | 15.44 | 31.48 | 14.13 | 33.79 | 16.23 | 30.92 | 14.07 | 30.11 | 13.08 | 0.016 | 0.275 | 0.167 | 1% | 8% | −1% | −3% |
| Rooibos | 27.22 | 8.58 | 26.15 | 7.29 | 28.94 | 7.54 | 26.55 | 6.65 | 28.02 | 11.80 | −4% | 6% | −2% | 3% | ||||
| ALT [U/L] | Placebo | 25.07 | 20.08 | 24.54 | 18.71 | 26.13 | 22.38 | 24.65 | 19.78 | 25.20 | 19.13 | 0.2 | 0.288 | 0.261 | −2% | 4% | −2% | 1% |
| Rooibos | 18.41 | 10.31 | 19.38 | 11.43 | 20.26 | 11.50 | 20.25 | 10.91 | 22.77 | 23.28 | 5% | 10% | 10% | 24% | ||||
| ALP [U/L] | Placebo | 77.28 | 23.78 | 77.21 | 21.38 | 82.54 | 25.21 | 75.90 | 21.71 | 75.83 | 22.60 | <0.001 | 0.200 | 0.903 | 0% | 7% | −2% | −2% |
| Rooibos | 73.74 | 21.36 | 75.42 | 21.85 | 83.28 | 24.68 | 77.00 | 23.30 | 75.84 | 20.90 | 2% | 13% | 4% | 3% | ||||
| GGT [U/L] | Placebo | 27.47 | 12.93 | 26.63 | 11.55 | 28.24 | 12.54 | 27.02 | 12.23 | 26.77 | 12.36 | <0.001 | 0.303 | 0.809 | −3% | 3% | −2% | −3% |
| Rooibos | 25.91 | 12.37 | 26.30 | 12.38 | 28.00 | 13.11 | 26.60 | 13.06 | 25.46 | 11.97 | 2% | 8% | 3% | −2% | ||||
| TBIL [µmol/L] | Placebo | 12.74 | 6.58 | 12.15 | 6.56 | 13.42 | 7.19 | 12.19 | 6.66 | 13.30 | 7.41 | 0.338 | 0.382 | 0.691 | −5% | 5% | −4% | 4% |
| Rooibos | 12.71 | 6.81 | 14.81 | 13.55 | 14.03 | 6.61 | 12.25 | 5.81 | 13.35 | 6.94 | 17% | 10% | −4% | 5% | ||||
| DBIL [µmol/L] | Placebo | 2.29 | 0.75 | 2.11 | 0.87 | 2.23 | 1.04 | 2.14 | 0.81 | 2.30 | 0.89 | 0.025 | 0.438 | 0.918 | −8% | −2% | −7% | 1% |
| Rooibos | 2.13 | 0.81 | 2.17 | 0.76 | 2.25 | 0.86 | 2.09 | 0.77 | 2.33 | 0.88 | 2% | 6% | −2% | 10% | ||||
| TP [g/L] | Placebo | 80.54 | 7.12 | 81.02 | 7.58 | 86.83 | 10.69 | 81.00 | 7.29 | 81.63 | 7.73 | <0.001 | 0.582 | 0.793 | 1% | 8% | 1% | 1% |
| Rooibos | 81.30 | 7.30 | 81.72 | 7.69 | 87.60 | 8.60 | 82.03 | 6.69 | 80.83 | 7.15 | 1% | 8% | 1% | −1% | ||||
| ALB [g/L] | Placebo | 51.13 | 3.26 | 51.73 | 3.33 | 54.27 | 5.48 | 51.65 | 2.64 | 50.45 | 7.29 | <0.001 | 0.599 | 0.822 | 1% | 6% | 1% | −1% |
| Rooibos | 50.52 | 7.74 | 51.35 | 3.24 | 54.23 | 5.64 | 52.47 | 4.39 | 51.68 | 2.96 | 2% | 7% | 4% | 2% | ||||
| BUN [mmol/L] | Placebo | 4.58 | 1.19 | 4.57 | 1.09 | 4.67 | 1.03 | 4.92 | 1.10 | 4.63 | 1.01 | <0.001 | 0.503 | 0.985 | 0% | 2% | 7% | 1% |
| Rooibos | 4.42 | 1.24 | 4.52 | 1.10 | 4.69 | 1.11 | 4.99 | 1.26 | 4.72 | 0.96 | 2% | 6% | 13% | 7% | ||||
| CREAT [µmol/L] | Placebo | 86.35 | 19.21 | 89.97 | 16.92 | 113.17 | 99.48 | 90.82 | 21.46 | 84.07 | 22.70 | 0.069 | 0.285 | 0.816 | 4% | 31% | 5% | −3% |
| Rooibos | 86.77 | 24.06 | 90.92 | 26.70 | 95.93 | 21.43 | 94.73 | 22.58 | 88.60 | 19.15 | 5% | 11% | 9% | 2% | ||||
| UA [µmol/L] | Placebo | 358.98 | 73.96 | 371.25 | 77.36 | 370.52 | 80.29 | 497.28 | 104.51 | 376.83 | 77.51 | <0.001 | 0.399 | 0.765 | 3% | 3% | 39% | 5% |
| Rooibos | 360.42 | 78.98 | 364.22 | 75.77 | 369.60 | 85.27 | 512.98 | 110.11 | 396.43 | 74.65 | 1% | 3% | 42% | 10% | ||||
| GLUC [nmol/L] | Placebo | 5.08 | 0.54 | 4.65 | 0.69 | 4.85 | 1.02 | 5.02 | 0.88 | 5.12 | 0.46 | <0.001 | 0.466 | 0.518 | −9% | −5% | −1% | 1% |
| Rooibos | 5.19 | 0.35 | 4.55 | 0.68 | 5.03 | 0.98 | 4.91 | 0.80 | 5.37 | 0.60 | −12% | −3% | −5% | 4% | ||||
| LDH [U/L] | Placebo | 184.87 | 47.65 | 188.84 | 40.23 | 203.00 | 53.38 | 190.00 | 44.15 | 193.22 | 48.20 | 0.063 | 0.464 | 0.569 | 2% | 10% | 3% | 5% |
| Rooibos | 188.07 | 47.93 | 180.37 | 44.06 | 191.71 | 32.61 | 186.71 | 40.28 | 184.83 | 35.19 | −4% | 2% | −1% | −2% | ||||
| CK [U/L] | Placebo | 510.21 | 567.18 | 528.27 | 577.61 | 552.40 | 643.99 | 510.96 | 579.06 | 450.04 | 453.91 | 0.283 | 0.172 | 0.149 | 4% | 8% | 0% | −12% |
| Rooibos | 390.67 | 401.41 | 312.28 | 241.13 | 356.57 | 259.29 | 327.76 | 237.30 | 355.02 | 268.70 | −20% | −9% | −16% | −9% | ||||
| LAC [U/L] | Placebo | 2.80 | 1.41 | 2.92 | 1.09 | 12.43 | 2.98 | 3.21 | 1.42 | 2.32 | 0.78 | <0.001 | 0.461 | 0.363 | 4% | 344% | 15% | −17% |
| Rooibos | 2.76 | 1.17 | 3.00 | 1.25 | 13.27 | 3.03 | 3.44 | 1.14 | 2.54 | 1.33 | 9% | 381% | 25% | −8% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamati, O.V.; Bragagna, L.; Bester, D.J.; Wagner, K.-H.; Stürmer, V.; Gassner, M.; Maqboul, L.; Louw, R.; West, S.; Davies, S.; et al. A Standardised Exercise Protocol to Induce Oxidative Stress in Humans: Validation with a Dietary Polyphenol Intervention. Nutrients 2025, 17, 2971. https://doi.org/10.3390/nu17182971
Kamati OV, Bragagna L, Bester DJ, Wagner K-H, Stürmer V, Gassner M, Maqboul L, Louw R, West S, Davies S, et al. A Standardised Exercise Protocol to Induce Oxidative Stress in Humans: Validation with a Dietary Polyphenol Intervention. Nutrients. 2025; 17(18):2971. https://doi.org/10.3390/nu17182971
Chicago/Turabian StyleKamati, Oiva V., Laura Bragagna, Dirk J. Bester, Karl-Heinz Wagner, Vera Stürmer, Markus Gassner, Lina Maqboul, Roan Louw, Sacha West, Simeon Davies, and et al. 2025. "A Standardised Exercise Protocol to Induce Oxidative Stress in Humans: Validation with a Dietary Polyphenol Intervention" Nutrients 17, no. 18: 2971. https://doi.org/10.3390/nu17182971
APA StyleKamati, O. V., Bragagna, L., Bester, D. J., Wagner, K.-H., Stürmer, V., Gassner, M., Maqboul, L., Louw, R., West, S., Davies, S., & Marnewick, J. L. (2025). A Standardised Exercise Protocol to Induce Oxidative Stress in Humans: Validation with a Dietary Polyphenol Intervention. Nutrients, 17(18), 2971. https://doi.org/10.3390/nu17182971








