The Ketogenic Diet Through a Metabolomic Lens: Biochemical Pathways, Therapeutic Applications, and Analytical Challenges
Abstract
1. Introduction
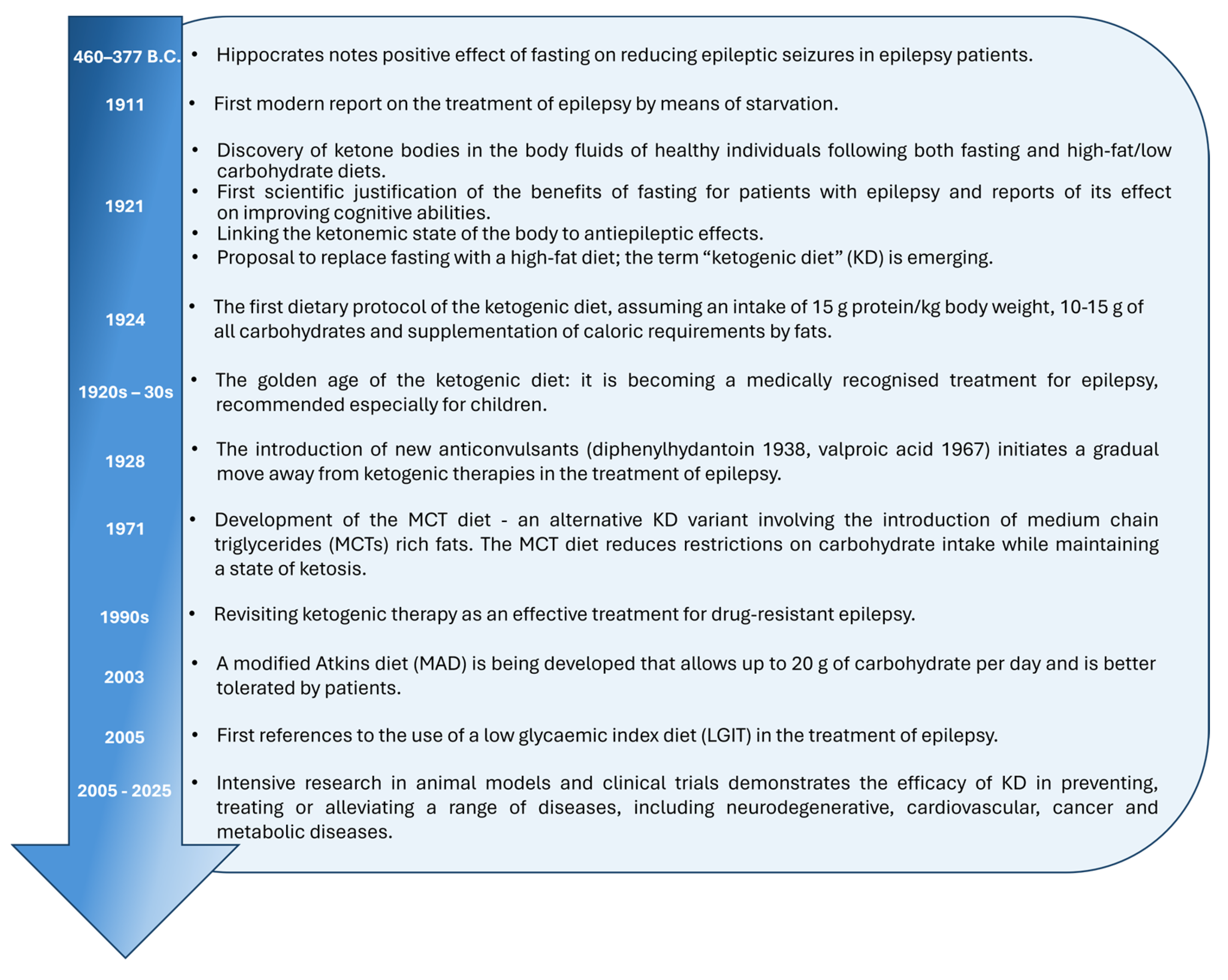
2. Milestones of the KD
2.1. Classic Ketogenic Diet
2.2. Modified Atkins Diet
2.3. Medium-Chain Fatty Acid Diet
2.4. Low Glycemic Index Diet and Low-Carbohydrate Diet
3. The Metabolic Perspective in the Context of a KD
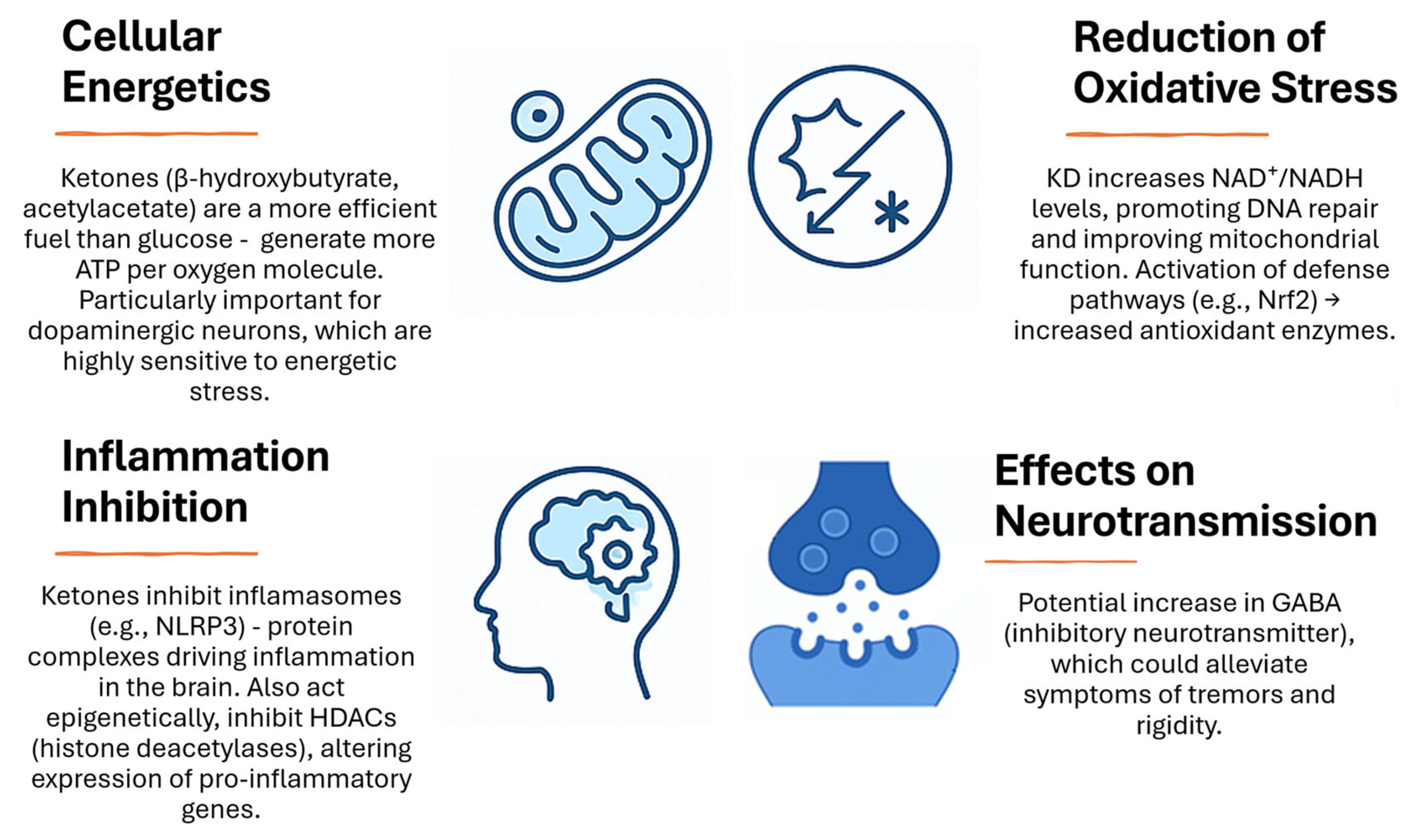
3.1. Energy Pathway: ATP and Krebs Cycle
3.2. One-Carbon Metabolism 1C
3.3. Selected Other Metabolic Pathways Involved in Antioxidant Activity
3.4. The Gut–Brain Axis and Neurotransmitters
3.5. Controversies Surrounding the KD in the Context of Selected Organs
4. Analytical Challenges and High-Throughput Metabolomic Approaches in KD Research
4.1. Analytical Challenges in KD Metabolomics
4.2. High-Throughput Metabolomic and Imaging Approaches
4.3. Clinical and Safety Considerations
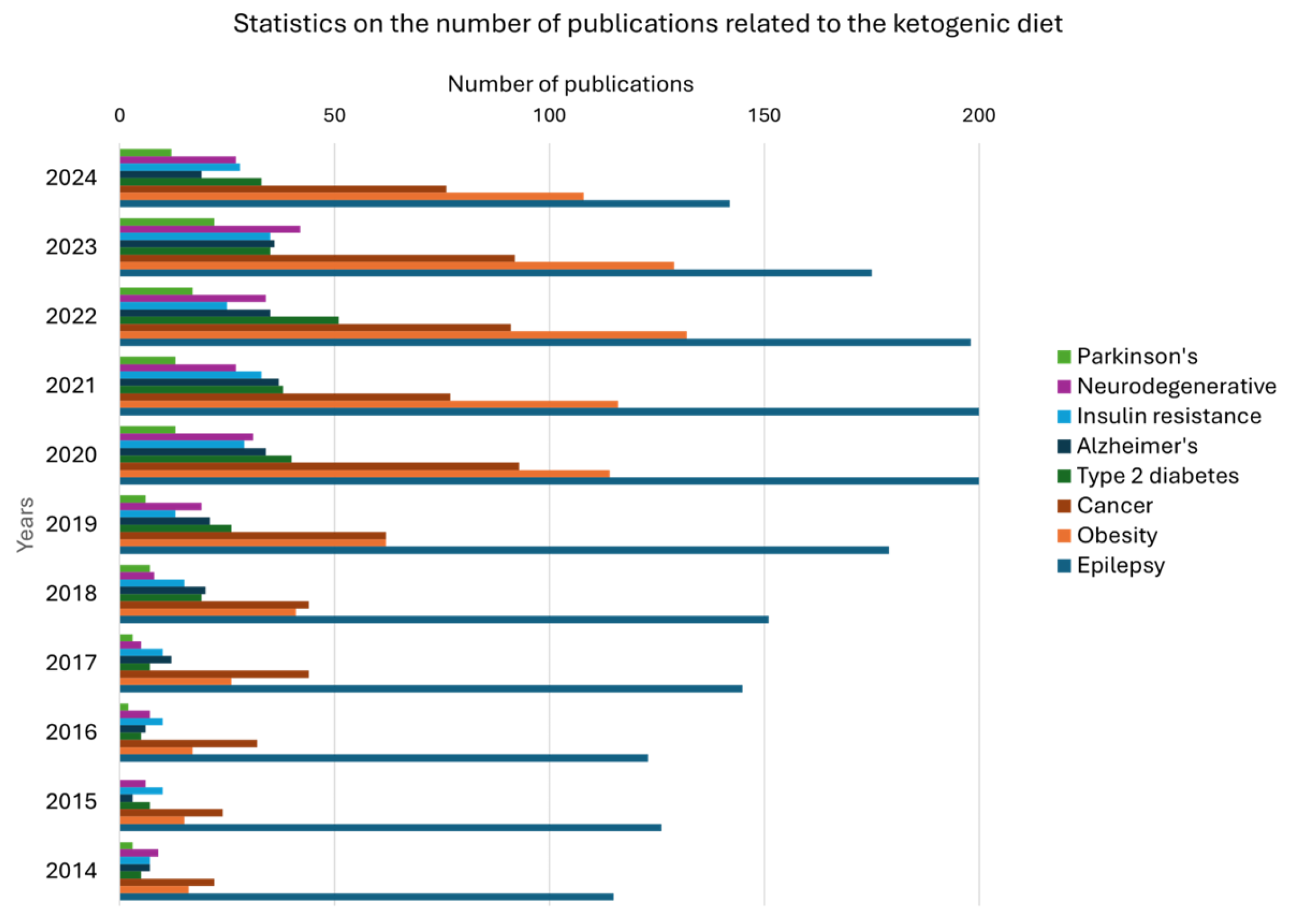
5. Potential of the KD in the Treatment of Various Diseases and Disorders
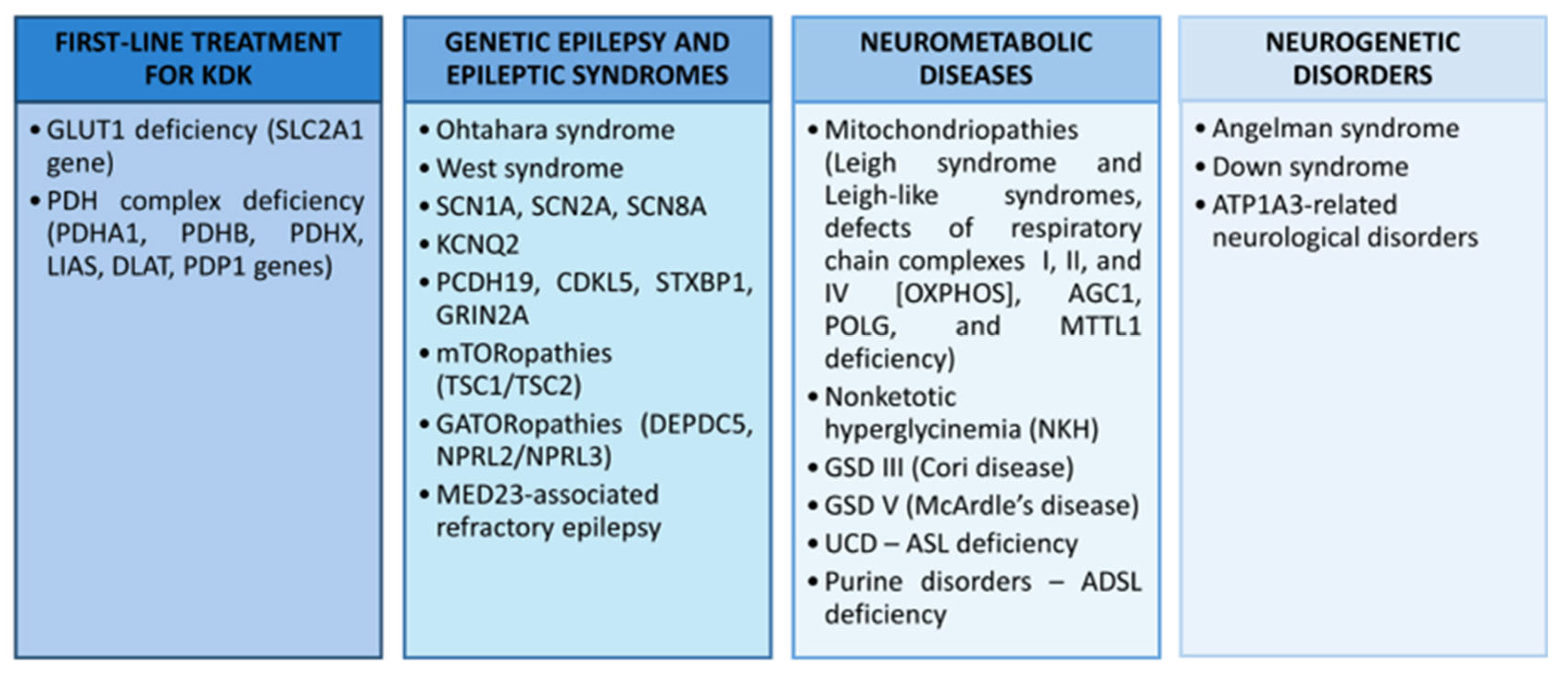
5.1. Drug-Resistant Epilepsy
5.2. Obesity, Type 2 Diabetes, and Insulin Resistance
5.3. Neurodegenerative Diseases (Parkinson’s and Alzheimer’s Disease)
5.4. Cancers
5.5. Ketogenic Therapy in Developmental Neurological Disorders
5.6. Critical Methodological Assessment of the Cited Studies
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1H-MRS5-MTHF | proton magnetic resonance spectroscopy5-methyltetrahydrofolate |
| 5-MTHF | 5-methyltetrahydrofolate |
| AcAc | acetoacetate |
| ACE-III | Addenbrooke’s Cognitive Examination–III scale |
| acetyl-CoA | acetyl coenzyme A |
| AD | Alzheimer’s disease |
| ADCS-ADL | AD Cooperative Study–Activities of Daily Living |
| ADSL | adenylosuccinate lyase |
| Akt | protein kinase B |
| ALS | amyotrophic lateral sclerosis |
| AMP | adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| anti-PD-1 | antibody against programmed cell death protein 1 |
| ASD | autism spectrum disorders |
| ASL | argininosuccinate lyase |
| ATP | adenosine triphosphate |
| Aβ | β-amyloid |
| BCAA | branched-chain amino acids |
| CDKL5 | cyclin-dependent kinase-like 5 deficiency disorder |
| CKD | classic ketogenic diet |
| CRP | c-reactive protein |
| CSF | cerebrospinal fluid |
| DEE | developmental and epileptic encephalopathies |
| DEE-STXBP1 | syntaxin-binding protein 1 |
| DI/LC-MS/MS | direct infusion/liquid chromatography–tandem mass spectrometry |
| DTC | differentiated thyroid carcinoma |
| dTMP | deoxythymidine monophosphate |
| FADH2 | flavin adenine dinucleotide reduced form |
| FGF21 | fibroblast growth factor 21 |
| FIRES | febrile infection-related epilepsy syndrome |
| GABA | gamma-aminobutyric acid |
| GCL | glutamate-cysteine synthase |
| GC-MS | gas chromatography–mass spectrometry |
| GCS | glycine breakdown system |
| GLUT1DS | glucose transporter type 1 deficiency syndrome |
| GRIN2A | glutamate receptor, ionotropic, N-methyl D-aspartate 2A |
| GSD | glycogen storage disease |
| GSH | glutathione |
| GTP | guanosine triphosphate |
| HbA1c | glycated hemoglobin |
| HDAC | histone deacetylases |
| HMG-CoA reductase | 3-hydroxy-3-methylglutaryl-coenzyme A reductase |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| KCNQ2 | potassium channelopathies |
| KD | ketogenic diet |
| LC | liquid chromatography |
| LC-MS | liquid chromatography–mass spectrometry |
| LDL-C | low-density lipoprotein cholesterol |
| LGIT | low glycemic index treatment |
| MAD | modified Atkins diet |
| MCT | medium-chain triglyceride diet |
| MCT1 | monocarboxylate transporter 1 |
| MCT-KD | ketogenic diet enriched in medium-chain fatty acids |
| MDA | malondialdehyde |
| MDS-UPDRS | Movement Disorder Society-Unified Parkinson’s Disease Rating Scale |
| MMKD | modified mediterranean ketogenic diet |
| MRS/MRI | magnetic resonance spectroscopy/imaging |
| MS | multiple sclerosis |
| MS/MS | tandem mass spectrometry |
| MTHFD2 | methylenetetrahydrofolate dehydrogenase 2 |
| mTOR | mechanistic target of rapamycin |
| NADH | nicotinamide adenine dinucleotide reduced form |
| NADPH | nicotinamide adenine dinucleotide phosphate reduced form |
| NAFLD | non-alcoholic fatty liver disease |
| NKH | non-ketotic hyperglycinemia |
| NMDA | N-methyl-D-aspartate |
| NMR | nuclear magnetic resonance spectroscopy |
| NORSE | new-onset refractory status epilepticus |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| OAA | oxaloacetate |
| PCDH19 | protocadherin 19 |
| PCOS | polycystic ovary syndrome |
| PD | Parkinson’s disease |
| PDAC | pancreatic ductal adenocarcinoma |
| PDCD | pyruvate dehydrogenase complex deficiency |
| PDH | pyruvate dehydrogenase |
| PI3K | reduced phosphoinositide 3-kinaze |
| QOL-AD | quality of life in AD |
| RAIR-DTC | radioiodine-refractory differentiated thyroid carcinoma |
| ROS | reactive oxygen species |
| SAM | S-adenosylmethionine |
| SCN1A | sodium voltage-gated channel alpha subunit 1 |
| SCOT | 3-oxoacyl-CoA transferase |
| SIRT1 | Sirtuin 1 |
| SMD | standardized mean difference |
| SOD | superoxide dismutase |
| TCA | Krebs cycle |
| TOF | time-of-flight analyzer |
| TSC1/TSC2 | tuberous sclerosis complex |
| UBE3A | ubiquitin protein ligase E3A |
| UCD | urea cycle disorder |
| UHPLC-MS | ultra-high performance liquid chromatography coupled with mass spectrometry |
| VLCKD | very low-carbohydrate ketogenic diets |
| βHB | β-hydroxybutyrate |
References
- Artati, A.; Prehn, C.; Adamski, J. LC-MS/MS-Based Metabolomics for Cell Cultures. In Cell-Based Assays Using iPSCs for Drug Development and Testing; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; Volume 1994, pp. 119–130. [Google Scholar]
- Ford, L.; Mitchell, M.; Wulff, J.; Evans, A.; Kennedy, A.; Elsea, S.; Wittmann, B.; Toal, D. Clinical metabolomics for inborn errors of metabolism. Adv. Clin. Chem. 2022, 107, 79–138. [Google Scholar]
- Li, X.; Zhao, G.; Zheng, Y.; Wang, Y.; Bai, X.; Li, F.; Gu, Y.; Zhu, C. Effects of single fermentation of Lactobacillus sakei and compound fermentation with Staphylococcus carnosus on the metabolomics of beef sausages. Food Chem. 2025, 464, 141728. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, Q.; Cheng, L.; Hu, G.; Liu, Z.; Lan, Y.; Cheng, Y. Metabolome and Transcriptome Combined Reveal the Main Floral Volatile Compounds and Key Regulatory Genes of Castanea mollissima. Plants 2024, 13, 2865. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, F.; Ma, Y.; Wang, W.; Guo, Y. Investigation of the regulatory mechanisms of Guiqi Yimu Powder on dairy cow fatty liver cells using a multi-omics approach. Front. Vet. Sci. 2024, 11, 1475564. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, F.; Wang, J.; Qin, Y.; Pan, Y. Unveiling the Metabolomic Profile of Oily Sensitive Skin: A Non-Invasive Approach. Int. J. Mol. Sci. 2024, 25, 11033. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies-Challenges and Emerging Directions. J. Am. Soc. Mass. Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Kim, S.J.; Song, H.E.; Lee, H.Y.; Yoo, H.J. Mass Spectrometry-based Metabolomics in Translational Research. Adv. Exp. Med. Biol. 2021, 1310, 509–531. [Google Scholar]
- Wu, Y.; Jin, X.; Wang, L.; Lei, J.; Chai, S.; Wang, C.; Zhang, W.; Yang, X. Integrated Transcriptional and Metabolomic Analysis of Factors Influencing Root Tuber Enlargement during Early Sweet Potato Development. Genes 2024, 15, 1319. [Google Scholar] [CrossRef]
- Feng, X.; Bai, S.; Zhou, L.; Song, Y.; Jia, S.; Guo, Q.; Zhang, C. Integrated Analysis of Transcriptome and Metabolome Pro-vides Insights into Flavonoid Biosynthesis of Blueberry Leaves in Response to Drought Stress. Int. J. Mol. Sci. 2024, 25, 11135. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Wang, Y.; Wang, J.; Hu, J.; Ji, Z.; Chao, T. Research Progress on Genomic Regions and Candidate Genes Related to Milk Composition Traits of Dairy Goats Based on Functional Genomics: A Narrative Review. Genes 2024, 15, 1341. [Google Scholar] [CrossRef]
- Hippocrates. Hippocratic Writings; Loeb Classical Library; Harvard University Press: Cambridge, MA, USA, 2010; Volume 10. [Google Scholar]
- Sadiq, J.; Maryam, M. The Role of Ketogenic Diets for Therapeutic Uses: A subject review. Lat. Am. J. Pharm. 2024, 43, 220–228. [Google Scholar]
- Wheless, J.W. History of the ketogenic diet. Epilepsia 2008, 49 (Suppl. S8), 3–5. [Google Scholar] [CrossRef] [PubMed]
- Guelpa, G.; Marie, A. La lutte contre l’e’pilepsie par la de’ sintoxication et par la re’e’ducation alimentaire. Rev. Ther. Medico-Chir. 1911, 78, 8–13. [Google Scholar]
- Geyelin, H.R. Fasting as a method for treating epilepsy. Med. Rec. 1921, 99, 1037–1039. [Google Scholar]
- Newburgh, L.H.; Marsh, P.L. The use of a high fat diet in the treatment of diabetes mellitus first paper. Arch. Intern. Med. 1920, 26, 647–662. [Google Scholar] [CrossRef][Green Version]
- Woodyatt, R.T. Objects and methods of diet adjustment in diabetes. Arch. Intern. Med. 1921, 28, 125–141. [Google Scholar] [CrossRef]
- Wilder, R.M. The effect of ketonemia on the course of epilepsy. Mayo Clin. Bull. 1921, 2, 307. [Google Scholar]
- Wilder, R.M. High fat diets in epilepsy. Mayo Clin. Bull. 1921, 2, 308. [Google Scholar]
- Peterman, M.G. The ketogenic diet in epilepsy. JAMA 1925, 84, 1979–1983. [Google Scholar] [CrossRef]
- Höhn, S.; Dozières-Puyravel, B.; Auvin, S. History of dietary treatment from Wilder’s hypothesis to the first open studies in the 1920s. Epilepsy Behav. 2019, 101 Pt A, 106588. [Google Scholar] [CrossRef]
- Huttenlocher, P.R.; Wilbourn, A.J.; Signore, J.M. Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology 1971, 21, 1097–1103. [Google Scholar] [CrossRef]
- Kossoff, E.H.; McGrogan, J.R.; Bluml, R.M.; Pillas, D.J.; Rubenstein, J.E.; Vining, E.P. A modified Atkins diet is effective for the treatment of intractable pediatric epilepsy. Epilepsia 2006, 47, 421–424. [Google Scholar] [CrossRef]
- Chen, W.; Kossoff, E.H. Long-term follow-up of children treated with the modified Atkins diet. J. Child Neurol. 2012, 27, 754–758. [Google Scholar] [CrossRef]
- Kossoff, E.H. The Modified Atkins Diet for Epilepsy: Two Decades of an “Alternative” Ketogenic Diet Therapy. Pediatr. Neurol. 2023, 147, 82–87. [Google Scholar] [CrossRef]
- Pfeifer, H.H.; Thiele, E.A. Low-glycemic-index treatment: A liberalized ketogenic diet for treatment of intractable epilepsy. Neurology 2005, 65, 1810–1812. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Qiu, J.; Li, H.; Guo, H.; Wang, S.; Ding, Y.; Xu, S.; Wang, Z.; Feng, J.; Zhang, P.; et al. Efficacy of the ketogenic diet in Chinese adults versus children with drug-resistant epilepsy: A pilot study. Epilepsy Behav. 2022, 134, 108820. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.S.; Turos, E. The Chemistry of the Ketogenic Diet: Updates and Opportunities in Organic Synthesis. Int. J. Mol. Sci. 2021, 22, 5230. [Google Scholar] [CrossRef] [PubMed]
- Çağıran, İ.H.; Yılmaz, D.A. Ketogenic diet in clinical practices. Hum. Nutr. Metab. 2024, 36, 200250. [Google Scholar] [CrossRef]
- Na, J.H.; Lee, H.; Lee, Y.M. Clinical Efficacy and Safety of the Ketogenic Diet in Patients with Genetic Confirmation of Drug-Resistant Epilepsy. Nutrients 2025, 17, 979. [Google Scholar] [CrossRef]
- Steinborn, B.; Mazurkiewicz-Bełdzińska, M.; Winczewska-Wiktor, A. Dietary treatment. In Developmental Neurology, 1st ed.; Steinborn, B., Ed.; PZWL Publishing House: Warszawa, Poland, 2020; pp. 524–527. [Google Scholar]
- Calderón, N.; Betancourt, L.; Hernández, L.; Rada, P. A ketogenic diet modifies glutamate, gamma-aminobutyric acid and agmatine levels in the hippocampus of rats: A microdialysis study. Neurosci. Lett. 2017, 642, 158–162. [Google Scholar] [CrossRef]
- Masino, S.A.; Ruskin, D.N.; Freedgood, N.R.; Lindefeldt, M.; Dahlin, M. Differential ketogenic diet-induced shift in CSF lipid/carbohydrate metabolome of pediatric epilepsy patients with optimal vs. no anticonvulsant response: A pilot study. Nutr. Metab. 2021, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, Q.; Xuan, Z.; Mao, Y.; Tang, X.; Yang, K.; Song, F.; Zhu, X. Metabolomics reveals the implication of acetoacetate and ketogenic diet therapy in radioiodine-refractory differentiated thyroid carcinoma. Oncologist 2024, 29, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Yin, X.; Wang, M.; Chen, T.; Wang, Y.; Gao, Z.; Wang, Z. Effects of Ketogenic Diet on Neuroinflammation in Neurodegenerative Diseases. Int. Soc. Aging Dis. 2022, 13, 1146–1165. [Google Scholar] [CrossRef] [PubMed]
- Inigo, M.; Deja, S.; Burgess, S.C. Ins and Outs of the TCA Cycle: The Central Role of Anaplerosis. Annu. Rev. Nutr. 2021, 41, 19–47. [Google Scholar] [CrossRef]
- Torres, J.A.; Holznecht, N.; Asplund, D.A.; Kroes, B.C.; Amarlkhagva, T.; Haeffner, M.M.; Sharpe, E.H.; Koestner, S.; Strubl, S.; Schimmel, M.F.; et al. β-hydroxybutyrate recapitulates the beneficial effects of ketogenic metabolic therapy in polycystic kidney disease. iScience 2024, 27, 110773. [Google Scholar] [CrossRef]
- Bellomo, F.; Pugliese, S.; Cairoli, S.; Krohn, P.; De Stefanis, C.; Raso, R.; Rega, L.R.; Taranta, A.; De Leo, E.; Ciolfi, A.; et al. Ketogenic Diet and Progression of Kidney Disease in Animal Models of Nephropathic Cystinosis. J. Am. Soc. Nephrol. 2024, 35, 1493–1506. [Google Scholar] [CrossRef]
- Wells, J.; Swaminathan, A.; Paseka, J.; Hanson, C. Efficacy and safety of a ketogenic diet in children and adolesents with refractory epilepsy—A review. Nutrients 2020, 12, 1809. [Google Scholar] [CrossRef]
- Armeno, M.; Calligaris, S.; Gagiulo, D.; Cresta, A.; Vaccarezza, M.M.; Diez, C.G.; Alberti, M.J.; Viollaz, R.; Vilavedra, F.; Caraballo, R.H. Use of ketogenic dietary therapy for drug-resistant epilepsy in early infancy. Epilepsia Open 2024, 9, 138–149. [Google Scholar] [CrossRef]
- Campbell, I.H.; Needham, N.; Grossi, H.; Kamenska, I.; Luz, S.; Sheehan, S.; Thompson, G.; Thrippleton, M.J.; Gibbs, M.C.; Leitao, J.; et al. A pilot study of a ketogenic diet in bipolar disorder: Clinical, metabolic and magnetic resonance spectroscopy findings. BJPsych Open 2025, 11, e34. [Google Scholar] [CrossRef]
- Parker, B.A.; Walton, C.M.; Carr, S.T.; Andrus, J.L.; Cheung, E.C.K.; Duplisea, M.J.; Wilson, E.K.; Draney, C.; Lathen, D.R.; Kenner, K.B.; et al. β-Hydroxybutyrate Elicits Favorable Mitochondrial Changes in Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 2247. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, S.Y.; Obaid, S.; Shahid, T.; Shah, F.U.; Samad, A. Changes in body composition and metabolic rate during a very low-calorie ketogenic diet and their association with weight loss. Int. J. Health Sci. 2023, 7, 690–697. [Google Scholar] [CrossRef]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B vitamins and one-carbon metabolism: Implications in human health and disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Y.; Seo, D.S.; Jang, Y. Metabolic Effects of Ketogenic Diets: Exploring Whole-Body Metabolism in Connection with Adipose Tissue and Other Metabolic Organs. Int. J. Mol. Sci. 2024, 25, 7076. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Saikia, G.; Zhang, X.; Shen, X.; Kahe, K. One-Carbon Metabolism Nutrients, Genetic Variation, and Diabetes Mellitus. Diabetes Metab. J. 2024, 48, 170–183. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, X.; Lu, L.; Hu, R.; Teng, Y.; Pan, L.; Zeng, X.; Jiang, W.; Li, W.; Dong, L.; et al. Assessment of blood one-carbon metabolism indexes during mid-to-late pregnancy in 397 Chinese pregnant women. Front. Nutr. 2024, 11, 1348930. [Google Scholar] [CrossRef]
- Hsu, F.Y.; Liou, J.Y.; Tang, F.Y.; Sou, N.L.; Peng, J.H.; Chiang, E.P.I. Ketogenic Diet Consumption Inhibited Mitochondrial One-Carbon Metabolism. Int. J. Mol. Sci. 2022, 23, 3650. [Google Scholar] [CrossRef]
- Boison, D. New insights into the mechanisms of the ketogenic diet. Curr. Opin. Neurol. 2017, 30, 187–192. [Google Scholar] [CrossRef]
- Joshi, S.M.; Jadavji, N.M. Deficiencies in one-carbon metabolism led to increased neurological disease risk and worse outcome: Homocysteine is a marker of disease state. Front. Nutr. 2024, 11, 1285502. [Google Scholar] [CrossRef]
- Williamson, J.M.; Arthurs, A.L.; Smith, M.D.; Roberts, C.T.; Jankovic-Karasoulos, T. High Folate, Perturbed One-Carbon Metabolism and Gestational Diabetes Mellitus. Nutrients 2022, 14, 3930. [Google Scholar] [CrossRef]
- Sampson, M.; Lathen, D.; Dallon, B.; Draney, C.; Ray, J.; Kener, K.; Parker, B.; Gibbs, J.; Gropp, J.; Tessem, J.; et al. β-Hydroxybutyrate improves β-cell mitochondrial function and survival. J. Insul. Resist. 2017, 2, a25. [Google Scholar] [CrossRef]
- Miller, V.J.; Villamena, F.A.; Volek, J.S. Nutritional Ketosis and Mitohormesis: Potential Implications for Mitochondrial Function and Human Health. J. Nutr. Metab. 2018, 2018, 5157645. [Google Scholar] [CrossRef]
- Xu, S.; Tao, H.; Cao, W.; Cao, L.; Lin, Y.; Zhao, S.; Xu, W.; Cao, J.; Zhao, J. Ketogenic diets inhibit mitochondrial biogenesis and induce cardiac fibrosis. Signal Transduct. Target. Ther. 2021, 6, 54. [Google Scholar] [CrossRef]
- Miller, V.J.; LaFountain, R.A.; Barnhart, E.; Sapper, T.S.; Short, J.; Arnold, W.D.; Hyde, P.N.; Crabtree, C.D.; Kackley, M.L.; Kraemer, W.J.; et al. A ketogenic diet combined with exercise alters mitochondrial function in human skeletal muscle while improving metabolic health. Am. J. Physiol. Endocrinol. Metab. 2020, 319, 995–1007. [Google Scholar] [CrossRef]
- Paoli, A.; Cerullo, G. Investigating the Link between Ketogenic Diet, NAFLD, Mitochondria, and Oxidative Stress: A Narrative Review. Antioxidants 2023, 12, 1065. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Tognini, P. Molecular Mechanisms Underlying the Bioactive Properties of a Ketogenic Diet. Nutrients 2022, 14, 782. [Google Scholar] [CrossRef] [PubMed]
- Drabińska, N.; Juśkiewicz, J.; Wiczkowski, W. The Effect of the Restrictive Ketogenic Diet on the Body Composition, Hematological and Biochemical Parameters, Oxidative Stress and Advanced Glycation End-Products in Young Wistar Rats with Diet-Induced Obesity. Nutrients 2022, 14, 4805. [Google Scholar] [CrossRef] [PubMed]
- Drabińska, N. Current Perspective About the Effect of a Ketogenic Diet on Oxidative Stress—A Review. Pol. J. Food Nutr. Sci. 2024, 74, 92–105. [Google Scholar] [CrossRef]
- Dyńka, D.; Kowalcze, K.; Charuta, A.; Paziewska, A. The Ketogenic Diet and Cardiovascular Diseases. Nutrients 2023, 15, 3368. [Google Scholar] [CrossRef]
- Hazany, S.; DeClouette, B.; Lowe, J.; Hwang, D.H.; Kim, P.E.; Bluml, S.; Partikian, A. Brain Glutathione Increase and Seizure Burden Decrease in Patients with Intractable Epilepsy on Ketogenic Diet. J. Epilepsy Res. 2023, 13, 1–6. [Google Scholar] [CrossRef]
- Napolitano, A.; Longo, D.; Lucignani, M.; Pasquini, L.; Rossi-Espagnet, M.C.; Lucignani, G.; Maiorana, A.; Elia, D.; De Liso, P.; Dionisi-Vici, C.; et al. The Ketogenic Diet Increases In Vivo Glutathione Levels in Patients with Epilepsy. Metabolites 2020, 10, 504. [Google Scholar] [CrossRef]
- Gzieło, K.; Janeczko, K.; Węglarz, W.; Jasiński, K.; Kłodowski, K.; Setkowicz, Z. MRI spectroscopic and tractography studies indicate consequences of long-term ketogenic diet. Brain Struct. Funct. 2020, 225, 2077–2089. [Google Scholar] [CrossRef]
- Qiao, Y.N.; Li, L.; Hu, S.H.; Yang, Y.X.; Ma, Z.Z.; Huang, L.; An, Y.P.; Yuan, Y.Y.; Lin, Y.; Xu, W.; et al. Ketogenic diet-produced β-hydroxybutyric acid accumulates brain GABA and increases GABA/glutamate ratio to inhibit epilepsy. Cell Discov. 2024, 10, 17. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kasperek, K.; Rękawek, P.; Piątkowska-Chmiel, I. The Therapeutic Role of Ketogenic Diet in Neuro-logical Disorders. Nutrients 2022, 14, 1952. [Google Scholar] [CrossRef]
- Dowis, K.; Banga, S. The potential health benefits of the ketogenic diet: A narrative review. Nutrients 2021, 13, 1654. [Google Scholar] [CrossRef]
- de la Rubia Ortí, J.E.; Fernández, D.; Platero, F.; García-Pardo, M.P. Can Ketogenic Diet Improve Alzheimer’s Disease? Association With Anxiety, Depression, and Glutamate System. Front. Nutr. 2021, 8, 744398. [Google Scholar] [CrossRef] [PubMed]
- Morscher, R.J.; Aminzadeh-Gohari, S.; Feichtinger, R.G.; Mayr, J.A.; Lang, R.; Neureiter, D.; Sperl, W.; Kofler, B. Inhibition of Neuroblastoma Tumor Growth by Ketogenic Diet and/or Calorie Restriction in a CD1-Nu Mouse Model. PLoS ONE 2015, 10, e0129802. [Google Scholar] [CrossRef] [PubMed]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Safety and metabolic implications of prolonged ketogenic diet use: A clinical perspective. Nutrients 2024, 16, 1152. [Google Scholar]
- Luong, T.V.; Abild, C.B.; Bangshaab, M.; Gormsen, L.C.; Søndergaard, E. Ketogenic Diet and Cardiac Substrate Metabolism. Nutrients 2022, 14, 1322. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J. Ketogenic diet and cardiovascular risk—State of the art review. Curr. Probl. Cardiol. 2024, 49, 102402. [Google Scholar] [CrossRef]
- Athinarayanan, S.J.; Roberts, C.G.P.; Vangala, C.; Shetty, G.K.; McKenzie, A.L.; Weimbs, T.; Volek, J.S. The case for a ketogenic diet in the management of kidney disease. BMJ Open Diabetes Res. Care 2024, 12, 004101. [Google Scholar] [CrossRef]
- Zemer, A.; Samaei, S.; Yoel, U.; Biderman, A.; Pincu, Y. Ketogenic diet in clinical populations-a narrative review. Front. Med. 2024, 11, 1432717. [Google Scholar] [CrossRef]
- García-Gorrita, C.; Soriano, J.; Merino-Torres, J.; Onofre, N. Anthropometric Trajectories and Dietary Compliance During a Personalized Ketogenic Program. Nutrients 2025, 17, 1475. [Google Scholar] [CrossRef] [PubMed]
- Boguszewicz, Ł.; Jamroz, E.; Ciszek, M.; Emich-Widera, E.; Kijonka, M.; Banasik, T.; Skorupa, A.; Sokół, M. NMR-based metabolomics in pediatric drug resistant epilepsy—Preliminary results. Sci. Rep. 2019, 9, 15035. [Google Scholar] [CrossRef] [PubMed]
- Effinger, D.; Hirschberger, S.; Yoncheva, P.; Schmid, A.; Heine, T.; Newels, P.; Schütz, B.; Meng, C.; Gigl, M.; Kleigrewe, K.; et al. A ketogenic diet substantially reshapes the human metabolome. Clin. Nutr. 2023, 42, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.M.; Celano, G.; Riezzo, G.; D’Attoma, B.; Ignazzi, A.; Di Chito, M.; Sila, A.; De Nucci, S.; Rinaldi, R.; Linsalata, M.; et al. Metabolomic Profiling of Obese Patients with Altered Intestinal Permeability Undergoing a Very Low-Calorie Ketogenic Diet. Nutrients 2023, 15, 5026. [Google Scholar] [CrossRef]
- Licha, D.; Vidali, S.; Aminzadeh-Gohari, S.; Alka, O.; Breitkreuz, L.; Kohlbacher, O.; Reischl, R.J.; Feichtinger, R.G.; Kofler, B.; Huber, C.G. Untargeted metabolomics reveals molecular effects of ketogenic diet on healthy and tumor xenograft mouse models. Int. J. Mol. Sci. 2019, 20, 3873. [Google Scholar] [CrossRef]
- AGRIS—International System for Agricultural Science and Technology. Available online: https://agris.fao.org/search/en/providers/125307/records/6748dc657625988a37211e6b (accessed on 2 May 2025).
- Qiu, H.; Kan, C.; Han, F.; Luo, Y.; Qu, N.; Zhang, K.; Ma, Y.; Hou, N.; Wu, D.; Sun, X.; et al. Metagenomic and metabolomic analysis showing the adverse risk–benefit trade-off of the ketogenic diet. Lipids Health Dis. 2024, 23, 207. [Google Scholar] [CrossRef]
- Kang, C.M.; Yun, B.; Kim, M.; Song, M.; Kim, Y.H.; Lee, S.H.; Lee, H.; Lee, S.M.; Lee, S.M. Postoperative serum metabolites of patients on a low carbohydrate ketogenic diet after pancreatectomy for pancreatobiliary cancer: A nontargeted metabolomics pilot study. Sci. Rep. 2019, 9, 16820. [Google Scholar] [CrossRef]
- Mukherjee, P.; Augur, Z.M.; Li, M.; Hill, C.; Greenwood, B.; Domin, M.A.; Kondakci, G.; Narain, N.R.; Kiebish, M.A.; Bronson, R.T.; et al. Therapeutic benefit of combining calorie-restricted ketogenic diet and glutamine targeting in late-stage experimental glioblastoma. Commun. Biol. 2019, 2, 200. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Akiyama, T.; Saigusa, D.; Hishinuma, E.; Matsukawa, N.; Shibata, T.; Tsuchiya, H.; Mori, A.; Fujii, Y.; Mogami, Y.; et al. Comprehensive study of metabolic changes induced by a ketogenic diet therapy using GC/MS- and LC/MS-based metabolomics. Seizure 2023, 107, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Saigusa, D.; Inoue, T.; Tokorodani, C.; Akiyama, M.; Michiue, R.; Mori, A.; Hishinuma, E.; Matsukawa, N.; Shibata, T.; et al. Exploration of urine metabolic biomarkers for new-onset, untreated pediatric epilepsy: A gas and liquid chromatography mass spectrometry-based metabolomics study. Brain Dev. 2024, 46, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Heischmann, S.; Gano, L.B.; Quinn, K.; Liang, L.P.; Klepacki, J.; Christians, U.; Reisdorph, N.; Patel, M. Regulation of kynurenine metabolism by a ketogenic diet. J. Lipid Res. 2018, 59, 958–966. [Google Scholar] [CrossRef]
- Lalwani, A.M.; Yilmaz, A.; Bisgin, H.; Ugur, Z.; Akyol, S.; Graham, S.F. The biochemical profile of post-mortem brain from people who suffered from epilepsy reveals novel insights into the etiopathogenesis of the disease. Metabolites 2020, 10, 261. [Google Scholar] [CrossRef]
- Schweickart, A.; Batra, R.; Neth, B.J.; Martino, C.; Shenhav, L.; Zhang, A.R.; Shi, P.; Karu, N.; Huynh, K.; Meikle, P.J.; et al. Serum and CSF metabolomics analysis shows Mediterranean Ketogenic Diet mitigates risk factors of Alzheimer’s disease. NPJ Metab. Health Dis. 2024, 2, 15. [Google Scholar] [CrossRef]
- Deja, S.; Kucejova, B.; Fu, X.; Browning, J.D.; Young, J.D.; Burgess, S. In vivo estimation of ketogenesis using metabolic flux analysis—Technical aspects and model interpretation. Metabolites 2021, 11, 293. [Google Scholar] [CrossRef]
- Mayengbam, S.; Ellegood, J.; Kesler, M.; Reimer, R.; Shearer, J.; Murari, K.; Rho, J.; Lerch, J.; Cheng, N. A ketogenic diet affects brain volume and metabolome in juvenile mice. NeuroImage 2021, 244, 118542. [Google Scholar] [CrossRef]
- Castro, R.; Kalecký, K.; Huang, N.; Petersen, K.; Singh, V.; Ross, A.; Neuberger, T.; Bottiglieri, T. A very-low carbohydrate content in a high-fat diet modifies the plasma metabolome and impacts systemic inflammation and experimental atherosclerosis. J. Nutr. Biochem. 2024, 126, 109562. [Google Scholar] [CrossRef]
- Dahlin, M.; Wheelock, C.E.; Prast-Nielsen, S. Association between seizure reduction during ketogenic diet treatment of epilepsy and changes in circulatory metabolites and gut microbiota composition. EBioMedicine 2024, 109, 105400. [Google Scholar] [CrossRef]
- Masino, S.A. Ketogenic Diet and Metabolic Therapies: Expanded Roles in Health and Disease, 2nd ed.; Oxford University Press: Oxford, UK, 2022; ISBN 978-0197501207. [Google Scholar]
- Jalal, R.; Qurat, U.A.; Mohd, S.I.; Mubashir, Y.S. Effects of ketogenic diet on body weight and histology of liver of male albino rats. J. Cardiovasc. Dis. Res. 2024, 15, 946–959. [Google Scholar]
- Joshi, S.; Shi, R.; Patel, J. Risks of the ketogenic diet in CKD—The con part. Clin. Kidney J. 2024, 17, 274. [Google Scholar] [CrossRef] [PubMed]
- Corsello, A.; Trovato, C.M.; Di Profio, E.; Cardile, S.; Campoy, C.; Zuccotti, G.; Verduci, E.; Diamanti, A. Ketogenic diet in children and adolescents: The effects on growth and nutritional status. Pharmacol. Res. 2023, 191, 106780. [Google Scholar] [CrossRef] [PubMed]
- Kosiek, W.; Rauk, Z.; Szulc, P.; Cichy, A.; Rugieł, M.; Chwiej, J.; Janeczko, K.; Setkowicz, Z. Ketogenic diet impairs neurological development of neonatal rats and affects biochemical composition of maternal brains: Evidence of functional recovery in pups. Brain Struct. Funct. 2022, 227, 1099–1113. [Google Scholar] [CrossRef]
- Miętkiewska, K.; Bogdański, P. Ketogenic diet in older adults: Potential benefits and risks. Geriatrics 2022, 7, 41. [Google Scholar]
- Almodallal, Y.; Cook, K.; Lammert, L.M.; Lee, M.; Le-Rademacher, J.G.; Jatoi, A. Can older patients adopt and maintain a ketogenic diet? An observational study in support of clinical trials in older patients. Medicine 2021, 100, 28033. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef]
- Shabbir, I.; Liu, K.; Riaz, B.; Rahim, M.F.; Zhong, S.; Aweya, J.J.; Cheong, K.L. Investigating the Therapeutic Potential of the Ketogenic Diet in Modulating Neurodegenerative Pathophysiology: An Interdisciplinary Approach. Nutrients 2025, 17, 1268. [Google Scholar] [CrossRef]
- Tao, Y.; Leng, S.X.; Zhang, H. Ketogenic Diet: An Effective Treatment Approach for Neurodegenerative Diseases. Curr. Neuropharmacol. 2022, 20, 2303–2319. [Google Scholar] [CrossRef]
- Shahpasand, S.; Khatami, S.H.; Ehtiati, S.; Alehossein, P.; Salmani, F.; Toutounchi, A.H.; Zarei, T.; Shahmohammadi, M.R.; Khodarahmi, R.; Aghamollaii, V.; et al. Therapeutic potential of the ketogenic diet: A metabolic switch with implications for neurological disorders, the gut-brain axis, and cardiovascular diseases. J. Nutr. Biochem. 2024, 132, 109693. [Google Scholar] [CrossRef]
- Rog, J.; Wingralek, Z.; Nowak, K.; Grudzień, M.; Grunwald, A.; Banaszek, A.; Karakula-Juchnowicz, H. The Potential Role of the Ketogenic Diet in Serious Mental Illness: Current Evidence, Safety, and Practical Advice. J. Clin. Med. 2024, 13, 2819. [Google Scholar] [CrossRef]
- Ułamek-Kozioł, M.; Czuczwar, S.J.; Pluta, R.; Januszewski, S. Ketogenic diet and epilepsy. Nutrients 2019, 11, 2510. [Google Scholar] [CrossRef]
- Rho, J.M.; Boison, D. The metabolic basis of epilepsy. Nat. Rev. Neurol. 2022, 18, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Skow, S.L.; Jha, R.K. A Ketogenic Diet is Effective in Improving Insulin Sensitivity in Individuals with Type 2 Diabetes. Curr. Diabetes Rev. 2023, 19, e250422203985. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L. European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes. Facts 2021, 14, 222–245. [Google Scholar] [CrossRef]
- O’Neill, B.J. Effect of low-carbohydrate diets on cardiometabolic risk, insulin resistance, and metabolic syn-drome. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 301–307. [Google Scholar] [CrossRef]
- Li, Z.; Li, A.; Liu, P.; Zhang, B.; Yan, Y. Mapping the evolution and impact of ketogenic diet research on diabetes management: A comprehensive bibliometric analysis from 2005 to 2024. Front. Nutr. 2024, 11, 1485642. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, M.; Liang, J.; He, G.; Chen, N. Ketogenic Diet Benefits to Weight Loss, Glycemic Control, and Lipid Profiles in Overweight Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trails. Int. J. Environ. Res. Public Health 2022, 19, 10429. [Google Scholar] [CrossRef]
- Merovci, A.; Finley, B.; Hansis-Diarte, A.; Neppala, S.; Abdul-Ghani, M.A.; Cersosimo, E.; Triplitt, C.; DeFronzo, R.A. Effect of weight-maintaining ketogenic diet on glycemic control and insulin sensitivity in obese T2D subjects. BMJ Open Diabetes Res. Care. 2024, 12, e004199. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Dangelo, K.; Couch, S.C.; Benoit, S.C.; Clegg, D.J. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol. Aging 2012, 33, 19–27. [Google Scholar] [CrossRef]
- Newport, M.T.; VanItallie, T.B.; Kashiwaya, Y.; King, M.T.; Veech, R.L. A new way to produce hyperketonemia: Use of ketone ester in a case of Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 99–103. [Google Scholar] [CrossRef]
- Roslund, K.J.; Ramsey, J.J.; Rutkowsky, J.M.; Zhou, Z.; Slupsky, C.M. Two-month ketogenic diet alters systemic and brain metabolism in middle-aged female mice. Geroscience 2025, 47, 935–952. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Janardhanam, H. Unveiling the Neuroprotective Power: Exploring the Therapeutic Benefits of the Ketogenic Diet in Epilepsy Management—A Review Article. Indian J. Nat. Sci. 2024, 15, 75392. [Google Scholar]
- Dilmore, A.H.; Martino, C.; Neth, B.J.; West, K.A.; Zemlin, J.; Rahman, G.; Panitchpakdi, M.; Meehan, M.J.; Weldon, K.C.; Blach, C.; et al. Alzheimer’s Gut Microbiome Project Consortium. Effects of a ketogenic and low-fat diet on the human metabolome, microbiome, and foodome in adults at risk for Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, 4805–4816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, S.; Huang, X.; Tong, H.; Niu, H.; Lu, L. Neuroprotective effect of a medium-chain triglyceride ketogenic diet on MPTP-induced Parkinson’s disease mice: A combination of transcriptomics and metabolomics in the substantia nigra and fecal microbiome. Cell Death Discov. 2023, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, D.; Żendzian-Piotrowska, M. Ketogenic Diet: A Review of Composition Diversity, Mechanism of Action and Clinical Application. J. Nutr. Metab. 2024, 2024, 6666171. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Cooper, J.M.; Dexter, D.; Clark, J.B.; Jenner, P.; Marsden, C.D. Mitochondrial Complex I Deficiency in Parkinson’s Disease. J. Neurochem. 1990, 54, 823–827. [Google Scholar] [CrossRef]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Zhou, Z.; Hagopian, K.; López-Domínguez, J.A.; Kim, K.; Jasoliya, M.; Roberts, M.N.; Cortopassi, G.A.; Showalter, M.R.; Roberts, B.S.; González-Reyes, J.A.; et al. A ketogenic diet impacts markers of mitochondrial mass in a tissue specific manner in aged mice. Aging 2021, 13, 7914–7930. [Google Scholar] [CrossRef]
- Tieu, K.; Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.C.; Yan, S.D.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; et al. D-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Investig. 2003, 112, 892–901. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; Murtagh, D.K.J.; Gilbertson, L.J.; Asztely, F.J.S.; Lynch, C.D.P. Low-fat versus ketogenic diet in Parkin-son’s disease: A pilot randomized controlled trial. Mov. Disord. 2018, 33, 1306–1314. [Google Scholar] [CrossRef]
- Yudkoff, M.; Daikhin, Y.; Melø, T.M.; Nissim, I.; Sonnewald, U.; Nissim, I. The Ketogenic Diet and Brain Metabolism of Amino Acids: Relationship to the Anticonvulsant Effect. Annu. Rev. Nutr. 2007, 27, 415–430. [Google Scholar] [CrossRef]
- VanItallie, T.B.; Nonas, C.; Di Rocco, A.; Boyar, K.; Hyams, K.; Heymsfield, S.B. Treatment of Parkinson disease with diet-induced hyperketonemia: A feasibility study. Neurology 2005, 64, 728–730. [Google Scholar] [CrossRef]
- Tidman, M. Effects of a Ketogenic Diet on Symptoms, Biomarkers, Depression, and Anxiety in Parkinson’s Disease: A Case Study. Cureus 2022, 14, 23684. [Google Scholar] [CrossRef] [PubMed]
- Tidman, M.M.; White, D.R.; White, T.A. Impact of a keto diet on symptoms of Parkinson’s disease, biomarkers, depression, anxiety and quality of life: A longitudinal study. Neurodegener. Dis. Manag. 2024, 14, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, Y.; Takeshima, T.; Mori, N.; Nakashima, K.; Clarke, K.; Veech, R.L. d-β-Hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 5440–5444. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Courchesne-Loyer, A.; St-Pierre, V.; Vandenberghe, C.; Pierotti, T.; Fortier, M.; Croteau, E.; Castellano, C.A. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2016, 1367, 12–20. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Wands, J.R. Alzheimer’s Disease is Type 3 Diabetes—Evidence Reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef]
- Manyevitch, R.; Protas, M.; Scarpiello, S.; Deliso, M.; Bass, B.; Nanajian, A.; Chang, M.; Thompson, S.M.; Khoury, N.; Gonnella, R.; et al. Evaluation of Metabolic and Synaptic Dysfunction Hypotheses of Alzheimer’s Disease (AD): A Meta-Analysis of CSF Markers. Curr. Alzheimer Res. 2018, 15, 164–181. [Google Scholar] [CrossRef]
- Cunnane, S.; Nugent, S.; Roy, M.; Courchesne-Loyer, A.; Croteau, E.; Tremblay, S.; Castellano, A.; Pifferi, F.; Bocti, C.; Paquet, N.; et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition 2011, 27, 3–20. [Google Scholar] [CrossRef]
- Meeusen, H.; Romagnolo, A.; Holsink, S.A.C.; van den Broek, T.J.M.; van Helvoort, A.; Gorter, J.A.; van Vliet, E.A.; Verkuyl, J.M.; Silva, J.P.; Aronica, E. A novel hepatocyte ketone production assay to help the selection of nutrients for the ketogenic diet treatment of epilepsy. Sci. Rep. 2024, 14, 11940. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, I.; Wera, S.; Van Leuven, F.; Henderson, S.T. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr. Metab. 2005, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Kashiwaya, Y.; Bergman, C.; Lee, J.H.; Wan, R.; King, M.T.; Mughal, M.R.; Okun, E.; Clarke, K.; Mattson, M.P.; Veech, R.L. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C.L.; Deprez, L.M.; Mortimer, G.M.N.; Murtagh, D.K.J.; McCoy, S.; Mylchreest, R.; Gilbertson, L.J.; Clark, K.M.; Simpson, P.V.; McManus, E.J.; et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimers Res. Ther. 2021, 13, 51. [Google Scholar] [CrossRef]
- Henderson, S.T.; Vogel, J.L.; Barr, L.J.; Garvin, F.; Jones, J.J.; Costantini, L.C. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled, multicenter trial. Nutr. Metab. 2009, 6, 31. [Google Scholar] [CrossRef]
- Pifferi, F.; Terrien, J.; Marchal, J.; Dal-Pan, A.; Djelti, F.; Hardy, I.; Chahory, S.; Cordonnier, N.; Desquilbet, L.; Hurion, M.; et al. Caloric restriction increases lifespan but affects brain integrity in grey mouse lemur primates. Commun. Biol. 2018, 1, 30. [Google Scholar] [CrossRef]
- Klement, R.J.; Champ, C.E.; Otto, C.; Kämmerer, U. Anti-Tumor Effects of Ketogenic Diets in Mice: A Meta-Analysis. PLoS ONE 2016, 11, e0155050. [Google Scholar] [CrossRef]
- Sandri, M.; Barberi, L.; Bijlsma, A.Y.; Blaauw, B.; Dyar, K.A.; Milan, G.; Mammucari, C.; Meskers, C.G.; Pallafacchina, G.; Paoli, A.; et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 2013, 14, 303–323. [Google Scholar] [CrossRef]
- Schwertfeger, K.L.; McManaman, J.L.; Palmer, C.A.; Neville, M.C.; Anderson, S.M. Expression of constitutively activated Akt in the mammary gland leads to excess lipid synthesis during pregnancy and lactation. J. Lipid Res. 2003, 44, 1100–1112. [Google Scholar] [CrossRef]
- Klement, R.J.; Kämmerer, U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr. Metab. 2011, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yamamoto, T.; Takahashi, Y.; Moro, T.; Tajima, T.; Sakaguchi, Y.; Sakata, N.; Yokoyama, A.; Hijioka, S.; Sada, A.; et al. Metabolic intervention by low carbohydrate diet suppresses the onset and progression of neuroendocrine tumors. Cell Death Dis. 2023, 14, 597. [Google Scholar] [CrossRef] [PubMed]
- Cortez, N.E.; Mackenzie, G.G. Ketogenic Diets in Pancreatic Cancer and Associated Cachexia: Cellular Mechanisms and Clinical Perspectives. Nutrients 2021, 13, 3202. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; TeSlaa, T.; Ng, S.; Nofal, M.; Wang, L.; Lan, T.; Zeng, X.; Cowan, A.; McBride, M.; Lu, W.; et al. Ketogenic diet and chemotherapy combine to disrupt pancreatic cancer metabolism and growth. Med 2022, 3, 119–136. [Google Scholar] [CrossRef]
- Radyk, M.D.; Kerk, S.A.; Lyssiotis, C.A. Ketotherapy: Cutting carbs to treat cancer. Med 2022, 3, 87–89. [Google Scholar] [CrossRef]
- Ferrere, G.; Tidjani Alou, M.; Liu, P.; Goubet, A.G.; Fidelle, M.; Kepp, O. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight 2021, 6, e145207. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Charlie Foundation; Matthew’s Friends; Practice Committee of the Child Neurology Society. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 3, 175–192. [Google Scholar] [CrossRef]
- Swaiman, K.F.; Ashwal, S.; Ferriero, D.M.; Schor, N.F.; Finkel, R.S.; Gropman, A.L.; Pearl, P.L.; Shevell, M. Swaiman’s Pediatric Neurology. Principles and Practice, 6th ed.; Elsevier: Philadelphia, PA, USA, 2017. [Google Scholar]
- Ko, A.; Jung, D.E.; Kim, S.H.; Kang, H.C.; Lee, J.S.; Lee, S.T.; Choi, J.R.; Kim, H.D. The Efficacy of Ketogenic Diet for Specific Genetic Mutation in Developmental and Epileptic Encephalopathy. Front. Neurol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Dahlin, M.; Stödberg, T.; Ekman, E.; Töhönen, V.; Wedell, A. Genetic aetiologies in relation to response to the ketogenic diet in 226 children with epilepsy. Brain Commun. 2025, 7, fcaf134. [Google Scholar] [CrossRef]
- Wickstrom, R.; Taraschenko, O.; Dilena, R.; Payne, E.T.; Specchio, N.; Nabbout, R.; Koh, S.; Gaspard, N.; Hirsch, L.J.; International NORSE Consensus Group. International consensus recommendations for management of New Onset Refractory Status Epilepticus (NORSE) including Febrile Infection-Related Epilepsy Syndrome (FIRES): Summary and Clinical Tools. Epilepsia 2022, 63, 2827–2839. [Google Scholar] [CrossRef]



| Regular Diet | CKD | MAD | LGIT | MCT | |
|---|---|---|---|---|---|
| fat | 20–40% | 90% | 60–70% | 60% | 30–60% |
| protein | 10–25% | 6–8% | 20–30% | 20–30% | 10% |
| carbohydrates | 45–65% | 2–4% | 6% | 10% | 15–19% |
| fat:protein + carbohydrate ratio | 0.2–0.3:1 | 3–4:1 | 1:1 | 1:1 | 1–2:1 |
| No. | Technique/Matrix | Column/Mobile Phase | Metabolite(s) | Sample Preparation | Source |
|---|---|---|---|---|---|
| 1 | LC-MS/MS QTOF blood | UPLC BEH Amide 5 mM ammonium acetate in water (eluent A) and 5 mM ammonium acetate in acetonitrile/water (95/5, v/v) (eluent B) | βHB, fatty acids, ETA, DHA | coagulation, centrifugation, serum collection, freezing | [15] |
| 2 | RP-HPLC plasma | Hypersil Gold aQ, octadecyl silica A and B were Millipore water and acetonitrile, both containing 0.10% formic acid | carnitines: N(5)-acetylornithine | extraction: with methanol containing 10 mol/L−1 ethylparaben, 2 mol/ L−1 1,3-nitro-L-tyrosine, 4 mol L−1 d4-succinate; deprotection; centrifugation, evaporation, redissolution in methanol | [16] |
| 3 | LC-MS/MS blood | - | lipids, carnitines, amino acids, structural analogues of γ-aminobutyric acid and lactic acid | coagulation (EDTA-2Na), centrifugation, serum collection, freezing | [70] |
| 4 | LC-MS/MS serum | C18 Mobile phase A: 0.1% formic acid in water, mobile phase B 0.1% formic acid in methanol | βHB | 100 µL serum + 800 µL (methanol: acetone = 7:3 v/v) and 50 µL IS, centrifuged, lyophilized, frozen, dissolved in 100 µL 10% methanol | [20] |
| 5 | LC-MS/MS QTOF brain tissue | Phenomenex Kinetex 2.6 μM F5 | DON—glutamine inhibitor | homogenized with 3 M HCl + butanol, carried out in the derivative incubated at 60 °C for 30 min, centrifuged, evaporated, resuspended in 50 µL dH2O containing 0.2% formic acid | [21] |
| 6 | LC-QTOF-MS hippocampus, frontal cortex, plasma | Acquity UPLC BEH Amide, A: 0.1% formic acid + 10 mM ammonium acetate in 20% acetonitrile B: 0.1% formic acid + 10 mM ammonium acetate in 95% acetonitrile | untargeted analysis > homostrachydrine | mixed with methanol at a ratio of 1:5 (v/v), internal standard: gabapentin, centrifugation | [71] |
| 7 | UPLC-ESI-MS/MS CSF | Waters BEH C18, A: water with 0.1% formic acid B: methanol | pyridoxal phosphate, pyridoxal, vitamin B6, pyridoxamine, pyridoxine acid | deproteinization: acetonitrile/methanol (9:1, v/v) + 0.1% formic acid, incubation in the dark for 20 min, centrifugation, evaporation in nitrogen 60 °C, reconstitution: 0.1% formic acid | [72] |
| 8 | HPLC-TOF-MS plasma hippocampus | Phenomenex Kinetex HILIC, A: 50% acetonitrile with 5 mM acetic acid B: 90% ACN with 5 mM acetic acid; pH 5.8 | nucleosides, nucleotides, metabolites of purines and pyrimidines, organic acids and their derivatives, peptides and metabolites related to amino acids | homogenization: sonication in 0.1% NH4Oac, modified liquid-liquid extraction (Matyash method) | [73] |
| 9 | UPLC-MS/MS CSF | Waters BEH C18, A: water + 0.1% formic acid, B: methanol | pyridoxal-5′-phosphate, pyridoxal, pyridoxine, pyridoxamine, pyridoxine acid | degranulation: 6.3% sulfosalicylic acid and acetonitrile, derivatization: 3 N HCl in n-butanol at 65 °C for 30 min, drying, reconstitution: water/methanol (70:30) SW: d9-pipecolic acid | [74] |
| 10 | DI/LC-MS/MS post-mortem brain tissue | Absolute IDQ p180 kit, Biocrates | adenosine monophosphate, o-acetylcholine, L-fucose, isobutyric acid, glycerol | extraction: methanol, sonication, centrifugation, evaporation, reconstitution with phosphate buffer | [75] |
| No. | Technique/Matrix | Column | Metabolite(s) | Sample Preparation | Source |
|---|---|---|---|---|---|
| 1 | GC-MS/MS urine | BPX-5 | 172 metabolites: amino acids, organic acids, fatty acids, carbohydrates, nitrogenous compounds, and polyamines | extraction with extraction solution, centrifugation, drying in a vacuum centrifuge, oximation with methoxylamine hydrochloride in pyridine | [76,77,78] |
| 2 | GC-MS CSF | BPX-5 | 56 metabolites, including glycine, xylose, ketoisocarpronic acid | extraction, derivatization | [79] |
| 3 | GC-MS dried blood spot | DB-5MS | glutamine, pyruvic acid, L-serine, oxalic acid, caprylic acid, palmitic acid | 6 mm circles of blotting paper were extracted with methanol/chloroform, dried under nitrogen, derivatized with MSTFA from TMCS at 70 °C for 1 h | [80] |
| 4 | GC-MS plasma | DB-5MS | phosphate, proline, lactic acid, alanine, glutamate, hexadecanoic acid | extraction: methanol, centrifugation drying under nitrogen, oximation with methoxyamine hydrochloride in pyridine 16 h at room temperature, trimethylsilylation of MTBSTFA with 1% TMCS 1 h at 37 °C | [81] |
| 5 | GC-TOF-MS plasma | DB-5MS | proline, glutamate, phenylalanine, methionine, lysine, tryptophan, citric acid, uric acid, cholesterol, palmitate, glucose, myo-inositol, creatinine | extraction, centrifugation, drying, oximation, trimethylsilylation | [82] |
| 6 | GC-MS/MS plasma | BPX-5 | taurine, quinolinic acid, N-acetylneuraminic acid, catechol | extraction SPME | [83] |
| 7 | GC-MS/MS serum | CP-SIL 8 CB | 3-hydroxybutyrate, acetoacetate, 2-hydroxybutyrate, 3-hydroxyisobutyrate, acetylglycine, decanoic acid, octanoic acid, isoleucine, adipic acid, uric acid, glyoxylic acid, citric acid, tartaric acid, glucosamine, galactose, mannitol, N-acetyl-lysine, 2-aminopimelanoate, 3-hydroxyanthranilate | separation on ion exchange column (elution with water/ hydrochloric acid/ NH4OH), freezing, lyophilization, derivatization with MTBSTFA | [84] |
| The Landscape of the Classic Ketogenic Diet (CKD) in 2025 | |||
|---|---|---|---|
| CKD modifications: | Patient groups to CKD: | CKD in other diseases: | CKD in the Intensive Care Unit: |
|
|
|
|
| The pleiotropic effect of ketosis involves the expression of genes and cellular pathways regulating inflammation, oxidative stress, immune function, cell membrane physiology, intracellular signaling, and intercellular communication. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idzikowska, K.; Gątarek, P.; Gajda, A.; Safiński, P.; Przyslo, L.; Kałużna-Czaplińska, J. The Ketogenic Diet Through a Metabolomic Lens: Biochemical Pathways, Therapeutic Applications, and Analytical Challenges. Nutrients 2025, 17, 2969. https://doi.org/10.3390/nu17182969
Idzikowska K, Gątarek P, Gajda A, Safiński P, Przyslo L, Kałużna-Czaplińska J. The Ketogenic Diet Through a Metabolomic Lens: Biochemical Pathways, Therapeutic Applications, and Analytical Challenges. Nutrients. 2025; 17(18):2969. https://doi.org/10.3390/nu17182969
Chicago/Turabian StyleIdzikowska, Katarzyna, Paulina Gątarek, Anna Gajda, Piotr Safiński, Lukasz Przyslo, and Joanna Kałużna-Czaplińska. 2025. "The Ketogenic Diet Through a Metabolomic Lens: Biochemical Pathways, Therapeutic Applications, and Analytical Challenges" Nutrients 17, no. 18: 2969. https://doi.org/10.3390/nu17182969
APA StyleIdzikowska, K., Gątarek, P., Gajda, A., Safiński, P., Przyslo, L., & Kałużna-Czaplińska, J. (2025). The Ketogenic Diet Through a Metabolomic Lens: Biochemical Pathways, Therapeutic Applications, and Analytical Challenges. Nutrients, 17(18), 2969. https://doi.org/10.3390/nu17182969