3.1. Overview
Iron accumulation, whether arising from genetic or acquired factors, may have distinct implications for ocular health. Primary iron overload includes systemic iron-loading inherited disorders such as hereditary hemochromatosis (HH) and aceruloplasminemia (ACP), leading to iron accumulation in various tissues. In contrast, pantothenate kinase-associated neurodegeneration (PKAN), and Friedreich’s Ataxia (FRDA) involve neuronal dysregulation at cellular or mitochondrial level classified as neurodegeneration with brain iron accumulation (NBIA) or mitochondrial Fe-S cluster disorders respectively [
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54]. Secondary iron overload can occur due to non-genetic causes such as repeated blood transfusions in thalassemia major or sickle cell disease, chronic hemolysis, excessive dietary or parenteral iron supplementation, inflammation, and certain chronic systemic diseases [
29]. These disturbances in iron homeostasis, whether primary or secondary, can lead to iron deposition within ocular tissues, initiating a cascade of cellular pathways that contribute to ocular pathology.
The principal mechanism of iron-mediated ocular injury is the generation of reactive oxygen species (ROS) through the iron-catalyzed Fenton reaction. Excess ferrous iron (Fe
2+) reacts with hydrogen peroxide (H
2O
2) to generate hydroxyl radicals, leading to lipid peroxidation (LPO), protein oxidation, and DNA damage. This oxidative stress compromises RPE [
55], PR [
20], and ganglion cells [
22], as observed in ocular siderosis [
56], age-related macular degeneration (AMD), and diabetic retinopathy (DR) [
57,
58]. While retinitis pigmentosa (RP) is genetically driven, preclinical data suggest iron dysregulation and ferroptosis may exacerbate its progression [
59,
60,
61]. Notably, oxidative stress is both a cause and consequence of iron overload, as it also upregulates TfR1 expression, further amplifying iron accumulation [
62].
Beyond oxidative stress, iron overload also activates inflammatory pathways in retinal cells, particularly through NF-κB and inflammasome signaling. Oxidative stress mediated phosphorylation and degradation of IκB, leads to nuclear translocation of NFκB, inducing the transcription of pro-inflammatory cytokines such as TNF-α, IL1-β, and IL-6. Further, chronic release of cytokines contributes to vascular dysfunction, which are commonly observed features in DR and glaucoma [
63,
64]. Pro-inflammatory cytokines, particularly IL-6 and toll-like receptor-4 (TLR-4) signaling activation in Müller glial and RPE cells have been shown to regulate the iron-regulatory hormone hepcidin transcription [
38,
65], exacerbating the internalization and degradation of the iron exporter ferroportin, leading to intracellular iron sequestration, disrupted iron efflux, enhanced oxidative stress and ferroptosis. This dysregulation is particularly detrimental to photoreceptors, with cones demonstrating greater susceptibility to iron-induced damage than rods, and subsequently exhibiting impaired phagocytosis in diabetic retinas [
60,
66]. Iron overload has additionally been linked to RPE degeneration through NLRP3 inflammasome activation, with direct implications in AMD pathogenesis [
67].
Another crucial mechanism is ferroptosis, an iron-dependent form of regulated cell death characterized by ROS-driven lipid peroxidation. Excess iron depletes intracellular glutathione (GSH), a critical antioxidant that compromises glutathione peroxidase 4 (GPX4) activity, further driving lipid peroxidation mediated ferroptotic cell death. Accumulating evidence implicates ferroptosis in retinal ganglion cell loss, RPE and PR degeneration, thereby contributing to the pathogenesis of RP [
59], DR [
68], AMD [
69], and inherited retinal degenerations [
23,
70,
71].
Furthermore, iron overload promotes the formation of new abnormal blood vessels known as angiogenesis, a major cause of vision loss in advanced DR and wet AMD [
72,
73,
74,
75,
76]. Iron overload activates G-protein coupled receptor 91 (GPR91), stimulating the release of vascular endothelial growth factor (VEGF) and other pro-angiogenic factors [
57]. Taken together, oxidative stress, inflammation, ferroptosis and angiogenesis represent interconnected pathways by which iron dysregulation drives ocular diseases. These mechanisms not only provide insight into disease pathogenesis but also present potential therapeutic targets for iron-induced retinal diseases.
3.2. Genetic Iron Overload
Hereditary hemochromatosis: HH is an autosomal recessive disorder characterized by dysregulated iron absorption and systemic iron overload. Late-onset or type 1 HH is commonly caused by mutations in the HFE gene. Non-HFE forms or atypical hemochromatosis involving HJV or hepcidin antimicrobial peptide (HAMP) mutations lead to juvenile or type 2 hemochromatosis, while the mutations in TfR2 cause an adult-onset form of type 3 hemochromatosis. Mutations in ferroportin/solute carrier family 40, member 1 (SLC40A1) instead cause ferroportin disease, an autosomal dominant condition sometimes classified as type 4 hemochromatosis as shown in
Table 1 [
28,
29,
30].
The most prevalent mutation in the HFE gene, C282Y, results from a substitution of cysteine by tyrosine at position 282, leading to impaired regulation of iron uptake by reducing the interaction of HFE with ß2-microglobulin and TfR1, and a subsequent decrease in hepcidin levels [
31]. Hepcidin is the key regulator of systemic iron homeostasis, and its deficiency leads to unregulated dietary iron absorption and tissue accumulation. Due to incomplete penetrance, the estimated prevalence of clinically diagnosed HH is approximately 1 in 200 to 250 individuals in Caucasian populations. However, ophthalmic manifestations are underreported, likely due to the delayed onset of symptoms and limited retinal screening in HH cohorts [
32]. Ocular iron overload can induce pathological changes in both the cornea and retina, contributing to reduced visual acuity, bull’s eye maculopathy, and photoreceptor (rod and cone) dysfunction. For instance, a 49-year-old patient homozygous for the C282Y mutation presented with progressive visual decline and abnormal changes in the RPE [
33], while a 39-year-old patient with the same genotype developed bilateral progressive blurry vision, photopsia, headaches, and bull’s eye maculopathy, suggesting retinal involvement [
34]. Nonetheless, such clinical reports remain sparse, confounded by age related and comorbid conditions, and lack validation from large cohort studies. To address the challenges of studying these slow-progressing disorders in humans, several murine models have been employed to explore retinal consequences of iron dysregulation. HFE knockout mice exhibit retinal iron accumulation, characterized by reduced ganglion cell density, disruption of the inner and outer nuclear layers, and hypertrophic changes in the RPE [
35,
36]. HJV knockout mice display defective retinal angiogenesis, increased vascular permeability, and reactive gliosis [
37]. The hepcidin knockout model, which mimics systemic hepcidin deficiency, exhibits age-dependent iron deposition in both the RPE and neural retina, leading to subsequent photoreceptor loss, lipofuscin accumulation, and subretinal neovascularization by 18 months of age [
38]. In summary, evidence from preclinical studies strongly supports a role for iron overload-mediated HH in driving ocular pathology, whereas clinical evidence remains limited to small, isolated case reports. The pathogenic significance of moderate iron accumulation is less clear, with conflicting findings. While experimental models show that even subclinical increases in systemic iron can exacerbate oxidative damage and retinal degeneration, most individuals with HH do not exhibit measurable impairments in visual function. Notably, large-scale investigations assessing retinal structure and function in HH patients are lacking. Moreover, HH associated iron overload predominantly affects the liver and other visceral organs, but the extent and conditions under which iron accumulation occurs and is retained within the human retina remains uncertain. Consequently, future studies are needed to establish clinically relevant thresholds of ocular iron overload in HH and to bridge the gap between preclinical evidence and clinical observations.
Pantothenate Kinase-Associated Neurodegeneration: PKAN is an early-onset autosomal-recessive monogenic disorder resulting from mutations in the pantothenate kinase 2 (PANK2) gene, which encodes pantothenate kinase 2, an enzyme that catalyzes the first step in coenzyme A (CoA) biosynthesis by phosphorylating pantothenic acid (Vitamin B5) to form 4’-phosphopantothenate [
39,
40]. Loss-of-function mutations in PANK2 lead to reduced production of 4-phosphopantothenate and CoA, with subsequent accumulation of cysteine containing compounds, which forms complexes with iron, thereby promoting pathological iron accumulation in the brains of PKAN patients. The hallmark of PKAN on magnetic resonance imaging (MRI) is the “eye-of-the-tiger” sign, characterized by bilateral hypointensity with central hyperintensity in the globus pallidus in most cases, although it is not entirely pathognomonic [
40]. Clinically, PKAN manifests with dystonia, dysarthria, retinitis pigmentosa, progressive movement disorder, and cognitive impairments. Approximately two-thirds of PKAN patients develop pigmentary retinal degeneration, typically presenting as early nyctalopia (night blindness) followed by progressive loss of peripheral visual fields [
41]. In preclinical studies, PKAN2-deficient mice replicate key retinal phenotypes observed in patients, including marked attenuation of scotopic a- and b- wave amplitudes on electroretinography, indicative of photoreceptor dysfunction and inner retinal degeneration, although these models do not exhibit brain iron accumulation [
77]. Furthermore, dietary supplementation with 4’-phosphopantetheine, which bypasses the enzymatic inhibition in CoA metabolism, ameliorates metabolic, structural and functional abnormalities in PKAN mouse models [
77]. Notably, patient-derived cellular models also strongly support CoA deficiency as a primary driver of PKAN pathology, with iron overload occurring as a downstream effect [
39]. Clinical and preclinical studies support the link between iron dysregulation and retinal pathology in PKAN disorder, however there are critical limitations. Clinically, phenotypic variability is high even among individuals with the same PANK2 mutation. Owing to the disorder’s rarity and reliance on small case reports, reliable estimates of the prevalence, onset, and progression of ocular changes remain elusive. Furthermore, animal models often fail to recapitulate the complete human phenotype, limiting translational relevance. Thus, patient-derived cellular systems offer a promising platform to further dissect the disease mechanisms and evaluate targeted therapies [
42].
Aceruloplasminemia: ACP is a rare adult-onset autosomal recessive disorder caused by mutations in the CP gene located on chromosome 3q23-q24. CP encodes ceruloplasmin, a multicopper oxidase primarily synthesized in hepatocytes, which facilitates the oxidation of Fe
2+ iron to its Fe
3+ form, a necessary step for iron export through ferroportin [
43,
44]. Mutations that abrogate ceruloplasmin function lead to intracellular iron sequestration and parenchymal iron overload, affecting the brain, liver, pancreas, and retina. Mouse models lacking CP recapitulate the iron-related retinal pathology of human ACP, with knockout mice aged 18 months or older showing excessive retinal iron deposition, retinal degeneration, and RPE hypertrophy [
45]. Clinical findings in ACP patients align with experimental evidence, showing RPE depigmentation, atrophy, hypertrophy, nodular and diffuse drusen, and accumulation of lipofuscin and melanolipofuscin granules. Histopathological studies have identified two distinct RPE cell populations, melanosome-rich cells containing abnormally high iron and degraded melanolipofuscin, and melanosome-poor RPE cells with iron-rich aggregates [
78]. Early diagnosis of ACP is challenging due to the delayed and non-specific onset of symptoms. However, a biochemical triad of microcytic anemia, low serum ceruloplasmin, and hypoferremia has emerged as a sensitive early indicator. In a cohort of 51 ACP patients, anemia was typically detected by age 30, diabetes mellitus by age 37, and neurological symptoms by age 50 [
46]. Additional case reports suggest that retinal changes may precede or occur alongside neurological manifestations, underscoring the importance of early ophthalmic evaluation [
47,
48]. Although both clinical and preclinical data consistently implicate iron accumulation in ACP retinal pathology, the full clinical spectrum and natural history of ACP-associated retinal disease remain incompletely characterized. Existing animal models do not fully reproduce the human neurological phenotype. Moreover, there is currently no standardized method to monitor ocular iron overload or to determine whether interventions such as iron chelation or antioxidant therapy can reliably arrest or reverse the progression of retinal damage in ACP.
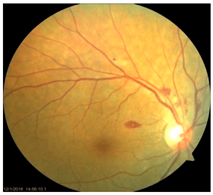
Friedreich’s Ataxia: FRDA is an autosomal recessive neurodegenerative disease caused by expanded GAA trinucleotide repeats in the frataxin (FXN) gene located on chromosome 9q13 with an estimated prevalence of ~1 in 40,000–50,000 in populations of European ancestry [
49]. This expansion leads to a reduction in the expression of frataxin, a mitochondrial protein involved in iron-sulfur (Fe-S) cluster biosynthesis and the regulation of oxidative metabolism. Frataxin deficiency disrupts mitochondrial iron handling, leading to mitochondrial iron accumulation, cytosolic iron depletion, impaired ATP production, and increased oxidative stress. FRDA predominantly affects the central nervous and cardiovascular systems, manifesting clinically with gait ataxia, scoliosis, diabetes mellitus, hypertrophic cardiomyopathy, and peripheral neuropathy [
50,
51]. Clinical studies report that 70–75% of FRDA patients exhibit subclinical neuro-ophthalmological abnormalities such as optic atrophy, oculomotor dysfunction, and RP-like retinal degeneration [
52]. Optical coherence tomography (OCT) frequently reveals thinning of the retinal fiber layer (RNFL) despite preserved visual acuity in many cases [
79]. Histopathological and imaging studies have documented degeneration of retinal ganglion cells (RGCs), PR, and RPE cells in affected individuals. A striking clinical example is a reported case of a 59-year-old FRDA patient who showed severe optic neuropathy with rapid-onset catastrophic visual impairment. Fundus examination revealed a pale optic disc and scattered fleck-like yellow deposits with autofluorescence, indicating lipofuscin-like deposits [
53]. Although preclinical ocular studies are limited, frataxin deficient cultured rat RGCs showed increased susceptibility to reactive oxygen species and cell death [
80]. Additionally, frataxin knockdown mice showed RPE loss and PR disruption [
54]. Taken together, clinical and preclinical findings demonstrate a strong association between FXN deficiency, mitochondrial dysfunction, and neuro-ophthalmic involvement, leading to retinal degeneration. Nonetheless, substantial uncertainties remain. There is currently limited evidence on the contribution of systemic iron dyshomeostasis to ocular pathology in FRDA, with mitochondrial iron misdistribution and impaired Fe-S cluster assembly being the more established drivers of oxidative stress in ocular tissues. Visual outcomes are heterogenous with significant variability and uncertainty, and ophthalmic involvement is incompletely defined. Longitudinal studies, ocular-specific iron imaging, and interventional trials including iron chelation therapies are needed to determine their potential role in modifying optic neuropathy in FRDA.
3.3. Posterior Segment Eye Diseases with Iron Accumulation
Retinitis Pigmentosa (RP): RP encompasses a clinically and genetically heterogenous group of inherited retinal dystrophies associated with mutations in more than 60 genes, including those involved in phototransduction, outer segment renewal, and the visual cycle. RP is characterized by the loss of rod photoreceptors, initially leading to nyctalopia (night blindness), followed by cone cell death, culminating in constricted visual fields and eventual blindness [
59]. Despite advancements in gene therapy and retinal implants, there is currently no definitive cure for RP due to irreversible photoreceptor degeneration. Emerging evidence suggests a role for dysregulated iron homeostasis in the progression of RP. Animal models such as rd10 mice and Royal College of Surgeons rats have demonstrated excessive retinal iron accumulation [
60]. In the rd1 mouse model of RP, retinal iron accumulation has been observed as early as postnatal day P10, preceding photoreceptor loss, suggesting a possible contributory role for iron in the disease progression [
61]. These rodent models also exhibit altered expression of iron regulatory proteins, including transferrin, TfR, ferritin, and CP, indicating disrupted iron metabolism [
81]. As discussed above in the overview, mechanistic studies further highlight ferroptosis, an iron-dependent form of cell death driven by lipid peroxidation, as a key contributing factor to photoreceptor loss in RP [
59]. Importantly, pharmacological interventions with iron chelators such as deferiprone (DFP), zinc-deferoxamine, VK28, and VAR10303 have been shown to mitigate the retinal iron burden, decrease oxidative stress and preserve cone function in an RP mouse model [
70]. In contrast, direct clinical evidence of iron overload in patients with classic RP is lacking. Limited clinical studies have led to uncertainty over whether iron overload is a primary driver or a secondary consequence of PR degeneration. Thus, while preclinical data robustly support iron dysregulation and ferroptosis as pathogenic mechanisms in RP, their clinical relevance remains uncertain. Future work should focus on elucidating retinal iron dynamics in RP patients and evaluating targeted neuroprotective chelation strategies in well-designed clinical studies.
Diabetic Retinopathy (DR): DR is the most prevalent microvascular complication of diabetes mellitus and a leading cause of blindness in working-age and elderly populations [
82]. Clinically, DR is stratified into non-proliferative and proliferative stages. The non-proliferative stage is characterized by microaneurysms, retinal hemorrhages, and capillary dropout, whereas proliferative DR is marked by microvascular abnormalities, neovascularization, cotton-wool spots, venous beading, and potential for vitreous hemorrhage [
83]. Diabetic macular edema (DME), associated with increased vascular permeability and deposition of hard exudates in the central macula, remains a major cause of vision impairment at any DR stage [
84]. While primarily viewed as a microvascular disease, DR also exhibits glial activation, oxidative stress, and early neurodegeneration. In early stages of DR, hyperglycemia-induced excitotoxicity through glutamate, upregulation of the renin-angiotensin system (RAS), and increased production of ROS contribute to retinal neuronal injury. Later stages of DR are associated with inflammatory cell adhesion, vascular hyperpermeability, and pathologic neovascularization with elevated markers, including VEGF and monocyte chemotactic protein-1 (MCP1) [
85,
86,
87].
Recent studies in our lab have demonstrated pathological iron accumulation in the retinas of diabetic animal models [
57]. Diabetes-induced hyperglycemia and associated microhemorrhage disrupts iron homeostasis, leading to inflammation [
60,
66], oxidative stress [
58], and ferroptosis [
68]. Both in vitro and in vivo studies suggest chronic hyperglycemia destabilizes heme-metabolism and promotes hemeoxygenase-1 upregulation, increasing the release of free heme and iron into the extracellular and interstitial compartments of retinal tissue. This results in mitochondrial and ER stress, sensitizing retinal cells to ferroptosis [
68,
88]. Hyperglycemia may also exacerbate iron burden through activation of the RAS [
89]. Angiotensin II upregulates genes associated with iron metabolism, including DMT1 and TfR1, enhancing iron uptake and intracellular accumulation in the retina [
90]. Interestingly, iron overload can also modulate the RAS through succinate receptor GPR91 signaling [
72]. Under hyperglycemic conditions, elevated succinate levels in the retina activate GPR91 in the RPE and ganglion cells, triggering downstream signaling through extracellular signal-regulated kinase 1/2 (ERK1/2), p38 mitogen-activated protein kinase (p38 MAPK), and c-Jun
N-terminal kinase (JNK) pathways [
73,
74]. This cascade increases the expression and secretion of VEGF, promoting pathological angiogenesis characteristic of proliferative DR [
75,
76]. Excess iron further promotes the expression of adhesion molecules, such as intercellular adhesion molecule 1 (ICAM1), and facilitates monocyte endothelial adhesion, both of which are critical early events in leukocytosis and microvascular dysfunction in DR [
91]. Systemic and retinal iron overload during DR also compromises the integrity of the BRB, exacerbating neurovascular damage through NLRP3 inflammasome activation and the subsequent release of pro-inflammatory cytokines [
57]. In contrast, clinical evidence remains more equivocal. Clinically, elevated levels of iron, ceruloplasmin, and transferrin have been detected in the retinas of DR patients, with vitreous iron accumulation particularly evident in proliferative stages [
89,
92]. DR patients also display higher serum levels of iron, ROS, and LPO, alongside decreased ferroptosis regulators like GPX4 and GSH, especially in non-proliferative DR [
93]. Large-scale epidemiological studies present conflicting results, with one study reporting that dietary iron intake was associated with reduced risk of vision-threatening DR [
94], whereas another study identified an inverse correlation between serum iron level and DR prevalence, suggesting iron deficiency may promote DR pathogenesis through hypoxia and inflammation [
95]. These conflicting observations highlight major clinical uncertainties. Although preclinical studies consistently implicate iron-induced oxidative stress and ferroptosis in DR, the clinical relevance remains ambiguous. Future trials are essential to understand whether iron dysregulation is a primary driver of retinal injury in DR or a secondary effect of vascular and metabolic dysfunction.
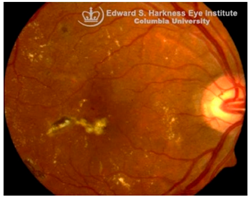
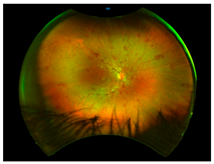
Age-related macular degeneration (AMD): AMD is a leading cause of irreversible vision loss in the elderly [
96]. It presents in two clinical forms: non-exudative (dry) AMD, which can progress to exudative (wet) AMD. Both forms are characterized by the early deposition of drusen, that are extracellular aggregates of proteins, lipids, and trace metals between the RPE and Bruch’s membrane. As the disease progresses, distinct pathological changes differentiate between the two forms: geographic atrophy (GA) of the RPE and PR in advanced dry AMD, and choroidal neovascularization (CNV) in wet AMD, marked by aberrant blood vessel growth from the choroid into the subretinal space [
96,
97]. Although accumulating evidence supports a correlation between iron dyshomeostasis and AMD pathogenesis, the precise mechanisms underlying iron accumulation in AMD retinas remain unresolved. Several biochemical pathways, including hypoxia, oxidative stress [
62], ferroptosis [
69], inflammation [
67,
98], and hemorrhage [
99], have been implicated in iron-mediated retinal injury. Hypoxic conditions, often present in degenerative retinal microenvironments, can activate hypoxia-inducible factors (HIFs), which transcriptionally upregulate iron import proteins, DMT1 and TfR1, enhancing intracellular iron uptake [
100]. In wet AMD, as in proliferative DR, subretinal hemorrhages introduce extracellular iron, further exacerbating local oxidative damage.
Histological studies of AMD-retinas consistently demonstrate elevated iron concentrations in the RPE, Bruch’s membrane and PR layers [
101] along with increased expression of ferritin and ferroportin in GA [
102], and higher aqueous humor iron levels in dry AMD [
103]. Some studies also revealed significantly higher retinal iron concentrations in donors over 65 years compared with those under 35, indicating age-dependent iron accumulation, though cellular localization varies [
104]. In contrast, in genetic disorders such as ACP, iron overload occurs in the RPE and exhibit AMD-like features, including drusen formation, even in younger patients [
105]. Transcriptomic and immunohistochemical data further suggest compensatory upregulation of transferrin localized to Müller glia and photoreceptors in AMD retinas to mitigate iron-induced oxidative stress [
106]. Despite this strong correlative evidence, a definitive causal relationship between iron accumulation and AMD remains unclear. Oral iron supplementation has been linked to subretinal hemorrhage in a dose-dependent manner in Comparison of AMD Treatment Trials (CATT) analysis [
107], while Mendelian randomization studies associated transferrin levels with wet AMD but not dry AMD [
108]. Similarly, NHANES data found no strong link between dietary or systemic iron intake and late AMD [
109]. Preclinical studies, however, strongly support iron-induced AMD-like pathology in animal models. Intravenous iron administration in mice leads to RPE hypertrophy, Bruch’s membrane thickening, and complement C3 deposition, mimicking features of rapid drusen formation observed clinically after IV iron therapy [
58,
110]. Similarly, genetic mouse models deficient in any of the iron regulatory proteins, including HFE, hemojuvelin, hepcidin or Cp/Hp knockout mice also exhibit retinal iron accumulation with several features reminiscent of human AMD, such as sub-RPE deposits resembling drusen, lysosomal inclusions, and even subretinal neovascularization, recapitulating both dry and wet AMD phenotypes [
111,
112]. Despite strong preclinical evidence supporting a causative role for iron overload in AMD pathogenesis, these models rely on induced iron overload and thus may not fully capture the multifactorial nature of human AMD. Additionally, clinical studies remain largely correlative, with inconsistencies across dietary and genetic studies. While elevated iron levels in AMD patients clearly suggest its involvement, the temporal sequence and mechanistic causality remains unresolved. Thus, the translational relevance of iron-targeted therapies in AMD requires carefully designed clinical studies to establish causality and to evaluate both the therapeutic potential and safety of iron-targeted interventions in AMD.
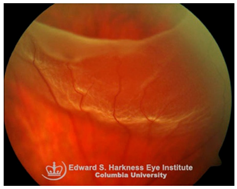
Retinal detachment (RD): RD is a sight-threatening condition in which separation of neurosensory retina from the underlying RPE leads to photoreceptor dysfunction and potential vision loss. Emerging evidence implicates iron overload in the retina as an exacerbating factor in RD-associated degeneration. Elevated iron levels and transferrin saturation (TSAT) have been detected in the vitreous and subretinal fluid of RD patients, correlating with prolonged detachment duration and poorer visual recovery [
113]. Under normal conditions, the RPE regulates retinal iron homeostasis through transferrin-mediated iron uptake and export through ferroportin. However, during RD, RPE dysfunction and breakdown of the BRB facilitate uncontrolled iron influx into the retina, driving ROS generation, oxidative stress and PR apoptosis. Preclinical models of RD demonstrate that iron chelation including local administration of transferrin or DFP protects against ferroptosis-mediated PR loss, indicating that iron overload is not merely a secondary consequence of RD but a modifiable driver of retinal degeneration [
113,
114]. Despite promising data from animal models, clinical translation of iron chelators or transferrin therapy in RD is still under investigation. No trials have yet defined the safety, optimal timing, dosing, or delivery strategies for chelation therapy. Thus, while preclinical evidence strongly supports iron overload as a therapeutic target, clinical validation is urgently needed.
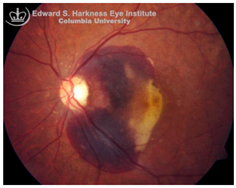
Subretinal hemorrhage (SH): SH results from the rupture of choroidal or retinal blood vessels, with blood accumulating between the neurosensory retina and RPE. This condition is observed in several ocular disorders, including AMD, myopic degeneration, angioid streaks, and ocular histoplasmosis [
115]. The extent of visual impairment is dictated by the hemorrhage volume, duration of exposure, and the efficacy of blood clearance by surrounding retinal cells [
116]. Multiple mechanisms contribute to vision loss following SH, including iron mediated toxicity to photoreceptors and the RPE, mechanical detachment of photoreceptors from the RPE disrupting metabolic support, migration and proliferation of subretinal cells, and/or fibrovascular membrane formation, all of which promote retinal scarring and irreversible structural damage [
116,
117]. Hemoglobin, which constitutes approximately 92% of red blood cell dry weight, undergoes breakdown following hemorrhage, releasing free heme and subsequently labile iron, both of which are cytotoxic when present in excess [
118,
119,
120]. Experimental models have shown that subretinal injection of fresh autologous blood into albino rats and rabbits induces rapid photoreceptor degeneration, edema in the outer retina, and marked iron accumulation in the photoreceptors and the RPE [
121]. In addition, oxyhemoglobin, a byproduct of red blood cell lysis, is also suspected to exacerbate retinal injury through lipid peroxidation and oxidative stress [
122]. Despite these preclinical insights using animal models, clinical evidence directly implicating iron toxicity as the dominant mechanism of SH-related damage remains limited. Future studies are needed to clarify the relative contribution of iron versus other pathogenic mechanisms in SH-induced retinal injury and to rigorously evaluate the therapeutic potential, safety and optimal dosing of iron-chelating agents.
A visual representation of all the posterior segment eye diseases related to iron overload discussed above are provided in
Table 2.
3.4. Anterior Segment Eye Diseases with Iron Deposition
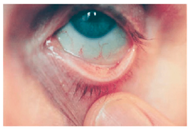
Ocular Siderosis: Ocular siderosis refers to the toxic accumulation of iron within the intraocular tissues, typically resulting from retention of iron-containing foreign bodies or intraocular hemorrhage. As discussed above, Fe
2+ released from these sources initiates Fenton reaction, leading to oxidative stress [
56]. Clinically and histologically, eyes affected by ocular siderosis demonstrate a wide spectrum of anterior and posterior segment alterations such as corneal iron deposition, iris heterochromia, pupillary mydriasis, loss of accommodation, anterior subcapsular cataract, lens discoloration, retinal arteriolar narrowing, retinal detachment, RPE clumping or atrophy, and progressive photoreceptor degeneration. It may also manifest as glaucoma when the trabecular meshwork and Schlemm’s canal are affected [
56,
129,
130]. Functional deterioration can be monitored using electroretinography (ERG), which typically shows an initial transient increase in a- and b-wave amplitudes, followed by a steady decline corresponding to progressive photoreceptor loss. Experimental and clinical observations suggest potential therapeutic benefits of iron chelation. For example, subconjunctival deferoxamine (DFO) has been reported to improve corneal siderosis and visual outcomes, although delayed administration failed to reverse established iron induced toxicity [
131]. Animal studies corroborate these observations. In rabbits, intravitreal placement of metallic foreign bodies led to rapid degeneration of the RPE and outer nuclear layer within ten days, accompanied by attenuated ERG responses under both photopic and scotopic conditions [
132]. Intravitreal deferoxamine treatment ameliorated these changes [
133]. Similarly, intravitreal iron injection in squirrel monkeys caused ophthalmoscopically visible RPE disruption and retinal whitening, accompanied by early reductions in ERG amplitude [
134]. Despite encouraging preclinical results, clinical evidence supporting iron chelation therapy remains limited. Concerns regarding optimal timing, safety and dosing of iron targeted interventions highlight the need for further translational and clinical research.
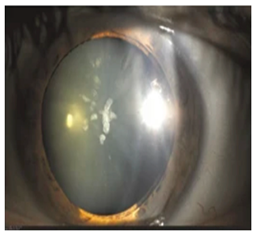
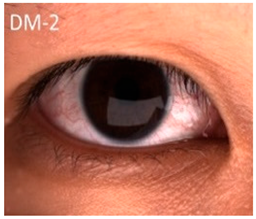
Hyperferritinemia-cataract syndrome (HCS): HCS is a rare autosomal-dominant anterior segment disorder characterized by elevated serum ferritin and early-onset bilateral cataracts, notably in the absence of systemic or tissue iron overload. HCS is caused by mutations in the iron-responsive element (IRE) of the ferritin light chain (FTL) mRNA, which impair the binding affinity of iron regulatory proteins (IRPs) to IRE, and lead to constitutive unregulated overproduction of L-ferritin irrespective of iron status. This dysregulation results in abnormally high serum ferritin concentrations and excessive ferritin accumulation in ocular tissues. Two main hypotheses have been proposed to explain the cataract formation in HCS. One suggests that aggregation of L-ferritin leads to crystalline deposits in the lens causing progressive opacification, while the other proposes that L-ferritin promotes iron accumulation leading to increased ROS and oxidative damage to the lens [
135,
136]. Clinical studies indicate that HCS demonstrates variable expressivity, with patients carrying identical mutations presenting with cataracts of differing severity during childhood, adolescence, or early adulthood, underscoring the influence of genetic and environmental modifiers. In some cases, individuals harboring both FTL and HFE mutations show elevated serum ferritin without evidence of systemic iron overload [
137]. This biochemical overlap often leads to misdiagnosis as HH, since both present with elevated serum ferritin. The misdiagnosis can lead to unnecessary and harmful phlebotomy, which can result in iron deficiency anemia. While cataract surgery is the current treatment for visual impairment in HCS, there are no pharmacological interventions addressing the underlying iron dysregulation. Additionally, preclinical models carrying analogous IRE mutations confirm the causal link but have not yet elucidated the precise pathogenic sequence from ferritin overexpression to lens opacification. Thus, key aspects of HCS pathophysiology remain poorly understood. Future directions include the application of advanced molecular diagnostics, such as next-generation sequencing panels for unexplained hyperferritinemia, to improve early and accurate diagnosis. Deeper mechanistic studies using cellular and animal models are needed to clarify how ferritin dysregulation alters lens proteostasis and oxidative balance. Such insights could ultimately support the development of targeted therapies beyond cataract surgery.
Dry eye disease (DED): Under physiological conditions, the tear film provides a mucin-rich protective layer that shields the cornea from oxidative damage. In DED, hyperosmolarity and chronic inflammation disrupt tear film stability, leading to increased ROS generation within the corneal epithelium [
138]. Emerging evidence implicates iron dysregulation in DED pathogenesis. A study by Lu et al. demonstrated that macrophage depletion using clodronate liposomes significantly reduced iron deposition in the ocular surface by targeting pro-inflammatory M1 macrophages and lowering IL-1β, IL-6, and TNF-α, thereby alleviating the symptoms and pathological changes of DED [
139]. Similarly, in a murine model, iron was shown to trigger ferroptosis in ocular epithelial and glandular cells, further contributing to DED pathogenesis [
140]. Although preclinical studies suggest that iron-mediated oxidative injury and macrophage activation play roles in DED, direct evidence of iron dysregulation in human DED is lacking. Consequently, the therapeutic potential of iron-targeted interventions remains largely experimental, with limited translational relevance. Future research should aim to determine whether iron metabolism is altered in human DED, and if confirmed, explore iron-modulating strategies as potential diagnostic or therapeutic avenues.
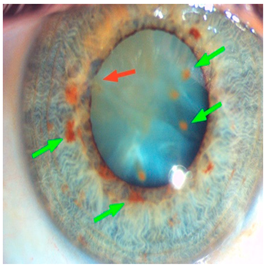
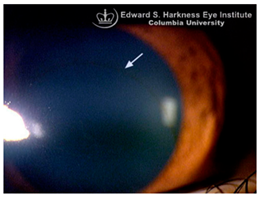
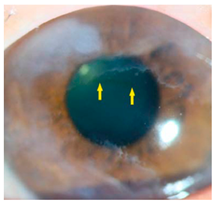
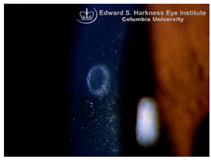
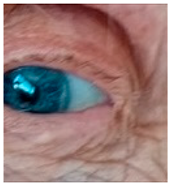
Corneal iron deposition lines (CIDL): Several distinctive CIDL, often referred to as “iron lines” have been identified in the cornea of the anterior segment, namely the Hudson-Stähli line, Fleischer’s ring, Stocker’s line, Ferry’s line, and Coats’ White Ring, each associated with distinct ocular conditions. The Hudson-Stähli line is a benign, pigmented, horizontal iron line within the corneal epithelium at the junction of the middle and lower thirds of the cornea. It is considered a physiological change caused by iron accumulation in the cytoplasm of corneal epithelial cells, most commonly seen in individuals over 50 years of age, and occasionally observed in patients with dry eye disease [
141]. Fleischer’s ring is a circumferential brownish iron line observed as a pigmented ring in keratoconus. Keratoconus is a bilateral ectatic disorder characterized by progressive corneal thinning and protrusion into a cone-like shape. Fleischer’s ring develops at the base of the cone as an iron line. Histopathological studies show ferritin deposits in the cytoplasm of corneal epithelial cells and in the intercellular spaces, likely secondary to altered corneal curvature and tear film dynamics [
142,
143,
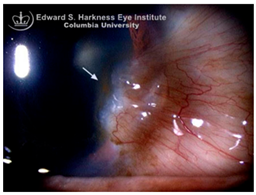
144]. Stocker’s line is a vertical iron line that develops at the leading edge of a pterygium, a fibrovascular growth of conjunctival tissue onto the cornea. Pterygium growth typically occurs on the lateral or nasal side and is hypothesized to be associated with prolonged ultraviolet (UV) exposure, as it is frequently observed in outdoor workers [
145,
146]. The presence of a Stocker’s line representing iron deposition suggests that the pterygium may be in a stationary or slow-growing phase. However, the precise mechanism of iron deposition in Stocker’s line remains unclear [
147]. Ferry’s line is a golden-brown iron line that occurs anterior to a filtration bleb following glaucoma surgery, most commonly trabeculectomy. Trabeculectomy creates an alternative drainage pathway that facilitates the outflow of aqueous humor from the anterior chamber into the subconjunctival space, forming a filtration bleb that subsequently decreases the intraocular pressure. In Ferry’s line, iron deposition occurs in the cytoplasm of basal epithelial cells. Ferry hypothesized that the repeated eyelid movement over an elevated bleb may cause mechanical trauma, eventually leading to iron deposition [
5,
148]. Coats’ white ring is a ring-shaped deposit found below the corneal epithelium or within the Bowman’s layer. It is widely believed to result from prior corneal trauma or retained iron-containing foreign bodies, with the deposits representing residual ferritin-laden macrophages or degenerated epithelial cells [
149,
150]. Collectively, these conditions highlight the diverse ocular manifestations associated with iron deposition, ranging from physiological processes to overt pathological changes. CIDL serve as valuable clinical biomarkers, often reflecting underlying or prior ocular disease, trauma, or surgical intervention. Nonetheless, the precise role of iron in corneal disease pathogenesis remains poorly defined. Most available studies suggest that iron deposition contributes to CIDL progression as an indirect correlation rather than acting as a primary driver. Moreover, the lack of animal models that faithfully replicate localized corneal iron deposition has limited mechanistic exploration. Future research should aim to elucidate the molecular pathways regulating corneal epithelial iron homeostasis and dysfunction, which may clarify whether iron is merely a secondary marker of disease or an active contributor to pathogenesis.
A visual representation of all the anterior segment diseases related to iron overload discussed above are provided in
Table 3.