Synbiotic Supplementation with Probiotics and Omega-3 Fatty Acids Enhances Upper-Body Muscle Strength in Elite Swimmers: Evidence for Gut–Muscle Axis Modulation During Race-Pace Training
Abstract
1. Introduction
2. Methodology
2.1. Participants
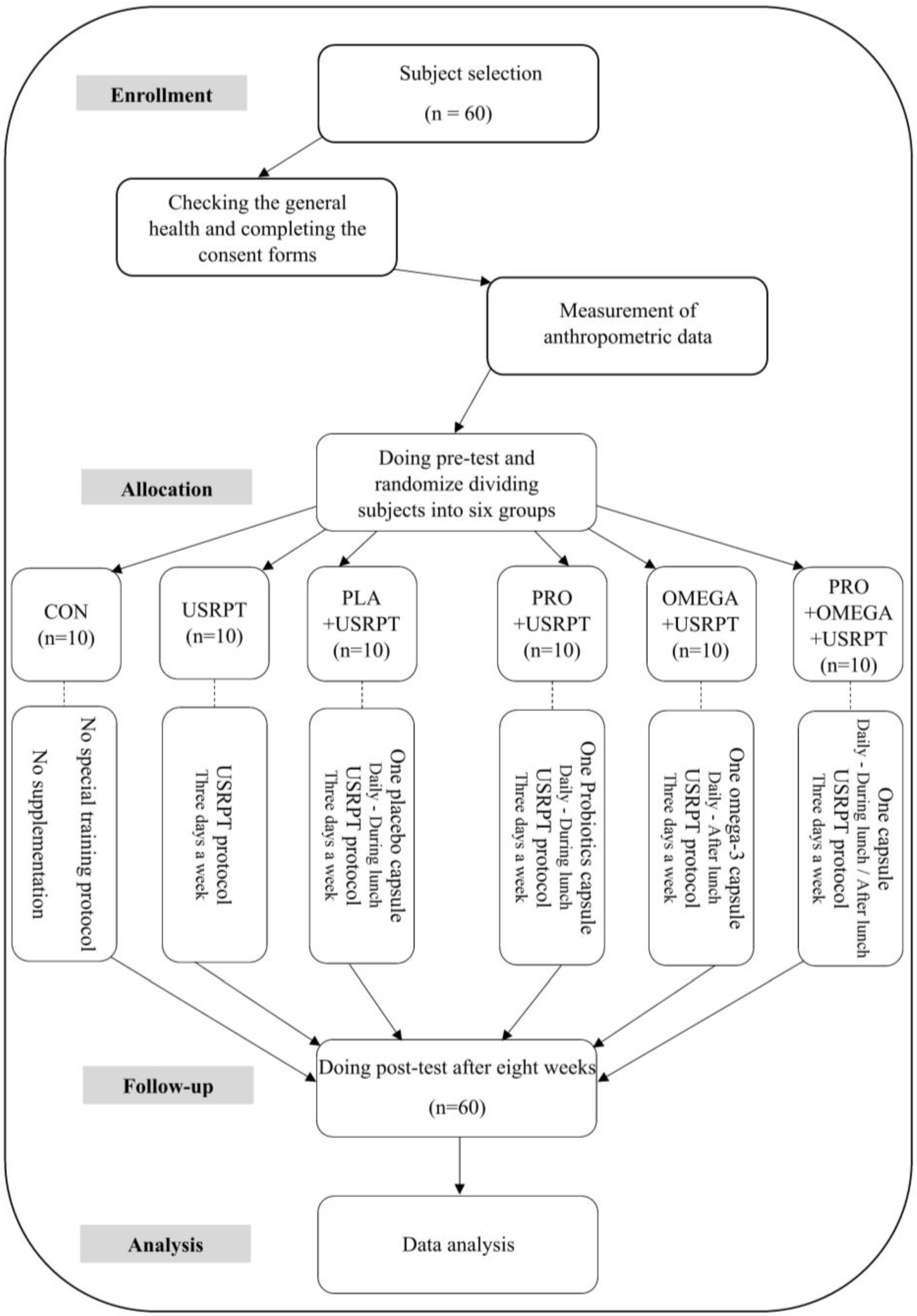
2.2. Sample Size Calculation and Study Design
2.3. Blinding Procedure
2.4. Training Protocol
2.5. Supplementation Protocol
3. Functional Tests
3.1. Handgrip Strength Test
3.2. Backward Overhead Throwing-Medicine-Ball Test
3.3. Dead-Hang Test
3.4. Isokinetic and Isometric Strength Tests
3.5. Statistical Analyses
4. Results
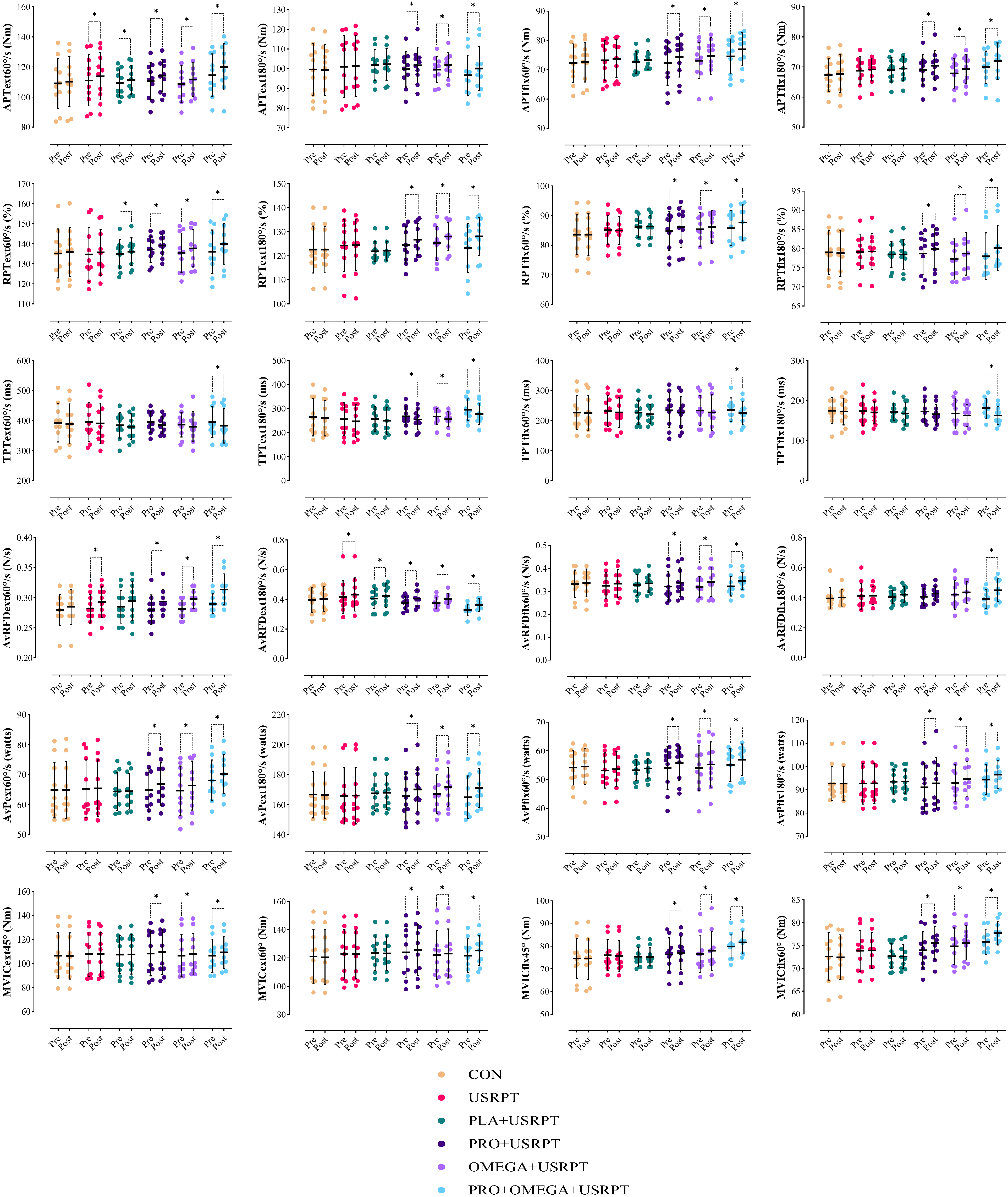
4.1. Functional Tests
4.2. Isokinetic and Isometric Strength Tests
5. Discussion
6. Limitations and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cengiz, Ş.Ş.; Coşkun, E.Ş. Swimming in The Olympics. Int. J. Sport Cult. Sci. 2023, 11, 56–70. [Google Scholar]
- Nugent, F.; Comyns, T.; Kearney, P.; Warrington, G. Ultra-short race-pace training (USRPT) In swimming: Current perspectives. Open Access J. Sports Med. 2019, 10, 133–144. [Google Scholar] [CrossRef]
- Papadimitriou, K. Intensity and Pace Calculation of Ultra Short Race Pace Training (USRPT) in Swimming—Take-Home Messages and Statements for Swimming Coaches. Sports 2024, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Fone, L.; van den Tillaar, R. Effect of different types of strength training on swimming performance in competitive swimmers: A systematic review. Sports Med. Open 2022, 8, 19. [Google Scholar] [CrossRef]
- González, L.R.; Melguizo-Ibáñez, E.; Martín-Moya, R.; González-Valero, G. Study of strength training on swimming performance. A systematic review. Sci. Sports 2023, 38, 217–231. [Google Scholar] [CrossRef]
- Barbosa, T.M.; Bragada, J.A.; Reis, V.M.; Marinho, D.A.; Carvalho, C.; Silva, A.J. Energetics and biomechanics as determining factors of swimming performance: Updating the state of the art. J. Sci. Med. Sport 2010, 13, 262–269. [Google Scholar] [CrossRef]
- Zamparo, P.; Pendergast, D.; Mollendorf, J.; Termin, A.; Minetti, A. An energy balance of front crawl. Eur. J. Appl. Physiol. 2005, 94, 134–144. [Google Scholar] [CrossRef]
- Crowley, E.; Harrison, A.J.; Lyons, M. Dry-land resistance training practices of elite swimming strength and conditioning coaches. J. Strength Cond. Res. 2018, 32, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Chiu, W.-C.; Hsu, Y.-P.; Lo, Y.-L.; Wang, Y.-H. Effects of omega-3 fatty acids on muscle mass, muscle strength and muscle performance among the elderly: A meta-analysis. Nutrients 2020, 12, 3739. [Google Scholar] [CrossRef] [PubMed]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C. α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, C.; Yu, X.; Guo, L.; Wan, X.; Xu, J.; Xiang, X.; Yang, J.; Kang, J.; Deng, Q. Conversion of α-linolenic acid into n-3 long-chain polyunsaturated fatty acids: Bioavailability and dietary regulation. Crit. Rev. Food Sci. Nutr. 2024, 1–33. [Google Scholar] [CrossRef]
- Covington, M.B. Omega-3 fatty acids. Am. Fam. Physician 2004, 70, 133–140. [Google Scholar] [PubMed]
- Rodacki, C.L.; Rodacki, A.L.; Pereira, G.; Naliwaiko, K.; Coelho, I.; Pequito, D.; Fernandes, L.C. Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 2012, 95, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: The STRENGTH randomized clinical trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.; Chan, E.-S.; Pushpamalar, J.; Choo, W.S. Advances in extrusion-dripping encapsulation of probiotics and omega-3 rich oils. Trends Food Sci. Technol. 2022, 123, 69–86. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Imanian, B.; Hemmatinafar, M.; Daryanoosh, F.; Koureshfard, N.; Sadeghi, R.; Niknam, A.; Rezaei, R.; Qashqaei, A. The effect of probiotics and casein supplementation on aerobic capacity parameters of male soccer players. J. Int. Soc. Sports Nutr. 2024, 21, 2382165. [Google Scholar] [CrossRef]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J.J. Gut-muscle axis exists and may affect skeletal muscle adaptation to training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; De Backer, F.; Sohet, F.M.; Dewulf, E.M.; Pachikian, B.D.; Neyrinck, A.M.; Thissen, J.-P.; et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE 2012, 7, e37971. [Google Scholar] [CrossRef]
- Lashkarbolouk, N.; Mazandarani, M.; Pakmehr, A.; Ejtahed, H.-S. Evaluating the role of probiotics, prebiotics, and synbiotics supplementation in age-related musculoskeletal disorders in older adults: A systematic review. Probiotics Antimicrob. Proteins 2024. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef]
- Giron, M.; Thomas, M.; Dardevet, D.; Chassard, C.; Savary-Auzeloux, I. Gut microbes and muscle function: Can probiotics make our muscles stronger? J. Cachexia Sarcopenia Muscle 2022, 13, 1460–1476. [Google Scholar] [CrossRef]
- Marttinen, M.; Ala-Jaakkola, R.; Laitila, A.; Lehtinen, M.J. Gut microbiota, probiotics and physical performance in athletes and physically active individuals. Nutrients 2020, 12, 2936. [Google Scholar] [CrossRef]
- Bielik, V.; Hric, I.; Ugrayová, S.; Kubáňová, L.; Putala, M.; Grznár, Ľ.; Penesová, A.; Havranová, A.; Šardzíková, S.; Grendar, M.; et al. Effect of High-intensity Training and Probiotics on Gut Microbiota Diversity in Competitive Swimmers: Randomized Controlled Trial. Sports Med.-Open 2022, 8, 64. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Kabasakalis, A.; Papadopoulos, A.; Mavridis, G.; Tsalis, G. Comparison of Ultra-Short Race Pace and High-Intensity Interval Training in Age Group Competitive Swimmers. Sports 2023, 11, 186. [Google Scholar] [CrossRef]
- Williamson, D.; McCarthy, E.; Ditroilo, M. Acute physiological responses to ultra short race-pace training in competitive swimmers. J. Hum. Kinet. 2020, 75, 95. [Google Scholar] [CrossRef]
- Rushall, B. Relevant training effects in pool swimming: Ultra-short race-pace training (Revised). Swim. Sci. Bull. 2013, 40b, 11. [Google Scholar]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’REilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K.; Wu, M. Associations among dietary omega-3 polyunsaturated fatty acids, the gut microbiota, and intestinal immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Bhagavathula, A.S.; Aldhaleei, W.A.; Rahmani, J.; Karam, G.; Rinaldi, G.; Clark, C.; Salehisahlabadi, A.; Yuan, Q. Effect of propolis supplementation on C-reactive protein levels and other inflammatory factors: A systematic review and meta-analysis of randomized controlled trials. J. King Saud Univ.-Sci. 2020, 32, 1694–1701. [Google Scholar] [CrossRef]
- Suzuki, K. Recent Progress in Applicability of Exercise Immunology and Inflammation Research to Sports Nutrition. Nutrients 2021, 13, 4299. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmi, M.; Foroumandi, E.; Asghari Jafarabadi, M. Effects of soy intake on circulating levels of TNF-α and interleukin-6: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2021, 60, 581–601. [Google Scholar] [CrossRef] [PubMed]
- Tingö, L.; Hutchinson, A.N.; Bergh, C.; Stiefvatter, L.; Schweinlin, A.; Jensen, M.G.; Krüger, K.; Bischoff, S.C.; Brummer, R.J. Potential Modulation of Inflammation by Probiotic and Omega-3 Supplementation in Elderly with Chronic Low-Grade Inflammation—A Randomized, Placebo-Controlled Trial. Nutrients 2022, 14, 3998. [Google Scholar] [CrossRef]
- Rajkumar, H.; Mahmood, N.; Kumar, M.; Varikuti, S.R.; Challa, H.R.; Myakala, S.P. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: A randomized, controlled trial. Mediat. Inflamm. 2014, 2014, 348959. [Google Scholar] [CrossRef]
- Carbuhn, A.F.; Reynolds, S.M.; Campbell, C.W.; Bradford, L.A.; Deckert, J.A.; Kreutzer, A.; Fry, A.C. Effects of probiotic (Bifidobacterium longum 35624) supplementation on exercise performance, immune modulation, and cognitive outlook in division I female swimmers. Sports 2018, 6, 116. [Google Scholar] [CrossRef]
- Maymandinejad, I.; Hemmatinafar, M.; Jäger, R.; Imanian, B.; Koushkie Jahromi, M.; Suzuki, K. Synergistic Effects of Probiotic and Omega-3 Supplementation with Ultra-Short Race Pace Training on Sprint Swimming Performance. Nutrients 2025, 17, 2296. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, H.; Zhao, L.; Liu, Z.; Chen, L.; Liu, C. Comparison of the Effects of Prebiotics and Synbiotics Supplementation on the Immune Function of Male University Football Players. Nutrients 2023, 15, 1158. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Purpura, M.; Stone, J.D.; Turner, S.M.; Anzalone, A.J.; Eimerbrink, M.J.; Pane, M.; Amoruso, A.; Rowlands, D.S.; Oliver, J.M. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 Supplementation Attenuates Performance and Range-of-Motion Decrements Following Muscle Damaging Exercise. Nutrients 2016, 8, 642. [Google Scholar] [CrossRef]
- Schulz, K.F.; Grimes, D.A. Allocation concealment in randomised trials: Defending against deciphering. Lancet 2002, 359, 614–618. [Google Scholar] [CrossRef]
- Rushall, B. Swimming energy training in the 21st century: The justification for radical changes. Swim. Sci. Bull. 2011, 39, 1–59. [Google Scholar]
- Sadeghi, R.; Hemmatinafar, M.; Eftekhari, F.; Imanian, B.; Koureshfard, N. Pre-sleep casein ingestion with probiotic strains improves anaerobic power and lower-body-specific strength and power performance in soccer players. J. Int. Soc. Sports Nutr. 2025, 22, 2505184. [Google Scholar] [CrossRef]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International society of sports nutrition position stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Guerra, R.; Amaral, T.F. Comparison of hand dynamometers in elderly people. J. Nutr. Health Aging 2009, 13, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, J.L.; Bird, M.; Cole, M.L.; Koch, A.J.; Jacques, J.A.; Ware, J.S.; Buford, B.N.; Fletcher, K.M. Comparison of the backward overhead medicine ball throw to power production in college football players. J. Strength Cond. Res. 2005, 19, 514–518. [Google Scholar]
- Stockbrugger, B.A.; Haennel, R.G. Contributing factors to performance of a medicine ball explosive power test: A comparison between jump and nonjump athletes. J. Strength Cond. Res. 2003, 17, 768–774. [Google Scholar] [CrossRef]
- Winnick, J.P.; Short, F.X. The Brockport Physical Fitness Test Manual; Human Kinetics: Champaign, IL, USA, 1999. [Google Scholar]
- Clemons, J.M. Construct Validity of a Modification of the Flexed Arm Hang Test. J. Strength Cond. Res. 2014, 28, 3523–3530. [Google Scholar] [CrossRef]
- Wang, A.; Doyle, T.; Cunningham, G.; Brutty, M.; Campbell, P.; Bharat, C.; Ackland, T. Isokinetic shoulder strength correlates with level of sports participation and functional activity after reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2016, 25, 1464–1469. [Google Scholar] [CrossRef]
- Ekstrand, E.; Lexell, J.; Brogårdh, C. Isometric and isokinetic muscle strength in the upper extremity can be reliably measured in persons with chronic stroke. J. Rehabil. Med. 2015, 47, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.K.; Watson, M.D.; Davies, G.J.; Riemann, B.L. Relationship between closed kinetic chain upper extremity stability test and isokinetic shoulder flexion and elbow extension work. Athl. Train. Sports Health Care 2021, 13, e383–e387. [Google Scholar] [CrossRef]
- Wiażewicz, A.; Eider, J. The relationship between swimming performance and isokinetic shoulder strength of elite swimmers. Hum. Mov. 2021, 22, 10–19. [Google Scholar] [CrossRef]
- Kuhlman, J.R.; Iannotti, J.P.; Kelly, M.J.; Riegler, F.X.; Gevaert, M.L.; Ergin, T.M. Isokinetic and isometric measurement of strength of external rotation and abduction of the shoulder. J. Bone Jt. Surg. Am. 1992, 74, 1320–1333. [Google Scholar] [CrossRef]
- Otis, J.C.; Warren, R.F.; Backus, S.I.; Santner, T.J.; Mabrey, J.D. Torque production in the shoulder of the normal young adult male: The interaction of function, dominance, joint angle, and angular velocity. Am. J. Sports Med. 1990, 18, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Collado-Mateo, D.; Dominguez-Muñoz, F.J.; Batalha, N.; Parraça, J.; Tomas-Carus, P.; Adsuar, J.C. Test-Retest Reliability of Isokinetic Arm Strength Measurements in Competitive Swimmers. J. Hum. Kinet. 2018, 65, 5–11. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Abingdon, UK, 2013. [Google Scholar]
- Chew, W.; Lim, Y.P.; Lim, W.S.; Chambers, E.S.; Frost, G.; Wong, S.H.; Ali, Y. Gut-muscle crosstalk. A perspective on influence of microbes on muscle function. Front. Med. 2023, 9, 1065365. [Google Scholar] [CrossRef]
- Fontana, F.; Longhi, G.; Tarracchini, C.; Mancabelli, L.; Lugli, G.A.; Alessandri, G.; Turroni, F.; Milani, C.; Ventura, M. The human gut microbiome of athletes: Metagenomic and metabolic insights. Microbiome 2023, 11, 27. [Google Scholar] [CrossRef]
- Aykut, M.N.; Erdoğan, E.N.; Çelik, M.N.; Gürbüz, M. An Updated View of the Effect of Probiotic Supplement on Sports Performance: A Detailed Review. Curr. Nutr. Rep. 2024, 13, 251–263. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Pulido-Mateos, E.C.; Bégin, F.; St-Arnaud, G.; Tinoco Mar, B.A.; Mayer, T.; Dumais, E.; Flamand, N.; Raymond, F.; Roy, D.; et al. Lactiplantibacillus plantarum strengthens the intestinal barrier: Involvement of the endocannabinoidome. Am. J. Physiol.-Gastrointest. Liver Physiol. 2025, 329, G245–G260. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tominaga, T.; Ruhee, R.T.; Ma, S. Characterization and Modulation of Systemic Inflammatory Response to Exhaustive Exercise in Relation to Oxidative Stress. Antioxidants 2020, 9, 401. [Google Scholar] [CrossRef]
- Tong, Y.; Ma, S.; Awa, R.; Tagawa, T.; Seki, Y.; Cao, T.; Kobori, H.; Suzuki, K. Effects of 3-(4-Hydroxy-3-methoxyphenyl)propionic Acid on Regulating Oxidative Stress and Muscle Fiber Composition. Nutrients 2025, 17, 668. [Google Scholar]
- Santibañez-Gutierrez, A.; Fernández-Landa, J.; Calleja-González, J.; Delextrat, A.; Mielgo-Ayuso, J. Effects of Probiotic Supplementation on Exercise with Predominance of Aerobic Metabolism in Trained Population: A Systematic Review, Meta-Analysis and Meta-Regression. Nutrients 2022, 14, 622. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Bo, L.; Zhou, E.; Chen, Y.; Naranmandakh, S.; Xie, W.; Ru, Q.; Chen, L.; Zhu, Z.; et al. Progress of linking gut microbiota and musculoskeletal health: Casualty, mechanisms, and translational values. Gut Microbes 2023, 15, 2263207. [Google Scholar] [CrossRef]
- Aparicio-Pascual, D.; Clemente-Suárez, V.J.; Tornero-Aguilera, J.F.; Rubio-Zarapuz, A. The Effect of Probiotic Supplementation on Cytokine Modulation in Athletes After a Bout of Exercise: A Systematic Review and Meta-Analysis. Sports Med.-Open 2025, 11, 58. [Google Scholar] [PubMed]
- Jäger, R.; Zaragoza, J.; Purpura, M.; Iametti, S.; Marengo, M.; Tinsley, G.M.; Anzalone, A.J.; Oliver, J.M.; Fiore, W.; Biffi, A.; et al. Probiotic administration increases amino acid absorption from plant protein: A placebo-controlled, randomized, double-blind, multicenter, crossover study. Probiotics Antimicrob. Proteins 2020, 12, 1330–1339. [Google Scholar]
- Nazari, M.; Faghfoori, Z.; As’Habi, A.; Arab, A.; Hajianfar, H. Probiotic consumption and inflammatory markers in athletes: A systematic review and meta-analysis. Int. J. Food Prop. 2020, 23, 1402–1415. [Google Scholar] [CrossRef]
- Nami, Y.; Barghi, A.; Shahgolzari, M.; Salehian, M.; Haghshenas, B. Mechanism of action and beneficial effects of probiotics in amateur and professional athletes. Food Sci. Nutr. 2025, 13, e4658. [Google Scholar] [PubMed]
- Jäger, R.; Heileson, J.L.; Abou Sawan, S.; Dickerson, B.L.; Leonard, M.; Kreider, R.B.; Kerksick, C.M.; Cornish, S.M.; Candow, D.G.; Cordingley, D.M.; et al. International society of sports nutrition position stand: Long-chain omega-3 polyunsaturated fatty acids. J. Int. Soc. Sports Nutr. 2025, 22, 2441775. [Google Scholar] [CrossRef] [PubMed]
- Therdyothin, A.; Prokopidis, K.; Galli, F.; Witard, O.C.; Isanejad, M. The effects of omega-3 polyunsaturated fatty acids on muscle and whole-body protein synthesis: A systematic review and meta-analysis. Nutr. Rev. 2025, 83, e131–e143. [Google Scholar]
- Ahmadi, M.; Hoorang, N.; Imanian, B.; Hemmatinafar, M.; Rezaei, R.; Nemati, J.; Eftekhari, F.; Alkasasbeh, W.J. Boosting Recovery: Omega-3 and Whey Protein Enhance Strength and Ease Muscle Soreness in Female Futsal Players. Nutrients 2024, 16, 4263. [Google Scholar] [CrossRef]
- Kopp, L.; Schweinlin, A.; Tingö, L.; Hutchinson, A.N.; Feit, V.; Jähnichen, T.; Lehnert, K.; Vetter, W.; Rings, A.; Jensen, M.G.; et al. Potential modulation of inflammation and physical function by combined probiotics, omega-3 supplementation and vitamin D supplementation in overweight/obese patients with chronic low-grade inflammation: A randomized, placebo-controlled trial. Int. J. Mol. Sci. 2023, 24, 8567. [Google Scholar] [CrossRef]
- Prokopidis, K.; Giannos, P.; Kirwan, R.; Ispoglou, T.; Galli, F.; Witard, O.C.; Triantafyllidis, K.K.; Kechagias, K.S.; Morwani-Mangnani, J.; Ticinesi, A.; et al. Impact of probiotics on muscle mass, muscle strength and lean mass: A systematic review and meta-analysis of randomized controlled trials. J. Cachexia Sarcopenia Muscle 2023, 14, 30–44. [Google Scholar] [CrossRef]
- Lee, C.-C.; Liao, Y.-C.; Lee, M.-C.; Cheng, Y.-C.; Chiou, S.-Y.; Lin, J.-S.; Huang, C.-C.; Watanabe, K. Different impacts of heat-killed and viable Lactiplantibacillus plantarum TWK10 on exercise performance, fatigue, body composition, and gut microbiota in humans. Microorganisms 2022, 10, 2181. [Google Scholar] [CrossRef]
- Li, Y.; Arai, S.; Kato, K.; Iwabuchi, S.; Iwabuchi, N.; Muto, N.; Motobayashi, H.; Ebihara, S.; Tanaka, M.; Hashimoto, S. The Potential Immunomodulatory Effect of Bifidobacterium longum subsp. longum BB536 on Healthy Adults through Plasmacytoid Dendritic Cell Activation in the Peripheral Blood. Nutrients 2023, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- Daneshpour, N. Bifidobacterium and the Immune System: A Key Player Against Gastrointestinal Cancers. J. Microbiota 2024, 1, e154264. [Google Scholar] [CrossRef]
- Yang, K.; Chen, Y.; Wang, M.; Zhang, Y.; Yuan, Y.; Hou, H.; Mao, Y.-H. The Improvement and Related Mechanism of Microecologics on the Sports Performance and Post-Exercise Recovery of Athletes: A Narrative Review. Nutrients 2024, 16, 1602. [Google Scholar] [CrossRef]
- Hasaniani, N.; Ghasemi-Kasman, M.; Halaji, M.; Rostami-Mansoor, S. Bifidobacterium breve probiotic compared to lactobacillus casei causes a better reduction in demyelination and oxidative stress in cuprizone-induced demyelination model of rat. Mol. Neurobiol. 2024, 61, 498–509. [Google Scholar] [CrossRef]
- Takayama, H.; Fukatsu, K.; Takahashi, K.; Noguchi, M.; Watkins, A.; Matsumoto, N.; Murakoshi, S. Influences of a fermented milk with Lactobacillus bulgaricus and Streptococcus thermophiles on gut associated lymphoid tissue, mucosal IgA, and gut flora in mice. Clin. Nutr. Open Sci. 2022, 42, 36–48. [Google Scholar] [CrossRef]
- Batista, R.A.; de Branco, F.M.; Nehme, R.; de Oliveira, E.P.; Pena, G.d.G. Association between plasma omega-3 and handgrip strength according to glycohemoglobin levels in older adults: Results from NHANES 2011–2012. Nutrients 2022, 14, 4060. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.R.; Bender, D.; Vantrease, W.C.; Sapp, P.A.; Toy, A.M.; Woods, C.A.; Johnson, K.D. Effects of probiotic (Bacillus subtilis DE111) supplementation on immune function, hormonal status, and physical performance in division I baseball players. Sports 2018, 6, 70. [Google Scholar] [CrossRef]
- de Paiva, A.K.; de Oliveira, E.P.; Mancini, L.; Paoli, A.; Mota, J.F. Effects of probiotic supplementation on performance of resistance and aerobic exercises: A systematic review. Nutr. Rev. 2023, 81, 153–167. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Arribalzaga, S.; Gutiérrez-Abejón, E.; Azarbayjani, M.A.; Mielgo-Ayuso, J.; Roche, E. Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults—A Systematic Review of Randomized Controlled Trials. Nutrients 2024, 16, 2044. [Google Scholar] [CrossRef]
- Sánchez Macarro, M.; Ávila-Gandía, V.; Pérez-Piñero, S.; Cánovas, F.; García-Muñoz, A.M.; Abellán-Ruiz, M.S.; Victoria-Montesinos, D.; Luque-Rubia, A.; Climent, E.; Genovés, S.; et al. Antioxidant effect of a probiotic product on a model of oxidative stress induced by high-intensity and duration physical exercise. Antioxidants 2021, 10, 323. [Google Scholar] [CrossRef]
- Mangine, G.T.; Hoffman, J.R.; Wang, R.; Gonzalez, A.M.; Townsend, J.R.; Wells, A.J.; Jajtner, A.R.; Beyer, K.S.; Boone, C.H.; Miramonti, A.A.; et al. Resistance training intensity and volume affect changes in rate of force development in resistance-trained men. Eur. J. Appl. Physiol. 2016, 116, 2367–2374. [Google Scholar] [CrossRef]


| Characteristic | Mean ± SD (n = 60) |
|---|---|
| Age (years) | 19.20 ± 3.64 |
| Height (cm) | 182.20 ± 5.21 |
| Weight (kg) | 81.60 ± 4.42 |
| Strains | Dosage (CFU) |
|---|---|
| Lactiplantibacillus plantarum BP06 | 0.43 × 1011 |
| Lacticaseibacillus casei BP07 | 0.65 × 1011 |
| Lactobacillus acidophilus BA05 | 0.94 × 1011 |
| Lactobacillus bulgaricus BD08 | 0.36 × 1011 |
| Bifidobacterium infantis BI04 | 0.57 × 1011 |
| Bifidobacterium longum BL03 | 0.73 × 1011 |
| Bifidobacterium breve BB02 | 0.62 × 1011 |
| Streptococcus thermophilus BT01 | 0.20 × 1011 |
| Total | 4.5 × 1011 |
| CON | USRPT | PLA + USRPT | OMEGA + USRPT | PRO + USRPT | PRO + OMEGA + USRPT | |||
|---|---|---|---|---|---|---|---|---|
| DHGS (kg) | Mean | Pre | 52.60 | 52.50 | 53.40 | 52.10 | 51.80 | 52.30 |
| SD | 4.37 | 4.08 | 5.77 | 4.48 | 5.32 | 6.05 | ||
| Mean | Post | 53.50 | 54.10 | 55.00 | 55.90 | 54.70 | 56.90 | |
| SD | 4.50 | 3.51 | 6.09 | 6.13 | 5.81 | 6.69 | ||
| %Δ (post vs. pre) | 1.71% | 3.05% | 3.00% | 7.29% | 5.60% | 8.80% | ||
| NDHGS (kg) | Mean | Pre | 50.10 | 50.60 | 50.10 | 50.80 | 50.50 | 50.40 |
| SD | 4.33 | 4.62 | 5.48 | 4.93 | 4.74 | 5.18 | ||
| Mean | Post | 51.10 | 51.20 | 49.90 | 53.10 | 50.80 | 52.40 | |
| SD | 5.21 | 4.36 | 6.96 | 5.36 | 5.32 | 5.23 | ||
| %Δ (post vs. pre) | 2.00% | 1.19% | −0.40% | 4.53% | 0.59% | 3.97% | ||
| TMB (m) | Mean | Pre | 9.85 | 9.74 | 9.73 | 9.79 | 9.84 | 9.71 |
| SD | 1.08 | 0.76 | 0.81 | 0.89 | 0.82 | 0.78 | ||
| Mean | Post | 10.00 | 9.87 | 9.83 | 10.01 | 10.02 | 9.94 | |
| SD | 0.96 | 0.89 | 0.76 | 0.83 | 0.76 | 0.77 | ||
| %Δ (post vs. pre) | 1.52% | 1.33% | 1.03% | 2.25% | 1.83% | 2.37% | ||
| SEDH (kg) | Mean | Pre | 98.30 | 95.50 | 97.90 | 96.40 | 93.10 | 96.60 |
| SD | 26.17 | 24.61 | 25.97 | 30.46 | 24.66 | 26.03 | ||
| Mean | Post | 98.70 | 97.00 | 99.40 | 98.20 | 94.90 | 98.90 | |
| SD | 25.57 | 23.68 | 24.81 | 28.84 | 23.71 | 25.97 | ||
| %Δ (post vs. pre) | 0.41% | 1.57% | 1.53% | 1.87% | 1.93% | 2.38% | ||
| FEDH (kg) | Mean | Pre | 50.10 | 48.40 | 49.80 | 49.80 | 47.40 | 48.20 |
| SD | 13.41 | 10.36 | 10.52 | 13.13 | 12.44 | 11.93 | ||
| Mean | Post | 50.80 | 49.90 | 51.40 | 51.80 | 49.00 | 51.50 | |
| SD | 12.34 | 10.20 | 10.27 | 13.03 | 12.58 | 11.83 | ||
| %Δ (post vs. pre) | 1.40% | 3.10% | 3.21% | 4.02% | 3.38% | 6.85% | ||
| APText60°/s (Nm) | Mean | Pre | 109.03 | 111.10 | 109.32 | 110.68 | 108.55 | 114.54 |
| SD | 16.79 | 17.0 | 9.31 | 12.20 | 12.23 | 14.11 | ||
| Mean | Post | 110.26 | 113.87 | 111.22 | 114.17 | 111.74 | 120.04 | |
| SD | 16.76 | 15.80 | 9.66 | 10.71 | 11.39 | 15.35 | ||
| %Δ (post vs. pre) | 1.13% | 2.49% | 1.74% | 3.15% | 2.94% | 4.80% | ||
| APText180°/s (Nm) | Mean | Pre | 99.70 | 101.06 | 101.92 | 99.84 | 99.49 | 96.73 |
| SD | 13.21 | 15.07 | 7.88 | 9.08 | 6.61 | 10.18 | ||
| Mean | Post | 99.45 | 101.43 | 102.29 | 101.81 | 101.92 | 100.14 | |
| SD | 12.90 | 15.37 | 8.03 | 8.99 | 6.24 | 11.05 | ||
| %Δ (post vs. pre) | −0.25% | 0.37% | 0.36% | 1.97% | 2.44% | 3.53% | ||
| APTflx60°/s (Nm) | Mean | Pre | 72.22 | 73.21 | 72.56 | 72.27 | 73.12 | 74.57 |
| SD | 6.60 | 6.76 | 3.67 | 7.53 | 6.14 | 5.97 | ||
| Mean | Post | 72.60 | 73.73 | 73.35 | 74.28 | 74.64 | 77.00 | |
| SD | 6.77 | 6.47 | 3.17 | 6.75 | 6.36 | 5.67 | ||
| %Δ (post vs. pre) | 0.53% | 0.71% | 1.09% | 2.78% | 2.08% | 3.26% | ||
| APTflx180°/s (Nm) | Mean | Pre | 67.36 | 68.76 | 69.03 | 69.01 | 67.88 | 69.93 |
| SD | 5.40 | 4.60 | 4.02 | 5.16 | 4.91 | 5.39 | ||
| Mean | Post | 67.75 | 69.18 | 69.49 | 70.40 | 69.26 | 71.99 | |
| SD | 6.31 | 3.80 | 3.92 | 5.00 | 4.87 | 5.99 | ||
| %Δ (post vs. pre) | 0.58% | 0.61% | 0.67% | 2.01% | 2.03% | 2.95% | ||
| RPText60°/s (%) | Mean | Pre | 135.16 | 134.77 | 134.87 | 137.25 | 135.60 | 136.05 |
| SD | 12.23 | 13.53 | 7.28 | 6.39 | 9.79 | 10.92 | ||
| Mean | Post | 135.93 | 135.76 | 136.08 | 139.20 | 137.81 | 140.03 | |
| SD | 12.31 | 11.44 | 6.91 | 5.31 | 9.31 | 10.16 | ||
| %Δ (post vs. pre) | 0.57% | 0.73% | 0.90% | 1.42% | 1.63% | 2.93% | ||
| RPText180°/s (%) | Mean | Pre | 122.64 | 124.31 | 121.87 | 124.55 | 125.26 | 123.22 |
| SD | 9.76 | 10.81 | 4.19 | 7.56 | 7.08 | 10.23 | ||
| Mean | Post | 122.59 | 124.53 | 122.13 | 126.75 | 128.06 | 128.14 | |
| SD | 9.66 | 10.74 | 3.89 | 6.73 | 5.97 | 7.81 | ||
| %Δ (post vs. pre) | −0.04% | 0.18% | 0.21% | 1.77% | 2.24% | 3.99% | ||
| RPTflx60°/s (%) | Mean | Pre | 83.55 | 85.15 | 86.23 | 84.77 | 85.37 | 85.77 |
| SD | 6.78 | 4.86 | 3.62 | 6.51 | 5.71 | 5.90 | ||
| Mean | Post | 83.55 | 84.95 | 86.25 | 86.13 | 86.22 | 87.71 | |
| SD | 7.12 | 4.61 | 3.87 | 6.92 | 5.51 | 6.13 | ||
| %Δ (post vs. pre) | 0.00% | −0.23% | 0.02% | 1.60% | 1.00% | 2.26% | ||
| RPTflx180°/s (%) | Mean | Pre | 79.00 | 79.12 | 78.49 | 78.69 | 77.29 | 78.05 |
| SD | 5.78 | 4.65 | 3.41 | 5.36 | 5.23 | 6.05 | ||
| Mean | Post | 78.82 | 79.24 | 78.54 | 79.97 | 78.67 | 80.14 | |
| SD | 6.01 | 4.80 | 3.89 | 5.16 | 5.55 | 5.84 | ||
| %Δ (post vs. pre) | −0.23% | 0.15% | 0.06% | 1.63% | 1.79% | 2.68% | ||
| TPText60°/s (ms) | Mean | Pre | 393.00 | 396.00 | 385.00 | 396.00 | 387.00 | 396.00 |
| SD | 63.77 | 64.67 | 43.01 | 35.33 | 41.10 | 51.03 | ||
| Mean | Post | 390.00 | 391.00 | 380.00 | 386.00 | 380.00 | 383.00 | |
| SD | 66.16 | 67.56 | 42.16 | 28.75 | 49.66 | 57.35 | ||
| %Δ (post vs. pre) | −0.76% | −1.26% | −1.30% | −2.53% | −1.81% | −3.28% | ||
| TPText180°/s (ms) | Mean | Pre | 265.00 | 256.00 | 258.00 | 268.00 | 267.00 | 296.00 |
| SD | 77.92 | 68.01 | 51.81 | 40.77 | 32.67 | 44.77 | ||
| Mean | Post | 260.00 | 248.00 | 250.00 | 255.00 | 256.00 | 279.00 | |
| SD | 73.78 | 69.08 | 55.57 | 40.62 | 31.69 | 44.33 | ||
| %Δ (post vs. pre) | −1.89% | −3.13% | −3.10% | −4.85% | −4.12% | −5.74% | ||
| TPTflx60°/s (ms) | Mean | Pre | 227.00 | 232.00 | 227.00 | 235.00 | 234.00 | 236.00 |
| SD | 55.38 | 50.50 | 39.45 | 57.59 | 50.15 | 39.77 | ||
| Mean | Post | 225.00 | 228.00 | 222.00 | 227.00 | 228.00 | 226.00 | |
| SD | 57.39 | 50.06 | 31.90 | 53.34 | 60.33 | 37.17 | ||
| %Δ (post vs. pre) | −0.88% | −1.72% | −2.20% | −3.40% | −2.56% | −4.24% | ||
| TPTflx180°/s (ms) | Mean | Pre | 175.00 | 174.00 | 172.00 | 173.00 | 168.00 | 181.00 |
| SD | 32.40 | 35.65 | 22.01 | 30.93 | 36.75 | 24.69 | ||
| Mean | Post | 173.00 | 171.00 | 168.00 | 166.00 | 163.00 | 163.00 | |
| SD | 33.35 | 27.26 | 28.59 | 25.03 | 28.30 | 22.63 | ||
| %Δ (post vs. pre) | −1.14% | −1.72% | −2.33% | −4.05% | −2.98% | −9.94% | ||
| AvRFDext60°/s (N/s) | Mean | Pre | 0.28 | 0.28 | 0.28 | 0.28 | 0.28 | 0.29 |
| SD | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | ||
| Mean | Post | 0.28 | 0.29 | 0.29 | 0.29 | 0.29 | 0.31 | |
| SD | 0.02 | 0.02 | 0.03 | 0.01 | 0.01 | 0.02 | ||
| %Δ (post vs. pre) | 0.00% | 3.57% | 3.57% | 3.57% | 3.57% | 6.90% | ||
| AvRFDext180°/s (N/s) | Mean | Pre | 0.39 | 0.41 | 0.40 | 0.37 | 0.37 | 0.33 |
| SD | 0.08 | 0.11 | 0.06 | 0.04 | 0.03 | 0.03 | ||
| Mean | Post | 0.40 | 0.43 | 0.42 | 0.40 | 0.40 | 0.36 | |
| SD | 0.08 | 0.11 | 0.08 | 0.05 | 0.03 | 0.04 | ||
| %Δ (post vs. pre) | 2.56% | 4.88% | 5.00% | 8.11% | 8.11% | 9.09% | ||
| AvRFDflx60°/s (N/s) | Mean | Pre | 0.33 | 0.32 | 0.32 | 0.32 | 0.32 | 0.32 |
| SD | 0.05 | 0.05 | 0.04 | 0.06 | 0.04 | 0.04 | ||
| Mean | Post | 0.33 | 0.33 | 0.33 | 0.33 | 0.34 | 0.34 | |
| SD | 0.05 | 0.06 | 0.04 | 0.06 | 0.06 | 0.03 | ||
| %Δ (post vs. pre) | 0.00% | 3.13% | 3.13% | 3.13% | 6.25% | 6.25% | ||
| AvRFDflx180°/s (N/s) | Mean | Pre | 0.39 | 0.41 | 0.40 | 0.40 | 0.42 | 0.39 |
| SD | 0.07 | 0.08 | 0.04 | 0.05 | 0.09 | 0.06 | ||
| Mean | Post | 0.40 | 0.41 | 0.42 | 0.42 | 0.43 | 0.45 | |
| SD | 0.05 | 0.05 | 0.05 | 0.04 | 0.07 | 0.07 | ||
| %Δ (post vs. pre) | 2.56% | 0.00% | 5.00% | 5.00% | 2.38% | 15.38% | ||
| AvPext60°/s (watts) | Mean | Pre | 64.87 | 65.32 | 64.41 | 64.93 | 64.66 | 68.09 |
| SD | 9.28 | 9.55 | 6.25 | 7.21 | 8.21 | 6.81 | ||
| Mean | Post | 64.94 | 65.44 | 64.49 | 66.86 | 66.36 | 70.16 | |
| SD | 9.46 | 9.40 | 5.95 | 7.08 | 8.18 | 6.51 | ||
| %Δ (post vs. pre) | 0.11% | 0.18% | 0.12% | 2.97% | 2.63% | 3.04% | ||
| AvPext180°/s (watts) | Mean | Pre | 166.68 | 165.97 | 167.57 | 165.63 | 167.08 | 165.06 |
| SD | 15.46 | 18.84 | 11.47 | 15.94 | 12.77 | 14.06 | ||
| Mean | Post | 166.33 | 166.01 | 167.98 | 170.17 | 171.77 | 171.16 | |
| SD | 15.44 | 18.80 | 11.22 | 15.08 | 13.17 | 13.04 | ||
| %Δ (post vs. pre) | −0.21% | 0.02% | 0.24% | 2.74% | 2.81% | 3.70% | ||
| AvPflx60°/s (watts) | Mean | Pre | 54.17 | 53.16 | 53.30 | 54.11 | 54.01 | 55.04 |
| SD | 6.04 | 6.11 | 3.69 | 7.52 | 7.96 | 5.74 | ||
| Mean | Post | 54.50 | 53.62 | 53.87 | 55.69 | 55.24 | 56.94 | |
| SD | 6.22 | 6.11 | 3.53 | 6.05 | 7.87 | 5.45 | ||
| %Δ (post vs. pre) | 0.61% | 0.87% | 1.07% | 2.92% | 2.28% | 3.45% | ||
| AvPflx180°/s (watts) | Mean | Pre | 92.69 | 92.64 | 93.50 | 91.05 | 92.94 | 94.41 |
| SD | 7.36 | 8.75 | 5.32 | 10.42 | 8.10 | 6.44 | ||
| Mean | Post | 92.75 | 92.77 | 93.59 | 92.84 | 94.58 | 96.54 | |
| SD | 7.41 | 8.70 | 5.21 | 11.02 | 7.59 | 5.95 | ||
| %Δ (post vs. pre) | 0.06% | 0.14% | 0.10% | 1.97% | 1.76% | 2.26% | ||
| MVICext45° (Nm) | Mean | Pre | 106.57 | 107.73 | 107.50 | 108.35 | 106.51 | 106.59 |
| SD | 19.13 | 18.51 | 15.80 | 18.63 | 17.63 | 13.73 | ||
| Mean | Post | 106.31 | 107.72 | 107.60 | 109.65 | 107.76 | 109.35 | |
| SD | 19.20 | 18.11 | 16.40 | 18.53 | 17.19 | 13.39 | ||
| %Δ (post vs. pre) | −0.24% | −0.01% | 0.09% | 1.20% | 1.17% | 2.59% | ||
| MVICext60° (Nm) | Mean | Pre | 121.01 | 122.63 | 123.38 | 124.08 | 122.16 | 121.55 |
| SD | 19.30 | 17.59 | 12.20 | 18.13 | 17.24 | 12.04 | ||
| Mean | Post | 120.55 | 122.60 | 123.26 | 125.58 | 123.14 | 125.39 | |
| SD | 19.34 | 17.25 | 12.31 | 18.17 | 17.38 | 10.51 | ||
| %Δ (post vs. pre) | −0.38% | −0.02% | −0.99% | 1.21% | 0.80% | 3.16% | ||
| MVICflx45° (Nm) | Mean | Pre | 74.53 | 76.06 | 75.35 | 76.38 | 76.64 | 79.83 |
| SD | 8.83 | 6.80 | 4.13 | 7.28 | 8.23 | 5.47 | ||
| Mean | Post | 74.51 | 75.68 | 75.28 | 77.13 | 77.96 | 81.71 | |
| SD | 9.11 | 6.94 | 3.97 | 7.29 | 8.53 | 4.93 | ||
| %Δ (post vs. pre) | −0.03% | −0.50% | −0.09% | 0.98% | 1.72% | 2.36% | ||
| MVICflx60° (Nm) | Mean | Pre | 72.61 | 73.86 | 72.68 | 73.96 | 74.83 | 75.80 |
| SD | 5.21 | 4.45 | 2.77 | 4.02 | 4.01 | 2.80 | ||
| Mean | Post | 72.42 | 73.89 | 72.58 | 75.54 | 75.60 | 77.70 | |
| SD | 4.90 | 3.95 | 2.68 | 3.65 | 3.77 | 2.57 | ||
| %Δ (post vs. pre) | −0.26% | 0.04% | −0.14% | 2.14% | 1.03% | 2.51% | ||
| Variables | CON | USRPT | PLA + USRPT | PRO + USRPT | OMEGA + USRPT | PRO + OMEGA + USRPT | ||
|---|---|---|---|---|---|---|---|---|
| Post | ||||||||
| DHGS (kg) | MD | Pre | 0.90 | 1.60 | 1.60 | 3.80 | 2.90 | 4.60 |
| Sig | 0.297 | 0.067 | 0.067 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −0.81–2.61 | −0.11–3.31 | −0.11–3.31 | 2.08–5.51 | 1.18–4.61 | 2.88–6.31 | ||
| NDHGS (kg) | MD | Pre | 1.00 | 0.60 | −0.20 | 2.30 | 0.30 | 2.00 |
| Sig | 0.266 | 0.466 | 0.807 | 0.007 | 0.715 | 0.018 | ||
| 95%CI | −0.63–2.63 | −1.03–2.23 | −1.83–1.43 | 0.66–3.93 | −1.33–1.93 | 0.36–3.63 | ||
| TMB (m) | MD | Pre | 0.14 | 0.13 | 0.10 | 0.22 | 0.18 | 0.23 |
| Sig | 0.114 | 0.156 | 0.273 | 0.018 | 0.041 | 0.014 | ||
| 95%CI | −0.03–0.32 | −0.05–0.31 | −0.08–0.28 | 0.03–0.40 | 0.01–0.36 | 0.04–0.41 | ||
| SEDH (kg) | MD | Pre | 0.40 | 1.50 | 1.50 | 1.80 | 1.80 | 2.30 |
| Sig | 0.678 | 0.123 | 0.123 | 0.065 | 0.065 | 0.020 | ||
| 95%CI | −1.51–2.31 | −0.41–3.41 | −0.41–3.41 | −0.11–3.71 | −0.11–3.71 | 0.38–4.21 | ||
| FEDH (kg) | MD | Pre | 0.70 | 1.50 | 1.60 | 2.00 | 1.60 | 3.30 |
| Sig | 0.337 | 0.043 | 0.031 | 0.008 | 0.031 | 0.001 | ||
| 95%CI | −0.74–2.14 | 0.05–2.94 | 0.15–3.04 | 0.55–3.44 | 0.15–3.04 | 1.85–4.74 | ||
| APText60°/s (Nm) | MD | Pre | 1.23 | 2.77 | 1.90 | 3.49 | 3.19 | 5.50 |
| Sig | 0.101 | 0.001 | 0.013 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −0.24–2.70 | 1.29–4.24 | 0.42–3.37 | 2.01–4.96 | 1.71–4.66 | 4.02–6.97 | ||
| APText180°/s (Nm) | MD | Pre | −0.25 | 0.37 | 0.37 | 1.97 | 2.43 | 3.41 |
| Sig | 0.601 | 0.440 | 0.440 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −1.20–0.70 | −0.58–1.32 | −0.58–1.32 | 1.01–2.92 | 1.45–3.38 | 2.45–4.36 | ||
| APTflx60°/s (Nm) | MD | Pre | 0.38 | 0.52 | 0.79 | 2.01 | 1.52 | 2.43 |
| Sig | 0.339 | 0.192 | 0.060 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −0.41–1.17 | −0.27–1.31 | −0.01–1.58 | 1.22–2.80 | 0.73–2.31 | 1.64–3.22 | ||
| APTflx180°/s (Nm) | MD | Pre | 0.39 | 0.42 | 0.46 | 1.39 | 1.38 | 2.06 |
| Sig | 0.366 | 0.330 | 0.287 | 0.002 | 0.002 | 0.001 | ||
| 95%CI | −0.46–1.24 | −0.43–1.27 | −0.39–1.31 | 0.53–2.24 | 0.52–2.23 | 1.20–2.91 | ||
| RPText60°/s (%) | MD | Pre | 0.77 | 0.99 | 1.21 | 1.95 | 2.21 | 3.98 |
| Sig | 0.150 | 0.066 | 0.026 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −0.28–1.82 | −0.06–2.04 | 0.15–2.26 | 0.89–3.00 | 1.15–3.26 | 2.92–5.03 | ||
| RPText180°/s (%) | MD | Pre | −0.05 | 0.22 | 0.26 | 2.20 | 2.80 | 4.92 |
| Sig | 0.927 | 0.689 | 0.636 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −1.14–1.04 | −0.87–1.31 | −0.83–1.35 | 1.10–3.29 | 1.70–3.89 | 3.82–6.01 | ||
| RPTflx60°/s (%) | MD | Pre | 0.00 | −0.20 | 0.02 | 1.36 | 0.85 | 1.94 |
| Sig | 1.000 | 0.466 | 0.942 | 0.001 | 0.003 | 0.001 | ||
| 95%CI | −0.54–0.54 | −0.74–0.34 | −0.52–0.56 | 0.81–1.90 | 0.30–1.39 | 1.39–2.48 | ||
| RPTflx180°/s (%) | MD | Pre | −0.18 | 0.12 | 0.05 | 1.28 | 1.38 | 2.09 |
| Sig | 0.358 | 0.539 | 0.798 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −0.56–0.20 | −0.50–0.26 | −0.43–0.33 | 0.89–1.66 | 0.99–1.76 | 1.70–2.47 | ||
| TPText60°/s (ms) | MD | Pre | −3.00 | −5.00 | −5.00 | −10.00 | −7.00 | −13.00 |
| Sig | 0.614 | 0.402 | 0.402 | 0.097 | 0.242 | 0.032 | ||
| 95%CI | −14.87–8.87 | −16.87–6.87 | −16.87–6.87 | −21.87–1.87 | −18.87–4.87 | −24.87–−1.13 | ||
| TPText180°/s (ms) | MD | Pre | −5.00 | −8.00 | −8.00 | −13.00 | −11.00 | −17.00 |
| Sig | 0.270 | 0.080 | 0.080 | 0.005 | 0.018 | 0.001 | ||
| 95%CI | −13.99–3.99 | −16.99–0.99 | −16.99–0.99 | −21.99–−4.00 | −19.99–−2.00 | −25.99–−8.00 | ||
| TPTflx60°/s (ms) | MD | Pre | −2.00 | −4.00 | −5.00 | −8.00 | −6.00 | −10.00 |
| Sig | 0.668 | 0.393 | 0.287 | 0.091 | 0.202 | 0.036 | ||
| 95%CI | −11.31–7.31 | −13.31–5.31 | −14.31–4.31 | −17.31–1.31 | −15.31–3.31 | −19.31–−0.68 | ||
| TPTflx180°/s (ms) | MD | Pre | −2.00 | −3.00 | −4.00 | −7.00 | −5.00 | −18.00 |
| Sig | 0.694 | 0.556 | 0.433 | 0.172 | 0.328 | 0.001 | ||
| 95%CI | −12.14–8.14 | −13.14–7.14 | −14.14–6.14 | −17.14–3.14 | −15.14–5.14 | −28.14–−7.85 | ||
| AvRFDext60°/s (N/s) | MD | Pre | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 |
| Sig | 0.347 | 0.042 | 0.063 | 0.010 | 0.002 | 0.001 | ||
| 95%CI | −0.01–0.01 | 0.01–0.02 | −0.01–0.02 | 0.01–0.02 | 0.01–0.02 | 0.01–0.03 | ||
| AvRFDext180°/s (N/s) | MD | Pre | 0.00 | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 |
| Sig | 0.520 | 0.043 | 0.017 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −0.01–0.02 | 0.01–0.03 | 0.01–0.03 | 0.01–0.04 | 0.01–0.04 | 0.01–0.04 | ||
| AvRFDflx60°/s (N/s) | MD | Pre | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.02 |
| Sig | 0.507 | 0.235 | 0.354 | 0.020 | 0.005 | 0.002 | ||
| 95%CI | −0.01–0.02 | −0.01–0.02 | −0.01–0.02 | 0.01–0.03 | 0.01–0.03 | 0.01–0.04 | ||
| AvRFDflx180°/s (N/s) | MD | Pre | 0.00 | 0.00 | 0.01 | 0.02 | 0.01 | 0.05 |
| Sig | 0.719 | 0.829 | 0.252 | 0.135 | 0.252 | 0.001 | ||
| 95%CI | −0.02–0.03 | −0.02–0.03 | −0.01–0.04 | −0.01–0.04 | −0.01–0.04 | 0.02–0.08 | ||
| AvPext60°/s (watts) | MD | Pre | 0.07 | 0.12 | 0.08 | 1.93 | 1.73 | 2.07 |
| Sig | 0.758 | 0.598 | 0.725 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −0.38–0.52 | −0.33–0.57 | −0.37–0.53 | 1.47–2.38 | 1.27–2.18 | 1.61–2.52 | ||
| AvPext180°/s (watts) | MD | Pre | −0.35 | 0.04 | 0.41 | 4.54 | 4.69 | 6.10 |
| Sig | 0.485 | 0.936 | 0.414 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −1.34–0.64 | −0.95–1.03 | −0.58–1.40 | 3.54–5.53 | 3.69–5.68 | 5.10–7.09 | ||
| AvPflx60°/s (watts) | MD | Pre | 0.33 | 0.46 | 0.57 | 1.58 | 1.23 | 1.90 |
| Sig | 0.313 | 0.161 | 0.084 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −0.32–0.98 | −0.19–1.11 | −0.08–1.22 | 0.93–2.23 | 0.58–1.88 | 1.25–2.55 | ||
| AvPflx180°/s (watts) | MD | Pre | 0.06 | 0.13 | 0.09 | 1.79 | 1.64 | 2.13 |
| Sig | 0.829 | 0.639 | 0.745 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −0.49–0.61 | −0.42–0.68 | −0.46–0.64 | 1.23–2.34 | 1.08–2.19 | 1.57–2.68 | ||
| MVICext45° (Nm) | MD | Pre | −0.26 | −0.01 | 0.10 | 1.30 | 1.25 | 2.76 |
| Sig | 0.256 | 0.965 | 0.660 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −0.71–0.19 | −0.46–0.44 | −0.35–0.55 | 0.84–1.75 | 0.79–1.70 | 2.30–3.21 | ||
| MVICext60° (Nm) | MD | Pre | −0.46 | −0.03 | −0.12 | 1.50 | 0.98 | 3.84 |
| Sig | 0.200 | 0.933 | 0.736 | 0.001 | 0.008 | 0.001 | ||
| 95%CI | −1.17–0.25 | −0.74–0.68 | −0.83–0.59 | 0.79–2.21 | 0.27–1.69 | 3.31–4.55 | ||
| MVICflx45° (Nm) | MD | Pre | −0.02 | −0.03 | −0.07 | 0.75 | 1.32 | 1.88 |
| Sig | 0.928 | 0.090 | 0.752 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −0.46–0.42 | −0.82–0.06 | −0.51–0.37 | 0.30–1.19 | 0.87–1.76 | 1.43–2.32 | ||
| MVICflx60° (Nm) | MD | Pre | −0.19 | 0.03 | −0.10 | 1.58 | 0.77 | 1.90 |
| Sig | 0.382 | 0.890 | 0.645 | 0.001 | 0.001 | 0.001 | ||
| 95%CI | −0.662–0.24 | −0.40–0.46 | −0.53–0.33 | 1.14–2.01 | 0.33–1.20 | 1.46–2.33 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imanian, B.; Hemmatinafar, M.; Maymandinejad, I.; Binazade, M.R.; Jäger, R.; Jahan, Z.; Naseri, K.; Rezaei, R.; Suzuki, K. Synbiotic Supplementation with Probiotics and Omega-3 Fatty Acids Enhances Upper-Body Muscle Strength in Elite Swimmers: Evidence for Gut–Muscle Axis Modulation During Race-Pace Training. Nutrients 2025, 17, 2959. https://doi.org/10.3390/nu17182959
Imanian B, Hemmatinafar M, Maymandinejad I, Binazade MR, Jäger R, Jahan Z, Naseri K, Rezaei R, Suzuki K. Synbiotic Supplementation with Probiotics and Omega-3 Fatty Acids Enhances Upper-Body Muscle Strength in Elite Swimmers: Evidence for Gut–Muscle Axis Modulation During Race-Pace Training. Nutrients. 2025; 17(18):2959. https://doi.org/10.3390/nu17182959
Chicago/Turabian StyleImanian, Babak, Mohammad Hemmatinafar, Ideh Maymandinejad, Mohammad Reza Binazade, Ralf Jäger, Zeinab Jahan, Kimia Naseri, Rasoul Rezaei, and Katsuhiko Suzuki. 2025. "Synbiotic Supplementation with Probiotics and Omega-3 Fatty Acids Enhances Upper-Body Muscle Strength in Elite Swimmers: Evidence for Gut–Muscle Axis Modulation During Race-Pace Training" Nutrients 17, no. 18: 2959. https://doi.org/10.3390/nu17182959
APA StyleImanian, B., Hemmatinafar, M., Maymandinejad, I., Binazade, M. R., Jäger, R., Jahan, Z., Naseri, K., Rezaei, R., & Suzuki, K. (2025). Synbiotic Supplementation with Probiotics and Omega-3 Fatty Acids Enhances Upper-Body Muscle Strength in Elite Swimmers: Evidence for Gut–Muscle Axis Modulation During Race-Pace Training. Nutrients, 17(18), 2959. https://doi.org/10.3390/nu17182959







