Abstract
Objective: The Mediterranean diet (MedDiet) is characterized by its emphasis on plant-based foods, olive oil, and fish products, and has been associated with providing relevant fatty acids (FAs) for adolescent physiology. This study aims to investigate the relationship between adherence to the MedDiet and the FA composition of red blood cell (RBC) membranes in an adolescent population. Methods: The current research examines the relationship between MedDiet adherence, assessed using the KIDMED questionnaire, and the composition of RBC membranes, specifically measuring 22 FAs in a cross-sectional analysis of adolescents from two cohorts (mean age = 14.55). Baseline data from 552 participants with complete dietary adherence and FA information were analyzed using multivariable regression models and principal component analysis (PCA) as confirmatory analysis. All regression models were adjusted by age, sex, body mass index, physical activity, maternal education and cohort enrollment. Results: Main results shown that “Good adherence” to the MedDiet was positively associated with omega-3 FAs, including eicosapentaenoic acid (β = 0.34; 95% CI: 0.17, 0.52; p-value < 0.001) and docosahexaenoic acid (β = 0.29; 95% CI: 0.11, 0.46; p-value = 0.001), and inversely associated with specific omega-6 FAs, such as arachidonic acid (β = −0.28; 95% CI: −0.46, −0.11; p-value = 0.002) and adrenic acid (β = −0.19; 95% CI: −0.30, −0.08; p-value < 0.001). PCA identified distinct FA patterns, with “Good adherence” to the MedDiet being associated with an increase in the omega-3 FAs pattern (β = 0.32; 95% CI: 0.14, 0.49; p-value < 0.001). These findings remained robust after multiple test comparisons. Conclusions: This study underscores the potential of the MedDiet to promote optimal RBC FA composition in healthy adolescents, characterized by high levels of omega-3 FAs and reduced levels of arachidonic acid and adrenic acid in RBC membranes.
1. Introduction
Adolescent development can be affected by cardiovascular diseases [1] and mental health disorders [2], with diet playing a crucial role in their progression [3]. Notably, the Mediterranean diet (MedDiet) has demonstrated a protective effect against these conditions during adolescence [4,5]. The MedDiet emphasizes plant-based foods, with olive oil as the primary fat source [6]. It includes moderate amounts of dairy, fish, and poultry, while eggs are consumed weekly and red meat sparingly [7,8].
The MedDiet provides essential fatty acids (FAs), characterized by low levels of saturated fatty acids (SFAs) and high levels of polyunsaturated fatty acids (PUFAs), particularly omega-3 FAs [9]. Omega-3 PUFAs, including alpha-linolenic acid (C18:3 n-3, ALA), eicosapentaenoic acid (20:5 n-3, EPA) and docosahexaenoic acid (22:6 n-3, DHA), play crucial roles in the structural, functional, and antioxidative processes of the cardiovascular system and adolescent brain [10]. These omega-3 species are obtained via dietary intake of fatty fish such as salmon, tuna and mackerel (EPA and DHA), as well as nuts, seeds, and their derivatives (ALA) [11]
The MedDiet has been associated with beneficial effects in adolescents, including a reduced risk of cardiovascular disease and milder depression symptoms, with lasting impacts into adulthood [12]. These effects may be partly explained by the MedDiet’s impact on the FA content in red blood cell (RBC) membranes [13,14]. RBCs play a crucial role in adolescent metabolism by delivering oxygen to the cardiovascular system and brain [15]. Maintaining membrane homeostasis is essential for their proper function, with FAs playing a key role in this process. There is growing interest in assessing FAs in RBCs due to their ability to provide an accurate reflection of dietary lipid intake [16,17]
Given that the MedDiet has been associated with better cardiovascular and mental health outcomes in adolescence, this dietary pattern may provide optimal levels of omega-3 FAs that are beneficial for overall adolescent health [18]. These beneficial effects may be mediated by omega-3 FAs in RBC membranes [13,14]. We aimed to investigate the association of adherence to the MedDiet and 22 RBC FAs through a cross-sectional and multi-cohort analysis of adolescents in Catalonia (Spain). We hypothesize that high adherence to the MedDiet during adolescence is positively associated with omega-3 FAs in RBC membranes.
2. Methods
The current study utilizes a cross-sectional design, drawing on baseline data from the Walnuts Smart Snack Dietary Intervention Trial (WSS) and the 14-year follow-up from the Spanish birth cohort of the Childhood and Environment (INMA) Project. The flowchart of WSS and INMA participants is presented in Supplementary Figure S1.
The WSS aimed to assess whether consuming 30 g of raw walnut kernels daily for six months could improve cognitive and socioemotional development in a group of healthy adolescents of Barcelona. The study involved 771 participants from 11 high schools, who were recruited over a year (2015–2016). Details of the clinical trial are outlined in the WSS protocol [19], which received approval from the Parc Salut Mar’s Clinical Research Ethics Committee (approval number: 2015/6026/I). In the current cross-sectional study, we analyzed the baseline data from WSS, focusing on a subsample of 14-year-olds with biological FA samples (n = 332).
The INMA study is a multi-centric study conducted across several Spanish cities. The project aims to investigate the impact of prenatal and postnatal environmental factors on fetal growth and childhood development, involving 3100 pregnant women and their children (2006–2008). Detailed information is available in the INMA protocol [20]. We based this study on the INMA-Sabadell cohort, which received permission from “Instituto Municipal de Asistencia Sanitaria” Research Ethic Committee (approval number: 2005/2106/I). Participants of the INMA project, which were enrolled in Sabadell (INMA-Sabadell, n = 657), have been assessed approximately every two years: at 6 and 14 months, and at 2, 4, 7, 9, 11, and 14 years of age. We focused on data from the 14th to 16th year of follow-up with biological FA determinations (n = 328).
2.1. Nutritional, Sociodemographic and Lifestyle Data
Nutritional, sociodemographic, clinical, and lifestyle data were collected through WSS baseline assessments and INMA-Sabadell follow up. Field technicians conducted in-person interviews with adolescent participants, while parents completed questionnaires at home, returning them through the school system in both studies.
Adherence to the MedDiet was assessed using the KIDMED questionnaire, which assigns a score based on intake of key food groups. These food groups include fruits, vegetables, legumes, seafood, cereals, nuts, dairy products, and olive oil. The 16-items KIDMED questionnaire was administered to the participants. Scores of 3 or below reflect poor adherence, 4 to 7 indicate average adherence, and scores of 8 or above signify good adherence [19,21]. Given the small number of participants with low adherence, the KIDMED index was dichotomized into two categories: “poor to average adherence” (scores 1–7) and “good adherence” (scores 8–12). Height (cm) and weight (kg) were measured following standard recommendations to compute the body mass index (BMI). BMI was computed using the reference population (z-score), and adjusting for age and sex according to the World Health Organization’s recommendations [22]. Physical activity and sociodemographic information (age, sex and maternal education) were collected by questionnaires. Physical activity can refer to any exercise or sports practiced out of school hours and was recorded in three categories: “sedentary to low physical activity”, “moderate”, and “active to quite active”. Age was recorded as a continuous variable, whereas sex (male/female) and maternal education (up to high school/university) were captured as dichotomous variables. “Maternal social class was recorded as a categorical variable: “High and upper-middle class”, “Middle class”, and “working class”. Cohort information (WSS/INMA-Sabadell) of participants was also collected.
2.2. Laboratory Protocol for Fatty Acids Analysis
Blood samples were collected from participants of both cohorts after an overnight fast. Subsequently, we subjected the samples to centrifugation at 2500× g for 20 min at 20 °C within 4 h of extraction. Packed RBCs were preserved at −80 °C until analysis of the FAs. The FA profile within the RBCs was determined using gas chromatography, coupled either to flame ionization detector (WSS) or to electron ionization mass spectrometry (INMA), as previously described [23,24]. In both cases, the measurement of each FA was expressed as a percentage of the twenty-two FAs identified in the sample (Supplementary Figure S2)
2.3. Principal Component Analysis
We performed Principal Component Analysis (PCA) on the FAs of adolescents from the WSS and INMA-Sabadell cohorts. This analysis included all FAs measured in both groups and those that were common to both cohorts. No variables or FAs were selectively chosen for convenience. PCA was utilized to address the high correlation among FAs in red blood cells (Supplementary Figure S3). Following the PCA, Principal Components (PCs) with eigenvalues greater than 2 were retained. A varimax rotation was then applied to these retained components to clarify the contribution of each variable within them, and loading scores were analyzed for each component. Components were described based on variable loadings exceeding |0.2|, with a detailed examination of variables with the highest loading scores. Standardized scores for each adolescent were calculated for each component to serve as “outcome variables” for subsequent regression analysis. Higher standardized scores reflect greater representation of participants in the PC with FAs that contribute positively to the component, while lower scores indicate greater representation of participants in the PC with FAs that contribute negatively to the component.
2.4. Statistical Analysis
Wilcoxon sum rank and Chi-square tests were employed to compare the KIDMED levels with sociodemographic characteristics and RBC FAs from both WSS and INMA-Sabadell participants. Additionally, associations between KIDMED, FAs, and FA PCs were evaluated using multivariable linear regression models. The KIDMED score was assessed as a dichotomous variable comparing “poor-to-average adherence” with “good adherence” to MedDiet (exposure), while FAs and PC standardized score were evaluated as continuous variables (outcomes). For all regression analyses, fully adjusted models were assessed with mandatory covariates, including age, biological sex, BMI z-score, physical activity, maternal education and cohort. Statistical analyses were exclusive to adolescents with complete information on the variables included in the models (n = 552). Missing data were not imputed.
For all regression models, a p-value below 0.05 was considered as statistically significant. We conducted the False Discovery Rate (FDR) test for multiple test comparisons on regression models. As the main criterion, we considered a threshold alpha value of 0.05 to calculate the q-values. After conducting FDR analyses, we considered exposure coefficients to be statistically significant when p-values were lower than q-values across all regression models.
Sex-stratified analyses were conducted to examine potential differences in associations by biological sex. Sensitivity analyses were performed to assess the influence of maternal social class and BMI on the observed relationships. Additionally, to evaluate potential cohort effects, interaction terms between MedDiet adherence (KIDMED categories) and cohort membership (KIDMED × cohort) were included in the models.
All analyses were conducted using R base (version 4.4.0) and R Studio (version 4.2.3). The “zscorer” library (version 0.3.1) was used to calculate BMI z-scores following World Organization Health’s recommendations [25], while the “factoextra” (version 1.0.7) and “psych” libraries (version 2.4.3) were utilized for performing PCA [26,27].
3. Results
The baseline characteristics of the study population are shown in Table 1. A large percentage of the mothers of participants with “good adherence” to MedDiet had a university-level education (60%), while only 45% of mothers in the “poor to average adherence” to MedDiet had university-level education. The majority of participants within the “good adherence” group to MedDiet engage in “active to quite active” physical activity (65%).

Table 1.
Descriptive analysis of covariates from the WSS and INMA-Sabadell participants.
The baseline characteristics of RBC FAs are shown in Table 2. While the percentage differences in the contribution of different FAs to RBC membranes were relatively small between the “poor to average adherence” and “good adherence” groups to the MedDiet, many of these differences were statistically significant. Several omega-6 FAs, such as C18:3 n-6 (gamma-linolenic acid, GLA), C20:2 n-6 (eicosadienoic acid, EDA), C20:4 n-6 (arachidonic acid, AA), and C22:4 n-6 (adrenic acid, AdA) showed slightly higher levels in the “poor to average adherence” to MedDiet group (p-value < 0.001). Meanwhile, certain specific omega-3 FAs, such as C20:5 n-3 (EPA) (p-value = 0.004), and C22:6 n-3 (DHA) (p-value = 0.001), showed higher levels in the “good adherence” to MedDiet group.

Table 2.
Descriptive analysis of fatty acids from the WSS and INMA-Sabadell participants (N = 629).
We present only the statistically significant associations (p-value < 0.05) of adherence to the MedDiet (exposure) with RBC FAs (outcomes) in Table 3, while all multivariate regression outputs are provided in Supplementary Table S1. Adherence to the MedDiet, as measured by the KIDMED score, was associated with significant changes in RBC FAs in the adolescent participants. “Good adherence” to MedDiet group was positively associated with an increase in C16:0 (palmitic acid) (β = 0.18; 95% CI: 0.01, 0.35; p = 0.041) and all-trans C18:1 FAs (β = 0.05; 95% CI: 0.01, 0.09; p = 0.016) compared to the “poor-to-average” adherence to MedDiet. However, the “good adherence” group was inversely associated with C20:4 n-6 (AA) (β = −0.19; 95% CI: −0.30, −0.08; p-value < 0.001), C22:4 n-6 (AdA) (β = −0.28; 95% CI: −0.46, −0.11; p-value = 0.002), and C22:5 n-6 (docosapentaenoic omega-6, DPA omega-6) (β = −0.22; 95% CI: −0.40, −0.04; p-value = 0.018) compared to the reference group (“poor-to-average adherence” to MedDiet). Additionally, the “good adherence” to MedDiet group was positively associated with C20:5 n-3 (EPA) (β = 0.34; 95% CI: 0.17, 0.52; p-value < 0.001) and C22:6 n-3 (DHA) (β = 0.29; 95% CI: 0.11, 0.46; p-value = 0.001) compared to the “poor-to-average adherence” group.

Table 3.
Multivariate linear regressions between KIDMED adherence group and fatty acids of red blood cell membranes.
Three PCs were extracted using PCA; eigenvalue > 2.0 criteria for PC retention and loadings greater than |0.4| were considered statistically relevant for the pattern analysis (Supplementary Table S2). The three PCs retained accounted for 57.20% of the total variance of the FA data. No additional relevant information was added in the subsequent PCs, as confirmed by the scree plot (Supplementary Figure S4). Factor loadings for the FA patterns are presented as their loading contributions in Figure 1. PC1 was named “very-long chain FAs” as it was primarily characterized by, ordered from largest to smallest loading, C24:1 n-9 (nervonic acid), C22:0 (behenic acid), and C24:0 (lignoceric acid). Both C16:1 n-7 (palmitoleic acid) and C18:0 (stearic acid) showed negative loadings. PC2 was labelled “long-chain omega-6” because it was characterized by C20:4 n-6 (AA), C22:4 n-6 (AdA), and C20:2 n-6 (EDA, omega-6) as positive loadings. The FAs with negative loadings were C18:1 n-9 cis (oleic acid), C16:0 (palmitic acid), C14:0 (myristic acid) and all-trans C18:1. PC3 was named “omega-3 FAs” since it was mainly characterized by positive loadings of C20:5 n-3 (EPA), C22:6 n-3 (DHA), and C22:5 n-3 (docosapentaenoic acid, DPA), while the FA with negative loadings was C22:4 n-6 (AdA). The summary of the variables’ loadings for each component (PC1, PC2, and PC3) is also displayed in the PCA biplot (Supplementary Figure S5). Additionally, the correlation between each FA and the PC scores was also confirmed using Spearman correlation (Supplementary Table S3).
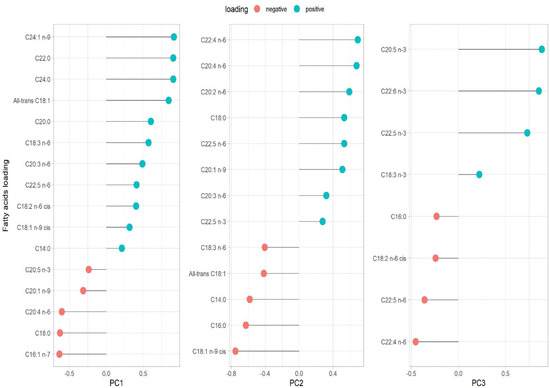
Figure 1.
Loading variable for each principal component. Eigenvalue criteria >2.0. The variance (%) of each component is presented as follows: PC1 (33.75%), PC2 (13.22%), and PC3 (10.21%). The total variance explained, by these three principal components, is 57.20%. PC1 very-long chain FAs; PC2 long-chain omega-6 FAs, PC3 omega-3 FAs.
Adherence to the MedDiet, as measured by the KIDMED score, was associated with distinct changes in FA patterns (Table 4). “Good adherence” to the MedDiet group was inversely associated with long-chain omega-6 FAs PC (PC2) (β = −0.19; 95% CI: −0.36, −0.03; p-value = 0.020) compared to reference group (“poor-to-average adherence” to MedDiet). “Good adherence” to the MedDiet group was positively associated with the omega-3 FAs PC (PC3) (β = 0.32; 95% CI: 0.14, 0.49; p-value < 0.001). No significant associations of adherence to the MedDiet with PC1 (long-chain FAs pattern) were found.

Table 4.
Multivariate linear regression models between KIDMED adherence group and the principal components of fatty acid.
FDR correction was applied to the significant associations of adherence to MedDiet with RBC FA composition (Supplementary Table S4). The following FAs remained statistically significant after FDR correction: C20:4 n-6 (p-value < 0.001; q-value = 0.004), C22:4 n-6 (p-value = 0.002, q-value = 0.010), C20:5 n-3 (p-value < 0.001; q-value = 0.002), C22:6 n-3 (p-value = 0.001; q-value = 0.008), and PC3 omega-3 FAs (p-value < 0.001; q-value = 0.006). These results indicate that the observed associations are unlikely to be due to multiple testing errors, reinforcing the robustness of the findings.
Our stratified analysis by sex revealed differential associations in the relationship between MedDiet adherence and fatty acid profiles (Supplementary Table S5). Significantly positive relationships were observed for long-chain omega-3 FAs in males compared to females (EPA: β = 0.46, 95% CI: 0.24, 0.68, p-value < 0.001; DHA: β = 0.32, 95% CI: 0.09, 0.54, p-value = 0.006; DPA: β = 0.26, 95% CI: 0.02, 0.49, p-value = 0.030). Conversely, inverse associations were found for specific omega-6 FAs in females (AA: β = −0.25, 95% CI: −0.42, −0.07, p-value = 0.005; AdA: β = −0.34, 95% CI: −0.61, −0.08, p-value = 0.012). Regarding sensitivity analyses, the addition of either BMI z-score or maternal social class to adjustment models resulted in coefficient changes of less than 20% for all FAs when comparing basic and fully adjusted models, indicating that these factors did not substantially influence the observed associations between MedDiet adherence and RBC FA concentrations (Supplementary Tables S6 and S7).
Finally, to evaluate potential cohort-related biases, we conducted an interaction analysis between MedDiet adherence and cohort (Supplementary Table S8). Statistically significant interaction effects (“Poor-to-average adherence” × “WSS cohort”) were observed for LA (β = 0.38, 95% CI: 0.03, 0.73, p-value = 0.032), DPA (β = −0.52, 95% CI: −0.86, −0.17, p-value = 0.004), DHA (β = −0.42, 95% CI: −0.78, −0.06, p-value = 0.022), and the omega-3 PC3 (β = −0.42, 95% CI: −0.78, −0.06, p-value = 0.021).
4. Discussion
In this cross-sectional study, we used data from adolescents in the WSS and INMA-Sabadell cohorts to analyze the associations of adherence to the MedDiet, as measured using the KIDMED score, with 22 FAs in RBC membranes. Our results show significant associations between the “good adherence” to the MedDiet group with FAs compared to the reference group (“poor-to-average adherence”) in fully adjusted models. Specifically, we observed inverse associations with omega-6 AA, and AdA, as well as positive associations with EPA, DHA, and the PC3 omega-3 FAs pattern. These findings remained consistent after correcting for multiple tests.
“Good adherence” to the MedDiet group was inversely associated with AA and AdA in RBCs. These inverse associations add to the growing evidence that individuals with good adherence to the MedDiet may exhibit lower levels of these omega-6 FAs in RBCs. A potential explanation for these findings lies in the dietary composition of the MedDiet, which emphasizes a high intake of omega-3 FA-rich foods, such as vegetables, fish, nuts, and seeds [28], while limiting the consumption of processed foods and vegetable oils high in omega-6 FAs [29]. At an epidemiological level, a cross-sectional study in children aged two to less than ten years old found an inverse association between total omega-6 FAs in whole blood with KIDMED scores [30]. Furthermore, an intervention promoting MedDiet adherence in adults with gingivitis, measured by FFQ before and after the intervention, showed decreased levels of LA, AA, and total omega-6 in the serum of the MedDiet intervention group [31]. Thus, our findings are consistent with previous research indicating that a good adherence to MedDiet is associated with lower levels of AA and AdA and replicates these results in healthy teenagers.
On the other hand, adherence to the MedDiet was positively associated with EPA and DHA. These findings are congruent with previous research showing that good adherence to MedDiet is associated with higher omega-3 composition in RBCs. EPA and DHA are predominantly found in fatty fish such as salmon, mackerel, sardines, and anchovies, as well as in fish oils and algae-based supplements [8,32]. These MedDiet food sources are important contributors to omega-3 FAs intake. Comparing our results with similar research, a report from HELENA study in European adolescents (n = 2330) showed a positive association between MedDiet score and omega-3 FAs in serum [33]. Other research using PCA-derived patterns found that a pattern characterized by the intake of fish, shrimp, crab, shellfish, leafy vegetables, nuts, and tubers was positively associated with omega-3 FAs and negatively associated with SFAs in a population of children aged 4 to 7 years with overweight or obesity [34]. In recent years, a systematic review on the relationship between MedDiet and omega-3 FAs was conducted which included 7 observational studies and 15 randomized controlled trials; all observational studies reported a positive relationship between adherence to the MedDiet and omega-3 PUFA tissue levels. Two-thirds of the randomized controlled trials showed significant increases in omega-3 FA concentrations [35].
Moreover, when focusing on highly correlated FAs, our findings revealed a positive association of adherence to the MedDiet with the PC3 omega-3 FAs pattern. This pattern was mainly enriched by C20:5 n-3 (EPA), C22:6 n-3 (DHA) and C22:5 n-3 (DPA). With a subtle differ-ence in the loading cut-off, 18:3 n-3 (ALA) is also present in the current PC. From a metabolic perspective, these FAs are interconnected through biosynthetic pathways. ALA serves as a precursor for the synthesis of longer-chain omega-3 FAs, including EPA, through a series of elongation and desaturation steps [36]. DPA, often considered an intermediary, can be converted to either EPA or DHA, depending on the metabolic demand and enzymatic activity [37]. The efficient metabolism of these PUFAs depends on factors such as dietary intake, genetic variation, and overall health, all of which can influence their relative abundance in the body [38,39]. In this manner, the association of adherence to MedDiet with the PC3 omega-3 pattern supports the idea that MedDiet foods intake is related to the percentage of related omega-3 FAs in RBC membranes. Furthermore, these PC3 omega-3 associations are consistent with the observed positive associations between adherence to the MedDiet and EPA and DHA in our previous regression models (Table 3).
Our sex-stratified analysis revealed notable differences in the associations between MedDiet adherence and FA profiles. In males, stronger positive relationships were observed for long-chain omega-3 FAs, including EPA, DHA, and DPA. In contrast, a more notable inverse relationship was observed in females for specific omega-6 fatty acids, including arachidonic acid (AA) and adrenic acid (AdA). Women may require higher amounts of omega-6, particularly AA, due to its crucial role in hormone synthesis. AA is an important precursor in the production of eicosanoids, compounds that regulate various physiological functions, including inflammatory responses and hormonal activity [40]. However, this is not observed in every case, underscoring the importance of considering sex-specific differences in fatty acid metabolism [41].
Moreover, maternal social class was included as a variable in the sensitivity analysis. No significant changes were observed in the coefficients of adherence to MedDiet exposure, either in the fully adjusted model or when additionally controlled for maternal social class. This demonstrates the robustness of variable selection in our model. However, it is important to note that previous studies analyzing the direct effect of social class on access to the MedDiet found that higher family income is associated with better adherence to this dietary pattern [42].
Finally, to evaluate potential cohort-related biases, we conducted an interaction analysis between MedDiet adherence and cohort. Significant interactions were observed for LA, DPA, DHA, and the omega-3 PC3, indicating that, relative to the reference group (“poor-to-average adherence”), the associations of good MedDiet adherence with these FAs differed between the WSS and INMA cohorts. These differences may reflect cohort-specific factors, including recruitment, socio-demographics, and RBC measurement protocols (flame ionization detector in WSS vs. electron ionization mass spectrometry in INMA). Despite this heterogeneity, the overall direction of associations remained consistent, supporting the robustness of our main findings.
Exploring the potential biological mechanisms, adolescence represents a period of increased metabolic demands where FA metabolism plays a central role. Adherence to the MedDiet may influence these processes through omega-3 FAs from fish, which support anti-inflammatory responses, membrane fluidity, and neurodevelopment. Olive oil, rich in omega-9, may improve lipid profiles and insulin sensitivity, and antioxidants from plant-based foods can reduce oxidative stress and modulate FA elongation and desaturation. Together, these mechanisms suggest that the MedDiet could play a key role in shaping lipid metabolism, cardiometabolic and neuropsychological health during this critical stage of development [43].
Although the differences in RBC FA levels between groups were statistically significant in our multivariate models (Table 3 and Table 4), the absolute changes reported in the descriptive Table 2 were modest (e.g., C20: 5 n-3: “poor-to-average adherence” 0.35% vs. “good adherence” 0.39%). The clinical and physiological relevance of such small variations in healthy adolescents remains uncertain. However, even subtle shifts in membrane FA composition may reflect longer-term dietary habits and could accumulate to exert metabolic effects over time, as suggested in both youth and adult populations. At cardiovascular level, a study assessed the Omega-3 Index in RBCs in a large sample of adolescents aged 13–15. Higher Omega-3 levels were associated with lower cardiovascular risk factors, including diastolic blood pressure and HDL cholesterol [44]. A study examined the association between RBC omega-3 fatty acids and cardiovascular risk in adults aged 30–74 without prior cardiovascular events. Higher RBC omega-3 levels were linked to lower odds of intermediate or high cardiovascular risk based on Framingham and Reynolds scores, suggesting that even modest changes in RBC fatty acid composition may have clinical relevance [45]. At mental health level. in a cross-sectional study using the WSS cohort, conducted in 372 adolescents (13.8 ± 0.9 years old), the RBC proportions of DHA and ALA were determined and found to be associated with attention scores [17]. In a cohort of postmenopausal women, lower RBC EPA + DHA levels correlated with smaller total and hippocampal brain volumes, the former being an indication of cognitive aging and the latter being centrally involved with Alzheimer’s disease pathology [46]. Therefore, despite our findings of only subtle differences between the MedDiet adherence groups and PUFAs in RBC, as part of our research question, these changes could still be associated at a physiological or clinical level. One key strength of this study lies in the analysis of lipidomics markers of diet, providing more precise information for the investigation of adherence to the MedDiet with RBCs FA composition. Using lipidomics techniques, we assessed 22 FAs in the RBC membrane, offering a more accurate long-term dietary indicator compared to total plasma or serum. This is because RBCs have a lifespan of approximately 120 days, allowing them to better reflect average dietary intake over time. From a metabolic perspective, FAs in RBC membranes exchange fatty acids with albumin, as well as high- and low-density lipoproteins. Since RBCs lack a nucleus, they continuously rely on FAs from dietary intake to maintain membrane homeostasis [15]. This reinforces the well-known metabolic relevance of measuring RBC FA composition as an indicator of physiological functioning during adolescence. Another strength of this study is the use of the KIDMED questionnaire, an adapted and validated instrument for the Spanish child and adolescent population [21]. Additionally, the use of PCA to create FA PC from highly correlated FAs enabled us to conduct confirmatory analyses based on the correlated FA approach. This method demonstrated statistical consistency with the individual associations observed between adherence to the MedDiet and FAs in the regression models. Finally, all analyzed associations were tested using multiple test comparison adjustments, with a maximum tolerance of 5% false positive results. This ensures the robustness of our findings [47].
Nevertheless, the current study also faced several limitations. The primary limitation is its cross-sectional design, which prevents the establishment of causality, as data were collected at a single time-point. The absence of longitudinal data also restricts the ability to comprehensively analyze the progression and dynamics of FAs and the MedDiet. Another limitation is the potential for selection bias, as the sample may not fully represent the broader population, potentially reflecting specific characteristics and inferences limited to the study sample. However, we used data from two different samples of two Spanish cities. Additionally, unmeasured environmental factors (e.g., environmental pollutants, family income) that influence both, adherence to the MedDiet and RBC FA composition may contribute to residual confounding making it challenging to account for the effects of omitted variables and thereby limiting the generalizability of the multivariate model results [48]. However, residual confounding is inherent to observational studies, and we included those confounders that we hypothesized may play the most important confounding role based on previous scientific literature. Nonetheless, the inclusion of two cohorts provided robust results regarding the association of MedDiet adherence with RBC FAs in adolescents. Unfortunately, using only overlapping data from WSS and INMA-Sabadell may have led to some loss of information regarding participants’ exposures, confounders, and outcomes in the study. Additionally, pooling baseline WSS data with 14-year INMA follow-up data may have introduced systematic bias due to differences in recruitment, socio-demographics, and measurement protocols. To account for this, cohort was included as a confounder and as an interaction term with MedDiet adherence in the analyses.
Future research should aim to provide further insight into the mechanisms that help explain the relationships between adherence to the MedDiet and saturated and polyunsaturated FA species in RBC as a reflection of dietary patterns in the adolescent population. This should be done taking into consideration a large amount of different fatty acids and adolescent samples from other countries and cultures with different dietary patterns. In addition, future longitudinal are warranted, potentially integrating FA biomarkers into long-term dietary surveillance systems to better monitor dieraty exposures in youth.
5. Conclusions
Overall, our findings suggest that a higher adherence to the MedDiet, as assessed by the KIDMED questionnaire, was directly associated with higher RBC EPA and DHA, as well as the PC primarily composed of omega-3 FAs (EPA, DHA, DPA, and ALA). In contrast, inverse associations were observed with AA and AdA. These association patterns are biological indicators of the potential influence of a healthy dietary pattern on adolescents during an important developing period.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17172888/s1, Figure S1: Flowchart of participants. WSS = Walnuts Smart Snack Intervention Trial; INMA = Childhood and Environment, “INfancia y Medio Ambiente”; Figure S2: Density plot of fatty acids from WSS and INMA-Sabadell cohorts; Figure S3: Correlation plot between fatty acids and continuous KIDMED score. Correlations correspond to Spearman’s correlation. Only p-values below 0.05 are shown; Figure S4: Scree plot of principal component analysis in fatty acids of red blood cell membrane; Figure S5: Biplot for fatty acids of principal component 1, 2 and 3. PC1 very-long chain FAs; PC2 long-chain omega-6 FAs; PC3 omega-3 FAs; Table S1: Multivariate linear regressions between KIDMED adherence group and fatty acids of red blood cell membranes; Table S2: Principal component, eigenvalues, and cumulative variance in principal components analysis; Table S3: Spearman correlation between fatty acid (%) and standardized scores of principal components. Table S4: Multivariate regression models (Table 2 and Table 3) corrected p-values for multiple testing using the Benjamini-Hochberg false discovery rate. Table S5: Stratification by sex: Multivariate linear regressions between KIDMED adherence group and fatty acids of red blood cell membranes. Table S6: Sensitivity analysis by body mass index: multivariate linear regressions between KIDMED adherence groups and fatty acids in red blood cell membranes. Table S7: Sensitivity analysis by maternal social class: multivariate linear regressions between KIDMED adherence groups and fatty acids in red blood cell membranes. Table S8: Interaction analysis by cohort: multivariate linear regressions between KIDMED adherence groups and fatty acids in red blood cell membranes.
Author Contributions
Author contributions included conceptualization N.A.-A., D.L., A.P.-M. and J.J.; data curation I.L. and A.S.-V.; and formal analysis N.A.-A. and D.L.; validation N.A.-A., D.L., I.L., A.P.-M., A.M., S.B.-C., D.R.H., A.S.-V. and J.J.; and writing—original draft N.A.-A., D.L., I.L., A.P.-M., S.F.-B., D.R.H., O.C.-R., A.S.-V., M.V. and J.J. All authors have read and agreed to the published version of the manuscript.
Funding
The WSS study was supported by “Instituto de Salud Carlos III” through the projects ‘CP14/00108, PI16/00261, PI21/00266’ (co-funded by European Regional development Fund ‘A way to make Europe’). The California Walnut Commission (CWC) has given support by supplying the walnuts for free for the WSS. The funders have no role in the study design, collection, management, analysis, and interpretation of data, writing of the report or decision to submit it for publication. The INMA-Sabadell study was funded by grants from Instituto de Salud Carlos III (Red INMA G03/176; CB06/02/0041; PI041436; PI081151 incl. FEDER funds; PI12/01890 incl. FEDER funds; CP13/00054 incl. FEDER funds; PI15/00118 incl. FEDER funds; CP16/00128 incl. FEDER funds; PI16/00118 incl. FEDER funds; PI16/00261 incl. FEDER funds; PI17/01194 incl. FEDER funds; PI17/01340 incl. FEDER funds; PI18/00547 incl. FEDER funds; PI20/01695 incl. FEDER funds), CIBERESP, Generalitat de Catalunya-CIRIT 1999SGR 00241, Generalitat de Catalunya-AGAUR (2009 SGR 501, 2014 SGR 822), Fundació La marató de TV3 (090430), Spanish Ministry of Economy and Competitiveness (SAF2012-32991 incl. FEDER funds), Agence Nationale de Securite Sanitaire de l’Alimentation de l’Environnement et du Travail (1262C0010; EST-2016 RF-21; EST-19 RF-04; 2019/1/233), EU Commission (261357, 308333, 603794; 634453; 825712 and 874583). We acknowledge support from the grant CEX2023-0001290-S funded by MCIN/AEI/ 10.13039/501100011033, and support from the “Generalitat de Catalunya” through the CERCA Program. NAA holds a pre-doctoral scholarship “Doctorado en el Extranjero” (number of resolution: 6775/2023) awarded by “Agencia Nacional de Investigacion y Desarrollo”—Government of Chile. APM holds a pre-doctoral research training (PFIS) contract (grant FI22/00119) awarded by the Instituto de Salud Carlos III. DRH is supported by the LongITools project which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 874739. JJ holds a Miguel Servet-II contract (grant CPII19/00015) awarded by the Instituto de Salud Carlos III (Co-funded by European Social Fund “Investing in your future”).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. WSS project received approval from the Parc Salut Mar’s Clinical Research Ethics Committee (approval number: 2015/6026/I, date: 2 May 2016). INMA-Sabadell project received permission from “Instituto Municipal de Asistencia Sanitaria” Research Ethic Committee (approval number: 2005/2106/I, date: 20 July 2005). The follow-up of the adolescent population (14–16 years old) from INMA Sabadell received permission from “Parc Salut Mar’s Clinical Research Ethics Committee” (approval number: 2019/8788/I, date: 26 September 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request. Further inquiries can be directed to the corresponding author.
Acknowledgments
We extend our gratitude to all study participants, their families and schools, as well as to the project investigators, fieldworkers, and contributors to the WSS and INMA projects for their collaboration.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AdA | Adrenic acid |
| ALA | Alpha-linolenic acid |
| BMI | Body mass index |
| DHA | Docosahexaenoic acid |
| DPA | Docosapentaenoic acid |
| DPA omega-6 | Docosapentaenoic omega-6 |
| EPA | Eicosapentaenoic acid |
| FA | Fatty acids |
| FDR | False discovery rate |
| GLA | Gamma-linolenic acid |
| INMA | Spanish birth cohort of the Childhood and Environment |
| LA | Linoleic acid |
| MedDiet | Mediterranean diet |
| PC | Principal component |
| PCA | Principal component analysis |
| PUFAs | Polyunsaturated fatty acids |
| RBC | Red blood cells |
| SFA | Saturated fatty acid |
| WSS | Walnuts Smart Snack Dietary Intervention Trial |
References
- Noubiap, J.J.; Nyaga, U.F. Cardiovascular disease prevention should start in early life. BMC Glob. Public Health 2023, 1, 14. [Google Scholar] [CrossRef]
- Kieling, C.; Buchweitz, C.; Caye, A.; Silvani, J.; Ameis, S.H.; Brunoni, A.R.; Cost, K.T.; Courtney, D.B.; Georgiades, K.; Merikangas, K.R.; et al. Worldwide Prevalence and Disability From Mental Disorders Across Childhood and Adolescence: Evidence From the Global Burden of Disease Study. JAMA Psychiatry 2024, 81, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Norris, S.A.; Frongillo, E.A.; Black, M.M.; Dong, Y.; Fall, C.; Lampl, M.; Liese, A.D.; Naguib, M.; Prentice, A.; Rochat, T.; et al. Nutrition in adolescent growth and development. Lancet 2022, 399, 172–184. [Google Scholar] [CrossRef] [PubMed]
- López-Gil, J.F.; García-Hermoso, A.; Martínez-González, M.Á.; Rodríguez-Artalejo, F. Mediterranean Diet and Cardiometabolic Biomarkers in Children and Adolescents: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2024, 7, e2421976. [Google Scholar] [CrossRef]
- Mongan, D.; Healy, C.; Jones, H.J.; Zammit, S.; Cannon, M.; Cotter, D.R. Plasma polyunsaturated fatty acids and mental disorders in adolescence and early adulthood: Cross-sectional and longitudinal associations in a general population cohort. Transl Psychiatry 2021, 11, 321. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Camprodon-Boadas, P.; Gil-Dominguez, A.; De la Serna, E.; Sugranyes, G.; Lázaro, I.; Baeza, I. Mediterranean Diet and Mental Health in Children and Adolescents: A Systematic Review. Nutr. Rev. 2025, 83, e343–e355. [Google Scholar] [CrossRef]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods- A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef] [PubMed]
- Saber, N.; Teymoori, F.; Kazemi Jahromi, M.; Mokhtari, E.; Norouzzadeh, M.; Farhadnejad, H.; Mirmiran, P.; Azizi, F. From adolescence to adulthood: Mediterranean diet adherence and cardiometabolic health in a prospective cohort study. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Pottala, J.V.; Talley, J.A.; Churchill, S.W.; Lynch, D.A.; von Schacky, C.; Harris, W.S. Red blood cell fatty acids are associated with depression in a case-control study of adolescents. Prostaglandins Leukot. Essent. Fatty Acids 2012, 86, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yan, Y.; Li, J.; Yang, B.; Zhao, X.; Wan, Y.; Zheng, J.-S.; Mi, J.; Li, D. Relationship between erythrocyte phospholipid fatty acid composition and obesity in children and adolescents. J. Clin. Lipidol. 2019, 13, 70–79.e1. [Google Scholar] [CrossRef]
- Chatzinikolaou, P.N.; Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Vrabas, I.S.; Kyparos, A.; D’Alessandro, A.; Nikolaidis, M.G. Erythrocyte metabolism. Acta Physiol. 2024, 240, e14081. [Google Scholar] [CrossRef]
- Harris, W.S.; Pottala, J.V.; Varvel, S.A.; Borowski, J.J.; Ward, J.N.; McConnell, J.P. Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: Observations from 160,000 patients. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 257–263. [Google Scholar] [CrossRef]
- Pinar-Martí, A.; Fernández-Barrés, S.; Gignac, F.; Persavento, C.; Delgado, A.; Romaguera, D.; Lázaro, I.; Ros, E.; López-Vicente, M.; Salas-Salvadó, J.; et al. Red blood cell omega-3 fatty acids and attention scores in healthy adolescents. Eur. Child Adolesc. Psychiatry 2023, 32, 2187–2195. [Google Scholar] [CrossRef]
- Frensham, L.J.; Bryan, J.; Parletta, N. Influences of micronutrient and omega-3 fatty acid supplementation on cognition, learning, and behavior: Methodological considerations and implications for children and adolescents in developed societies. Nutr. Rev. 2012, 70, 594–610. [Google Scholar] [CrossRef]
- Julvez, J.; Gignac, F.; Fernández-Barrés, S.; Romaguera, D.; Sala-Vila, A.; Ranzani, O.T.; Persavento, C.; Delgado, A.; Carol, A.; Torrent, J.; et al. Walnuts, Long-Chain Polyunsaturated Fatty Acids, and Adolescent Brain Development: Protocol for the Walnuts Smart Snack Dietary Intervention Trial. Front. Pediatr. 2021, 9, 593847. [Google Scholar] [CrossRef]
- Ribas-Fitó, N.; Ramón, R.; Ballester, F.; Grimalt, J.; Marco, A.; Olea, N.; Posada, M.; Rebagliato, M.; Tardón, A.; Torrent, M.; et al. Child health and the environment: The INMA Spanish Study. Paediatr. Perinat. Epidemiol. 2006, 20, 403–410. [Google Scholar] [CrossRef]
- Serra Majem, L.L.; Ribas Barba, L.; Ngo de la Cruz, J.; Ortega Anta, R.M.; Pérez Rodrigo, C.; Aranceta Bartrina, J. Alimentación, jóvenes y dieta mediterránea en España. In Desarrollo del KIDMED, Índice de Calidad de la Dieta Mediterránea en la Infancia y la Adolescencia; Serra Majem, L., Aranceta Bartrina, J., Eds.; Aliment Infant y Juv Estud enKid 1a Edición; Masson: Barcelona, Spain, 2002; pp. 51–59. [Google Scholar]
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Cofán, M.; Covas, M.-I.; Estruch, R.; Harris, W.S.; Lamuela-Raventós, R.M.; Pintó, X.; Pérez-Heras, A.M.; Ros, E.; Sala-Vila, A. Determinants of the omega-3 index in a Mediterranean population at increased risk for CHD. Br. J. Nutr. 2011, 106, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, I.; Grau-Rivera, O.; Suárez-Calvet, M.; Fauria, K.; Minguillón, C.; Shekari, M.; Falcón, C.; García-Prat, M.; Huguet, J.; Molinuevo, J.L.; et al. Omega-3 blood biomarkers relate to brain glucose uptake in individuals at risk of Alzheimer’s disease dementia. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2024, 16, e12596. [Google Scholar] [CrossRef] [PubMed]
- Guevarra, E. Nutriverse/Zscorer: Zscorer v0.3.1. In Zenodo 2019. Available online: https://zenodo.org/records/3510075 (accessed on 1 December 2024).
- Kassambara, A.; Mundt, F. CRAN-Package Factoextra 2020. Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 1 December 2024).
- Revelle, W. CRAN-Package Psych 2014. Available online: https://doi.org/10.32614/CRAN.package.psych (accessed on 1 December 2024).
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their “Balanced Antagonistic Metabolic Functions” in the Human Body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef] [PubMed]
- Syrén, M.-L.; Turolo, S.; de Marco, E.A.; De Cosmi, V.; Risé, P.; Marangoni, F.; Minoli, D.G.; Manzoni, G.; Agostoni, C. Whole blood fatty acid profile of young subjects and adherence to the Mediterranean diet: An observational cohort study. Lipids Health Dis. 2022, 21, 23. [Google Scholar] [CrossRef]
- Bartha, V.; Exner, L.; Basrai, M.; Bischoff, S.C.; Schweikert, D.; Adolph, M.; Bruckner, T.; Grueninger, D.; Klein, D.; Meller, C.; et al. Changes in serum omega fatty acids on a Mediterranean diet intervention in patients with gingivitis: An exploratory study. J. Periodontal Res. 2022, 57, 1198–1209. [Google Scholar] [CrossRef]
- Ortega, R.M. Importance of functional foods in the Mediterranean diet. Public Health Nutr. 2006, 9, 1136–1140. [Google Scholar] [CrossRef]
- Aparicio-Ugarriza, R.; Cuenca-García, M.; Gonzalez-Gross, M.; Julián, C.; Bel-Serrat, S.; Moreno, L.A.; Breidenassel, C.; Kersting, M.; Arouca, A.B.; Michels, N.; et al. Relative validation of the adapted Mediterranean Diet Score for Adolescents by comparison with nutritional biomarkers and nutrient and food intakes: The Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study. Public Health Nutr. 2019, 22, 2381–2397. [Google Scholar] [CrossRef]
- Huang, Z.; Ma, K.; Yin, X.; Li, Z.; Chen, M.; Duan, Y.; Li, L.; Hu, Y. The associations of fatty acids related dietary patterns with overweight and obesity among Chinese children. J. Health Popul. Nutr. 2024, 43, 54. [Google Scholar] [CrossRef]
- Mantzioris, E.; Muhlhausler, B.S.; Villani, A. Impact of the Mediterranean Dietary pattern on n-3 fatty acid tissue levels–A systematic review. Prostaglandins Leukot. Essent. Fat. Acids 2022, 176, 102387. [Google Scholar] [CrossRef]
- Turchini, G.M.; Francis, D.S.; De Silva, S.S. A Whole Body, In Vivo, Fatty Acid Balance Method to Quantify PUFA Metabolism (Desaturation, Elongation and Beta-oxidation). Lipids 2007, 42, 1065–1071. [Google Scholar] [CrossRef]
- Kaur, G.; Cameron-Smith, D.; Garg, M.; Sinclair, A.J. Docosapentaenoic acid (22:5n-3): A review of its biological effects. Prog. Lipid Res. 2011, 50, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tong, W.; Ruan, Y.; Sinclair, A.J.; Li, D. Different metabolism of EPA, DPA and DHA in humans: A double-blind cross-over study. Prostaglandins Leukot. Essent. Fat. Acids 2020, 158, 102033. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Sandhu, K.S.; Siroha, A.K.; Dhull, S.B. Omega 3-metabolism, absorption, bioavailability and health benefits—A review. PharmaNutrition 2019, 10, 100162. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Komorniak, N.; Bilicki, J.; Celewicz, Z.; Ziętek, M. The Role of Arachidonic and Linoleic Acid Derivatives in Pathological Pregnancies and the Human Reproduction Process. Int. J. Mol. Sci. 2020, 21, 9628. [Google Scholar] [CrossRef]
- Decsi, T.; Kennedy, K. Sex-specific differences in essential fatty acid metabolism. Am. J. Clin. Nutr. 2011, 94, S1914–S1919. [Google Scholar] [CrossRef] [PubMed]
- Kontele, I.; Panagiotakos, D.; Yannakoulia, M.; Vassilakou, T. Socio-Demographic Determinants of Mediterranean Diet Adherence: Results of the EU-National Health Interview Survey (EHIS-3). J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2025, 38, e70023. [Google Scholar] [CrossRef]
- Di Nucci, A.; Silano, M.; Cardamone, E. Adherence to Mediterranean Diet and Health Outcomes in Adolescents: An Umbrella Review. Nutr. Rev. 2025, 83, e1329–e1342. [Google Scholar] [CrossRef]
- O’Sullivan, T.A.; Ambrosini, G.L.; Mori, T.A.; Beilin, L.J.; Oddy, W.H. Omega-3 Index Correlates with Healthier Food Consumption in Adolescents and with Reduced Cardiovascular Disease Risk Factors in Adolescent Boys. Lipids 2011, 46, 59–67. [Google Scholar] [CrossRef]
- Gonçalinho, G.H.; Sampaio, G.R.; Soares-Freitas, R.A.; Damasceno, N.R. Omega-3 Fatty Acids in Erythrocyte Membranes as Predictors of Lower Cardiovascular Risk in Adults without Previous Cardiovascular Events. Nutrients 2021, 13, 1919. [Google Scholar] [CrossRef]
- Pottala, J.V.; Yaffe, K.; Robinson, J.G.; Espeland, M.A.; Wallace, R.; Harris, W.S. Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes. Neurology 2014, 82, 435–442. [Google Scholar] [CrossRef]
- Glickman, M.E.; Rao, S.R.; Schultz, M.R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 2014, 67, 850–857. [Google Scholar] [CrossRef]
- Fewell, Z.; Davey Smith, G.; Sterne, J.A.C. The Impact of Residual and Unmeasured Confounding in Epidemiologic Studies: A Simulation Study. Am. J. Epidemiol. 2007, 166, 646–655. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).