Sex-Based Associations Between Education Level, EAT–Lancet Diet, and 20-Year Cardiovascular Risk: The ATTICA Study (2002–2022)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Compliance and Protection of Personal Data
2.2. Design, Setting, and Participant Recruitment
2.3. Data Collection at 2001/2002 Baseline
2.3.1. Ascertainment of Socio-Demographic Factors, Including Educational Attainment
2.3.2. Assessment of Other Explanatory Variables
2.4. Primary Endpoints Measures at the 2006, 2011/2012, and 2022 Follow-Up Examinations
2.5. Statistical Analysis
3. Results
3.1. Crude Analysis of CVD Outcomes by Educational Level
3.2. Differences on Baseline CVD Predisposing Factors by Educational Level
3.3. Moderation and Mediation Analysis of the Role of Education on the 20-Year CVD Risk
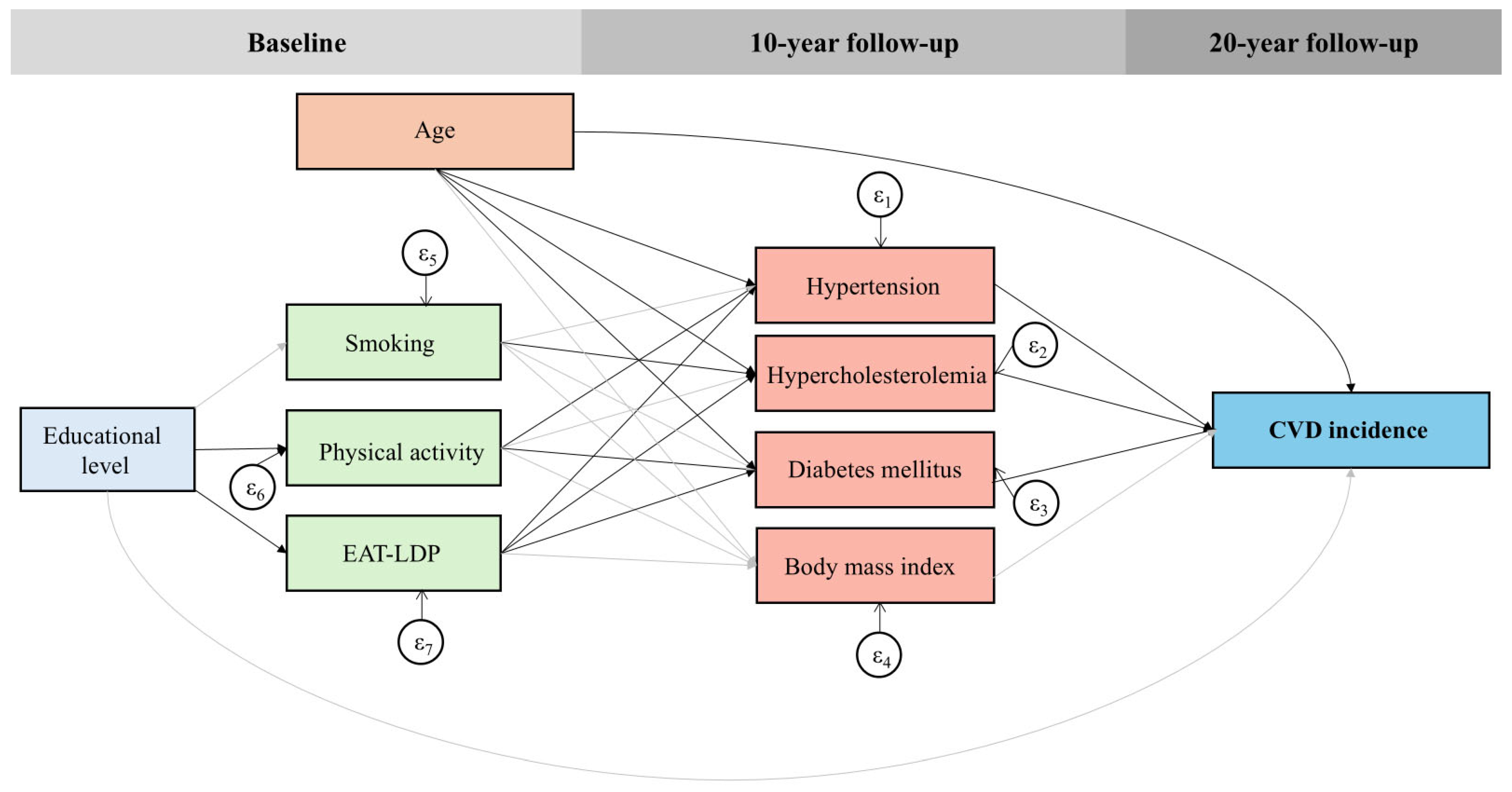
3.4. Generalized Structural Equation Model (GSEM)
4. Discussion
4.1. Main Epidemiological Findings
4.2. The Pathways Between Educational Attainment and CVD Outcomes
4.3. The Interplay of Education, Healthy and Sustainable Dietary Habits, and CVD
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular diseases |
| BMI | Body mass index |
| BMR | Basal metabolic rate |
| DALYs | Disability-adjusted life years |
| EAT-LDP | EAT-Lancet diet pattern |
| FFQ | Food frequency questionnaire |
| GSEM | Generalized structural equation model |
| HR | Hazards ratio |
| hs-CRP | High-sensitivity C-reactive protein |
| ICD | International Classification of Diseases |
| OR | Odds ratio |
| RR | Risk ratio |
| RRR | Ratio of relative risks |
| SD | Standard deviation |
| SDI | Socio-demographic index |
| WC | Waist circumference |
| WHO | World Health Organization |
| WHtR | Waist-to-height ratio |
| WHR | Waist-to-hip ratio |
| 95% CI | 95% confidence interval |
References
- GBD 2021 Diseases Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Baumer, Y.; Baah, F.O.; Baez, A.S.; Farmer, N.; Mahlobo, C.T.; Pita, M.A.; Potharaju, K.A.; Tamura, K.; Wallen, G.R. Social Determinants of Cardiovascular Disease. Circ. Res. 2022, 130, 782–799. [Google Scholar] [CrossRef]
- Kubota, Y.; Heiss, G.; MacLehose, R.F.; Roetker, N.S.; Folsom, A.R. Association of Educational Attainment with Lifetime Risk of Cardiovascular Disease: The Atherosclerosis Risk in Communities Study. JAMA Intern. Med. 2017, 177, 1165–1172. [Google Scholar] [CrossRef]
- Jeong, C.; Lee, K.N.; Jung, J.H.; Sohn, T.S.; Kwon, H.S.; Han, K.; Lee, S.H. Socioeconomic gradients and inequalities in all-cause mortality and cardiovascular diseases: A retrospective cohort study using Korean NHANES-mortality linkage data. Public Health 2025, 244, 105767. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Paultre, F.; Mosca, L. The association between educational level and risk of cardiovascular disease fatality among women with cardiovascular disease. Women’s Health Issues Off. Publ. Jacobs Inst. Women’s Health 2005, 15, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Khaing, W.; Vallibhakara, S.A.; Attia, J.; McEvoy, M.; Thakkinstian, A. Effects of education and income on cardiovascular outcomes: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Backholer, K.; Peters, S.A.E.; Bots, S.H.; Peeters, A.; Huxley, R.R.; Woodward, M. Sex differences in the relationship between socioeconomic status and cardiovascular disease: A systematic review and meta-analysis. J. Epidemiol. Community Health 2017, 71, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Magnani, J.W.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Allen, N.B. Educational Attainment and Lifetime Risk of Cardiovascular Disease. JAMA Cardiol. 2024, 9, 45–54. [Google Scholar] [CrossRef]
- Merz, E.C.; Myers, B.; Hansen, M.; Simon, K.R.; Strack, J.; Noble, K.G. Socioeconomic Disparities in Hypothalamic-Pituitary-Adrenal Axis Regulation and Prefrontal Cortical Structure. Biol. Psychiatry Glob. Open Sci. 2024, 4, 83–96. [Google Scholar] [CrossRef]
- de Mestral, C.; Stringhini, S. Socioeconomic Status and Cardiovascular Disease: An Update. Curr. Cardiol. Rep. 2017, 19, 115. [Google Scholar] [CrossRef]
- WHO; FAO. Sustainable Healthy Diets-Guiding Principles; FAO: Rome, Italy, 2019. [Google Scholar]
- Willett, W.; Rockstrom, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Food security. In Climate Change and Land: IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Cambridge, UK, 2019; pp. 437–550. [Google Scholar] [CrossRef]
- World Health Organization. Gender. Available online: https://www.who.int/health-topics/gender#tab=tab_1 (accessed on 3 August 2024).
- Damigou, E.; Kouvari, M.; Chrysohoou, C.; Barkas, F.; Kravvariti, E.; Pitsavos, C.; Skoumas, J.; Michelis, E.; Liberopoulos, E.; Tsioufis, C.; et al. Lifestyle Trajectories Are Associated with Incidence of Cardiovascular Disease: Highlights from the ATTICA Epidemiological Cohort Study (2002–2022). Life 2023, 13, 1142. [Google Scholar] [CrossRef]
- Panagiotakos, D.; Georgousopoulou, E.; Notara, V.; Pitaraki, E.; Kokkou, E.; Chrysohoou, C.; Skoumas, Y.; Metaxa, V.; Pitsavos, C.; Stefanadis, C.; et al. Education status determines 10-year (2002–2012) survival from cardiovascular disease in Athens metropolitan area: The ATTICA study, Greece. Health Soc. Care Community 2016, 24, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Pitsavos, C.; Panagiotakos, D.B.; Chrysohoou, C.; Stefanadis, C. Epidemiology of cardiovascular risk factors in Greece: Aims, design and baseline characteristics of the ATTICA study. BMC Public Health 2003, 3, 32. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Skoumas, I.; Stefanadis, C. Five-year incidence of cardiovascular disease and its predictors in Greece: The ATTICA study. Vasc. Med. 2008, 13, 113–121. [Google Scholar] [CrossRef]
- Katsouyanni, K.; Rimm, E.B.; Gnardellis, C.; Trichopoulos, D.; Polychronopoulos, E.; Trichopoulou, A. Reproducibility and relative validity of an extensive semi-quantitative food frequency questionnaire using dietary records and biochemical markers among Greek schoolteachers. Int. J. Epidemiol. 1997, 26 (Suppl. 1), S118–S127. [Google Scholar] [CrossRef] [PubMed]
- Stubbendorff, A.; Sonestedt, E.; Ramne, S.; Drake, I.; Hallstrom, E.; Ericson, U. Development of an EAT-Lancet index and its relation to mortality in a Swedish population. Am. J. Clin. Nutr. 2022, 115, 705–716. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Papathanasiou, G.; Georgoudis, G.; Papandreou, M.; Spyropoulos, P.; Georgakopoulos, D.; Kalfakakou, V.; Evangelou, A. Reliability measures of the short International Physical Activity Questionnaire (IPAQ) in Greek young adults. Hellenic. J. Cardiol. 2009, 50, 283–294. [Google Scholar]
- Georgoulis, M.; Damigou, E.; Chrysohoou, C.; Barkas, F.; Kravvariti, E.; Tsioufis, C.; Pitsavos, C.; Liberopoulos, E.; Sfikakis, P.P.; Panagiotakos, D.B.; et al. Increased body weight and central adiposity markers are positively associated with the 20-year incidence of cardiovascular disease: The ATTICA epidemiological study (2002–2022). Nutr. Res. 2024, 121, 1–15. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Stefanadis, C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J. Am. Coll. Cardiol. 2004, 44, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Huffman, M.D.; Karmali, K.N.; Sanghavi, D.M.; Wright, J.S.; Pelser, C.; Gulati, M.; Masoudi, F.A.; Goff, D.C., Jr. Estimating longitudinal risks and benefits from cardiovascular preventive therapies among Medicare patients: The million hearts longitudinal ASCVD risk assessment tool: A special report from the American heart association and American College of cardiology. J. Circ. 2017, 135, e793–e813. [Google Scholar] [CrossRef] [PubMed]
- Beiser, A.; D’Agostino, R.B., Sr.; Seshadri, S.; Sullivan, L.M.; Wolf, P.A. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat. Med. 2000, 19, 1495–1522. [Google Scholar] [CrossRef]
- Kushner, R.F. Clinical assessment and management of adult obesity. Circulation 2012, 126, 2870–2877. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Browning, L.M.; Hsieh, S.D.; Ashwell, M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value. Nutr. Res. Rev. 2010, 23, 247–269. [Google Scholar] [CrossRef]
- WHO. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Sigala, E.G.; Vaina, S.; Chrysohoou, C.; Dri, E.; Damigou, E.; Tatakis, F.P.; Sakalidis, A.; Barkas, F.; Liberopoulos, E.; Sfikakis, P.P.; et al. Sex-related differences in the 20-year incidence of CVD and its risk factors: The ATTICA study (2002–2022). Am. J. Prev. Cardiol. 2024, 19, 100709. [Google Scholar] [CrossRef]
- Alemu, Y.M.; Bagheri, N.; Wangdi, K.; Richardson, A.; Chateau, D.J.C.P.H. Disparities in primary cardiovascular risks and social determinants: Multilevel analysis of national surveys. Crit. Public Health 2025, 35, 2507229. [Google Scholar] [CrossRef]
- Petrelli, A.; Sebastiani, G.; Di Napoli, A.; Macciotta, A.; Di Filippo, P.; Strippoli, E.; Mirisola, C.; d’Errico, A. Education inequalities in cardiovascular and coronary heart disease in Italy and the role of behavioral and biological risk factors. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 918–928. [Google Scholar] [CrossRef]
- Powell, K.L.; Stephens, S.R.; Stephens, A.S. Cardiovascular risk factor mediation of the effects of education and Genetic Risk Score on cardiovascular disease: A prospective observational cohort study of the Framingham Heart Study. BMJ Open 2021, 11, e045210. [Google Scholar] [CrossRef]
- Kontele, I.; Panagiotakos, D.; Yannakoulia, M.; Vassilakou, T. Socio-Demographic Determinants of Mediterranean Diet Adherence: Results of the EU-National Health Interview Survey (EHIS-3). J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2025, 38, e70023. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Abreu, S.; Liz Martins, M. Determinants of adherence to sustainable healthy diets among Portuguese adults. NFS J. 2024, 37, 100200. [Google Scholar] [CrossRef]
- Carvalho, C.; Correia, D.; Lopes, C.; Torres, D. Adherence to the EAT-Lancet Planetary Health Diet in Portugal and its associations with socioeconomic and lifestyle factors. Eur. J. Nutr. 2025, 64, 152. [Google Scholar] [CrossRef]
- Boujelbane, M.A.; Ammar, A.; Salem, A.; Kerkeni, M.; Trabelsi, K.; Bouaziz, B.; Masmoudi, L.; Heydenreich, J.; Schallhorn, C.; Müller, G.; et al. Sex-Specific Insights into Adherence to Mediterranean Diet and Lifestyle: Analysis of 4000 Responses from the MEDIET4ALL Project. Front. Nutr. 2025, 12, 1570904. [Google Scholar] [CrossRef]
- Liu, J.; Shen, Q.; Wang, X. Emerging EAT-Lancet planetary health diet is associated with major cardiovascular diseases and all-cause mortality: A global systematic review and meta-analysis. Clin. Nutr. 2024, 43, 167–179. [Google Scholar] [CrossRef]
- Sawicki, C.M.; Ramesh, G.; Bui, L.; Nair, N.K.; Hu, F.B.; Rimm, E.B.; Stampfer, M.J.; Willett, W.C.; Bhupathiraju, S.N. Planetary health diet and cardiovascular disease: Results from three large prospective cohort studies in the USA. Lancet Planet Health 2024, 8, e666–e674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dukuzimana, J.; Stubbendorff, A.; Ericson, U.; Borne, Y.; Sonestedt, E. Adherence to the EAT-Lancet diet and risk of coronary events in the Malmo Diet and Cancer cohort study. Am. J. Clin. Nutr. 2023, 117, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Marken, I.; Stubbendorff, A.; Ericson, U.; Qi, L.; Sonestedt, E.; Borne, Y. The EAT-Lancet Diet Index, Plasma Proteins, and Risk of Heart Failure in a Population-Based Cohort. JACC Heart Fail 2024, 12, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Stubbendorff, A.; Ericson, U.; Wandell, P.; Niu, K.; Qi, L.; Borne, Y.; Sonestedt, E. The EAT-Lancet diet, genetic susceptibility and risk of atrial fibrillation in a population-based cohort. BMC Med. 2023, 21, 280. [Google Scholar] [CrossRef]
- Ibsen, D.B.; Christiansen, A.H.; Olsen, A.; Tjonneland, A.; Overvad, K.; Wolk, A.; Mortensen, J.K.; Dahm, C.C. Adherence to the EAT-Lancet Diet and Risk of Stroke and Stroke Subtypes: A Cohort Study. Stroke 2022, 53, 154–163. [Google Scholar] [CrossRef]
- Shan, Y.; Bertrand, K.A.; Petrick, J.L.; Sheehy, S.; Palmer, J.R. Planetary Health Diet Index in relation to mortality in a prospective cohort study of United States Black females. Am. J. Clin. Nutr. 2025, 121, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.B.; Gamba, M.; Stubbendorff, A.; Gasser, N.; Lobl, L.; Stern, F.; Ericson, U.; Marques-Vidal, P.; Vuilleumier, S.; Chatelan, A. Association between the EAT-Lancet Diet, Incidence of Cardiovascular Events, and All-Cause Mortality: Results from a Swiss Cohort. J. Nutr. 2025, 155, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Castellanos, K.B.; Zazpe, I.; Santiago, S.; Bes-Rastrollo, M.; Martinez-Gonzalez, M.A. Planetary Health Diet and Cardiovascular Disease Risk in the Seguimiento Universidad de Navarra (SUN) Cohort. Nutrients 2024, 17, 27. [Google Scholar] [CrossRef]
- Colizzi, C.; Harbers, M.C.; Vellinga, R.E.; Verschuren, W.M.M.; Boer, J.M.A.; Biesbroek, S.; Temme, E.H.M.; van der Schouw, Y.T. Adherence to the EAT-Lancet Healthy Reference Diet in Relation to Risk of Cardiovascular Events and Environmental Impact: Results From the EPIC-NL Cohort. J. Am. Heart Assoc. 2023, 12, e026318. [Google Scholar] [CrossRef] [PubMed]
| Total Sample | Females | Males | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidemiological Indices | Low (n = 422) | Medium (n = 847) | High (n = 719) | p-Value for Trend | Low (n = 225) | Medium (n = 431) | High (n = 345) | p-Value for Trend | Low (n = 197) | Medium (n = 416) | High (n = 374) | p-Value for Trend |
| Age at first CVD event, years | 75 (15) | 65 (16) | 61 (14) | <0.001 | 75 (12) | 65 (12) | 59 (24) | <0.001 | 72 (19) | 65 (18) | 61 (13) | <0.001 |
| 20-year CVD incidence, % | 62.9 | 30.9 | 26.6 | <0.001 | 62.7 | 26.5 | 19.1 | <0.001 | 62.9 | 35.6 | 33.4 | <0.001 |
| ≤35 years old | 10.7 | 5.6 | 4.1 | 0 | 3.4 | 3.7 | 14.3 | 8.3 | 4.7 | |||
| 35–45 years old | 6.7 | 6.2 | 7.6 | 5.4 | 3.1 | 4.5 | 8.1 | 10.0 | 10.1 | |||
| 45–55 years old | 51.6 | 53.6 | 52.1 | 40.3 | 55.0 | 41.2 | 64.4 | 52.4 | 59.2 | |||
| 55–65 years old | 97.8 | 95.0 | 96.3 | 96.5 | 90.2 | 91.3 | 100.0 | 100.0 | 100.0 | |||
| >65 years old | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||
| 20-year CVD mortality, % | 11.2 | 3.2 | 2.2 | <0.001 | 5.4 | 0.9 | 0.8 | <0.001 | 18.2 | 5.7 | 3.6 | <0.001 |
| Lifetime CVD risk, % (95% CI) | ||||||||||||
| 40–50 years old | 72.3 (70.4, 74.2) | 71.0 (69.7, 72.2) | 69.3 (67.9, 70.7) | 0.050 | 66.6 (64.0, 69.3) | 65.7 (64.0, 67.4) | 61.8 (59.6, 63.9) | 0.004 | 79.2 (77.4, 81.1) | 76.9 (75.5, 78.4) | 74.3 (72.8, 75.8) | 0.001 |
| 50–60 years old | 65.7 (63.9, 66.7) | 65.8 (63.9, 66.7) | 63.5 (61.9, 65.2) | 0.091 | 63.7 (61.3, 66.2) | 63.8 (61.7, 65.9) | 58.6 (56.1, 61.1) | 0.002 | 67.7 (65.2, 70.3) | 66.8 (65.1, 68.6) | 67.0 (65.1, 68.9) | 0.684 |
| 60–70 years old | 66.5 (64.5, 68.5) | 65.3 (63.2, 67.4) | 62.5 (58.8, 66.1) | 0.186 | 66.2 (63.5, 68.9) | 66.6 (63.7, 69.6) | 64.1 (63.9, 70.0) | 0.808 | 67.0 (63.9, 70.0) | 64.0 (61.0, 66.9) | 61.0 (56.2, 65.9) | 0.070 |
| CVD burden, DALYs (95% CI) | 10.2 (9.1, 11.4) | 13.4 (12.0, 14.8) | 13.3 (11.7, 14.9) | 0.006 | 9.7 (8.3, 11.1) | 13.8 (12.0, 15.5) | 14.5 (11.4, 17.5) | <0.001 | 11.0 (9.0, 13.0) | 13.1 (11.0, 15.2) | 12.7 (10.8, 14.6) | 0.604 |
| Total Sample | Females | Males | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Low (n = 422) | Medium (n = 847) | High (n = 719) | p-Value for Trend | Low (n = 225) | Medium (n = 431) | High (n = 345) | p-Value for Trend | Low (n = 197) | Medium (n = 416) | High (n = 374) | p-Value for Trend |
| Age, years | 54 (19) | 43 (18) | 41 (17) | <0.001 | 56 (19) | 42 (18) | 39 (17) | <0.001 | 52 (20) | 44 (18) | 43 (15) | <0.001 |
| Financial status, % low income | 77.7 | 62.1 | 33.8 | <0.001 | 88.8 | 68.7 | 48.3 | <0.001 | 66.4 | 55.6 | 21.6 | <0.001 |
| Socio-economic status, % | <0.001 | <0.001 | <0.001 | |||||||||
| Low class | 81.0 | 0 | 0 | 83.2 | 0 | 0 | 78.9 | 0 | 0 | |||
| Middle class | 19.0 | 94.6 | 13.1 | 16.8 | 95.5 | 18.5 | 21.6 | 93.7 | 8.6 | |||
| High class | 0 | 5.4 | 86.9 | 0 | 4.5 | 81.5 | 0 | 6.3 | 91.4 | |||
| Residential setting, % urban | 79.2 | 77.1 | 77.2 | 0.680 | 79.6 | 77.3 | 78.3 | 0.794 | 78.7 | 76.9 | 76.2 | 0.799 |
| Hypertension, % | 40.2 | 30.6 | 26.6 | <0.001 | 41.0 | 22.6 | 13.9 | <0.001 | 39.4 | 39.3 | 38.4 | 0.957 |
| Treated | 48.4 | 27.3 | 21.7 | 53.5 | 33.3 | 24.4 | 42.7 | 23.5 | 20.7 | |||
| Untreated but aware | 51.6 | 72.7 | 78.3 | 46.5 | 66.7 | 75.6 | 57.3 | 76.5 | 79.3 | |||
| Unaware | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Hypercholesterolemia, % | 56.6 | 39.9 | 37.3 | <0.001 | 55.6 | 34.3 | 31.3 | <0.001 | 59.0 | 45.7 | 42.9 | <0.001 |
| Treated | 16.1 | 9.5 | 10.1 | 16.0 | 9.1 | 8.3 | 16.1 | 12.1 | 11.2 | |||
| Untreated but aware | 83.9 | 90.5 | 89.9 | 84.0 | 90.9 | 81.7 | 83.9 | 87.9 | 88.8 | |||
| Unaware | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Diabetes mellitus, % | 15.6 | 5.8 | 3.8 | <0.001 | 12.0 | 4.9 | 2.3 | <0.001 | 19.8 | 6.7 | 5.1 | <0.001 |
| Treated | 51.5 | 40.8 | 22.2 | 59.3 | 47.6 | 25.0 | 46.2 | 35.7 | 21.1 | |||
| Untreated but aware | 43.9 | 51.0 | 74.1 | 33.3 | 47.6 | 75.0 | 51.3 | 53.6 | 73.7 | |||
| Unaware | 5.6 | 8.2 | 3.7 | 7.4 | 4.8 | 0 | 2.5 | 10.7 | 5.2 | |||
| Fibrinogen, mg/dL | 328 ± 72 | 305 ± 65 | 306 ± 71 | <0.001 | 340 ± 71 | 316 ± 66 | 307 ± 74 | <0.001 | 312 ± 70 | 293 ± 63 | 305 ± 68 | 0.006 |
| hs-CRP, mg/L | 1.34 (2.11) | 1.03 (1.85) | 0.88 (1.80) | <0.001 | 1.62 (2.39) | 0.88 (2.06) | 0.75 (1.73) | <0.001 | 1.22 (1.77) | 1.16 (1.66) | 1.04 (179) | 0.472 |
| Overweight and/or obesity, % | 71.7 | 54.9 | 52.0 | <0.001 | 65.8 | 40.8 | 30.9 | <0.001 | 78.5 | 69.5 | 71.6 | 0.069 |
| Increased WC, % | 64.0 | 51.5 | 48.6 | <0.001 | 68.9 | 46.9 | 39.1 | <0.001 | 58.4 | 56.3 | 57.4 | 0.876 |
| BMI, kg/m2 | 27.6 ± 4.6 | 26.1 ± 4.5 | 25.8 ± 4.4 | <0.001 | 27.4 ± 4.9 | 25.0 ± 4.7 | 24.0 ± 4.4 | <0.001 | 27.8 ± 4.2 | 27.2 ± 3.9 | 27.3 ± 3.8 | 0.236 |
| WC, cm | 94 ± 14 | 90 ± 14 | 89 ± 16 | <0.001 | 89 ± 14 | 82 ± 13 | 79 ± 13 | <0.001 | 99 ± 12 | 97 ± 12 | 98 ± 14 | 0.218 |
| WHtR | 0.57 ± 0.08 | 0.53 ± 0.08 | 0.52 ± 0.09 | <0.001 | 0.56 (0.11) | 0.49 (0.11) | 0.47 (0.09) | <0.001 | 0.57 (0.09) | 0.55 (0.08) | 0.56 (0.09) | <0.001 |
| WHR | 0.89 (0.14) | 0.85 (0.14) | 0.86 (0.16) | <0.001 | 0.83 (0.09) | 0.79 (0.08) | 0.78 (0.09) | <0.001 | 0.95 (0.08) | 0.92 (0.09) | 0.93 (0.09) | <0.001 |
| Energy intake adjusted to ΒMR | 1.62 (0.38) | 1.42 (0.30) | 1.41 (0.24) | <0.001 | 1.66 (0.31) | 1.44 (0.31) | 1.44 (0.24) | <0.001 | 1.58 (0.44) | 1.41 (0.29) | 1.40 (0.24) | <0.001 |
| EAT-LDP, 0–42 units | 16.5 (13.0) | 18.8 (6.3) | 20.4 (6.3) | <0.001 | 8.6 (11.5) | 19.6 (6.3) | 22.7 (6.3) | <0.001 | 16.5 (14.6) | 18.8 (6.3) | 18.8 (6.3) | <0.001 |
| Adherence to EAT-LDP, % low | 72.5 | 45.0 | 39.8 | <0.001 | 76.4 | 45.2 | 35.7 | <0.001 | 68.0 | 44.7 | 43.6 | <0.001 |
| MedDietScore, 0–55 units | 25.7 (3.0) | 26.8 (2.8) | 26.8 (3.0) | <0.001 | 26.3 (3.3) | 28.0 (2.4) | 28.3 (2.1) | <0.001 | 25.1 (2.7) | 25.7 (1.9) | 25.7 (1.9) | <0.001 |
| Adherence to MDP, % low | 80.3 | 54.9 | 54.1 | <0.001 | 68.9 | 29.5 | 21.2 | <0.001 | 93.4 | 81.3 | 84.5 | <0.001 |
| Smoking, % ever | 52.1 | 60.1 | 51.8 | 0.001 | 34.7 | 51.3 | 42.3 | <0.001 | 72.1 | 69.2 | 60.6 | 0.007 |
| Physically inactivity, % | 61.4 | 60.6 | 56.5 | 0.157 | 67.1 | 61.5 | 57.4 | 0.066 | 54.8 | 59.6 | 55.6 | 0.401 |
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Females | ||||
| Educational level, per 1 year | 0.81 (0.77, 0.84) *** | 0.99 (0.94, 1.06) | 1.02 (0.95, 1.08) | 1.02 (0.95, 1.09) |
| Age, per 1 year | 1.30 (1.25, 1.35) *** | 1.28 (1.23, 1.34) *** | 1.21 (1.16, 1.27) *** | |
| Hypertension, ref: normal | 1.35 (0.78, 2.33) | 1.37 (0.78, 2.42) | ||
| Hypercholesterolemia, ref: normal | 3.72 (2.30, 6.02) *** | 3.28 (2.00, 5.39) *** | ||
| Diabetes mellitus, ref: normal | 8.41 (1.78, 39.8) ** | 9.89 (2.05, 47.7) ** | ||
| WHtR, per 1 unit | 1.30 (0.07, 25.1) | 0.79 (0.04, 16.7) | ||
| Smoking, ref: never smokers | 0.77 (0.46, 1.27) | |||
| EAT-LDP, per 1/42 unit | 0.85 (0.79, 0.92) *** | |||
| Physically inactivity, ref: yes | 1.13 (0.68, 1.90) | |||
| p-value (omnibus test) | <0.001 | <0.001 | <0.001 | <0.001 |
| p-value (likelihood ratio test) | <0.001 | <0.001 | <0.001 | <0.001 |
| Males | ||||
| Educational level, per 1 year | 0.89 (0.86, 0.93) *** | 0.97 (0.92, 1.03) | 0.99 (0.93, 1.05) | 0.99 (0.93, 1.05) |
| Age, per 1 year | 1.29 (1.24, 1.34) *** | 1.27 (1.23, 1.33) *** | 1.21 (1.16, 1.27) *** | |
| Hypertension, ref: normal | 1.35 (0.87, 2.09) | 1.43 (0.91, 2.24) | ||
| Hypercholesterolemia, ref: normal | 1.69 (1.11, 2.57) * | 1.90 (1.23, 2.93) ** | ||
| Diabetes mellitus, ref: normal | 4.27 (1.70, 10.7) ** | 4.78 (1.84, 12.4) ** | ||
| WHtR, per 1 unit | 2.21 (0.10, 47.6) | 2.41 (0.09, 67.1) | ||
| Smoking, ref: never smokers | 1.42 (0.92, 2.19) | |||
| EAT-LDP, per 1/42 unit | 0.84 (0.78, 0.90) *** | |||
| Physically inactivity, ref: yes | 0.97 (0.92, 2.19) | |||
| p-value (omnibus test) | <0.001 | <0.001 | <0.001 | <0.001 |
| p-value (likelihood ratio test) | <0.001 | <0.001 | 0.002 | <0.001 |
| Outcome Variables | Manifest Variables | Path OR (95% CI) |
|---|---|---|
| 20-year CVD incidence, ref.: no | ||
| Age at baseline, per 1 year | 0.29 (0.24, 0.33) *** | |
| Hypertension, ref.: no | 0.83 (0.38, 1.29) *** | |
| Hypercholesterolemia, ref.: no | 1.06 (0.56, 1.56) *** | |
| Diabetes mellitus, ref.: no | 1.43 (0.84, 2.01) *** | |
| BMI, per 1 kg/m2 | −0.02 (−0.07, 0.03) | |
| Educational level, per 1 year | −0.02 (−0.08, 0.05) | |
| Hypertension, ref.: no | ||
| Age at baseline, per 1 year | 0.012 (0.010, 0.015) *** | |
| Smoking, ref.: never smokers | 0.02 (−0.02, 0.07) | |
| EAT-LDP, per 1/42 unit | −0.007 (−0.012, −0.002) * | |
| Physically inactivity, ref.: yes | −0.04 (−0.085, −0.001) * | |
| Hypercholesterolemia, ref.: no | ||
| Age at baseline, per 1 year | 0.005 (0.003, 0.007) *** | |
| Smoking, ref: never smokers | 0.048 (0.006, 0.090) * | |
| EAT-LDP, per 1/42 unit | −0.012 (−0.017, −0.007) *** | |
| Physically inactivity, ref.: yes | −0.03 (−0.07, 0.02) | |
| Diabetes mellitus, ref.: no | ||
| Age at baseline, per 1 year | 0.007 (0.005, 0.009) *** | |
| Smoking, ref.: never smokers | −0.009 (−0.050, 0.032) | |
| EAT-LDP, per 1/42 unit | −0.012 (−0.016, −0.007) *** | |
| Physically inactivity, ref.: yes | −0.06 (−0.10, −0.01) ** | |
| BMI, per 1 kg/m2 | ||
| Age at baseline, per 1 year | 0.005 (−0.019, 0.030) | |
| Smoking, ref.: never smokers | 0.40 (−0.02, 0.82) | |
| EAT-LDP, per 1/42 unit | −0.027 (−0.079, 0.026) | |
| Physically inactivity, ref.: yes | 0.003 (−0.441, 0.447) | |
| EAT-LDP, per 1/42 unit | ||
| Educational level, per 1 year | 0.45 (0.40, 0.50) *** | |
| Smoking, ref.: never smokers | ||
| Educational level, per 1 year | 0.001 (−0.004, 0.006) | |
| Physically inactivity, ref.: yes | ||
| Educational level, per 1 year | 0.009 (0.004, 0.013) *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigala, E.G.; Pitsavos, C.; Barkas, F.; Liberopoulos, E.; Sfikakis, P.P.; Tsioufis, C.; Panagiotakos, D. Sex-Based Associations Between Education Level, EAT–Lancet Diet, and 20-Year Cardiovascular Risk: The ATTICA Study (2002–2022). Nutrients 2025, 17, 2827. https://doi.org/10.3390/nu17172827
Sigala EG, Pitsavos C, Barkas F, Liberopoulos E, Sfikakis PP, Tsioufis C, Panagiotakos D. Sex-Based Associations Between Education Level, EAT–Lancet Diet, and 20-Year Cardiovascular Risk: The ATTICA Study (2002–2022). Nutrients. 2025; 17(17):2827. https://doi.org/10.3390/nu17172827
Chicago/Turabian StyleSigala, Evangelia G., Christos Pitsavos, Fotios Barkas, Evangelos Liberopoulos, Petros P. Sfikakis, Costas Tsioufis, and Demosthenes Panagiotakos. 2025. "Sex-Based Associations Between Education Level, EAT–Lancet Diet, and 20-Year Cardiovascular Risk: The ATTICA Study (2002–2022)" Nutrients 17, no. 17: 2827. https://doi.org/10.3390/nu17172827
APA StyleSigala, E. G., Pitsavos, C., Barkas, F., Liberopoulos, E., Sfikakis, P. P., Tsioufis, C., & Panagiotakos, D. (2025). Sex-Based Associations Between Education Level, EAT–Lancet Diet, and 20-Year Cardiovascular Risk: The ATTICA Study (2002–2022). Nutrients, 17(17), 2827. https://doi.org/10.3390/nu17172827







