The Relationship Between Nutritional Status, Micronutrient Deficiency, and Disease Activity in IBD Patients: A Multicenter Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
- -
- Adult patients (≥18 years old)
- -
- As per study protocol, no nutritional supplementation was allowed at the baseline scheduled visit
- -
- Established diagnosis of IBD (either CD or UC), according to current guidelines [27]
- -
- Biochemical evaluation performed within two weeks of the outpatient visit
- -
- Written informed consent for data processing
- -
- Biochemical evaluation performed more than two weeks prior to the outpatient visit
2.1. Disease Activity
- -
- Clinical activity:
- -
- Active disease based on calprotectin levels≥ 150 mcg/kg
2.2. Aim
2.3. Statistical Analysis
3. Results
3.1. Micronutrients Serum Level and Disease Activity
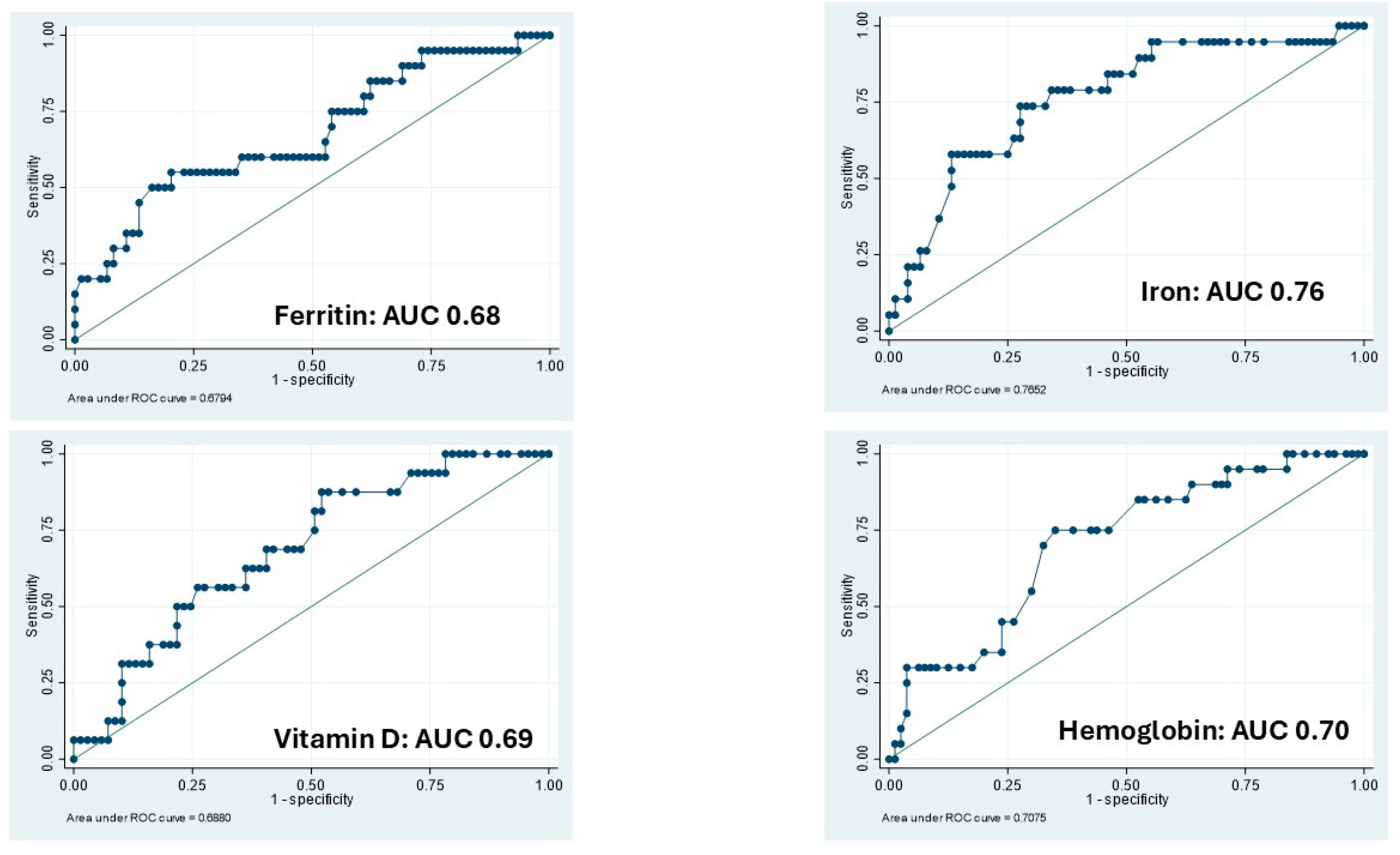
3.2. Clinical Activity: Logistic Regressions and ROC Analysis
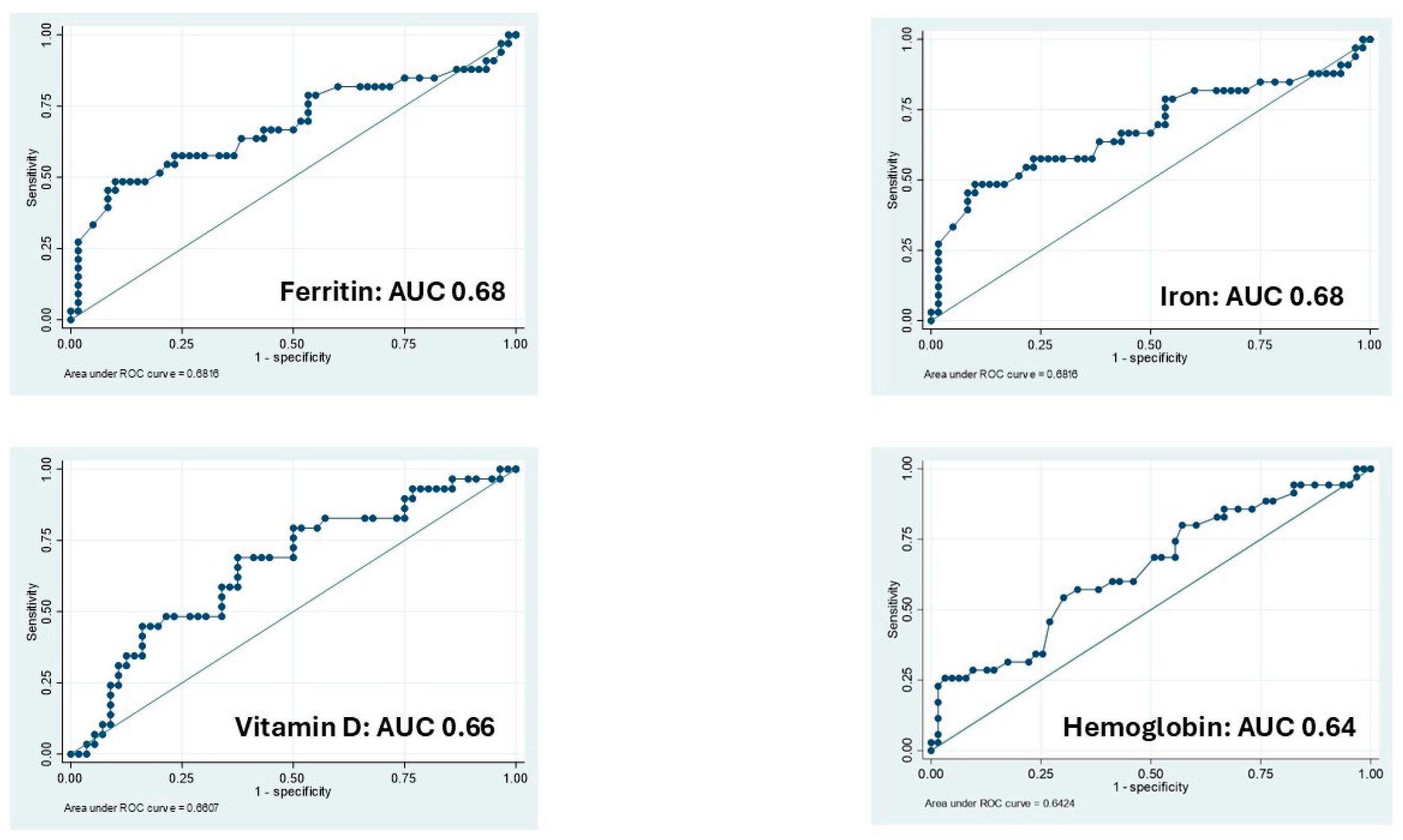
3.3. Calprotectin: Logistic Regressions and ROC Analysis
3.4. Multivariate Analysis of Micronutrients and Disease Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBD | Inflammatory Bowel Diseases |
| ROC | Receiver Operating Characteristics |
| CD | Crohn’s Disease |
| UC | Ulcerative Colitis |
| OR | Odds Ratio |
| PMS | Partial Mayo clinical score |
| HBI | Harvey-Bradshaw Index |
| SD | standard deviations |
| AUC | Area Under the Curve |
References
- Gkikas, K.; Gerasimidis, K.; Milling, S.; Ijaz, U.Z.; Hansen, R.; Russell, R.K. Dietary Strategies for Maintenance of Clinical Remission in Inflammatory Bowel Diseases: Are We There Yet? Nutrients 2020, 12, 2018. [Google Scholar] [CrossRef]
- Pasternak, G.; Chrzanowski, G.; Aebisher, D.; Myśliwiec, A.; Dynarowicz, K.; Bartusik-Aebisher, D.; Sosna, B.; Cieślar, G.; Kawczyk-Krupka, A.; Filip, R. Crohn’s Disease: Basic Characteristics of the Disease, Diagnostic Methods, the Role of Biomarkers, and Analysis of Metalloproteinases: A Review. Life 2023, 13, 2062. [Google Scholar] [CrossRef]
- Li, L.; Cheng, R.; Wu, Y.; Lin, H.; Gan, H.; Zhang, H. Diagnosis and Management of Inflammatory Bowel Disease. J. Evid. Based Med. 2024, 17, 409–433. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, Regional and National Burden of Inflammatory Bowel Disease in 204 Countries and Territories from 1990 to 2019: A Systematic Analysis Based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef]
- Aebisher, D.; Bartusik-Aebisher, D.; Przygórzewska, A.; Oleś, P.; Woźnicki, P.; Kawczyk-Krupka, A. Key Interleukins in Inflammatory Bowel Disease—A Review of Recent Studies. Int. J. Mol. Sci. 2024, 26, 121. [Google Scholar] [CrossRef]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.G.; Rochev, Y. IBD Disease-Modifying Therapies: Insights from Emerging Therapeutics. Trends Mol. Med. 2023, 29, 241–253. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s Disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Flynn, S.; Eisenstein, S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg. Clin. N. Am. 2019, 99, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Froehlich-Grobe, K.; Jones, D.; Businelle, M.S.; Kendzor, D.E.; Balasubramanian, B.A. Impact of Disability and Chronic Conditions on Health. Disabil. Health J. 2016, 9, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Chimienti, M.; Morlino, G.; Ingravalle, F.; Vinci, A.; Colarusso, E.; De Santo, C.; Formosa, V.; Gentile, L.; Lorusso, G.; Mosconi, C.; et al. Unemployment Status Subsequent to Cancer Diagnosis and Therapies: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 1513. [Google Scholar] [CrossRef]
- Bisgaard, T.H.; Allin, K.H.; Keefer, L.; Ananthakrishnan, A.N.; Jess, T. Depression and Anxiety in Inflammatory Bowel Disease: Epidemiology, Mechanisms and Treatment. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 717–726. [Google Scholar] [CrossRef]
- Yanai, H.; Feakins, R.; Allocca, M.; Burisch, J.; Ellul, P.; Iacucci, M.; Maaser, C.; Zilli, A.; Zidar, N.; Wilkens, R.; et al. ECCO-ESGAR-ESP-IBUS Guideline on Diagnostics and Monitoring of Patients with Inflammatory Bowel Disease: Part 2. J. Crohn’s Colitis 2025, 19, jjaf106. [Google Scholar] [CrossRef]
- De Bernardi, A.; Bezzio, C.; Puricelli, M.; Gilardi, D.; Saibeni, S. Combining Advanced Targeted Therapy in Inflammatory Bowel Disease: Current Practice and Future Directions. J. Clin. Med. 2025, 14, 590. [Google Scholar] [CrossRef]
- Plevris, N.; Lees, C.W. Disease Monitoring in Inflammatory Bowel Disease: Evolving Principles and Possibilities. Gastroenterology 2022, 162, 1456–1475.e1. [Google Scholar] [CrossRef]
- Colombel, J.-F.; D’haens, G.; Lee, W.-J.; Petersson, J.; Panaccione, R. Outcomes and Strategies to Support a Treat-to-Target Approach in Inflammatory Bowel Disease: A Systematic Review. J. Crohn’s Colitis 2020, 14, 254–266. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial Diagnosis, Monitoring of Known IBD, Detection of Complications. J. Crohn’s Colitis 2019, 13, 144–164K. [Google Scholar] [CrossRef] [PubMed]
- Weisshof, R.; Chermesh, I. Micronutrient Deficiencies in Inflammatory Bowel Disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 576–581. [Google Scholar] [CrossRef]
- Svolos, V.; Gordon, H.; Lomer, M.C.E.; Aloi, M.; Bancil, A.; Day, A.S.; Day, A.S.; Fitzpatrick, J.A.; Gerasimidis, K.; Gkikas, K.; et al. ECCO Consensus on Dietary Management of Inflammatory Bowel Disease. J. Crohn’s Colitis 2025, jjaf122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Gao, X.; Dai, C.; Huang, Y.; Wu, Y.; Zhou, W.; Cao, Q.; Jing, X.; Jiang, H.; et al. Impact of Malnutrition and Sarcopenia on Quality of Life in Patients with Inflammatory Bowel Disease: A Multicentre Study. J. Cachexia Sarcopenia Muscle 2023, 14, 2663–2675. [Google Scholar] [CrossRef]
- Hashash, J.G.; Elkins, J.; Lewis, J.D.; Binion, D.G. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients with Inflammatory Bowel Disease: Expert Review. Gastroenterology 2024, 166, 521–532. [Google Scholar] [CrossRef]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association Between Inflammatory Bowel Disease and Vitamin D Deficiency. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef]
- Valvano, M.; Magistroni, M.; Cesaro, N.; Carlino, G.; Monaco, S.; Fabiani, S.; Vinci, A.; Vernia, F.; Viscido, A.; Latella, G. Effectiveness of Vitamin D Supplementation on Disease Course in Inflammatory Bowel Disease Patients: Systematic Review with Meta-Analysis. Inflamm. Bowel Dis. 2024, 30, 281–291. [Google Scholar] [CrossRef]
- Wallace, C.; Gordon, M.; Sinopoulou, V.; Limketkai, B.N. Vitamin D for the Treatment of Inflammatory Bowel Disease. Cochrane Database Syst. Rev. 2023, 2023, CD011806. [Google Scholar] [CrossRef] [PubMed]
- Valvano, M.; Capannolo, A.; Cesaro, N.; Stefanelli, G.; Fabiani, S.; Frassino, S.; Monaco, S.; Magistroni, M.; Viscido, A.; Latella, G. Nutrition, Nutritional Status, Micronutrients Deficiency, and Disease Course of Inflammatory Bowel Disease. Nutrients 2023, 15, 3824. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Kucharzik, T.; Taylor, S.; Allocca, M.; Burisch, J.; Ellul, P.; Iacucci, M.; Maaser, C.; Baldin, P.; Bhatnagar, G.; Ben-Horin, S.; et al. ECCO-ESGAR-ESP-IBUS Guideline on Diagnostics and Monitoring of Patients with Inflammatory Bowel Disease: Part 1. J. Crohn’s Colitis 2025, 19, jjaf107. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN Guideline on Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Moss, A.C. Vitamin D in Inflammatory Bowel Disease. Curr. Opin. Gastroenterol. 2018, 34, 217–225. [Google Scholar] [CrossRef]
- Massironi, S.; Viganò, C.; Palermo, A.; Pirola, L.; Mulinacci, G.; Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Inflammation and Malnutrition in Inflammatory Bowel Disease. Lancet Gastroenterol. Hepatol. 2023, 8, 579–590. [Google Scholar] [CrossRef]
- Cesaro, N.; Valvano, M.; Monaco, S.; Stefanelli, G.; Fabiani, S.; Vernia, F.; Necozione, S.; Viscido, A.; Latella, G. The Role of New Inflammatory Indices in the Prediction of Endoscopic and Histological Activity in Inflammatory Bowel Disease Patients. Eur. J. Gastroenterol. Hepatol. 2025, 37, 24–32. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Higuchi, L.M.; Bao, Y.; Korzenik, J.R.; Giovannucci, E.L.; Richter, J.M.; Fuchs, C.S.; Chan, A.T. Higher Predicted Vitamin D Status Is Associated with Reduced Risk of Crohn’s Disease. Gastroenterology 2012, 142, 482–489. [Google Scholar] [CrossRef] [PubMed]
- MacMaster, M.J.; Damianopoulou, S.; Thomson, C.; Talwar, D.; Stefanowicz, F.; Catchpole, A.; Gerasimidis, K.; Gaya, D.R. A Prospective Analysis of Micronutrient Status in Quiescent Inflammatory Bowel Disease. Clin. Nutr. 2021, 40, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Valvano, M.; Magistroni, M.; Mancusi, A.; D’Ascenzo, D.; Longo, S.; Stefanelli, G.; Vernia, F.; Viscido, A.; Necozione, S.; Latella, G. The Usefulness of Serum Vitamin D Levels in the Assessment of IBD Activity and Response to Biologics. Nutrients 2021, 13, 323. [Google Scholar] [CrossRef]
- Battistini, C.; Ballan, R.; Herkenhoff, M.E.; Saad, S.M.I.; Sun, J. Vitamin D Modulates Intestinal Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2020, 22, 362. [Google Scholar] [CrossRef] [PubMed]

| Clinical Active Disease (n = 25) | Clinical Inactive Disease (n = 85) | |
|---|---|---|
| Disease | ||
| CD | 9 | 33 |
| UC | 16 | 52 |
| Sex gender | ||
| Male | 12 | 52 |
| Female | 13 | 33 |
| Therapy | ||
| Advanced therapies | 12 | 49 |
| Conventional therapies | 13 | 36 |
| Disease duration (months) | Median: 88.5 (Range: 2–300) | Median: 96.0 (Range: 6–588) |
| BMI | ||
| CD | 23.35 (4.41) | 24.29 (2.56) |
| UC | 24.5 (3.23) | 24.63 (2.90) |
| Localization | ||
| CD | ||
| Ileal | 4 | 18 |
| Ileo-colic | 5 | 13 |
| Colic | 0 | 2 |
| UC | ||
| Ulcerative proctitis | 7 | 19 |
| Left side colitis | 5 | 20 |
| Extensive colitis | 4 | 13 |
| Item | Missing Data (N) | Mean Difference Among Clinical Active/Inactive Groups (Standard Error) | p-Value (95% CI) | Mean (SD) |
|---|---|---|---|---|
| Hemoglobin | 10 (100) | ∆ + 1.02 mg/dL (0.39) | p = 0.007 (13.45–14.26) | Active: 13.05 (1.42) Inactive: 14.07 (2.13) |
| Iron | 15 (95) | ∆ + 33.03 μg/dL (8.82) | p < 0.001 (79.46–97.13) | Active: 61.88 (31.73) Inactive: 94.90 (43.55) |
| Ferritin | 16 (94) | ∆ + 44.59 ng/dL (16.90) | p = 0.005 (70.25–107.68) | Active: 53.87 (56.40) Inactive: 98.46 (96.87) |
| Vitamin D | 25 (85) | ∆ + 6.84 mg/dL (2.35) | p = 0.003 (2.2.79–27.68) | Active: 19.67 (7.61) Inactive: 26.51 (11.59) |
| Vitamin B12 | 57 (53) | ∆ + 107.3 pg/dL (102.90) | p = 0.16 (−336.72–122.12) | Active: 370.00 (136.62) Inactive: 477.30 (318.67) |
| Folic Acid | 47 (63) | ∆ − 1.90 ng/dL (2.98) | p = 0.267 (−8.30–4.49) | Active: 8.43 (10.40) Inactive: 6.53 (5.39) |
| Albumin | 45 (65) | ∆ + 0.12 g/dL (0.13) | p = 0.203 (−0.16–0.39) | Active: 4.12 (4.05) Inactive: 4.23 (3.90) |
| Calprotectin | 11 (99) | ∆ − 917.44 μg/gr (285.27) | p = 0.002 (186.10–474.20) | Active: 1062.25 (1266.58) Inactive: 144.81 (303.93) |
| Item | Missing Data (N) | Mean Difference Among Low/High Calprotectin Groups (Standard Error) | p Value (95% CI) | Mean (SD) |
| Hemoglobin | 12 (98) | ∆ + 0.79 mg/dL (0.31) | p = 0.006 (0.18–1.42) | High: 13.49 (1.60) Low: 14.29 (1.41) |
| Iron | 17 (93) | ∆ + 17.04 μg/dL (9.18) | p = 0.033 (−1.21–35.29) | High: 77.87 (49.56) Low: 94.91 (37.96) |
| Ferritin | 19 (91) | ∆ + 66.32 ng/dL (18.76) | p < 0.001 (29.03–103.60) | High: 50.83 (40.98) Low: 117.14 (104.54) |
| Vitamin D | 25 (85) | ∆ + 5.51 mg/dL (2.49) | p = 0.015 (0.56–10.47) | High: 21.48 (8.72) Low: 26.99 (11.83) |
| Vitamin B12 | 59 (51) | ∆ + 72.66 pg/dL (52.63) | p = 0.08 (−33.09–178.41) | High: 352.09 (123.65) Low: 424.75 (225.85) |
| Folic Acid | 49 (61) | ∆ + 0.42 ng/dL (1.77) | p = 0.407 (−3.12–3.96) | High: 6.79 (7.76) Low: 7.21 (6.05) |
| Albumin | 48 (62) | ∆ + 0.01 g/dL (0.17) | p = 0.466 (−0.33–0.36) | High: 4.19 (0.35) Low: 4.21 (0.81) |
| Item | Missing Data (N) | Mean Difference Among Active/Inactive Groups † (Standard Error) | pValue (95% CI) | Mean (SD) |
| Hemoglobin | 8 (102) | ∆ + 0.48 mg/dL (0.20) | p = 0.106 (−0.28–1.23) | Healthy: 14.04 (2.29) Diseased: 13.56 (1.55) |
| Iron | 13 (97) | ∆+ 14.78 μg/dL (9.43) | p = 0.061 (−4.06–33.61) | Healthy: 94.23 (38.37) Diseased: 79.45 (48.77) |
| Ferritin | 14 (96) | ∆ + 48.66 ng/mL (16.06) | p = 0.002 (16.74–80.58) | Healthy: 108.61 (105.09) Diseased: 59.95 (50.66) |
| Vitamin D | 23 (87) | ∆ + 5.47 mg/dL (2.25) | p = 0.009 (0.98–9.95) | Healthy: 27.24 (11.80) Diseased: 21.77 (9.09) |
| Vitamin B12 | 57 (53) | ∆ − 5.14 pg/dL (52.83) | p = 0.462 (−111.91–101.64) | Healthy: 387.82 (144.51) Diseased: 392.96 (226.11) |
| Folic Acid | 47 (63) | ∆ + 0.79 ng/mL (1.72) | p = 0.322 (−2.64–4.24) | Healthy: 7.29 (6.22) Diseased: 6.49 (7.25) |
| Albumin | 45 (65) | ∆ − 0.01 g/dL (.15) | p = 0.469 (−0.31–0.28) | Healthy: 4.20 (1.81) Diseased: 4.22 (0.33) |
| Calprotectin | 9 (101) | ∆ − 734.12 μg/gr (156.87) | p < 0.001 (−1051.39–416.85) | Healthy: 41.15 (39.15) Diseased: 775.28 (991.61) |
| Clinical Activity | Calprotectin | |||
|---|---|---|---|---|
| Micronutrients and Hemoglobin | Odds Ratio (95% CI) | AUC | Odds Ratio (95% CI) | AUC |
| Hemoglobin | 0.80 (95% CI: 0.62–1.03) | 0.70 (p = 0.06) | 0.69 (95% CI: 0.51–0.93) | 0.64; p = 0.01 |
| Iron | 0.97 (95% CI: 0.95–0.98) | 0.76 (p < 0.001) | 0.98 (95% CI: 0.97–1.00) | 0.68; p = 0.05 |
| Ferritin | 0.98 (95% CI: 0.97–1.00) | 0.68 (p = 0.018) | 0.94 (95% CI: 0.89–0.99) | 0.68; p = 0.19 |
| Vitamin D | 0.92 (95% CI: 0.86–0.99) | 0.69 (p = 0.013) | 0.98 (95% CI: 0.97–0.99) | 0.66; p < 0.001 |
| Micronutrients and Hemoglobin | Odds Ratio (95% CI) | p Value |
|---|---|---|
| Hemoglobin | 0.75 (0.50–1.12) | 0.170 |
| Iron | 0.99 (0.98–1.00) | 0.655 |
| Ferritin | 0.99 (0.92–1.02) | 0.082 |
| Vitamin D | 0.97 (0.93–1.02) | 0.270 |
| CRP | 1.07 (0.93–1.24) | 0.314 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valvano, M.; Faenza, S.; Cortellini, F.; Vinci, A.; Ingravalle, F.; Calabrò, M.; Scurti, L.; Di Nezza, M.; Valerio, S.; Viscido, A.; et al. The Relationship Between Nutritional Status, Micronutrient Deficiency, and Disease Activity in IBD Patients: A Multicenter Cross-Sectional Study. Nutrients 2025, 17, 2690. https://doi.org/10.3390/nu17162690
Valvano M, Faenza S, Cortellini F, Vinci A, Ingravalle F, Calabrò M, Scurti L, Di Nezza M, Valerio S, Viscido A, et al. The Relationship Between Nutritional Status, Micronutrient Deficiency, and Disease Activity in IBD Patients: A Multicenter Cross-Sectional Study. Nutrients. 2025; 17(16):2690. https://doi.org/10.3390/nu17162690
Chicago/Turabian StyleValvano, Marco, Susanna Faenza, Fabio Cortellini, Antonio Vinci, Fabio Ingravalle, Mauro Calabrò, Lorenza Scurti, Mariagiulia Di Nezza, Sergio Valerio, Angelo Viscido, and et al. 2025. "The Relationship Between Nutritional Status, Micronutrient Deficiency, and Disease Activity in IBD Patients: A Multicenter Cross-Sectional Study" Nutrients 17, no. 16: 2690. https://doi.org/10.3390/nu17162690
APA StyleValvano, M., Faenza, S., Cortellini, F., Vinci, A., Ingravalle, F., Calabrò, M., Scurti, L., Di Nezza, M., Valerio, S., Viscido, A., & Latella, G. (2025). The Relationship Between Nutritional Status, Micronutrient Deficiency, and Disease Activity in IBD Patients: A Multicenter Cross-Sectional Study. Nutrients, 17(16), 2690. https://doi.org/10.3390/nu17162690






