Dietary Patterns and Nutritional Status of Polish Elite Athletes
Abstract
1. Introduction
- What are the nutritional status indicators observed in the studied athletes?
- Which dietary patterns are characteristic of Polish athletes?
- Do the identified dietary patterns correlate with nutritional status indicators?
- Do dietary patterns and nutritional status differ according to sex?
- The identified dietary patterns (DPs) among Polish athletes exhibit similarities to those previously described in the literature.
- Non-healthy dietary patterns are positively associated with higher anthropometric indicators (BMI, WHR, AMC, %FM) and negatively associated with the slenderness index.
- Non-healthy dietary patterns occur more frequently in men than in women.
- Prudent dietary patterns are positively correlated with indicators of proper nutritional status.
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Dietary Assessment
2.3. Anthropometric Assessment and Blood and Urine Biomarkers
2.4. Derivation of Dietary Patterns
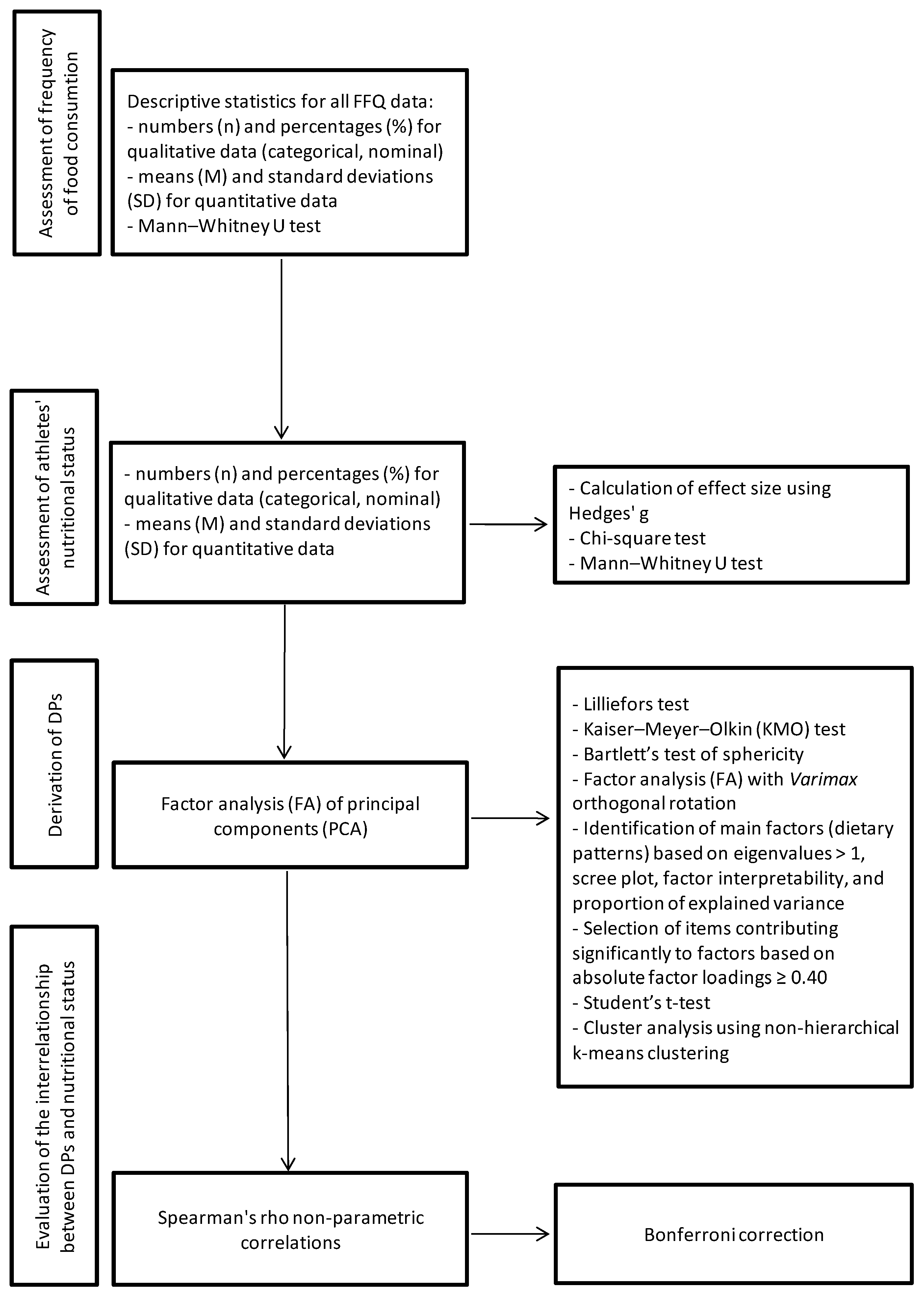
2.5. Statistical Analyses
3. Results
3.1. Nutritional Status
3.2. Frequency of Food Consumtion
3.3. Dietary Pattern Characterization
3.4. Dietary Patterns and Nutritional Status of Athletes
4. Discussion
4.1. Dietary Patterns and Nutritional Status of Polish Athletes
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| %FM | Fat mass percentage |
| ALT | Alanine aminotransferase |
| AMC | Arm muscle circumference |
| AST | Aspartate transaminase |
| BASOs | Basophils |
| BMI | Body mass index |
| DPs | Dietary patterns |
| EOSs | Eosinophils |
| FA | Factor analysis |
| FFQ | Food frequency questionnaire |
| GGTP | γ-glutamyl transferase |
| HCT | Hematocrit |
| HDL-c | High-density lipoprotein cholesterol |
| HGB | Hemoglobin |
| KMO | Kaiser–Meyer–Olkin |
| LDL-c | Low-density lipoprotein cholesterol |
| LYMPHs | Lymphocytes |
| MAC | Midarm circumference |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| MCV | Mean corpuscular volume |
| MONOs | Monocytes |
| NEUT s | Neutrophils |
| PCA | Principal component analysis |
| PLT | Platelet count |
| RBCs | Red blood cells |
| RDW-CV | Red blood cell distribution width |
| SG | Specific gravity |
| TGs | Triglycerides |
| WBCs | White blood cells |
| WHR | Waist–hip ratio |
References
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef]
- Aragon, A.A.; Schoenfeld, B.J.; Wildman, R.; Kleiner, S.; VanDusseldorp, T.; Taylor, L.; Earnest, C.P.; Arciero, P.J.; Wilborn, C.; Kalman, D.S.; et al. International society of sports nutrition position stand: Diets and body composition. J. Int. Soc. Sports Nutr. 2017, 14, 16. [Google Scholar] [CrossRef]
- Park, K. Nutrition and health. In Textbook of Preventive and Social Medicine; Park, K., Ed.; Banarsidas Bhanot: Mumbai, India, 2009; pp. 562–564. [Google Scholar]
- Tucker, L.; Tucker, J.; Bailey, B.; LeCheminant, J. Dietary patterns as predictors of body fat and BMI in women: A factor analytic study. Am. J. Health Promot. 2015, 29, 136–146. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef]
- Dion, S.; Walker, G.; Lambert, K.; Stefoska-Needham, A.; Craddock, J.C. The diet quality of athletes as measured by diet quality indices: A scoping review. Nutrients 2025, 17, 89. [Google Scholar] [CrossRef]
- Capling, L.; Tam, R.; Beck, K.L.; Slater, G.J.; Flood, V.M.; O’connor, H.T.; Gifford, J.A. Diet quality of elite Australian athletes evaluated using the Athlete Diet Index. Nutrients 2021, 13, 126. [Google Scholar] [CrossRef]
- Miguel-Ortega, Á.; Calleja-González, J.; Mielgo-Ayuso, J. Longitudinal comparison of the relationship of energy intake with body composition and physical performance in elite female basketball and volleyball players. Sport Sci. Health 2025, 21, 301–319. [Google Scholar] [CrossRef]
- Gronowska-Senger, A. Zarys Oceny Żywienia, 2nd ed.; Wydawnictwo SGGW: Warszawa, Poland, 2013. [Google Scholar]
- Wądołowska, L. Zasady obliczania i interpretacji wyników. In Przewodnik Metodyczny Badań Sposobu Żywienia; Gronowska-Senger, A., Ed.; Komitet Nauki o Żywieniu Człowieka PAN: Warszawa, Poland, 2013; pp. 38–67. [Google Scholar]
- Bronkowska, M. Badanie Wzorów Żywienia Osób Zdrowych Oraz Obciążonych Otyłością i Jej Wybranymi Powikłaniami w Aspekcie Stanu Odżywienia; Wydawnictwo Uniwersytetu Przyrodniczego: Wrocław, Poland, 2012. [Google Scholar]
- Newby, P.K.; Muller, D.; Hallfrisch, J.; Qiao, N.; Andres, R.; Tucker, K.L. Dietary patterns and changes in body mass index and waist circumference in adults. Am. J. Clin. Nutr. 2003, 77, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Boggs, D.A.; Palmer, J.R.; Spiegelman, D.; Stampfer, M.J.; Adams-Campbell, L.L.; Rosenberg, L. Dietary patterns and 14-y weight gain in African American women. Am. J. Clin. Nutr. 2011, 94, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, A.O.; Koh, W.-P.; Butler, L.M.; Duval, S.; Gross, M.D.; Yu, M.C.; Yuan, J.-M.; Pereira, M.A. Dietary patterns and incident type 2 diabetes in Chinese men and women: The Singapore Chinese Health Study. Diabetes Care 2011, 34, 880–885. [Google Scholar] [CrossRef]
- Burke, L.M.; Slater, G.; Broad, E.M.; Haukka, J.; Modulon, S.; Hopkins, W.G. Eating patterns and meal frequency of elite Australian athletes. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 521–538. [Google Scholar] [CrossRef]
- Frączek, B. Wzory Żywienia Polskich Sportowców w Kontekście Częstości Spożycia i Preferencji Pokarmowych; Wydawnictwo AWF: Kraków, Poland, 2013. [Google Scholar]
- Gillen, J.B.; Trommelen, J.; Wardenaar, F.C.; Brinkmans, N.Y.; Versteegen, J.J.; Jonvik, K.L.; Kapp, C.; de Vries, J.; Borne, J.J.v.D.; Gibala, M.J.; et al. Dietary protein intake and distribution patterns of well-trained Dutch athletes. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Noll, M.; Rodrigues, A.P.; Silveira, E.A. Sport types and time spent playing sport are associated with eating pattern among young Brazilian athletes. Asian J. Sports Med. 2019, 10, e96561. [Google Scholar] [CrossRef]
- Debnath, M.; Chatterjee, S.; Bandyopadhyay, A.; Datta, G.; Kumar, S. Prediction of athletic performance through nutrition knowledge and practice: A cross-sectional study among young team athletes. Sport Mont. 2019, 17, 13–20. [Google Scholar] [CrossRef]
- Noormohammadpour, P.; Mazaheri, R.; Abarashi, M.; Halabchi, F.; Barghi, T.; Alizadeh, Z. Body composition and dietary pattern of Iranian male soccer players, a large national study. Asian J. Sports Med. 2019, 10, e83684. [Google Scholar] [CrossRef]
- Bettonviel, A.E.O.; Brinkmans, N.Y.J.; Russcher, K.; Wardenaar, F.C.; Witard, O.C. Nutritional status and daytime pattern of protein intake on match, post-match, rest and training days in senior professional and youth elite soccer players. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 285–293. [Google Scholar] [CrossRef]
- Ferro, A.; Garrido, G.; Villacieros, J.; Pérez, J.; Grams, L. Nutritional habits and performance in male elite wheelchair basketball players during a precompetitive period. Adapt. Phys. Act. Q. 2017, 34, 295–310. [Google Scholar] [CrossRef]
- Tawfik, S.; El Koofy, N.; Moawad, E.M. Patterns of nutrition and dietary supplements use in young Egyptian athletes: A community-based cross-sectional survey. PLoS ONE 2016, 11, e0161252. [Google Scholar] [CrossRef] [PubMed]
- Viner, R.T.; Harris, M.; Berning, J.R.; Meyer, N.L. Energy availability and dietary patterns of adult male and female competitive cyclists with lower than expected bone mineral density. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Erdman, K.A.; Tunnicliffe, J.; Lun, V.M.; Reimer, R.A. Eating patterns and composition of meals and snacks in elite Canadian athletes. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 210–219. [Google Scholar] [CrossRef]
- Singh, R.; Hwa, O.C.; Roy, J.; Jin, C.W.; Ismail, S.M.; Lan, M.F.; Hiong, L.L.; Aziz, A.R. Subjective Perception of Sports Performance, Training, Sleep and Dietary Patterns of Malaysian Junior Muslim Athletes during Ramadan Intermittent Fasting. Asian J. Sports Med. 2011, 2, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Drenowatz, C.; Eisenmann, J.C.; Carlson, J.J.; Pfeiffer, K.A.; Pivarnik, J.M. Energy expenditure and dietary intake during high-volume and low-volume training periods among male endurance athletes. Appl. Physiol. Nutr. Metab. 2012, 37, 199–205. [Google Scholar] [CrossRef]
- Kirwan, R.D.; Kordick, L.K.; McFarland, S.; Lancaster, D.; Clark, K.; Miles, M.P. Dietary, anthropometric, blood-lipid, and performance patterns of American college football players during 8 weeks of training. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 444–451. [Google Scholar] [CrossRef]
- Ghloum, K.; Hajji, S. Comparison of diet consumption, body composition and lipoprotein lipid values of Kuwaiti fencing players with international norms. J. Int. Soc. Sports Nutr. 2011, 8, 13. [Google Scholar] [CrossRef]
- Riebl, S.K.; Subudhi, A.W.; Broker, J.P.; Schenck, K.; Berning, J.R. The prevalence of subclinical eating disorders among male cyclists. J. Am. Diet. Assoc. 2007, 107, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Camacho, J.D.; Fuentes-Lorca, E.; Moya-Amaya, H. Anthropometric characteristics, somatotype and dietary patterns in youth soccer players. Rev. Andal. Med. Deporte 2017, 10, 192–196. [Google Scholar] [CrossRef]
- Kopiczko, A.; Bałdyka, J.; Adamczyk, J.G.; Nyrć, M.; Gryko, K. Association between long-term exercise with different osteogenic index, dietary patterns, body composition, biological factors, and bone mineral density in female elite masters athletes. Sci. Rep. 2025, 15, 9167. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: Anthropometry Procedures Manual; CDC: Atlanta, GA, USA, 2007; pp. 3–15. Available online: https://stacks.cdc.gov/view/cdc/50334 (accessed on 27 September 2018).
- Gibson, R.S. Principles of Nutritional Assessment, 2nd ed.; Oxford University Press: New York, NY, USA, 2005; Available online: https://global.oup.com/academic/product/principles-of-nutritional-assessment-9780195171693?cc=pl&lang=en& (accessed on 27 September 2018).
- Frisancho, A.R. Nutritional anthropometry. J. Am. Diet. Assoc. 1988, 88, 553–555. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry; Technical Report Series; World Health Organization: Geneva, Switzerland, 1995; p. 854. Available online: https://apps.who.int/iris/handle/10665/37003 (accessed on 13 January 2023).
- Charzewski, J. Zarys Antropologii Dla Studiujących Wychowanie Fizyczne; Wydawnictwo AWF: Warszawa, Poland, 1999. [Google Scholar]
- Siri, W.E. Body composition from fluid spaces and density: Analysis of methods. Nutrition 1993, 9, 480–491. [Google Scholar] [PubMed]
- Durnin, J.V.; Womersley, J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br. J. Nutr. 1974, 32, 77–97. [Google Scholar] [CrossRef]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Pearson: New York, NY, USA, 2010. [Google Scholar]
- Newby, P.K.; Tucker, K.L. Empirically derived eating patterns using factor or cluster analysis: A review. Nutr. Rev. 2004, 62, 177–203. [Google Scholar] [CrossRef]
- Stanisz, A. Przystępny Kurs Statystyki Z Zastosowaniem STATISTICA PL na Przykładach z Medycyny; tom 1–3; StatSoft: Kraków, Poland, 2006. [Google Scholar]
- Bland, J.M.; Altman, D.G. Multiple significance tests: The Bonferroni method. BMJ 1995, 310, 170. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Podgórski, T. Biochemia … Czytasz i Rozumiesz; Wydawnictwo Akademii Wychowania Fizycznego: Poznań, Poland, 2016. [Google Scholar]
- Esmaillzadeh, A.; Mirmiran, P.; Azizi, F. Whole-grain consumption and the metabolic syndrome: A favorable association in Tehranian adults. Eur. J. Clin. Nutr. 2005, 59, 353–362. [Google Scholar] [CrossRef]
- Freire, R.D.; Cardoso, M.A.; Gimeno, S.G.; Ferreira, S.R.; Japanese-Brazilian Diabetes Study Group. Dietary fat is associated with metabolic syndrome in Japanese Brazilians. Diabetes Care 2005, 28, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Kesse-Guyot, E.; Bertrais, S.; Péneau, S.; Estaquio, C.; Dauchet, L.; Vergnaud, A.-C.; Czernichow, S.; Galan, P.; Hercberg, S.; Bellisle, F. Dietary patterns and their sociodemographic and behavioural correlates in French middle-aged adults from the SU.VI.MAX cohort. Eur. J. Clin. Nutr. 2009, 63, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, E.S.; França-Santos, D.; Magliano, E.d.S.; Bloch, K.V.; Barufaldi, L.A.; de Freitas Cunha, C.; de Vasconcellos, M.T.L.; Szklo, M. ERICA: Patterns of alcohol consumption in Brazilian adolescents. Rev. Saude Publica 2016, 50 (Suppl. S1), 8s. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Suliburska, J.; Jeszka, J. Ocena stanu odżywienia i nawyków żywieniowych wybranej grupy zawodników uprawiających wioślarstwo. Bromatol. Chem. Toksykol. 2011, 44, 262–270. [Google Scholar]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Brantsæter, A.L.; Haugen, M.; Samuelsen, S.O.; Torjusen, H.; Trogstad, L.; Alexander, J.; Magnus, P.; Meltzer, H.M. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J. Nutr. 2009, 139, 1162–1168. [Google Scholar] [CrossRef]
- Shi, Z.; Yuan, B.; Hu, G.; Dai, Y.; Zuo, H.; Holmboe-Ottesen, A. Dietary pattern and weight change in a 5-year follow-up among Chinese adults: Results from the Jiangsu Nutrition Study. Br. J. Nutr. 2011, 105, 1047–1054. [Google Scholar] [CrossRef]
- Mullie, P.; Aerenhouts, D.; Clarys, P. Demographic, socioeconomic and nutritional determinants of daily versus non-daily sugar-sweetened and artificially sweetened beverage consumption. Eur. J. Clin. Nutr. 2012, 66, 150–155. [Google Scholar] [CrossRef]
- Kim, J.; Jo, I.; Joung, H. A rice-based traditional dietary pattern is associated with obesity in Korean adults. J. Acad. Nutr. Diet. 2012, 112, 246–253. [Google Scholar] [CrossRef]
- Demetriou, C.A.; Hadjisavvas, A.; Loizidou, M.A.; Loucaides, G.; Neophytou, I.; Sieri, S.; Kakouri, E.; Middleton, N.; Vineis, P.; Kyriacou, K. The Mediterranean dietary pattern and breast cancer risk in Greek-Cypriot women: A case-control study. BMC Cancer 2012, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Farajian, P.; Kavouras, S.A.; Yannakoulia, M.; Sidossis, L.S. Dietary intake and nutritional practices of elite Greek aquatic athletes. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 574–585. [Google Scholar] [CrossRef]
- Machefer, G.; Groussard, C.; Zouhal, H.; Vincent, S.; Youssef, H.; Faure, H.; Malardé, L.; Gratas-Delamarche, A. Nutritional and plasmatic antioxidant vitamins status of ultra endurance athletes. J. Am. Coll. Nutr. 2007, 26, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Aerenhouts, D.; Hebbelinck, M.; Poortmans, J.R.; Clarys, P. Nutritional habits of Flemish adolescent sprint athletes. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 509–523. [Google Scholar] [CrossRef]
- Gacek, M.; Frączek, B. Nutritional evaluation of junior football players depending on the global level of self-efficacy of the athletes. Med. Sport 2013, 17, 72–75. [Google Scholar]
- Anyżewska, A.; Dzierżanowski, I.; Woźniak, A.; Leonkiewicz, M.; Wawrzyniak, A. Rapid weight loss and dietary inadequacies among martial arts practitioners from Poland. Int. J. Environ. Res. Public Health 2018, 15, 2476. [Google Scholar] [CrossRef]
- Gacek, M. Selected personal conditions determining the frequency of consuming groups of products among athletes professionally training individual sports disciplines. Hum. Mov. 2019, 20, 56–65. [Google Scholar] [CrossRef]
- Schulze, M.B.; Fung, T.T.; Manson, J.E.; Willett, W.C.; Hu, F.B. Dietary patterns and changes in body weight in women. Obesity 2006, 14, 1444–1453. [Google Scholar] [CrossRef]
- Agurs-Collins, T.; Rosenberg, L.; Makambi, K.; Palmer, J.R.; Adams-Campbell, L. Dietary patterns and breast cancer risk in women participating in the Black Women’s Health Study. Am. J. Clin. Nutr. 2009, 90, 621–628. [Google Scholar] [CrossRef]
- Paradis, A.M.; Godin, G.; Pérusse, L.; Vohl, M.C. Associations between dietary patterns and obesity phenotypes. Int. J. Obes. 2009, 33, 1419–1426. [Google Scholar] [CrossRef]
- Sarkhosh-Khorasani, S.; Mozaffari-Khosravi, H.; Mirzaei, M.; Nadjarzadeh, A.; Hosseinzadeh, M. Empirically derived dietary patterns and obesity among Iranian adults: Yazd Health Study–TAMYZ and Shahedieh cohort study. Food Sci. Nutr. 2020, 8, 2478–2489. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Rashidkhani, B. The association of general and central obesity with major dietary patterns of adult women living in Tehran, Iran. J. Nutr. Sci. Vitaminol. 2010, 56, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Mirzababaei, A.; Sajjadi, S.F.; Ghodoosi, N.; Pooyan, S.; Arghavani, H.; Yekaninejad, M.S.; Mirzaei, K. Relations of major dietary patterns and metabolically unhealthy overweight/obesity phenotypes among Iranian women. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Rezagholizadeh, F.; Djafarian, K.; Khosravi, S.; Shab-Bidar, S. A posteriori healthy dietary patterns may decrease the risk of central obesity: Findings from a systematic review and meta-analysis. Nutr. Res. 2017, 41, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bertin, M.; Touvier, M.; Dubuisson, C.; Dufour, A.; Havard, S.; Lafay, L.; Volatier, J.; Lioret, S. Dietary patterns of French adults: Associations with demographic, socio-economic and behavioural factors. J. Hum. Nutr. Diet. 2016, 29, 241–254. [Google Scholar] [CrossRef]
- Walter, P.; Infager, E.; Muhlemann, P. Food pyramid of the Swiss Society for nutrition. Ann. Nutr. Metab. 2007, 51 (Suppl. S2), 15–20. [Google Scholar] [CrossRef]
- Burke, L.M. A food pyramid for Swiss athletes. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 430–437. [Google Scholar] [CrossRef]
- Orzeł, D.; Kosendiak, A.; Bronkowska, M. Comparison of vegetables and fruit consumption frequency by athletes before and after marathon. Rocz. Państw. Zakł. Hig. 2018, 69, 267–272. [Google Scholar]
- Tukhtarov, B.E. [Comparative assessment of the biological value of average daily diets in professional athletes of Uzbekistan]. Gig. Sanit. 2010, 2, 65–67. (In Russian) [Google Scholar] [PubMed]
- Ubeda, N.; Palacios Gil-Antuñano, N.; Montalvo Zenarruzabeitia, Z.; García Juan, B.; García, A.; Iglesias-Gutiérrez, E. [Food habits and body composition of Spanish elite athletes in combat sports]. Nutr. Hosp. 2010, 25, 414–421. (In Spanish) [Google Scholar] [PubMed]
- Parnell, J.A.; Wiens, K.P.; Erdman, K.A. Dietary intakes and supplement use in pre-adolescent and adolescent Canadian athletes. Nutrients 2016, 8, 526. [Google Scholar] [CrossRef]
- Alacid, F.; Vaquero-Cristóbal, R.; Sánchez-Pato, A.; Muyor, J.M.; López-Miñarro, P.Á. Habit-based consumption in the Mediterranean diet and the relationship with anthropometric parameters in young female kayakers. Nutr. Hosp. 2014, 29, 121–127. [Google Scholar] [CrossRef]
- Kałużny, K.; Śpica, D.; Drobik, P.; Michalska, A.; Kałużna, A.; Kochański, B.; Zukow, W. Evaluation and comparison of nutritional behavior of people practicing professional and amateur sport. J. Educ. Health Sport 2016, 6, 301–310. [Google Scholar]
- Markaki, I.; Linos, D.; Linos, A. The influence of dietary patterns on the development of thyroid cancer. Eur. J. Cancer 2003, 39, 1912–1919. [Google Scholar] [CrossRef]
- DiBello, J.R.; Kraft, P.; McGarvey, S.T.; Goldberg, R.; Campos, H.; Baylin, A. Comparison of 3 methods for identifying dietary patterns associated with risk of disease. Am. J. Epidemiol. 2008, 168, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Cutler, G.J.; Flood, A.; Hannan, P.J.; Slavin, J.L.; Neumark-Sztainer, D. Association between major patterns of dietary intake and weight status in adolescents. Br. J. Nutr. 2012, 108, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Novotny, R.; Tasaki, K. Dietary patterns are associated with body mass index in multiethnic women. J. Nutr. 2000, 130, 3068–3072. [Google Scholar] [CrossRef]
- López-Sánchez, G.F.; Radzimiński, Ł.; Skalska, M.; Jastrzębska, J.; Smith, L.; Wakuluk, D.; Jastrzębski, Z. Body composition, physical fitness, physical activity and nutrition in Polish and Spanish male students of sports sciences: Differences and correlations. Int. J. Environ. Res. Public Health 2019, 16, 1148. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.; Baxter, V.; Spaccarotella, K.; Andzel, W. College students’ knowledge of recovery beverage serving sizes. Int. J. Exerc. Sci. 2017, 10, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Nikpartow, N.; Danyliw, A.D.; Whiting, S.J.; Lim, H.J.; Vatanparast, H. Beverage consumption patterns of Canadian adults aged 19 to 65 years. Public Health Nutr. 2012, 15, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, F.T. A Manual of Laboratory and Diagnostic Tests, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
| Anthropometric Parameters | Total (n = 226) | Women (n = 87) | Men (n = 139) | p Values | |||
|---|---|---|---|---|---|---|---|
| x− | SD | x− | SD | x− | SD | ||
| Age (years) | 22.8 | 5.5 | 21.7 | 4.6 | 23.5 | 5.9 | 0.330 |
| Body height (cm) | 177.5 | 9.2 | 170.8 | 7.8 | 181.7 | 7.2 | <0.001 |
| Body weight (kg) | 69.9 | 12.2 | 61.6 | 8.3 | 75.0 | 11.3 | <0.001 |
| Circumference (cm) | |||||||
| Arm at rest | 29.9 | 3.3 | 28.2 | 2.6 | 30.6 | 3.3 | <0.001 |
| Waist | 78.4 | 6.9 | 73.2 | 5.3 | 80.7 | 6.2 | <0.001 |
| Hip | 91.6 | 6.0 | 91.3 | 5.7 | 91.7 | 6.2 | 0.706 |
| Skin-fat folds (mm) | |||||||
| Over the triceps arm muscle | 9.6 | 4.9 | 12.8 | 5.1 | 8.2 | 4.1 | <0.001 |
| Over the biceps muscle of the arm | 5.4 | 2.9 | 7.0 | 3.7 | 4.8 | 2.3 | <0.001 |
| Under the shoulder blade | 10.7 | 4.7 | 10.4 | 3.9 | 10.8 | 5.0 | 0.870 |
| Over the iliac crest | 11.4 | 6.4 | 11.7 | 5.3 | 11.3 | 6.9 | 0.115 |
| Sum of four skin-fat folds | 37.2 | 16.3 | 41.9 | 15.2 | 35.2 | 16.3 | <0.001 |
| Anthropometric indices | |||||||
| BMI (kg/m2) | 22.1 | 2.5 | 21.1 | 2.1 | 22.7 | 2.6 | <0.001 |
| WHR | 0.9 | 0.1 | 0.8 | 0.1 | 0.9 | 0.0 | <0.001 |
| Slenderness | 43.3 | 1.5 | 43.4 | 1.5 | 43.2 | 1.6 | 0.838 |
| AMC (cm) | 26.9 | 3.3 | 24.2 | 2.3 | 28.0 | 3.0 | <0.001 |
| %FM | 17.3 | 6.3 | 23.5 | 5.2 | 14.6 | 4.5 | <0.001 |
| Indices | % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Women | Men | ||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | p Values | |
| HGB (g/dL/g/L/mmol/L) | 0.6 | 98.8 | 0.6 | 0.0 | 100.0 | 0.0 | 0.9 | 98.2 | 0.9 | 0.261 |
| HCT (%) | 0.6 | 97.6 | 1.8 | 0.0 | 98.1 | 1.8 | 0.9 | 97.3 | 1.8 | 0.427 |
| RBC (mln/μL/T/L) | 3.0 | 94.6 | 2.4 | 0.0 | 96.3 | 3.7 | 4.5 | 93.7 | 1.8 | 0.289 |
| MCV (fl) | 1.8 | 91.0 | 7.2 | 3.7 | 83.3 | 13.0 | 0.9 | 94.6 | 4.5 | 0.005 |
| MCH (pg/fmol) | 0.0 | 97.6 | 2.4 | 0.0 | 98.1 | 1.9 | 0.0 | 97.3 | 2.7 | 0.792 |
| MCHC (g/dL/g/L/mmol/L) | 4.8 | 94.5 | 0.6 | 3.8 | 94.3 | 1.9 | 5.4 | 94.6 | 0.0 | 0.061 |
| RDW-CV (%) | 4.4 | 91.2 | 4.4 | 6.0 | 84.0 | 10.0 | 3.6 | 94.5 | 1.8 | 0.005 |
| PLT (tys/μL/G/L) | 3.0 | 95.8 | 1.2 | 1.8 | 98.1 | 0.0 | 3.6 | 94.6 | 1.8 | 0.208 |
| WBC (tys/μL/G/L) | 9.0 | 88.5 | 2.4 | 5.6 | 90.7 | 3.7 | 10.7 | 87.5 | 1.8 | 0.109 |
| NEUT (tys/μL/G/L) | 15.1 | 84.8 | 0.0 | 20.4 | 79.6 | 0.0 | 12.6 | 87.4 | 0.0 | 0.122 |
| LYMPH (tys/μL/G/L) | 0.0 | 90.8 | 9.1 | 0.0 | 90.7 | 9.3 | 0.0 | 90.9 | 9.1 | 0.968 |
| MONO (tys/μL/G/L) | 0.0 | 98.2 | 1.8 | 0.0 | 100.0 | 0.0 | 0.0 | 97.3 | 2.7 | 0.110 |
| EOS (tys/μL/G/L) | 0.6 | 97.5 | 1.8 | 1.9 | 98.1 | 0.0 | 0.0 | 97.3 | 2.7 | 0.017 |
| BASO (tys/μL/G/L) | 12.8 | 85.4 | 1.8 | 9.3 | 90.7 | 0.0 | 14.5 | 82.7 | 2.7 | 0.044 |
| Serum sodium (mmol/L) | 0.0 | 98.7 | 1.3 | 0.0 | 100.0 | 0.0 | 0.0 | 98.1 | 1.9 | 0.168 |
| Serum potassium (mmol/L) | 0.6 | 96.7 | 2.6 | 0.0 | 98.0 | 2.0 | 0.9 | 96.2 | 2.9 | 0.402 |
| Serum chloride (mmol/L/mg/dL) | 0.9 | 97.3 | 1.8 | 0.0 | 97.4 | 2.6 | 1.3 | 97.3 | 1.3 | 0.224 |
| Total serum calcium (mmol/L/mg/dL) | 0.7 | 86.6 | 12.7 | 2.5 | 87.5 | 10.0 | 0.0 | 86.3 | 13.7 | 0.055 |
| Serum inorganic phosphorus (mmol/L/mg/dL) | 0.9 | 87.4 | 11.7 | 0.0 | 83.8 | 16.2 | 1.3 | 89.2 | 9.5 | 0.063 |
| Serum magnesium (mmol/L/mg/dL) | 1.3 | 94.7 | 4.0 | 4.3 | 93.6 | 2.1 | 0.0 | 95.2 | 4.8 | 0.065 |
| Serum iron (mmol/L/μmol/L/mol/L/U/L/μg/dL) | 7.3 | 87.1 | 5.6 | 8.9 | 88.9 | 2.2 | 6.3 | 86.1 | 7.6 | 0.062 |
| Serum ferritin (mmol/L/ng/mL/μg/L) | 4.0 | 89.7 | 6.3 | 8.3 | 81.2 | 10.4 | 1.3 | 94.9 | 3.8 | 0.001 |
| Serum vitamin B12 (pg/mL/μg/L) | 1.6 | 85.4 | 13.0 | 2.2 | 82.2 | 15.6 | 1.3 | 87.2 | 11.5 | 0.278 |
| Total serum protein (g/L/g/dL) | 1.3 | 95.3 | 3.4 | 2.3 | 97.7 | 0.0 | 0.9 | 94.3 | 4.7 | 0.019 |
| Serum albumin (g/L/g/dL) | 0.0 | 73.9 | 26.1 | 0.0 | 89.2 | 10.8 | 0.0 | 68.3 | 31.7 | <0.001 |
| Serum urea (μmol/L/mg/dL) | 3.3 | 89.5 | 7.19 | 8.5 | 91.5 | 0.0 | 0.9 | 88.7 | 10.4 | <0.001 |
| Serum creatinine (μmol/L/μg/dL) | 2.6 | 83.8 | 13.7 | 0.0 | 80.5 | 19.5 | 3.9 | 85.5 | 10.5 | 0.013 |
| Serum uric acid (mmol/L/mg/dL) | 2.9 | 91.4 | 5.7 | 4.2 | 91.7 | 4.2 | 2.2 | 91.3 | 6.5 | 0.246 |
| Blood glucose (mmol/L/mg/dL) | 0.0 | 92.8 | 7.2 | 0.0 | 100.0 | 0.0 | 0.0 | 89.6 | 10.4 | 0.002 |
| Triglycerides level (mmol/L/mg/dL) | 0.0 | 96.6 | 3.4 | 0.0 | 100.0 | 0.00 | 0.0 | 95.1 | 4.8 | 0.034 |
| Total cholesterol level (mmol/L/mg/dL) | 5.3 | 74.7 | 20.0 | 4.4 | 77.8 | 17.8 | 5.7 | 73.3 | 21.0 | 0.417 |
| LDL cholesterol level (mmol/L/mg/dL) | 0.0 | 87.8 | 12.2 | 0.0 | 97.7 | 2.3 | 0.0 | 83.6 | 16.3 | 0.001 |
| HDL cholesterol level (mmol/L/mg/dL) | 3.4 | 95.2 | 1.4 | 2.3 | 95.3 | 2.3 | 3.9 | 95.1 | 1.0 | 0.256 |
| Total serum bilirubin (μmol/L/mg/dL) | 0.0 | 87.4 | 12.6 | 0.0 | 95.8 | 4.2 | 0.0 | 83.5 | 16.5 | 0.007 |
| AST (U/L) | 0.0 | 82.7 | 17.3 | 0.0 | 71.1 | 28.9 | 0.0 | 87.6 | 12.4 | 0.002 |
| ALT (U/L) | 0.0 | 95.3 | 4.7 | 0.0 | 95.6 | 4.4 | 0.0 | 95.2 | 4.8 | 0.882 |
| GGTP (Ul/L) | 3.4 | 95.3 | 1.35 | 2.3 | 95.5 | 2.3 | 3.8 | 95.2 | 1.0 | 0.256 |
| Urine color | 0.0 | 59.4 | 40.6 | 0.0 | 65.2 | 34.9 | 0.0 | 56.5 | 43.5 | 0.195 |
| Urine pH | 0.0 | 97.1 | 2.9 | 0.0 | 97.8 | 2.2 | 0.0 | 96.8 | 3.2 | 0.792 |
| Urine specific gravity (g/mL/kg/L/mg/dL) | 8.0 | 84.0 | 8.0 | 11.1 | 84.4 | 4.4 | 6.2 | 83.8 | 10.0 | 0.057 |
| Protein in urine | 0.0 | 98.5 | 1.4 | 0.0 | 100.0 | 0.0 | 0.0 | 97.8 | 2.2 | 0.168 |
| Glucose in urine | 0.0 | 99.3 | 0.7 | 0.0 | 100.0 | 0.0 | 0.0 | 98.9 | 1.1 | 0.261 |
| Ketone bodies in urine | 0.0 | 91.2 | 8.8 | 0.0 | 87.0 | 13.0 | 0.0 | 93.4 | 6.6 | 0.112 |
| Bilirubin in urine | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 1.000 |
| Urobilinogen in urine | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 1.000 |
| Nitrites in urine | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 1.000 |
| Dietary Patterns (n = 226). | Food and Food Products | Factor Loadings | Variance Explained (%) |
|---|---|---|---|
| High-fat | Light bread | 0.71 | 8.7 |
| Potatoes | 0.64 | ||
| Cold cuts, wieners, sausages | 0.63 | ||
| Cheese, processed cheese, moldy cheese | 0.61 | ||
| Butter | 0.49 | ||
| Sweets and beverages | Sweetened carbonated and non-carbonated drinks | 0.67 | 6.8 |
| Sweets | 0.58 | ||
| Alcoholic beverages | 0.55 | ||
| Energy drinks | 0.55 | ||
| Powdered or ready-made soups | 0.54 | ||
| Potentially rational | Eggs | 0.69 | 6.2 |
| Legume seeds | 0.68 | ||
| White rice, pasta, small groats | 0.57 | ||
| Canned, marinated, or pickled vegetables | 0.44 | ||
| Buckwheat groats, flakes, whole-grain pasta | 0.42 | ||
| Vegetables and fruits | Vegetables | 0.71 | 5.7 |
| Fruits | 0.69 | ||
| Meat and flour | White meat | 0.74 | 5.6 |
| Fried foods | 0.61 | ||
| Low-fat | Red meat | 0.71 | 5.4 |
| Fish | 0.59 | ||
| Lard | 0.58 | ||
| Fast food | 0.42 | ||
| Dairy | Fermented milk drinks | 0.79 | 5.3 |
| Cottage cheese | 0.69 | ||
| Milk | 0.54 | ||
| Juices | Vegetable juices, vegetable and fruit juices | 0.75 | 4.7 |
| Fruit juices | 0.63 |
| DPs. | Positive Relationship | Negative Relationship |
|---|---|---|
| High-fat | slenderness index 1, HCT 1, RBC 1, serum iron 1, serum urea concentration 1, TG 1, SG 1 | BMI 1, serum uric acid 1, AST 1 |
| Sweets and beverages | PLT 1, WBC 1, NEUT 1, serum albumin 1, serum uric acid 1, GGTP 1 | AST 1 |
| Potentially rational | BMI 1, BASO 1 | slenderness index 1, serum chloride 1, ketone bodies 1 |
| Vegetables and fruits | - | BMI 1, AMC 1, HGB 1, HCT 1, RBC 1, serum calcium 1, serum uric acid 1, SG 1 |
| Meat and flour | HGB 1, RBC 1, LYMPH 1, MONO 1, serum ferritin 1, serum uric acid 1, SG 1 | %FM 1 |
| Low-fat | BMI 1, WHR 1, HCT 1, RBC 1, serum inorganic phosphorus 1, serum ferritin 1, serum vit. B12 1, ALT 1, GGTP 1, serum urea 2, SG 1 | %FM 1, total serum protein 1 |
| Dairy | - | serum creatinine 1 |
| Juices | serum magnesium 1, serum iron 1, serum urea 1 | BMI 1, serum chlorine 1, total serum protein 1, serum creatinine 1, serum uric acid 1 |
| DPs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Nutritional Status Indicators | High-Fat | Sweets and Beverages | Potentially Rational | Vegetables and Fruits | Meat and Flour | Low-Fat | Dairy | Juices |
| BMI [kg/m2] | −0.17 * | 0.06 | 0.17 ** | −0.14 * | 0.05 | 0.15 * | −0.06 | −0.18 ** |
| WHR | 0.02 | 0.05 | 0.04 | −0.04 | 0.07 | 0.22** | −0.07 | −0.07 |
| Slenderness | 0.24 ** | 0.00 | −0.16 * | 0.06 | 0.10 | −0.11 | 0.05 | 0.12 |
| AMC [cm] | −0.02 | 0.02 | 0.15 | −0.25 ** | 0.13 | 0.10 | −0.09 | −0.13 |
| %FM | −0.13 | 0.01 | −0.02 | 0.13 | −0.16 * | −0.16 * | 0.13 | −0.09 |
| HGB (g/dL/g/L/mmol/L) | 0.10 | 0.10 | −0.05 | −0.26 ** | 0.16 * | 0.14 | −0.11 | −0.06 |
| HCT (%) | 0.16 * | 0.07 | 0.02 | −0.27 ** | 0.18 * | 0.18 * | −0.13 | −0.02 |
| RBC (mln/μL/T/L) | 0.16 * | 0.07 | 0.02 | −0.27 ** | 0.18 * | 0.18 * | −0.13 | −0.02 |
| MCV (fl) | 0.04 | −0.12 | 0.03 | −0.04 | −0.04 | 0.05 | 0.11 | 0.09 |
| MCH (pg/fmol) | 0.01 | −0.08 | −0.03 | −0.05 | −0.02 | 0.03 | 0.00 | 0.10 |
| MCHC (g/dL/g/L/mmol/L) | −0.01 | 0.10 | −0.10 | 0.01 | 0.09 | −0.13 | −0.05 | −0.04 |
| RDW-CV (%) | 0.13 | −0.15 | 0.04 | 0.02 | −0.12 | −0.02 | 0.09 | 0.03 |
| PLT (tys/μL/G/L) | −0.05 | 0.18 * | 0.06 | 0.04 | −0.11 | 0.14 | −0.03 | −0.05 |
| Triglycerides level (mmol/L/mg/dL) | 0.13 | 0.17 * | 0.05 | −0.01 | 0.14 | 0.03 | 0.00 | −0.12 |
| Total cholesterol level (mmol/L/mg/dL) | 0.09 | 0.18 * | 0.05 | 0.00 | 0.02 | 0.00 | 0.03 | −0.09 |
| LDL cholesterol level (mmol/L/mg/dL) | 0.13 | 0.08 | −0.06 | −0.06 | 0.17 * | 0.07 | −0.01 | −0.09 |
| HDL cholesterol level (mmol/L/mg/dL) | 0.00 | 0.11 | 0.13 | −0.09 | 0.21 ** | −0.09 | −0.01 | −0.07 |
| EOS (tys/μL/G/L) | −0.01 | 0.07 | 0.01 | −0.11 | 0.10 | 0.13 | −0.07 | −0.07 |
| BASO (tys/μL/G/L) | −0.01 | 0.07 | 0.18 * | 0.01 | 0.12 | −0.05 | 0.00 | −0.10 |
| Serum sodium (mmol/L) | −0.04 | 0.09 | −0.011 | 0.03 | 0.12 | −0.05 | −0.15 | −0.15 |
| Serum potassium (mmol/L) | −0.15 | −0.11 | 0.06 | 0.07 | 0.07 | 0.15 | −0.05 | −0.03 |
| Serum chloride (mmol/L/mg/dL) | 0.15 | −0.05 | −0.25 ** | −0.04 | −0.01 | −0.04 | −0.08 | −0.28 ** |
| Total serum calcium (mmol/L/mg/dL) | 0.08 | −0.01 | 0.03 | −0.19 * | −0.13 | 0.14 | 0.06 | 0.10 |
| Serum inorganic phosphorus (mmol/L/mg/dL) | 0.10 | −0.10 | −0.02 | −0.09 | −0.04 | 0.21 * | 0.08 | 0.15 |
| Serum magnesium (mmol/L/mg/dL) | −0.12 | −0.02 | −0.10 | 0.01 | −0.13 | 0.14 | 0.11 | 0.20 * |
| Serum iron (mmol/L/μmol/L/mol/L/U/L/μg/dL) | 0.22 * | −0.05 | −0.07 | −0.05 | −0.06 | 0.13 | 0.13 | 0.22 * |
| Serum ferritin (mmol/L/ng/mL/μg/L) | 0.16 | 0.04 | −0.03 | −0.16 | 0.18 * | 0.23 ** | −0.11 | −0.07 |
| Serum vitamin B12 (pg/mL/μg/L) | −0.08 | −0.08 | 0.07 | −0.06 | 0.01 | 0.25 ** | 0.09 | 0.10 |
| Total serum protein (g/L/g/dL) | −0.05 | 0.14 | 0.05 | −0.03 | 0.05 | −0.20 * | 0.05 | −0.21 * |
| Serum albumin (g/L/g/dL) | −0.15 | 0.17 * | 0.02 | −0.08 | 0.00 | –0.08 | 0.06 | −0.16 |
| Serum urea (μmol/L/mg/dL) | 0.19 * | −0.12 | 0.04 | −0.08 | 0.07 | 0.32 ** | 0.11 | 0.17 * |
| Serum creatinine (μmol/L/μg/dL) | −0.09 | 0.14 | 0.00 | −0.10 | 0.12 | −0.01 | −0.20 * | −0.20 * |
| Serum uric acid (mmol/L/mg/dL) | −0.17 * | 0.19 * | −0.02 | −0.18 * | 0.25 ** | −0.03 | −0.15 | −0.29 ** |
| Blood glucose (mmol/L/mg/dL) | 0.10 | −0.08 | −0.14 | 0.00 | 0.08 | 0.07 | −0.08 | −0.05 |
| Triglycerides level (mmol/L/mg/dL) | 0.18 * | −0.08 | 0.01 | −0.08 | −0.05 | 0.15 | 0.05 | 0.07 |
| Total cholesterol level (mmol/L/mg/dL) | 0.05 | −0.15 | 0.06 | 0.02 | −0.12 | 0.10 | 0.00 | 0.09 |
| LDL cholesterol level (mmol/L/mg/dL) | 0.07 | −0.15 | 0.05 | −0.04 | −0.10 | 0.08 | 0.01 | 0.10 |
| HDL cholesterol level (mmol/L/mg/dL) | 0.12 | −0.11 | 0.15 | 0.14 | −0.13 | 0.10 | 0.01 | 0.16 |
| AST (U/L) | −0.23 ** | −0.17 * | 0.11 | −0.04 | 0.08 | 0.02 | 0.12 | 0.02 |
| ALT (U/L) | −0.08 | −0.09 | 0.10 | −0.07 | 0.12 | 0.19 * | 0.00 | −0.06 |
| GGTP (Ul/L) | 0.09 | 0.21 * | 0.02 | −0.06 | 0.08 | 0.24 ** | −0.02 | −0.09 |
| Urine color | 0.13 | −0.01 | −0.11 | −0.14 | 0.04 | 0.07 | 0.03 | 0.09 |
| Urine pH | −0.11 | −0.05 | −0.03 | 0.05 | 0.10 | −0.12 | −0.13 | 0.05 |
| Urine specific gravity (g/mL/kg/L/mg/dL) | 0.17 * | 0.16 | −0.04 | −0.23 ** | 0.22 * | 0.18 * | –0.10 | −0.02 |
| Protein in urine | −0.01 | −0.09 | 0.02 | 0.00 | 0.02 | 0.06 | 0.04 | 0.05 |
| Glucose in urine | −0.04 | 0.14 | 0.08 | −0.08 | 0.11 | −0.11 | −0.14 | 0.12 |
| Ketone bodies in urine | −0.01 | 0.11 | −0.17 * | −0.03 | 0.13 | −0.07 | 0.08 | −0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyrała, F.; Frączek, B. Dietary Patterns and Nutritional Status of Polish Elite Athletes. Nutrients 2025, 17, 2685. https://doi.org/10.3390/nu17162685
Tyrała F, Frączek B. Dietary Patterns and Nutritional Status of Polish Elite Athletes. Nutrients. 2025; 17(16):2685. https://doi.org/10.3390/nu17162685
Chicago/Turabian StyleTyrała, Florentyna, and Barbara Frączek. 2025. "Dietary Patterns and Nutritional Status of Polish Elite Athletes" Nutrients 17, no. 16: 2685. https://doi.org/10.3390/nu17162685
APA StyleTyrała, F., & Frączek, B. (2025). Dietary Patterns and Nutritional Status of Polish Elite Athletes. Nutrients, 17(16), 2685. https://doi.org/10.3390/nu17162685






