Ultra-Processed Foods, Gut Microbiota, and Inflammatory Bowel Disease: A Critical Review of Emerging Evidence
Abstract
1. Introduction
2. Ultra-Processed Foods
3. Impact of Ultra-Processed Foods on Gut Microbiota and Intestinal Homeostasis
4. Impact of Food Additives on Gut Microbiota
4.1. Emulsifiers
4.2. Non-Caloric Artificial Sweeteners
4.3. Maltodextrin
4.4. Carrageenan
4.5. Synthetic Colorants (Azo Dyes)
4.6. Nanoparticles and Microparticles
5. Dysbiosis and Modulation of the Intestinal Microbiota in IBD
5.1. Microbial Dysbiosis in IBD
5.2. Gut Microbiota Modulation in IBD
5.3. Dietary Patterns
5.4. Prebiotics
5.5. Probiotics
5.6. Symbiotics
5.7. Postbiotics
5.8. Fecal Microbiota Transplantation (FMT)
6. The Complex Relationship: UPF, Gut Microbiota, and IBD
7. Final Considerations
8. Study Limitations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CMC | Carboxymethylcellulose |
| CD | Crohn’s disease |
| CDED | Crohn’s Disease Exclusion Diet |
| IBD | Inflammatory bowel disease |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-18 | Interleukin-18 |
| LPS | Lipopolysaccharide |
| MASLD | Metabolic-dysfunction-associated steatohepatitis |
| NAS | Non-caloric artificial sweetener |
| NCDs | Non-communicable chronic diseases |
| NF-kB | Nuclear factor kappa B |
| SCFAs | Short-chain fatty acids |
| SFA | Saturated fatty acid |
| TIO2 | Titanium dioxide |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor |
| UC | Ulcerative colitis |
| UPF | Ultra-processed food |
References
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-Processed Foods and the Nutrition Transition: Global, Regional and National Trends, Food Systems Transformations and Political Economy Drivers. Obes. Rev. 2020, 21, e13126. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.-C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-Processed Products Are Becoming Dominant in the Global Food System. Obes. Rev. 2013, 14, 21–28. [Google Scholar] [CrossRef]
- Srour, B.; Kordahi, M.C.; Bonazzi, E.; Deschasaux-Tanguy, M.; Touvier, M.; Chassaing, B. Ultra-Processed Foods and Human Health: From Epidemiological Evidence to Mechanistic Insights. Lancet Gastroenterol. Hepatol. 2022, 7, 1128–1140. [Google Scholar] [CrossRef]
- Jalali, M.; Bahadoran, Z.; Mirmiran, P.; Khalili, D.; Symonds, M.E.; Azizi, F.; Faghih, S. Higher Ultra-Processed Food Intake Is Associated with an Increased Incidence Risk of Cardiovascular Disease: The Tehran Lipid and Glucose Study. Nutr. Metab. 2024, 21, 14. [Google Scholar] [CrossRef]
- Monda, A.; de Stefano, M.I.; Villano, I.; Allocca, S.; Casillo, M.; Messina, A.; Monda, V.; Moscatelli, F.; Dipace, A.; Limone, P.; et al. Ultra-Processed Food Intake and Increased Risk of Obesity: A Narrative Review. Foods 2024, 13, 2627. [Google Scholar] [CrossRef]
- Jardim, M.Z.; Costa, B.V.; de Lima Costa, B.V.; Pessoa, M.C.; Duarte, C.K. Ultra-Processed Foods Increase Noncommunicable Chronic Disease Risk. Nutr. Res. 2021, 95, 19–34. [Google Scholar] [CrossRef]
- Babaei, A.; Pourmotabbed, A.; Talebi, S.; Mehrabani, S.; Bagheri, R.; Ghoreishy, S.M.; Amirian, P.; Zarpoosh, M.; Mohammadi, H.; Kermani, M.A.H.; et al. The Association of Ultra-Processed Food Consumption with Adult Inflammatory Bowel Disease Risk: A Systematic Review and Dose-Response Meta-Analysis of 4 035 694 Participants. Nutr. Rev. 2024, 82, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Christensen, C.; Knudsen, A.; Arnesen, E.K.; Hatlebakk, J.G.; Sletten, I.S.; Fadnes, L.T. Diet, Food, and Nutritional Exposures and Inflammatory Bowel Disease or Progression of Disease: An Umbrella Review. Adv. Nutr. 2024, 15, 100219. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Gamage, E.; Du, S.; Ashtree, D.N.; McGuinness, A.J.; Gauci, S.; Baker, P.; Lawrence, M.; Rebholz, C.M.; Srour, B.; et al. Ultra-Processed Food Exposure and Adverse Health Outcomes: Umbrella Review of Epidemiological Meta-Analyses. BMJ 2024, 384, e077310. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Magro, D.O.; Rossoni, C.; Saad-Hossne, R.; Santos, A. INTERACTION BETWEEN FOOD PYRAMID AND GUT MICROBIOTA. A NEW NUTRITIONAL APPROACH. Arq. Gastroenterol. 2023, 60, 132–136. [Google Scholar] [CrossRef]
- Racine, A.; Carbonnel, F.; Chan, S.S.M.; Hart, A.R.; Bueno-de-Mesquita, H.B.; Oldenburg, B.; van Schaik, F.D.M.; Tjønneland, A.; Olsen, A.; Dahm, C.C.; et al. Dietary Patterns and Risk of Inflammatory Bowel Disease in Europe: Results from the EPIC Study. Inflamm. Bowel Dis. 2016, 22, 345–354. [Google Scholar] [CrossRef]
- Louzada, M.L.D.C.; Gabe, K.T. Classificação de alimentos Nova: Uma contribuição da epidemiologia brasileira. Rev. Bras. Epidemiol. 2025, 28. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-Processed Foods: What They Are and How to Identify Them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Hracs, L.; Windsor, J.W.; Gorospe, J.; Cummings, M.; Coward, S.; Buie, M.J.; Quan, J.; Goddard, Q.; Caplan, L.; Markovinović, A.; et al. Global Evolution of Inflammatory Bowel Disease across Epidemiologic Stages. Nature 2025, 642, 458–466. [Google Scholar] [CrossRef]
- Cox, S.; Sandall, A.; Smith, L.; Rossi, M.; Whelan, K. Food Additive Emulsifiers: A Review of Their Role in Foods, Legislation and Classifications, Presence in Food Supply, Dietary Exposure, and Safety Assessment. Nutr. Rev. 2021, 79, 726–741. [Google Scholar] [CrossRef]
- Whelan, K.; Bancil, A.S.; Lindsay, J.O.; Chassaing, B. Ultra-Processed Foods and Food Additives in Gut Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 406–427. [Google Scholar] [CrossRef]
- Rondinella, D.; Raoul, P.C.; Valeriani, E.; Venturini, I.; Cintoni, M.; Severino, A.; Galli, F.S.; Mora, V.; Mele, M.C.; Cammarota, G.; et al. The Detrimental Impact of Ultra-Processed Foods on the Human Gut Microbiome and Gut Barrier. Nutrients 2025, 17, 859. [Google Scholar] [CrossRef] [PubMed]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet–Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Sonnenburg, J.L. The Ancestral and Industrialized Gut Microbiota and Implications for Human Health. Nat. Rev. Microbiol. 2019, 17, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Vanuytsel, T.; Tack, J.; Farre, R. The Role of Intestinal Permeability in Gastrointestinal Disorders and Current Methods of Evaluation. Front. Nutr. 2021, 8, 717925. [Google Scholar] [CrossRef]
- Moens, E.; Veldhoen, M. Epithelial Barrier Biology: Good Fences Make Good Neighbours. Immunology 2012, 135, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Llavero-Valero, M.; Martín, J.E.-S.; Martínez-González, M.A.; Basterra-Gortari, F.J.; de la Fuente-Arrillaga, C.; Bes-Rastrollo, M. Ultra-Processed Foods and Type-2 Diabetes Risk in the SUN Project: A Prospective Cohort Study. Clin. Nutr. 2021, 40, 2817–2824. [Google Scholar] [CrossRef] [PubMed]
- LaFata, E.M.; Allison, K.C.; Audrain-McGovern, J.; Forman, E.M. Ultra-Processed Food Addiction: A Research Update. Curr. Obes. Rep. 2024, 13, 214–223. [Google Scholar] [CrossRef]
- Kendig, M.D.; Hasebe, K.; McCague, R.; Lee, F.; Leigh, S.-J.; Arnold, R.; Morris, M.J. Adolescent Exposure to a Solid High-Fat, High-Sugar ‘Cafeteria’ Diet Leads to More Pronounced Changes in Metabolic Measures and Gut Microbiome Composition than Liquid Sugar in Female Rats. Appetite 2022, 172, 105973. [Google Scholar] [CrossRef] [PubMed]
- Mignogna, C.; Costanzo, S.; Castelnuovo, A.D.; Ruggiero, E.; Shivappa, N.; Hebert, J.R.; Esposito, S.; Curtis, A.D.; Persichillo, M.; Cerletti, C.; et al. The Inflammatory Potential of the Diet as a Link between Food Processing and Low-Grade Inflammation: An Analysis on 21,315 Participants to the Moli-Sani Study. Clin. Nutr. 2022, 41, 2226–2234. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-Term Dietary Patterns Are Associated with pro-Inflammatory and Anti-Inflammatory Features of the Gut Microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- Martínez Leo, E.E.; Segura Campos, M.R. Effect of Ultra-Processed Diet on Gut Microbiota and Thus Its Role in Neurodegenerative Diseases. Nutrition 2020, 71, 110609. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Andreu-Sánchez, S.; Vogl, T.; Hu, S.; Vila, A.V.; Gacesa, R.; Leviatan, S.; Kurilshikov, A.; Klompus, S.; Kalka, I.N.; et al. Phage-Display Immunoprecipitation Sequencing of the Antibody Epitope Repertoire in Inflammatory Bowel Disease Reveals Distinct Antibody Signatures. Immunity 2023, 56, 1393–1409.e6. [Google Scholar] [CrossRef]
- Morgan, N.N.; Duck, L.W.; Wu, J.; Rujani, M.; Thomes, P.G.; Elson, C.O.; Mannon, P.J. Crohn’s Disease Patients Uniquely Contain Inflammatory Responses to Flagellin in a CD4 Effector Memory Subset. Inflamm. Bowel Dis. 2022, 28, 1893–1903. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Grillo, T.G.; De Oliveira, E.C.S.; Di Stasi, L.C.; Sassaki, L.Y. Gut Microbiota, Inflammatory Bowel Disease and Colorectal Cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef] [PubMed]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; Lindsay, J.O.; Whelan, K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Pacifico, T.; Monteleone, G.; Laudisi, F. Impact of Western Diet and Ultra-Processed Food on the Intestinal Mucus Barrier. Biomedicines 2023, 11, 2015. [Google Scholar] [CrossRef]
- He, Z.; Chen, L.; Catalan-Dibene, J.; Bongers, G.; Faith, J.J.; Suebsuwong, C.; DeVita, R.J.; Shen, Z.; Fox, J.G.; Lafaille, J.J.; et al. Food Colorants Metabolized by Commensal Bacteria Promote Colitis in Mice with Dysregulated Expression of Interleukin-23. Cell Metab. 2021, 33, 1358–1371.e5. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Laudisi, F.; Di Fusco, D.; Dinallo, V.; Stolfi, C.; Di Grazia, A.; Marafini, I.; Colantoni, A.; Ortenzi, A.; Alteri, C.; Guerrieri, F.; et al. The Food Additive Maltodextrin Promotes Endoplasmic Reticulum Stress–Driven Mucus Depletion and Exacerbates Intestinal Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2018, 7, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Pinget, G.; Tan, J.; Janac, B.; Kaakoush, N.O.; Angelatos, A.S.; O’Sullivan, J.; Koay, Y.C.; Sierro, F.; Davis, J.; Divakarla, S.K.; et al. Impact of the Food Additive Titanium Dioxide (E171) on Gut Microbiota-Host Interaction. Front. Nutr. 2019, 6, 57. [Google Scholar] [CrossRef]
- Santos, F.S.D.; Mintem, G.C.; de Oliveira, I.O.; Horta, B.L.; Ramos, E.; Lopes, C.; Gigante, D.P. Consumption of Ultra-Processed Foods and IL-6 in Two Cohorts from High- and Middle-Income Countries. Br. J. Nutr. 2023, 129, 1552–1562. [Google Scholar] [CrossRef]
- Chassaing, B.; Compher, C.; Bonhomme, B.; Liu, Q.; Tian, Y.; Walters, W.; Nessel, L.; Delaroque, C.; Hao, F.; Gershuni, V.; et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology 2022, 162, 743–756. [Google Scholar] [CrossRef]
- Araújo, J.R.; Tomas, J.; Brenner, C.; Sansonetti, P.J. Impact of High-Fat Diet on the Intestinal Microbiota and Small Intestinal Physiology before and after the Onset of Obesity. Biochimie 2017, 141, 97–106. [Google Scholar] [CrossRef]
- Sieg, H.; Schaar, C.; Fouquet, N.; Böhmert, L.; Thünemann, A.F.; Braeuning, A. Particulate Iron Oxide Food Colorants (E 172) during Artificial Digestion and Their Uptake and Impact on Intestinal Cells. Toxicol. In Vitro 2024, 96, 105772. [Google Scholar] [CrossRef]
- Han, Y.; Guo, X.; Thanuphol, P.; Ji, R.; Zhu, Z.; Wu, Y.; Du, H.; Xiao, H. Gut Microbiota-Mediated Degradation of Food-Grade Lambda-Carrageenan by Bacteroides Xylanisolvens and Its Role in Inflammation. J. Agric. Food Chem. 2025, 73, 4288–4298. [Google Scholar] [CrossRef]
- Elmén, L.; Zlamal, J.E.; Scott, D.A.; Lee, R.B.; Chen, D.J.; Colas, A.R.; Rodionov, D.A.; Peterson, S.N. Dietary Emulsifier Sodium Stearoyl Lactylate Alters Gut Microbiota in Vitro and Inhibits Bacterial Butyrate Producers. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.; Poole, A.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary Emulsifiers Impact the Mouse Gut Microbiota Promoting Colitis and Metabolic Syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Roberts, C.L.; Keita, Å.V.; Duncan, S.H.; O’Kennedy, N.; Söderholm, J.D.; Rhodes, J.M.; Campbell, B.J. Translocation of Crohn’s Disease Escherichia Coli across M-Cells: Contrasting Effects of Soluble Plant Fibres and Emulsifiers. Gut 2010, 59, 1331–1339. [Google Scholar] [CrossRef]
- Swidsinski, A.; Ung, V.; Sydora, B.C.; Loening-Baucke, V.; Doerffel, Y.; Verstraelen, H.; Fedorak, R.N. Bacterial Overgrowth and Inflammation of Small Intestine after Carboxymethylcellulose Ingestion in Genetically Susceptible Mice. Inflamm. Bowel Dis. 2009, 15, 359–364. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; Leblanc, J.; et al. Re-evaluation of Xanthan Gum (E 415) as a Food Additive. EFSA J. 2017, 15, e04909. [Google Scholar] [CrossRef]
- Pan, H.; Xu, X.; Qian, Z.; Cheng, H.; Shen, X.; Chen, S.; Ye, X. Xanthan Gum-Assisted Fabrication of Stable Emulsion-Based Oleogel Structured with Gelatin and Proanthocyanidins. Food Hydrocoll. 2021, 115, 106596. [Google Scholar] [CrossRef]
- Thymann, T.; Møller, H.K.; Stoll, B.; Støy, A.C.F.; Buddington, R.K.; Bering, S.B.; Jensen, B.B.; Olutoye, O.O.; Siggers, R.H.; Mølbak, L.; et al. Carbohydrate Maldigestion Induces Necrotizing Enterocolitis in Preterm Pigs. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 297, G1115–G1125. [Google Scholar] [CrossRef]
- Almutairi, R.; Basson, A.R.; Wearsh, P.; Cominelli, F.; Rodriguez-Palacios, A. Validity of Food Additive Maltodextrin as Placebo and Effects on Human Gut Physiology: Systematic Review of Placebo-Controlled Clinical Trials. Eur. J. Nutr. 2022, 61, 2853–2871. [Google Scholar] [CrossRef]
- Udo, T.; Mummaleti, G.; Mohan, A.; Singh, R.K.; Kong, F. Current and Emerging Applications of Carrageenan in the Food Industry. Food Res. Int. 2023, 173, 113369. [Google Scholar] [CrossRef]
- David, S.; Shani Levi, C.; Fahoum, L.; Ungar, Y.; Meyron-Holtz, E.G.; Shpigelman, A.; Lesmes, U. Revisiting the Carrageenan Controversy: Do We Really Understand the Digestive Fate and Safety of Carrageenan in Our Foods? Food Funct. 2018, 9, 1344–1352. [Google Scholar] [CrossRef]
- de Barros Brito, A.K.; Cardoso, K.G.; Soares, S.D.; Chisté, R.C. Corantes Artificiais Permitidos No Brasil: Principais Características E Efeitos Toxicológicos; Editora Científica Digital: São Paulo, Brazil, 2021; Volume 2, pp. 428–444. [Google Scholar]
- Vojdani, A.; Vojdani, C. Immune Reactivity to Food Coloring. Altern. Ther. Health Med. 2015, 21 (Suppl. 1), 52–62. [Google Scholar]
- Elder, R.; Vancuren, S.J.; Botschner, A.J.; Josephy, P.D.; Allen-Vercoe, E. Metabolism of Azo Food Dyes by Bacterial Members of the Human Gut Microbiome. Anaerobe 2023, 83, 102783. [Google Scholar] [CrossRef]
- Kulkarni, A.; Jung, S. Food Colors Caught Red-Handed. Cell Metab. 2021, 33, 1267–1269. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives. Risk Assessment of Titanium Dioxide Risk Released—Background Information. Available online: https://www.who.int/publications/m/item/jecfa-risk-assessment-of-titanium-dioxide-risk-released-background-information (accessed on 9 July 2025).
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-Grade TiO2 Impairs Intestinal and Systemic Immune Homeostasis, Initiates Preneoplastic Lesions and Promotes Aberrant Crypt Development in the Rat Colon. Sci. Rep. 2017, 7, 40373. [Google Scholar] [CrossRef]
- Proquin, H.; Rodríguez-Ibarra, C.; Moonen, C.G.J.; Urrutia Ortega, I.M.; Briedé, J.J.; de Kok, T.M.; van Loveren, H.; Chirino, Y.I. Titanium Dioxide Food Additive (E171) Induces ROS Formation and Genotoxicity: Contribution of Micro and Nano-Sized Fractions. Mutagenesis 2017, 32, 139–149. [Google Scholar] [CrossRef]
- Giambra, V.; Pagliari, D.; Rio, P.; Totti, B.; Di Nunzio, C.; Bosi, A.; Giaroni, C.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Gut Microbiota, Inflammatory Bowel Disease, and Cancer: The Role of Guardians of Innate Immunity. Cells 2023, 12, 2654. [Google Scholar] [CrossRef]
- Alam, M.T.; Amos, G.C.A.; Murphy, A.R.J.; Murch, S.; Wellington, E.M.H.; Arasaradnam, R.P. Microbial Imbalance in Inflammatory Bowel Disease Patients at Different Taxonomic Levels. Gut Pathog. 2020, 12, 1. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-Chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- De Preter, V.; Arijs, I.; Windey, K.; Vanhove, W.; Vermeire, S.; Schuit, F.; Rutgeerts, P.; Verbeke, K. Impaired Butyrate Oxidation in Ulcerative Colitis Is Due to Decreased Butyrate Uptake and a Defect in the Oxidation Pathway. Inflamm. Bowel Dis. 2012, 18, 1127–1136. [Google Scholar] [CrossRef]
- Tacke, F.; Horn, P.; Wong, V.W.-S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and Diagnostic Criteria of Clinical Obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The Interplay between Diet and the Gut Microbiome: Implications for Health and Disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M. Gut Microbiota in Overweight and Obesity: Crosstalk with Adipose Tissue. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 164–183. [Google Scholar] [CrossRef]
- Nesci, A.; Carnuccio, C.; Ruggieri, V.; D’Alessandro, A.; Di Giorgio, A.; Santoro, L.; Gasbarrini, A.; Santoliquido, A.; Ponziani, F.R. Gut Microbiota and Cardiovascular Disease: Evidence on the Metabolic and Inflammatory Background of a Complex Relationship. Int. J. Mol. Sci. 2023, 24, 9087. [Google Scholar] [CrossRef]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota–Gut–Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef]
- Gill, P.A.; Inniss, S.; Kumagai, T.; Rahman, F.Z.; Smith, A.M. The Role of Diet and Gut Microbiota in Regulating Gastrointestinal and Inflammatory Disease. Front. Immunol. 2022, 13, 866059. [Google Scholar] [CrossRef]
- Ferenc, K.; Sokal-Dembowska, A.; Helma, K.; Motyka, E.; Jarmakiewicz-Czaja, S.; Filip, R. Modulation of the Gut Microbiota by Nutrition and Its Relationship to Epigenetics. Int. J. Mol. Sci. 2024, 25, 1228. [Google Scholar] [CrossRef]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- García-Gavilán, J.F.; Atzeni, A.; Babio, N.; Liang, L.; Belzer, C.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Moreno-Indias, I.; et al. Effect of 1-Year Lifestyle Intervention with Energy-Reduced Mediterranean Diet and Physical Activity Promotion on the Gut Metabolome and Microbiota: A Randomized Clinical Trial. Am. J. Clin. Nutr. 2024, 119, 1143–1154. [Google Scholar] [CrossRef]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean Diet Intervention in Overweight and Obese Subjects Lowers Plasma Cholesterol and Causes Changes in the Gut Microbiome and Metabolome Independently of Energy Intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef]
- Haskey, N.; Estaki, M.; Ye, J.; Shim, R.K.; Singh, S.; Dieleman, L.A.; Jacobson, K.; Gibson, D.L. A Mediterranean Diet Pattern Improves Intestinal Inflammation Concomitant with Reshaping of the Bacteriome in Ulcerative Colitis: A Randomised Controlled Trial. J. Crohns Colitis 2023, 17, 1569–1578. [Google Scholar] [CrossRef]
- Godny, L.; Elial-Fatal, S.; Arrouasse, J.; Fischler, T.S.; Reshef, L.; Kutukov, Y.; Cohen, S.; Pfeffer-Gik, T.; Barkan, R.; Shakhman, S.; et al. Mechanistic Implications of the Mediterranean Diet in Patients With Newly Diagnosed Crohn’s Disease: Multiomic Results From a Prospective Cohort. Gastroenterology 2025, 168, 952–964.e2. [Google Scholar] [CrossRef]
- Hashash, J.G.; Elkins, J.; Lewis, J.D.; Binion, D.G. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients With Inflammatory Bowel Disease: Expert Review. Gastroenterology 2024, 166, 521–532. [Google Scholar] [CrossRef]
- Erol Doğan, Ö.; Karaca Çelik, K.E.; Baş, M.; Alan, E.H.; Çağın, Y.F. Effects of Mediterranean Diet, Curcumin, and Resveratrol on Mild-to-Moderate Active Ulcerative Colitis: A Multicenter Randomized Clinical Trial. Nutrients 2024, 16, 1504. [Google Scholar] [CrossRef]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm. Bowel Dis. 2021, 27, 1–9. [Google Scholar] [CrossRef]
- Randeni, N.; Bordiga, M.; Xu, B. A Comprehensive Review of the Triangular Relationship among Diet–Gut Microbiota–Inflammation. Int. J. Mol. Sci. 2024, 25, 9366. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN Practical Guideline: Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef]
- Sinopoulou, V.; Gordon, M.; Gregory, V.; Saadeh, A.; Akobeng, A.K. Prebiotics for Induction and Maintenance of Remission in Ulcerative Colitis; Cochrane Library: London, UK, 2024. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Iheozor-Ejiofor, Z.; Kaur, L.; Gordon, M.; Baines, P.A.; Sinopoulou, V.; Akobeng, A.K. Probiotics for Maintenance of Remission in Ulcerative Colitis; Cochrane Library: London, UK, 2020. [Google Scholar]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN Guideline on Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.N.; da Costa, A.L.; Jorge, E.N.; Paiano, V.F.; Camparoto, M.L.; Keller, R.; Bremer-Neto, H. Synbiotics Improve Clinical Indicators of Ulcerative Colitis: Systematic Review with Meta-Analysis. Nutr. Rev. 2022, 80, 157–164. [Google Scholar] [CrossRef]
- Roy, S.; Dhaneshwar, S. Role of Prebiotics, Probiotics, and Synbiotics in Management of Inflammatory Bowel Disease: Current Perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef]
- Lê, A.; Mantel, M.; Marchix, J.; Bodinier, M.; Jan, G.; Rolli-Derkinderen, M. Inflammatory Bowel Disease Therapeutic Strategies by Modulation of the Microbiota: How and When to Introduce Pre-, pro-, Syn-, or Postbiotics? Am. J. Physiol.-Gastrointest. Liver Physiol. 2022, 323, G523–G553. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Kavita; Om, H.; Chand, U.; Kushawaha, P.K. Postbiotics: An Alternative and Innovative Intervention for the Therapy of Inflammatory Bowel Disease. Microbiol. Res. 2024, 279, 127550. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Wang, D.; Shen, S.; Wang, S.; Li, Y.; Chen, H. Postbiotics in Inflammatory Bowel Disease: Efficacy, Mechanism, and Therapeutic Implications. J. Sci. Food Agric. 2025, 105, 721–734. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European Consensus Conference on Faecal Microbiota Transplantation in Clinical Practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wellens, J.; Kalla, R.; Fu, T.; Deng, M.; Zhang, H.; Yuan, S.; Wang, X.; Theodoratou, E.; Li, X.; et al. Intake of Ultra-Processed Foods Is Associated with an Increased Risk of Crohn’s Disease: A Cross-Sectional and Prospective Analysis of 187 154 Participants in the UK Biobank. J. Crohns Colitis 2022, 17, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Peery, A.F.; Kelly, C.R.; Kao, D.; Vaughn, B.P.; Lebwohl, B.; Singh, S.; Imdad, A.; Altayar, O. AGA Clinical Practice Guideline on Fecal Microbiota–Based Therapies for Select Gastrointestinal Diseases. Gastroenterology 2024, 166, 409–434. [Google Scholar] [CrossRef]
- Halkjær, S.I.; Lo, B.; Cold, F.; Højer Christensen, A.; Holster, S.; König, J.; Brummer, R.J.; Aroniadis, O.C.; Lahtinen, P.; Holvoet, T.; et al. Fecal Microbiota Transplantation for the Treatment of Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. World J. Gastroenterol. 2023, 29, 3185–3202. [Google Scholar] [CrossRef]
- Imdad, A.; Pandit, N.G.; Zaman, M.; Minkoff, N.Z.; Tanner-Smith, E.E.; Gomez-Duarte, O.G.; Acra, S.; Nicholson, M.R. Fecal Transplantation for Treatment of Inflammatory Bowel Disease. Cochrane Database Syst. Rev. 2023, 4, CD012774. [Google Scholar] [CrossRef]
- Yan, J.; Wang, L.; Gu, Y.; Hou, H.; Liu, T.; Ding, Y.; Cao, H. Dietary Patterns and Gut Microbiota Changes in Inflammatory Bowel Disease: Current Insights and Future Challenges. Nutrients 2022, 14, 4003. [Google Scholar] [CrossRef]
- Juul, F.; Vaidean, G.; Parekh, N. Ultra-Processed Foods and Cardiovascular Diseases: Potential Mechanisms of Action. Adv. Nutr. 2021, 12, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Vissers, E.; Wellens, J.; Sabino, J. Ultra-Processed Foods as a Possible Culprit for the Rising Prevalence of Inflammatory Bowel Diseases. Front. Med. 2022, 9, 1058373. [Google Scholar] [CrossRef] [PubMed]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; Mateos, D.; Ugarriza, L.; Gómez, C.; Tur, J.A.; Sureda, A. Oxidative Stress and Inflammatory Biomarkers Are Related to High Intake of Ultra-Processed Food in Old Adults with Metabolic Syndrome. Antioxidants 2023, 12, 1532. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, G.; Lou, P.; Zhang, M.; Yao, K.; Xiao, J.; Chen, Y.; Xu, J.; Tian, S.; Deng, M.; et al. Excessive Nucleic Acid R-Loops Induce Mitochondria-Dependent Epithelial Cell Necroptosis and Drive Spontaneous Intestinal Inflammation. Proc. Natl. Acad. Sci. USA 2024, 121, e2307395120. [Google Scholar] [CrossRef]
- Dang, P.M.-C.; Rolas, L.; El-Benna, J. The Dual Role of Reactive Oxygen Species-Generating Nicotinamide Adenine Dinucleotide Phosphate Oxidases in Gastrointestinal Inflammation and Therapeutic Perspectives. Antioxid. Redox Signal. 2020, 33, 354–373. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Tristan Asensi, M.; Napoletano, A.; Sofi, F.; Dinu, M. Low-Grade Inflammation and Ultra-Processed Foods Consumption: A Review. Nutrients 2023, 15, 1546. [Google Scholar] [CrossRef]
- Song, Z.; Song, R.; Liu, Y.; Wu, Z.; Zhang, X. Effects of Ultra-Processed Foods on the Microbiota-Gut-Brain Axis: The Bread-and-Butter Issue. Food Res. Int. 2023, 167, 112730. [Google Scholar] [CrossRef]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of Ultra-Processed Food Intake with Risk of Inflammatory Bowel Disease: Prospective Cohort Study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef]
- Meyer, A.; Dong, C.; Casagrande, C.; Chan, S.S.M.; Huybrechts, I.; Nicolas, G.; Rauber, F.; Levy, R.B.; Millett, C.; Oldenburg, B.; et al. Food Processing and Risk of Crohn’s Disease and Ulcerative Colitis: A European Prospective Cohort Study. Clin. Gastroenterol. Hepatol. 2023, 21, 1607–1616.e6. [Google Scholar] [CrossRef]
- Preda, C.M.; Istratescu, D.; Nitescu, M.; Manuc, T.; Manuc, M.; Stroie, T.; Tieranu, C.; Meianu, C.G.; Andrei, A.; Ciora, C.A.; et al. Diet Optimization in Inflammatory Bowel Disease: Impact on Disease Relapse and Inflammatory Markers. A 1-Year Prospective Trial. J. Gastrointestin. Liver Dis. 2024, 33, 184–193. [Google Scholar] [CrossRef]
- Svolos, V.; Gordon, H.; Lomer, M.C.E.; Aloi, M.; Bancil, A.; Day, A.S.; Day, A.S.; Fitzpatrick, J.A.; Gerasimidis, K.; Gkikas, K.; et al. ECCO Consensus on Dietary Management of Inflammatory Bowel Disease. J. Crohns Colitis 2025, jjaf122. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Li, F.; Zhang, D. Dietary Fiber Intake Reduces Risk of Inflammatory Bowel Disease: Result from a Meta-Analysis. Nutr. Res. 2015, 35, 753–758. [Google Scholar] [CrossRef]
- Sigall Boneh, R.; Westoby, C.; Oseran, I.; Sarbagili-Shabat, C.; Albenberg, L.G.; Lionetti, P.; Manuel Navas-López, V.; Martín-de-Carpi, J.; Yanai, H.; Maharshak, N.; et al. The Crohn’s Disease Exclusion Diet: A Comprehensive Review of Evidence, Implementation Strategies, Practical Guidance, and Future Directions. Inflamm. Bowel Dis. 2023, 30, 1888–1902. [Google Scholar] [CrossRef] [PubMed]
- Strisciuglio, C.; Cenni, S.; Serra, M.R.; Dolce, P.; Martinelli, M.; Staiano, A.; Miele, E. Effectiveness of Mediterranean Diet’s Adherence in Children with Inflammatory Bowel Diseases. Nutrients 2020, 12, 3206. [Google Scholar] [CrossRef] [PubMed]
- Martín-Masot, R.; Herrador-López, M.; Navas-López, V.M. Dietary Habit Modifications in Paediatric Patients after One Year of Treatment with the Crohn’s Disease Exclusion Diet. Nutrients 2023, 15, 554. [Google Scholar] [CrossRef] [PubMed]
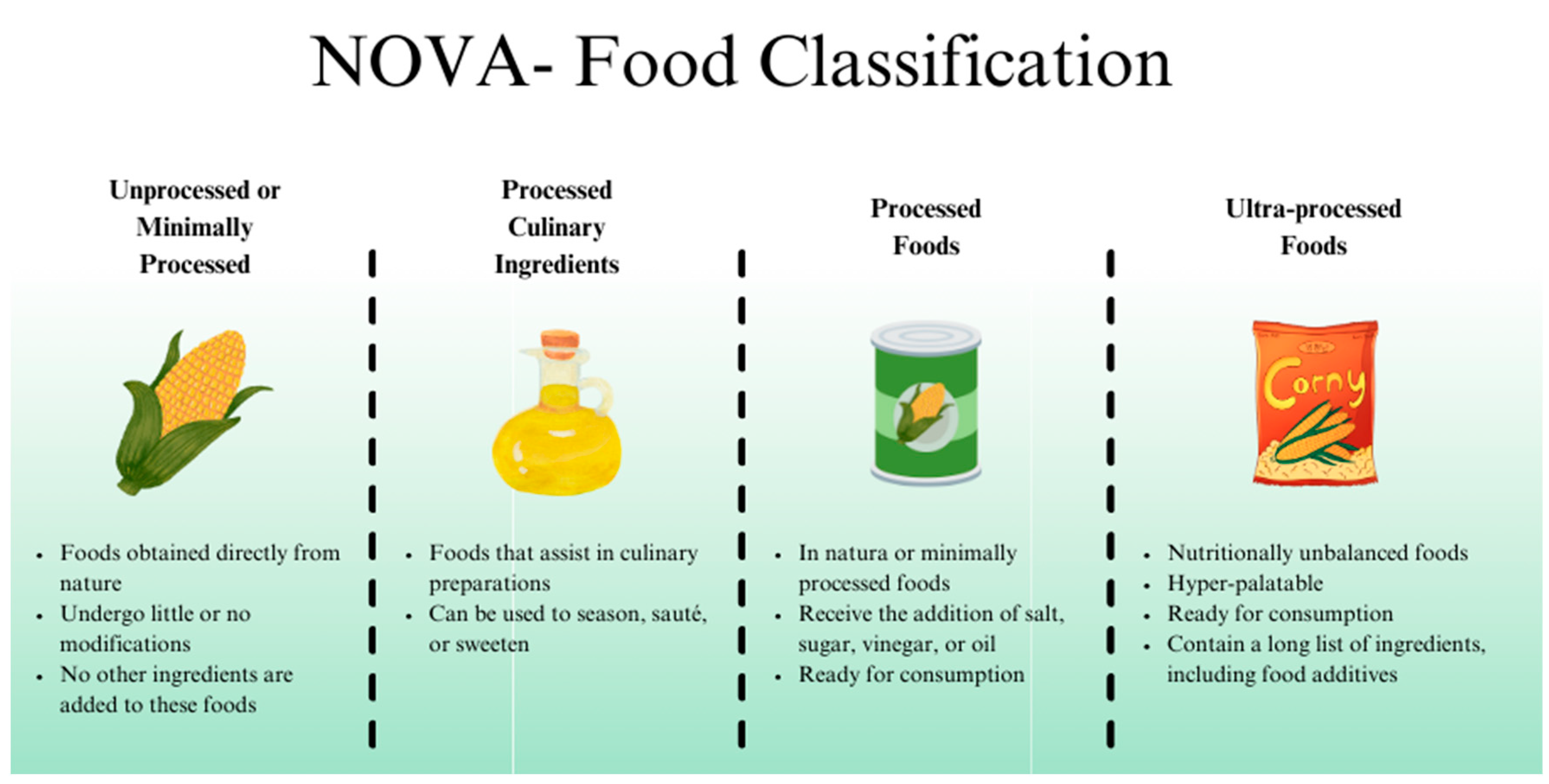
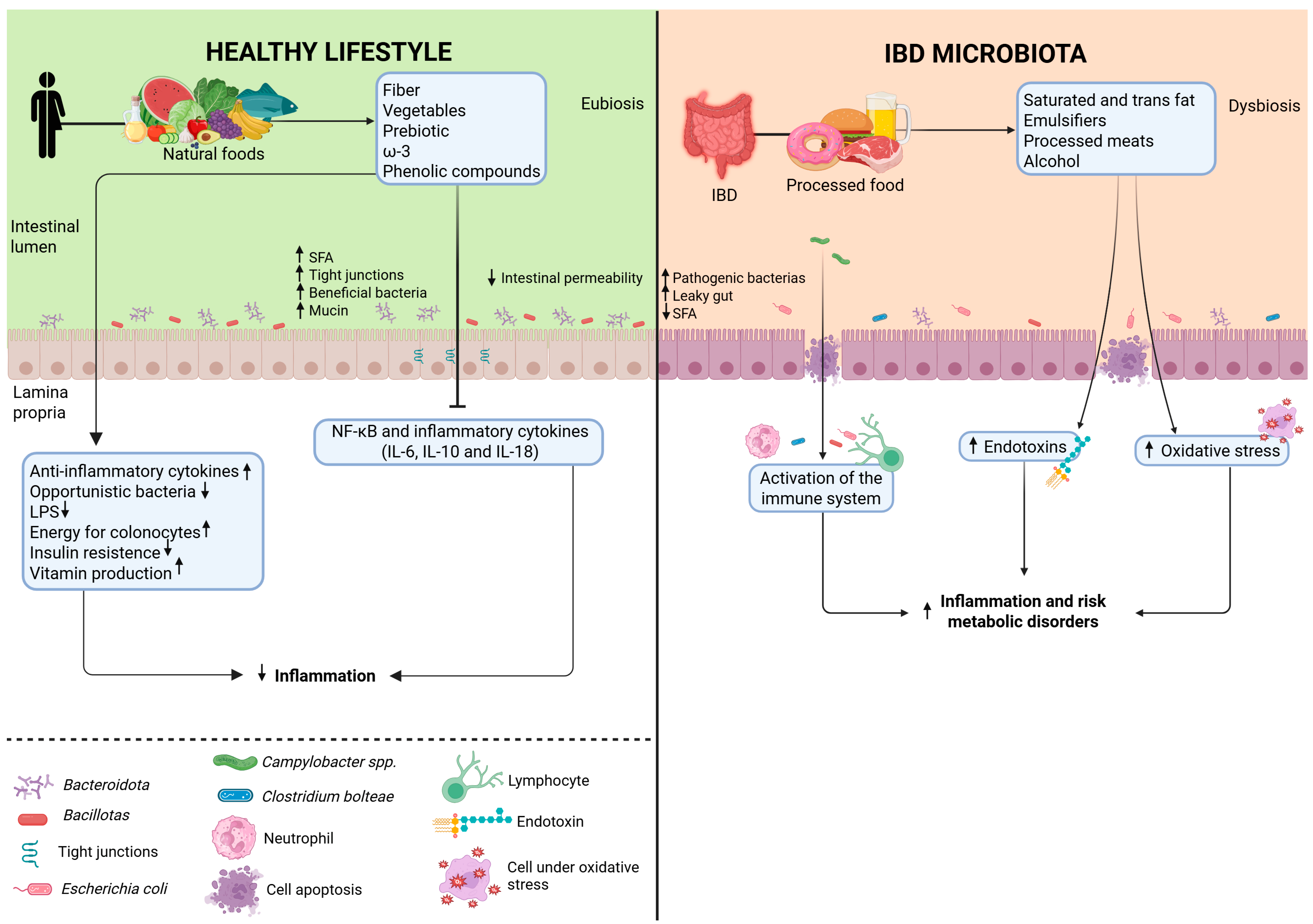
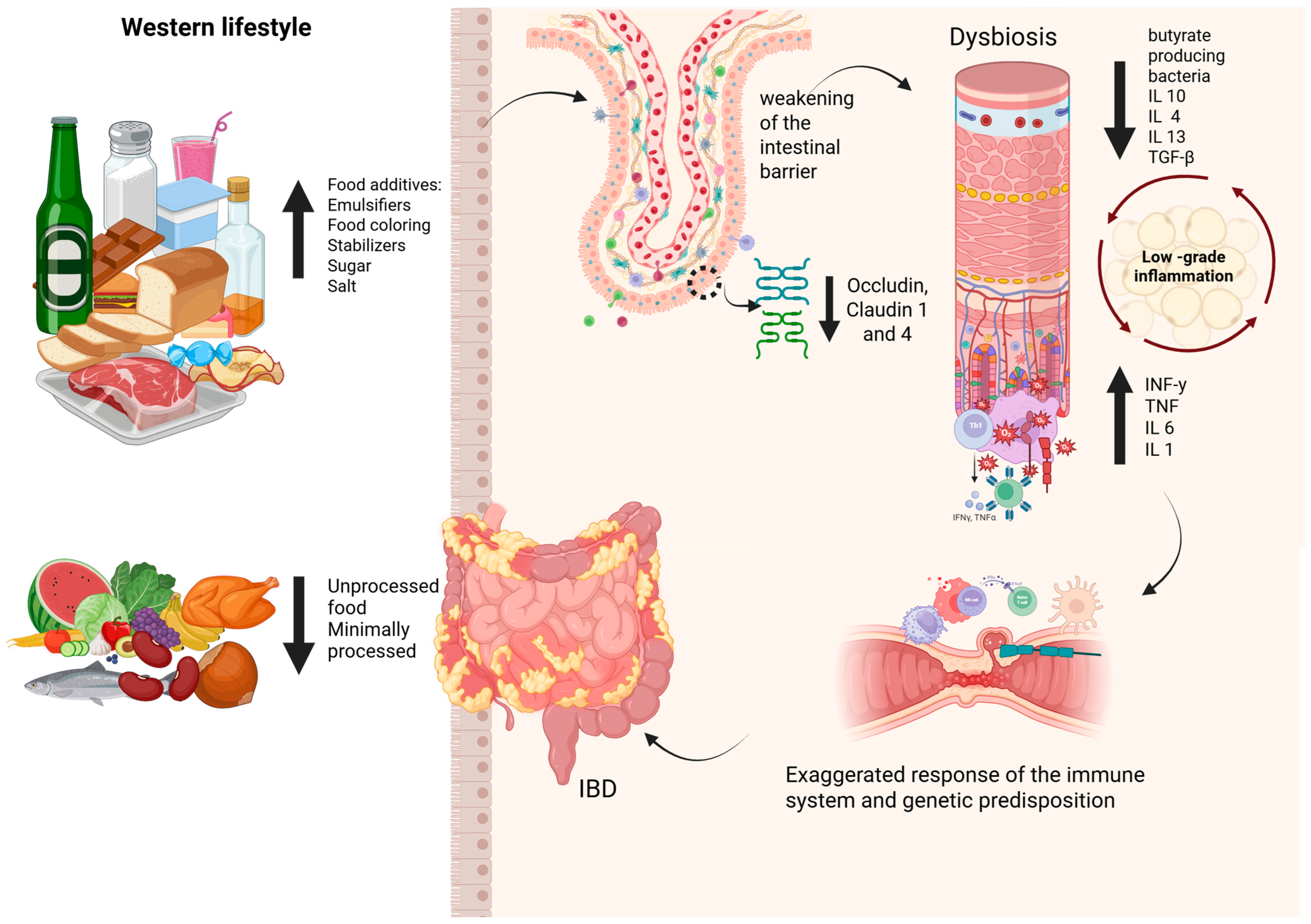
| Study (Year) | Model | Additive(s) | Key Findings Related to Gut Microbiota/Health |
|---|---|---|---|
| Suez et al., 2014 [36] | Germ-free mice | Non-caloric artificial sweeteners (NASs) | Excessive consumption may promote glucose intolerance, dysbiosis, and metabolic alteration. |
| Laudisi, 2018 [37] | Mice | Maltodextrin | Decreased Muc-2 results in greater adhesion of pathogenic bacteria. |
| Pinget et al., 2019 [38] | Mice | Titanium dioxide (TiO2) | TiO2 may impair intestinal homeostasis, increase inflammatory cytokine expression, and decrease crypt length. |
| He et al., 2021 [35] | Mice | Colorants Red 40 and Yellow 6 | Can intensify intestinal inflammation and induce colitis. |
| Santos, 2023 [39] | Mice | Xanthan gum | Continuous consumption increases proinflammatory cytokines (TNF-α, IL-6, and IL-10) and alters intestinal barrier integrity. |
| Chassaing et al., 2022 [40] | Humans, 16 adults | Carboxymethylcellulose (CMC) | Alteration in gut microbiota composition and reduction of metabolites like SCFAs. |
| Araújo, 2017 [41] | Humans, ages 18–60 years | Carboxymethylcellulose (CMC) | Increased bacterial proliferation and infiltration, with an increase in Roseburia spp. and Lachnospiraceae bacterium. |
| Sieg et al., 2024 [42] | In vitro | Iron oxide food colorants (E 172) | E 172 showed strong interaction with intestinal cells, though no toxic effects were observed. |
| Han et al., 2025 [43] | In vitro | Carrageenan | Degraded carrageenan generates proinflammatory cytokines, such as IL-1β and TNF-α, which are related to the development of IBD. |
| Phylum | Description | Reference |
|---|---|---|
| Bacteroidota and Bacillotas | Comprise 90% of the gut microbiota and are often reduced, potentially impairing the inflammatory response and short-chain fatty acid production. | Giambra et al. [61]; Santana et al. [64] |
| Proteobacteria | Typically increased, including opportunistic pathogens, such as Enterobacteriaceae and Burkholderiaceae, that can exacerbate inflammation. | Alam et al. [62] |
| Actinobacteria | In patients with Crohn’s disease, they are increased, which influences dysbiosis and intestinal inflammation. | Takahashi et al. [63] |
| Increased in IBD | Decrease in IBD | ||
|---|---|---|---|
| Phylum | Species | Phylum | Species |
| Proteobacteria [66] | E. coli Campylobacter spp. H. parainfluenzae E. corrodens | Verrucomicrobia [66] | A. muciniphila |
| Bacteroidota [64] | B. fragilis | Bacillota [65,67] | F. prausnitzii R. albus Eubacterium spp. |
| Bacillota [66] | R. torques Ruminococcus spp. C. hathewayi C. bolteae R. gnavus | ||
| Study (Year) | Model | Objectives | Main Findings |
|---|---|---|---|
| Vanuytsel, 2021 [21] | Review | To review the role of the intestinal epithelial barrier in the pathophysiology of IBD. | Importance of epithelial integrity in preventing bacterial translocation and modulating the inflammatory response. |
| Babaei et al., 2022 [7] | Review | Evaluate the relationship between the intestinal microbiota and IBD. | Reduction of beneficial species and increase in pathogens in patients with IBD. |
| Whelean et al., 2024 [17] | Review | Explore mechanisms by which diet influences the intestinal microbiota and immunity. | Diets rich in sugars, saturated fat, and food additives promote dysbiosis and inflammation. |
| Cox et al., 2021 [16] | Review | Review the impact of additives and ultra-processed foods on the gut microbiota. | Emulsifiers, artificial sweeteners, and other additives can compromise the intestinal barrier and promote inflammation. |
| Zinöcker, 2018 [19] | Review | Integrate evidence on Western diet, microbiota, and IBD risk. | Reducing ultra-processed foods and increasing natural/minimally processed foods can contribute to the prevention and management of IBD. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiller, A.L.; Costa, B.G.d.; Yoshihara, R.N.Y.; Nogueira, E.J.Z.; Castelhano, N.S.; Santos, A.; Brusco De Freitas, M.; Magro, D.O.; Yukie Sassaki, L. Ultra-Processed Foods, Gut Microbiota, and Inflammatory Bowel Disease: A Critical Review of Emerging Evidence. Nutrients 2025, 17, 2677. https://doi.org/10.3390/nu17162677
Spiller AL, Costa BGd, Yoshihara RNY, Nogueira EJZ, Castelhano NS, Santos A, Brusco De Freitas M, Magro DO, Yukie Sassaki L. Ultra-Processed Foods, Gut Microbiota, and Inflammatory Bowel Disease: A Critical Review of Emerging Evidence. Nutrients. 2025; 17(16):2677. https://doi.org/10.3390/nu17162677
Chicago/Turabian StyleSpiller, Amanda Luísa, Beatriz Gabriela da Costa, Ryan Nunes Yoshio Yoshihara, Enya Julia Zucari Nogueira, Natalia Salvador Castelhano, Andrey Santos, Maiara Brusco De Freitas, Daniéla Oliveira Magro, and Ligia Yukie Sassaki. 2025. "Ultra-Processed Foods, Gut Microbiota, and Inflammatory Bowel Disease: A Critical Review of Emerging Evidence" Nutrients 17, no. 16: 2677. https://doi.org/10.3390/nu17162677
APA StyleSpiller, A. L., Costa, B. G. d., Yoshihara, R. N. Y., Nogueira, E. J. Z., Castelhano, N. S., Santos, A., Brusco De Freitas, M., Magro, D. O., & Yukie Sassaki, L. (2025). Ultra-Processed Foods, Gut Microbiota, and Inflammatory Bowel Disease: A Critical Review of Emerging Evidence. Nutrients, 17(16), 2677. https://doi.org/10.3390/nu17162677







