The Role of Zinc in Pediatric Asthma and Allergic Rhinitis: Mechanisms and Clinical Implications
Abstract
1. Introduction
1.1. Dietary Interventions in Pediatric Allergic Diseases
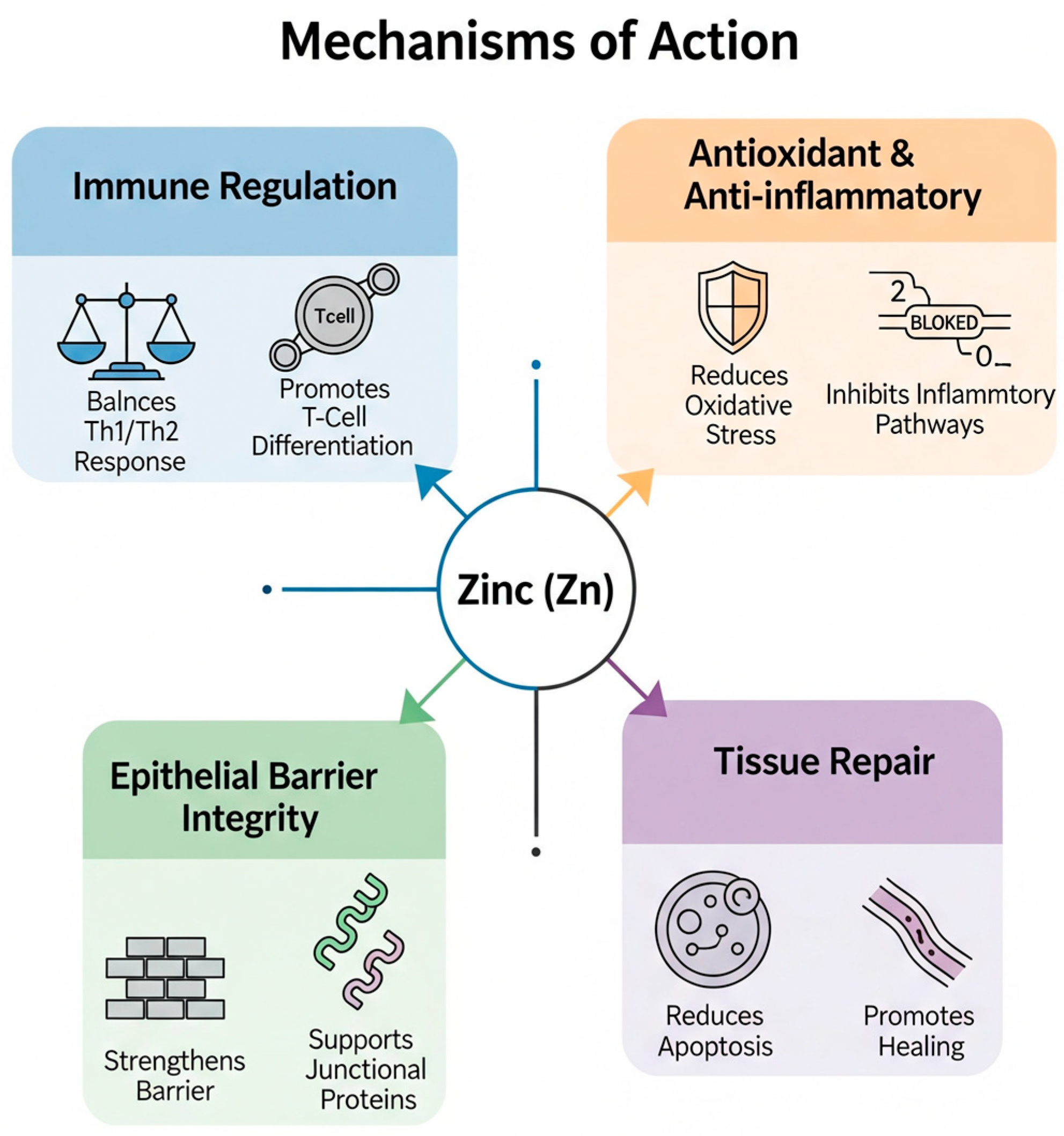
1.2. Zinc Mechanism
1.3. Rationale and Objectives
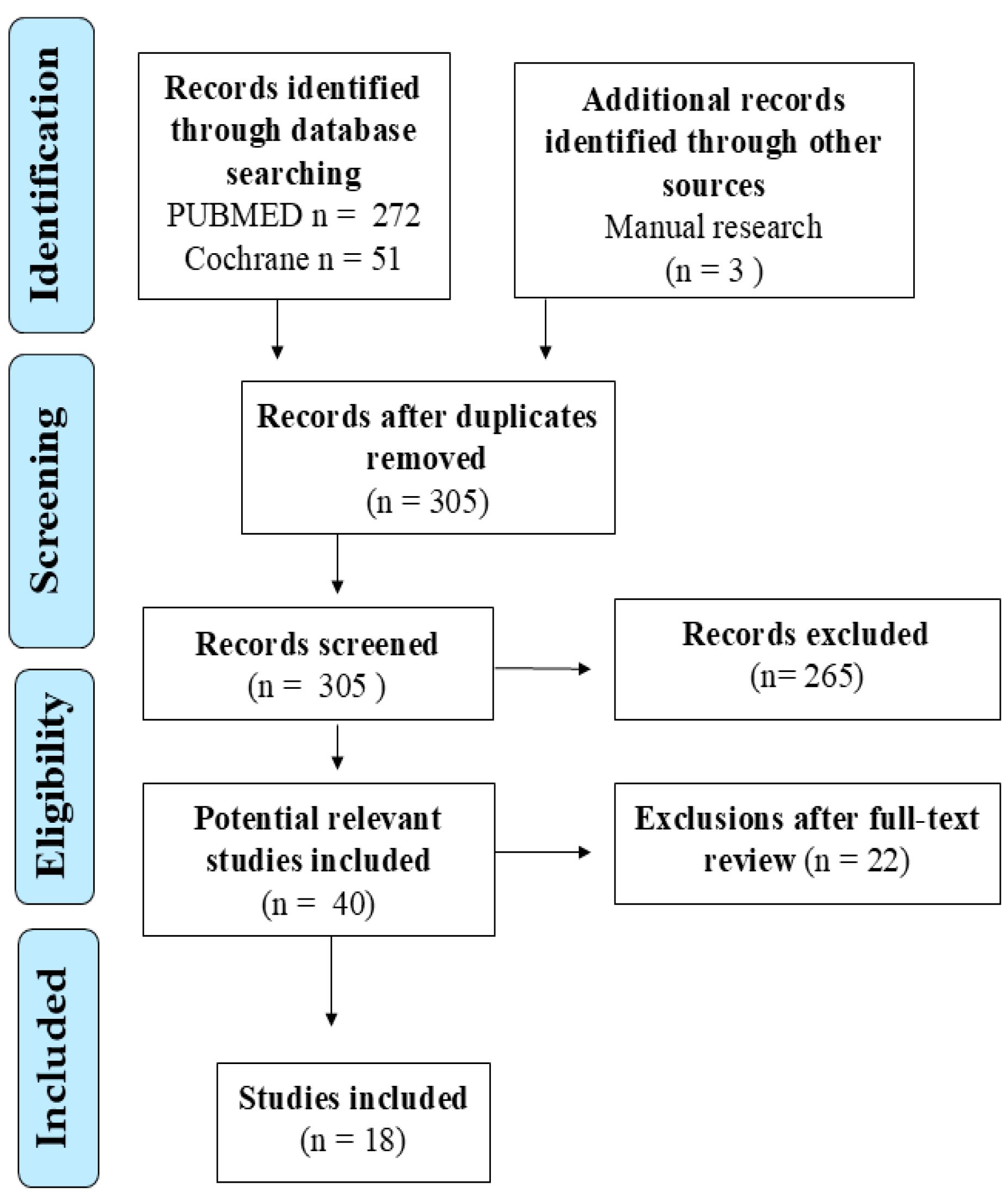
2. Materials and Methods
3. Results
3.1. Zinc in Clinical Studies on Asthma
3.2. Zinc Status in Children with Asthma
3.3. Relationship Between Zinc Levels and Asthma Control
3.4. Clinical Impact of Zinc Supplementation in Pediatric Asthma
3.5. Zinc in Clinical Studies on Rhinitis
3.6. Zinc Homeostasis in Rhinitis: A Localized Paradox
3.7. Correlation with Pathophysiological Features
3.8. Effects of Zinc Supplementation on Animal Models
3.9. Mechanistic Insights from Preclinical Studies
4. Discussion
4.1. Zinc in Asthma
4.2. Zinc in Rhinitis
4.3. Clinical Implications in Asthma and Rhinitis
4.4. Limitations of Current Studies
4.5. Future Perspectives and Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Maywald, M.; Rink, L. Zinc deficiency and zinc supplementation in allergic diseases. Biomolecules 2024, 14, 863. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Barbarot, S.; Gadkari, A.; Simpson, E.L.; Weidinger, S.; Mina-Osorio, P.; Rossi, A.B.; Brignoli, L.; Saba, G.; Guillemin, I.; et al. Atopic dermatitis in the pediatric population: A cross-sectional, international epidemiologic study. Ann. Allergy Asthma Immunol. 2021, 126, 417–428.e2. [Google Scholar] [CrossRef]
- CDC. Most Recent National Asthma Data. Available online: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm (accessed on 22 June 2025).
- Global Initiative for Asthma—GINA. 2025 GINA Strategy Report. Available online: https://ginasthma.org/2025-gina-strategy-report/ (accessed on 22 June 2025).
- Khan, A.H.; Gouia, I.; Jacob-Nara, J.; Kamat, S.; Jaffe, D.; Mackie, D.; Balkaran, B.L.; Wisnivesky, J. Prevalence and burden of asthma in five European countries: A retrospective cross-sectional study. BMJ Open 2025, 15, e085175. [Google Scholar] [CrossRef]
- Ciprandi, G.; Daglia, M.; Brindisi, G.; Brunese, F.P.; Dinardo, G.; Gori, A.; Indolfi, C.; Naso, M.; Tondina, E.; Trincianti, C.; et al. Attitude to Food Supplement Use: A Survey Promoted by the Italian Society of Pediatric Allergy and Immunology. Ital. J. Pediatr. 2024, 50, 118. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Aragona, S.E.; Drago, L.; Mantia, I.L. The Nutraceuticals: A New Therapeutic Strategy in the Management of Digestive and Respiratory Disorders. Acta Biomed. 2019, 90 (Suppl. 7), 5–7. [Google Scholar] [CrossRef]
- Peroni, D.G.; Hufnagl, K.; Comberiati, P.; Roth-Walter, F. Lack of iron, zinc, and vitamins as a contributor to the etiology of atopic diseases. Front. Nutr. 2023, 9, 1032481. [Google Scholar] [CrossRef]
- Indolfi, C.; Klain, A.; Bencivenga, C.L.; Dinardo, G.; Ferrara, S.; Marrapodi, M.M.; Decimo, F.; del Giudice, M.M. Allergie in Età Pediatrica: Il Ruolo Fisiologico della Vitamina D. Ital. J. Pediatr. Allergy Immunol. 2024, 38, 17–22. [Google Scholar] [CrossRef]
- Gori, A.; Brindisi, G.; Daglia, M.; del Giudice, M.M.; Dinardo, G.; Di Minno, A.; Drago, L.; Indolfi, C.; Naso, M.; Trincianti, C.; et al. Exploring the Role of Lactoferrin in Managing Allergic Airway Diseases among Children: Unrevealing a Potential Breakthrough. Nutrients 2024, 16, 1906. [Google Scholar] [CrossRef] [PubMed]
- Dinardo, G.; del Giudice, M.M.; Drago, L.; Daglia, M.; Gori, A.; Varricchio, A.; Trincianti, C.; Tondina, E.; Brunese, F.P.; Brindisi, G.; et al. A Review of Clinical and Preclinical Data Supporting a Role for Resveratrol in the Treatment of Common Respiratory Tract Pathogens. NFS J. 2024, 37, 100194. [Google Scholar] [CrossRef]
- Indolfi, C.; Dinardo, G.; Klain, A.; Grella, C.; Marrapodi, M.M.; Decimo, F.; Ciprandi, G.; del Giudice, M.M. Resveratrol plus Carboxymethyl-β-Glucan for Children with Respiratory Diseases. Allergol. Immunopathol. 2024, 52, 91–95. [Google Scholar] [CrossRef]
- Naso, M.; Trincianti, C.; Drago, L.; Daglia, M.; Brindisi, G.; Brunese, F.P.; Dinardo, G.; Gori, A.; Indolfi, C.; Tondina, E.; et al. Resveratrol: Immunological Activity and Possible Application in Children and Adolescents with Allergic Rhinitis. Expert Rev. Clin. Immunol. 2025, 21, 1–3. [Google Scholar] [CrossRef]
- Seo, H.-M.; Kim, Y.H.; Lee, J.H.; Kim, J.S.; Park, Y.M.; Lee, J.Y. Serum Zinc Status and Its Association with Allergic Sensitization: The Fifth Korea National Health and Nutrition Examination Survey. Sci. Rep. 2017, 7, 12637. [Google Scholar] [CrossRef]
- Prasad, A.S. Impact of the Discovery of Human Zinc Deficiency on Health. J. Am. Coll. Nutr. 2009, 28, 257–265. [Google Scholar] [CrossRef]
- Zajac, D. Mineral Micronutrients in Asthma. Nutrients 2021, 13, 4001. [Google Scholar] [CrossRef]
- Vural, H.; Uzun, K.; Uz, E.; Koçyigit, A.; Çigli, A.; Akyol, Ö. Concentrations of Copper, Zinc and Various Elements in Serum of Patients with Bronchial Asthma. J. Trace Elements Med. Biol. 2000, 14, 88–91. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc: An Antioxidant and Anti-Inflammatory Agent: Role of Zinc in Degenerative Disorders of Aging. J. Trace Elem. Med. Biol. 2014, 28, 364–371. [Google Scholar] [CrossRef]
- Vuralli, D.; Tumer, L.; Hasanoglu, A. Zinc deficiency in the pediatric age group is common but underevaluated. World J. Pediatr. 2017, 13, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Hennigar, S.R.; Lieberman, H.R.; Fulgoni, V.L., III; McClung, J.P. Serum Zinc Concentrations in the US population are related to sex, age, and time of blood draw but not dietary or supplemental zinc. J. Nutr. 2018, 148, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Truong-Tran, A.Q.; Carter, J.; Ruffin, R.; Zalewski, P.D. New Insights into the Role of Zinc in the Respiratory Epithelium. Immunol. Cell Biol. 2001, 79, 170–177. [Google Scholar] [CrossRef]
- Kahmann, L.; Uciechowski, P.; Warmuth, S.; Malavolta, M.; Mocchegiani, E.; Rink, L. Effect of Improved Zinc Status on T Helper Cell Activation and TH1/TH2 Ratio in Healthy Elderly Individuals. Biogerontology 2006, 7, 429–435. [Google Scholar] [CrossRef]
- Murdoch, J.R.; Lloyd, C.M. Chronic Inflammation and Asthma. Mutat. Res. Mol. Mech. Mutagen. 2010, 690, 24–39. [Google Scholar] [CrossRef]
- Habib, N.; Pasha, M.A.; Tang, D.D. Current Understanding of Asthma Pathogenesis and Biomarkers. Cells 2022, 11, 2764. [Google Scholar] [CrossRef]
- Rajkumar, S.; Bhat, N.K.; Kumar, V.; Bolia, R.; Verma, P.K.; Kumar, M.; Chacham, S.; Mirza, A.A. Association of Serum Zinc Levels and Symptom Control of Asthma in Children and Adolescents—A Prospective Observational Study. Eur. J. Pediatr. 2023, 182, 141–147. [Google Scholar] [CrossRef]
- Suzuki, M.; Suzuki, T.; Watanabe, M.; Hatakeyama, S.; Kimura, S.; Nakazono, A.; Honma, A.; Nakamaru, Y.; Vreugde, S.; Homma, A. Role of Intracellular Zinc in Molecular and Cellular Function in Allergic Inflammatory Diseases. Allergol. Int. 2021, 70, 190–200. [Google Scholar] [CrossRef]
- Zalewski, P.D.; Truong-Tran, A.Q.; Grosser, D.; Jayaram, L.; Murgia, C.; Ruffin, R.E. Zinc Metabolism in Airway Epithelium and Airway Inflammation: Basic Mechanisms and Clinical Targets. A Review. Pharmacol. Ther. 2005, 105, 127–149. [Google Scholar] [CrossRef]
- Roscioli, E.; Hamon, R.; Lester, S.; Murgia, C.; Grant, J.; Zalewski, P. Zinc-Rich Inhibitor of Apoptosis Proteins (IAPs) as Regulatory Factors in the Epithelium of Normal and Inflamed Airways. BioMetals 2013, 26, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2018, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Rushdy, M.; Abdel-Rehim, A.S.M. The Immunomodulatory Role of Zinc in Asthmatic Patients. Cytokine 2018, 110, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, J.; Alizadeh-Navaei, R.; Dabaghzadeh, A.; Ghaffari, N. Serum Zinc Level and Children’s Asthma: A Systematic and Meta-Analysis Review Article. Casp. J. Intern. Med. 2021, 12, 236. [Google Scholar] [CrossRef]
- Chen, M.; Sun, Y.; Wu, Y. Lower Circulating Zinc and Selenium Levels Are Associated with an Increased Risk of Asthma: Evidence from a Meta-Analysis. Public Health Nutr. 2020, 23, 1555–1562. [Google Scholar] [CrossRef]
- Xue, M.; Wang, Q.; Pang, B.; Zhang, X.; Zhang, Y.; Deng, X.; Zhang, Z.; Niu, W. Association between circulating zinc and risk for childhood asthma and wheezing: A meta-analysis on 21 articles and 2205 children. Biol. Trace Element Res. 2024, 202, 442–453. [Google Scholar] [CrossRef]
- Kuti, B.P.; Kuti, D.K.; Smith, O.S. Serum Zinc, Selenium and Total Antioxidant Contents of Nigerian Children with Asthma: Association with Disease Severity and Symptoms Control. J. Trop. Pediatr. 2021, 66, 395–402. [Google Scholar] [CrossRef]
- Srivastava, S.; Tiwari, V.; Singh, S.; Karoli, R.; Bhattacharya, P.; Gupta, N. Low Serum Levels of Zinc, Selenium, and Vitamin D3 Are Biomarkers of Airway Inflammation and Poor Asthma Control: A Two-Centre Study. Cureus 2023, 15, e41082. [Google Scholar] [CrossRef] [PubMed]
- Andino, D.; Moy, J.; Gaynes, B.I. Serum Vitamin A, Zinc and Visual Function in Children with Moderate to Severe Persistent Asthma. J. Asthma 2019, 56, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Abdulwahab, A.; Zeidan, A.; Avades, T.; Chandra, P.; Soliman, A. Serum Zinc Level in Asthmatic and Non-Asthmatic School Children. Children 2018, 5, 42. [Google Scholar] [CrossRef]
- Siripornpanich, S.; Chongviriyaphan, N.; Manuyakorn, W.; Matangkasombut, P. Zinc and Vitamin C Deficiencies Associate with Poor Pulmonary Function in Children with Persistent Asthma. Asian Pacific J. Allergy Immunol. 2022, 40, 103–110. [Google Scholar] [CrossRef]
- Rerksuppaphol, S.; Rerksuppaphol, L. Zinc Supplementation in Children with Asthma Exacerbation. Pediatr. Rep. 2016, 8, 63–67. [Google Scholar] [CrossRef]
- Cheng, C.; Lin, J.; Zhang, Z.; Zhang, L. Association between Dietary Zinc Intake and Asthma in Overweight or Obese Children and Adolescents: A Cross-Sectional Analysis of NHANES. World Allergy Organ. J. 2024, 17, 100900. [Google Scholar] [CrossRef]
- Xu, H.; Tong, K.; Iwasaki, N.; Ohgami, N.; Tazaki, A.; Kagawa, T.; Gao, Y.; Nishadhi, D.A.S.M.; Harusato, A.; Sakashita, M.; et al. Alleviating Effect of Intranasal Zinc on Symptoms of Allergic Rhinitis. J. Allergy Clin. Immunol. Glob. 2025, 4, 100408. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Shen, X.; Long, C.; Mi, Z.; Li, Y.; Ma, R. Zinc Supplement Reduces Allergic Responses through Modulating the P38 MAPK Pathway Activation in an Allergic Rhinitis Mouse Model. J. Trace Elem. Med. Biol. 2023, 75, 127094. [Google Scholar] [CrossRef] [PubMed]
- Changhai, L.; Zaichun, W.; Bo, Y.; Dan, L.; Shaohua, W. Micronutrients and allergic diseases: A Mendelian randomization study. Int. Arch. Allergy Immunol. 2024, 186, 41–51. [Google Scholar] [CrossRef]
- Agrawal, A. Unveiling Childhood Asthma: Exploring Biomarkers, Zinc, and Beyond. World J. Clin. Pediatr. 2024, 13, 91699. [Google Scholar] [CrossRef]
- Suzuki, M.; Ramezanpour, M.; Cooksley, C.; Lee, T.J.; Jeong, B.; Kao, S.; Suzuki, T.; Psaltis, A.J.; Nakamaru, Y.; Homma, A.; et al. Zinc-Depletion Associates with Tissue Eosinophilia and Collagen Depletion in Chronic Rhinosinusitis. Rhinology 2020, 58, 451–459. [Google Scholar] [CrossRef]
- Razi, C.H.; Akelma, A.Z.; Akin, O.; Kocak, M.; Ozdemir, O.; Celik, A.; Kislal, F.M. Hair Zinc and Selenium Levels in Children with Recurrent Wheezing. Pediatr. Pulmonol. 2012, 47, 1185–1191. [Google Scholar] [CrossRef]
| First Author (Year) | Study Type | Population/Model | Zinc Assessment Method | Zinc Levels (Patient Group vs. Control Group) | Key Findings & Conclusion |
|---|---|---|---|---|---|
| Asthma Studies | |||||
| Maywald, M. (2024) [3] | Review | Allergic diseases | N/A | N/A | Zinc deficiency promotes a Th2-dominant immune status and impairs epithelial barriers, contributing to allergy development. |
| Peroni, D.G. (2023) [10] | Review | Atopic diseases | N/A | N/A | Posits that deficiencies of zinc, iron, and vitamins are key contributors to the etiology of atopic diseases by skewing immune responses. |
| Rajkumar, S. (2023) [27] | Cross-sectional | 67 children with asthma (6–18 years) | Serum; Photometry | Controlled: 158.06 µg/dL; Uncontrolled: 129.23 µg/dL (p = 0.006) | Serum zinc is significantly higher in controlled asthma. A weak positive correlation exists between zinc levels and asthma control scores. |
| Ghaffari, J. (2021) [33] | Systematic Review and Meta-analysis | 21 articles (pediatric asthma) | Serum, Hair, Nail, Erythrocyte | Serum: No significant difference (pooled data); Hair: Consistently lower in asthmatics | While serum zinc levels are inconsistent, hair zinc may be a better marker. Zinc supplementation appears to improve clinical symptoms. |
| Chen, M. (2020) [34] | Meta-analysis | 26 studies | Circulating (Serum/Plasma) | Effect Size: SMD = −0.40 (95% CI: −0.77 to −0.03) | Asthma patients have significantly lower circulating zinc levels compared to healthy controls. |
| Xue, M. (2024) [35] | Meta-analysis | 21 articles (2205 children) | Circulating (Serum/Plasma) | Effect Size (Asthma): SMD = −0.41 (95% CI: −0.65 to −0.16) | Lower circulating zinc is significantly associated with an increased risk of childhood asthma. |
| Kuti, B.P. (2020) [36] | Cross-sectional | 80 asthmatic vs. 80 control children (Nigeria) | Serum | Asthmatics: 71.0 ± 30.3 µg/dL; Controls: 84.2 ± 31.7 µg/dL (p = 0.008) | Asthmatic children have significantly lower serum zinc levels, but no association was found between this and disease severity or symptom control. |
| Srivastava, S. (2023) [37] | Cross-sectional | 100 asthmatic children (avg. 8.7 yrs) vs. 75 controls | Serum | Asthmatics: 51 ± 12.8 µg/dL; Controls: 60 ± 18.2 µg/dL (p = 0.0002) | Asthmatic children have significantly lower serum levels of zinc, selenium, and vitamin D3. Low zinc is associated with poorer asthma control. |
| Andino, D. (2019) [38] | Case–control | 12 children with moderate–severe persistent asthma vs. 12 controls | Serum | Asthmatics: 759 µg/L (median); Controls: 910 µg/L (median) (p = 0.011) | Asthmatics have statistically lower serum zinc levels, though not meeting the threshold for clinical deficiency. |
| AbdulWahab, A. (2018) [39] | Cross-sectional | 40 asthmatic vs. 40 control children (Qatar) | Serum | Asthmatics: 12.78 ± 1.8 µmol/L; Controls: 13.0 ± 1.52 µmol/L (p > 0.05) | No significant difference in serum zinc levels between groups. No association was found between zinc and asthma control. |
| Siripornpanich, S. (2021) [40] | Cross-sectional | 76 children with persistent asthma (Thailand) | Plasma; Atomic Absorption Spectrophotometry | Mean: 54.1 µg/dL (all participants below normal range) | All participants were zinc deficient. Plasma zinc positively correlated with lung function (FEV1 and FEV1/FVC ratio). |
| Rerksuppaphol, S. (2016) [41] | RCT | 42 children with acute asthma exacerbation | Serum | Baseline (avg.): 63.8 µg/dL in both groups | Zinc supplementation (30 mg/day) significantly accelerated clinical improvement (PRAM score) at 24 and 48 h. |
| Cheng, C. (2024) [42] | Cross-sectional (NHANES) | 4597 overweight/obese children and adolescents | Dietary Recall (24 h) | Intake assessed by quartiles (mg/day) | Higher dietary zinc intake is inversely associated with asthma prevalence in a dose-response manner (OR = 0.71 for Q4 vs. Q1). |
| Rhinitis Studies | |||||
| Suzuki, M. (2020) [28] | Human Study | CRS patients vs. controls | Serum, Mucus, Mucosal Tissue | Serum: No difference; Mucus: Increased in inflamed sites; Tissue: Decreased in CRSwNP | Mucosal tissue zinc depletion in CRSwNP correlates with eosinophilia and collagen depletion, suggesting a role in pathophysiology. |
| Xu, H. (2025) [43] | Human Observational and Animal Study | 44 AR patients vs. 57 controls and mouse model | Serum, Nasal Epithelial Lining Fluid (ELF) | Serum: Decreased in patients; Nasal ELF: Increased in patients | Paradoxical zinc distribution found during allergic inflammation. Intranasal zinc application alleviates allergic symptoms in mice. |
| Changhai, L. (2025) [45] | Mendelian Randomization | General population (European ancestry) | Genetic Proxies (from GWAS) | N/A | No evidence was found for a causal association between genetically predicted serum zinc levels and the risk of allergic rhinitis. |
| Shi, Q. (2023) [44] | Animal Study | OVA-induced allergic rhinitis mouse model | N/A | N/A | Zinc supplementation reverses high IgE and inflammatory cytokines by downregulating the p38 MAPK pathway. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinardo, G.; Indolfi, C.; Klain, A.; Grella, C.; Tosca, M.A.; Ruocco, E.; Miraglia del Giudice, M.; Ciprandi, G. The Role of Zinc in Pediatric Asthma and Allergic Rhinitis: Mechanisms and Clinical Implications. Nutrients 2025, 17, 2660. https://doi.org/10.3390/nu17162660
Dinardo G, Indolfi C, Klain A, Grella C, Tosca MA, Ruocco E, Miraglia del Giudice M, Ciprandi G. The Role of Zinc in Pediatric Asthma and Allergic Rhinitis: Mechanisms and Clinical Implications. Nutrients. 2025; 17(16):2660. https://doi.org/10.3390/nu17162660
Chicago/Turabian StyleDinardo, Giulio, Cristiana Indolfi, Angela Klain, Carolina Grella, Maria Angela Tosca, Eleonora Ruocco, Michele Miraglia del Giudice, and Giorgio Ciprandi. 2025. "The Role of Zinc in Pediatric Asthma and Allergic Rhinitis: Mechanisms and Clinical Implications" Nutrients 17, no. 16: 2660. https://doi.org/10.3390/nu17162660
APA StyleDinardo, G., Indolfi, C., Klain, A., Grella, C., Tosca, M. A., Ruocco, E., Miraglia del Giudice, M., & Ciprandi, G. (2025). The Role of Zinc in Pediatric Asthma and Allergic Rhinitis: Mechanisms and Clinical Implications. Nutrients, 17(16), 2660. https://doi.org/10.3390/nu17162660








