Gender and Age-Specific Responses to Non-Invasive Body-Contouring Interventions and Their Impact on Body Composition—Pilot Study
Abstract
1. Introduction
2. Materials and Methods
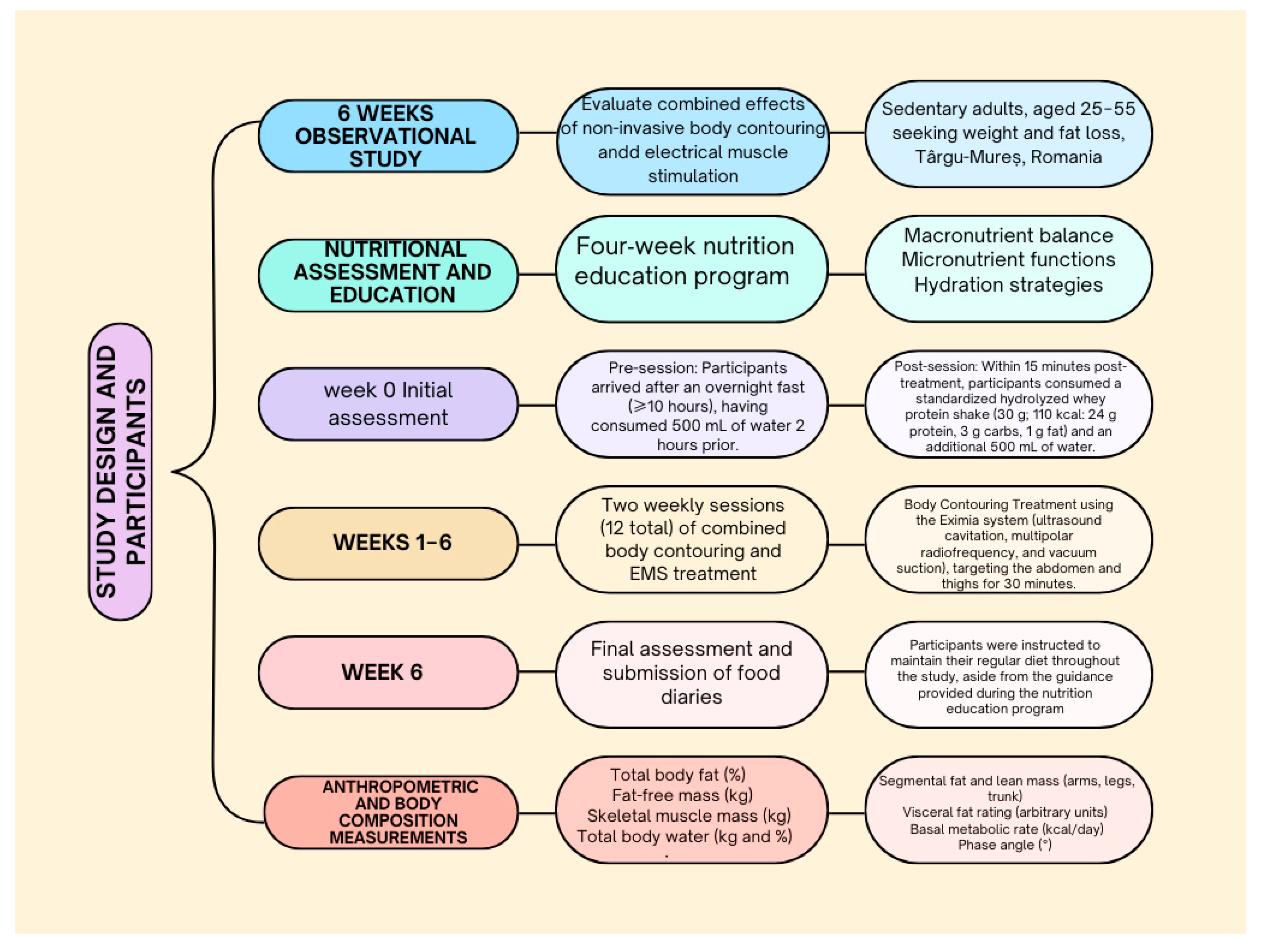
2.1. Study Design and Participants
2.2. Intervention Protocol
2.3. Each Intervention Session Included
2.4. Peri-Procedural Standardization
2.5. Nutritional Assessment and Education
2.6. Dietary Controls
2.7. Physical Activity Assessment
2.8. Anthropometric and Body Composition Measurements
2.9. Anthropometric Measures
2.10. Body Composition Analysis
2.11. Statistical Analysis
3. Results
Age Analyses
4. Discussion
5. Practical Applications
Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, L. The impact of nutrition and lifestyle modification on health. Eur. J. Intern. Med. 2022, 97, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yu, D.; Fan, J.; Yu, C.; Guo, Y.; Pei, P.; Yang, L.; Chen, Y.; Du, H.; Yang, X.; et al. China Kadoorie Biobank Collaborative Group. Healthy lifestyle and life expectancy at age 30 years in the Chinese population: An observational study. Lancet Public Health 2022, 7, e994–e1004. [Google Scholar] [CrossRef]
- Sohn, Y.J.; Chun, H. Ultrasound and high frequency equipment efficacy for abdominal obesity reduction in women. Sci. Rep. 2025, 15, 17500. [Google Scholar] [CrossRef]
- Tang, J.; Chen, L.; Zhang, S.; Shao, H.; Wang, L.; Wang, C.; Chen, Q.; Hou, Y.; Liao, X.; Song, M.; et al. Efficacy and safety of non-focused low-intensity ultrasound technology for subcutaneous lipolysis in the lower abdomen: A clinical study. Postgrad. Med. J. 2025, 22, qgaf008. [Google Scholar] [CrossRef]
- Santos, A.F.; Fernández, A.I.; Fernández, L.S.; Zapico, L.H.; Freitag, S.V. Effectiveness of Body Remodeling and Cellulite Appearance Improvement Treatments in the Thighs Using Symmed Radiofrequency Device. J. Cosmet. Dermatol. 2025, 24, e16796. [Google Scholar] [CrossRef]
- Mazzoni, D.; Lin, M.J.; Dubin, D.P.; Khorasani, H. Review of non-invasive body contouring devices for fat reduction, skin tightening and muscle definition. Australas. J. Dermatol. 2019, 60, 278–283. [Google Scholar] [CrossRef]
- Marques, L.; Neves, J.; Pereira, A.; Santiago, A.; Troia, S.; Vilarinho, R.; Amorim, M.M.; Noites, A. Effects of Combining Shockwaves or Radiofrequency with Aerobic Exercise on Subcutaneous Adipose Tissue and Lipid Mobilization: A Randomized Controlled Trial. Obesities 2025, 5, 31. [Google Scholar] [CrossRef]
- Bani, D.; Li, A.Q.; Freschi, G.; Russo, G.L. Histological and Ultrastructural Effects of Ultrasound-induced Cavitation on Human Skin Adipose Tissue. Plast. Reconstr. Surg. Glob. Open 2013, 1, e41. [Google Scholar] [CrossRef]
- Arent, S.M.; Cintineo, H.P.; McFadden, B.A.; Chandler, A.J.; Arent, M.A. Nutrient Timing: A Garage Door of Opportunity? Nutrients 2020, 12, 1948. [Google Scholar] [CrossRef]
- Ullmann, P.; Viol, M.; Schleicher, W. Mitteilung zur Objektivierung der reaktiven Wirksamkeit mittelfrequenter Elektromyostimulation mit Hilfe muskelmechanischer Diagnostik (Myomechanographie) [Objective assessment of the reactive effectiveness of middle frequency electromyostimulation using mechanical muscle diagnosis (myomechanography)]. Beitr. Orthop. Traumatol. 1989, 36, 91–96. [Google Scholar] [PubMed]
- Happ, K.A.; Behringer, M. Neuromuscular Electrical Stimulation Training vs. Conventional Strength Training: A Systematic Review and Meta-Analysis of the Effect on Strength Development. J. Strength Cond. Res. 2022, 36, 3527–3540. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Park, S.; Oh, S.; Kang, M.; Seo, Y.; Kim, B.G.; Lee, S.H. Effects of electrical muscle stimulation on core muscle activation and physical performance in non-athletic adults: A randomized controlled trial. Medicine 2023, 102, e32765. [Google Scholar] [CrossRef] [PubMed]
- Maffiuletti, N.A.; Herrero, A.J.; Jubeau, M.; Impellizzeri, F.M.; Bizzini, M. Differences in electrical stimulation thresholds between men and women. Ann. Neurol. 2008, 63, 507–512. [Google Scholar] [CrossRef]
- Enoka, R.M.; Amiridis, I.G.; Duchateau, J. Electrical Stimulation of Muscle: Electrophysiology and Rehabilitation. Physiology 2020, 35, 40–56. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Aragon, A.A. Is There a Postworkout Anabolic Anabolic Window of Opportunity for Nutrient Consumption? Clearing up Controversies. J. Orthop. Sports Phys. Ther. 2018, 48, 911–914. [Google Scholar] [CrossRef]
- Aragon, A.A.; Schoenfeld, B.J. Nutrient timing revisited: Is there a post-exercise anabolic window? J. Int. Soc. Sports Nutr. 2013, 10, 5. [Google Scholar] [CrossRef]
- Kyle, U.G.; Genton, L.; Hans, D.; Karsegard, L.; Slosman, D.O.; Pichard, C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur. J. Clin. Nutr. 2001, 55, 663–672. [Google Scholar] [CrossRef]
- Jakicic, J.M.; Rogers, R.J.; Davis, K.K.; Collins, K.A. Role of Physical Activity and Exercise in Treating Patients with Overweight and Obesity. Clin. Chem. 2018, 64, 99–107. [Google Scholar] [CrossRef]
- Rennie, M.J. Control of muscle protein synthesis as a result of contractile activity and amino acid availability: Implications for protein requirements. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, S170–S176. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E.; Brambilla, E.; Luzi, L.; Landaker, E.J.; Kahn, C.R. Bidirectional modulation of insulin action by amino acids. J. Clin. Investig. 1998, 101, 1519–1529. [Google Scholar] [CrossRef]
- Meijer, A.J.; Dubbelhuis, P.F. Amino acid signaling and the integration of metabolism. Biochem. Biophys. Res. Commun. 2004, 313, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Thornton, S.N. Increased Hydration Can Be Associated with Weight Loss. Front. Nutr. 2016, 3, 18. [Google Scholar] [CrossRef]
- Ruta, F.; Avram, C.; Maior, R. Physico-Chemical and Microbiological Differences between Mains and Bottled Water, in an Area in the Central Area of Romania. Int. J. Environ. Res. Public Health. 2023, 20, 1115. [Google Scholar] [CrossRef]
- Jee, Y.S. The efficacy and safety of whole-body electromyostimulation in applying to human body: Based from graded exercise test. J. Exerc. Rehabil. 2018, 14, 49–57. [Google Scholar] [CrossRef]
- Sara, J.; Rajai, N.; Olson, T.; Lerman, L.O.; Lerman, A. Physical training augmented with whole body electronic muscle stimulation is superior to conventional training alone in healthy subjects, a pilot randomized controlled trial. J. Am. Coll. Cardiol. 2023, 81 (Suppl. 8), 2153. [Google Scholar] [CrossRef]
- Bucurica, S.; Nancoff, A.S.; Dutu, M.; Mititelu, M.R.; Gaman, L.E.; Ioniță-Radu, F.; Jinga, M.; Maniu, I.; Ruța, F. Exploring the Relationship between Lipid Profile, Inflammatory State and 25-OH Vitamin D Serum Levels in Hospitalized Patients. Biomedicines 2024, 12, 1686. [Google Scholar] [CrossRef]
- Lee, M.C.; Ho, C.S.; Hsu, Y.J.; Wu, M.F.; Huang, C.C. Effect of 8-week frequency-specific electrical muscle stimulation combined with resistance exercise training on muscle mass, strength, and body composition in men and women: A feasibility and safety study. PeerJ 2023, 11, e16303. [Google Scholar] [CrossRef]
- Bera, T.K. Bioelectrical Impedance Methods for Noninvasive Health Monitoring: A Review. J. Med. Eng. 2014, 2014, 381251. [Google Scholar] [CrossRef]
- Taniguchi, M.; Yamada, Y.; Yagi, M.; Nakai, R.; Tateuchi, H.; Ichihashi, N. Estimating thigh skeletal muscle volume using multi-frequency segmental-bioelectrical impedance analysis. J. Physiol. Anthropol. 2021, 40, 13. [Google Scholar] [CrossRef] [PubMed]
- Cherecheș, M.C.; Finta, H.; Prisada, R.M.; Rusu, A. Pharmacists’ Professional Satisfaction and Challenges: A Netnographic Analysis of Reddit and Facebook Discussions. Pharmacy 2024, 12, 155. [Google Scholar] [CrossRef]
- Chereches, M.C.; Popa, C.O.; Finta, H. The dynamics of food for special medical purposes (FSMPs) utilization in cancer care: From doctor recommendations to online pharmacy procurement. Front. Pharmacol. 2024, 15, 1393784. [Google Scholar] [CrossRef] [PubMed]
- Avram, C.; Gligor, A.; Avram, L. A Formal Model Based Automated Decision Makin. Procedia Manuf. 2020, 46, 573–579. [Google Scholar] [CrossRef]
- Avram, C.; Gligor, A.; Roman, D.; Soylu, A.; Nyulas, V.; Avram, L. Machine learning based assessment of preclinical health questionnaires. Int. J. Med. Inform. 2023, 180, 105248. [Google Scholar] [CrossRef] [PubMed]
| Variable | Before | After | p Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Muscle mass % | 37.34 | 5.09 | 38.13 | 5.94 | 0.02 * |
| Muscle mass in kg | 25.88 | 5.04 | 26.71 | 4.487 | 0.74 ** |
| Phase Angle | 6.22 | 0.61 | 6.40 | 0.64 | 0.16 * |
| Intracellular water | 19.78 | 3.39 | 19.96 | 3.29 | 0.72 ** |
| Extracellular water | 15.46 | 2.12 | 15.28 | 2.09 | 0.04 * |
| Metabolic age | 36.00 | 12.79 | 35.97 | 12.78 | 0.95 * |
| Bone density | 2.61 | 0.34 | 2.61 | 0.33 | 0.99 * |
| Visceral fat | 4.97 | 2.88 | 4.74 | 2.99 | 0.08 * |
| Total body water | 35.82 | 6.01 | 35.14 | 5.15 | 0.25 * |
| BMR kj | 6457.65 | 876.69 | 6418.15 | 841.49 | 0.45 ** |
| Bioimpedance | 614.90 | 64.03 | 618.45 | 73.19 | 0.77 * |
| Fat-free mass | 52.02 | 7.21 | 51.67 | 7.02 | 0.39 ** |
| Fat % | 26.11 | 8.13 | 25.04 | 9.02 | 0.04 * |
| BMI body mass index | 26.03 | 4.26 | 25.68 | 4.16 | 0.054 * |
| Waist–hip ratio | 0.87 | 0.07 | 0.87 | 0.07 | 0.38 * |
| Weight | 71.24 | 12.37 | 70.02 | 11.75 | 0.024 * |
| Fat kg | 19.21 | 8.23 | 18.19 | 8.42 | 0.01 * |
| Variable | Before | After | p Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Muscle mass % | 36.34 | 4.673 | 37.19 | 5.82 | 0.27 * |
| Muscle mass in kg | 47.27 | 5.29 | 46.83 | 4.27 | 0.29 * |
| Phase angle | 6.13 | 0.56 | 6.35 | 0.68 | 0.13 * |
| Intracellular water | 18.41 | 1.37 | 18.59 | 1.18 | 0.47 * |
| Extracellular water | 14.87 | 1.78 | 14.6 | 1.55 | 0.007 * |
| Metabolic age | 35.41 | 12.44 | 35.52 | 12.12 | 0.84 * |
| Bone density | 2.51 | 0.26 | 2.49 | 0.21 | 0.45 * |
| Visceral fat | 4.48 | 2.4 | 4.24 | 2.49 | 0.16 ** |
| Total body water | 33.97 | 4.63 | 33.06 | 2.29 | 0.12 ** |
| BMR kj | 6193 | 684.1 | 6122 | 552.2 | 0.12 * |
| Bioimpedance | 621.3 | 67.14 | 629.5 | 73.17 | 0.38 ** |
| Fat-free mass | 49.78 | 5.55 | 49.13 | 4.46 | 0.13 * |
| Fat % | 27.08 | 8.26 | 25.98 | 9.16 | 0.06 ** |
| BMI body mass index | 26.16 | 4.48 | 25.72 | 4.39 | 0.004 * |
| Waist–hip ratio | 0.85 | 0.06 | 0.84 | 0.06 | 0.098 * |
| Weight | 72.24 | 12.37 | 69.31 | 12.21 | 0.003 ** |
| Fat kg | 19.53 | 8.63 | 18.41 | 8.75 | 0.014 * |
| Variable | Before | After | p Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Muscle mass % | 42.17 | 4.56 | 42.72 | 4.43 | 0.041 * |
| Muscle mass in kg | 59.75 | 3.12 | 60.75 | 2.25 | 0.29 * |
| Phase angle | 6.70 | 0.63 | 6.65 | 0.29 | 0.75 * |
| Intracellular water | 26.43 | 1.99 | 26.62 | 1.35 | 0.64 * |
| Extracellular water | 18.37 | 0.88 | 18.6 | 0.72 | 0.20 * |
| Metabolic age | 38.83 | 15.33 | 38.17 | 16.77 | 0.61 * |
| Bone density | 3.13 | 0.15 | 3.2 | 0.10 | 0.23 * |
| Visceral fat | 7.33 | 4.03 | 7.16 | 4.30 | 0.61 * |
| Total body water | 44.8 | 2.82 | 45.22 | 2.05 | 0.45 * |
| BMR kj | 7734 | 494.9 | 7850 | 369.8 | 0.46 ** |
| Bioimpedance | 584.2 | 35.68 | 565.1 | 48.29 | 0.14 * |
| Fat-free mass | 62.88 | 3.27 | 63.95 | 2.35 | 0.29 * |
| Fat % | 21.47 | 6.02 | 20.55 | 7.37 | 0.32 * |
| BMI body mass index | 25.38 | 3.22 | 25.55 | 3.12 | 0.61 * |
| Waist–hip ratio | 0.96 | 0.04 | 0.97 | 0.04 | 0.48 * |
| Weight | 80.6 | 8.93 | 81.08 | 8.46 | 0.64 * |
| Fat kg | 17.72 | 6.31 | 17.13 | 7.41 | 0.48 * |
| Parameters | >40 Years (n = 35) | <40 Years (n = 42) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p Value | Before | After | p Value | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Muscle mass % | 36.92 | 5.23 | 37.55 | 5.79 | 0.58 * | 37.68 | 5.09 | 38.62 | 6.17 | 0.18 * |
| Muscle mass in kg | 26.44 | 4.98 | 25.85 | 3.81 | 0.71 * | 38.62 | 5.20 | 27.25 | 4.96 | 0.91 ** |
| Phase Angle | 6.37 | 0.67 | 6.51 | 0.62 | 0.48 * | 6.10 | 0.53 | 6.30 | 0.65 | 0.30 * |
| Intracellular water | 19.53 | 3.01 | 19.79 | 2.84 | 0.67 ** | 19.99 | 3.75 | 20.10 | 3.70 | 0.97 ** |
| Extracellular water | 15.44 | 2.07 | 15.18 | 1.99 | 0.07 * | 15.48 | 2.22 | 15.37 | 2.23 | 0.32 * |
| Metabolic age | 37.56 | 10.75 | 37.62 | 10.78 | 0.91 * | 34.68 | 14.45 | 34.57 | 14.4 | 0.89 * |
| Bone density | 2.63 | 0.30 | 2.61 | 0.29 | 0.68 * | 2.61 | 0.37 | 2.61 | 0.37 | 0.85 * |
| Visceral fat | 5.43 | 2.92 | 5.12 | 3.05 | 0.13 * | 4.57 | 2.87 | 4.42 | 2.98 | 0.38 * |
| Total body water | 36.22 | 6.36 | 34.73 | 4.61 | 0.18 ** | 35.48 | 5.84 | 35.48 | 5.67 | 0.89 * |
| BMR kj | 6419.46 | 804.19 | 6362.42 | 738.42 | 0.37 * | 6489.82 | 954.18 | 6465.08 | 937.05 | 0.45 ** |
| Bioimpedance | 594.99 | 49.31 | 599.58 | 52.75 | 0.02 * | 631.67 | 71.20 | 634.34 | 84.95 | 0.98 ** |
| Fat-free mass | 52.32 | 6.51 | 51.65 | 6.15 | 0.26 * | 51.77 | 7.92 | 51.68 | 7.84 | 0.92 ** |
| Fat % | 70.64 | 12.08 | 69.09 | 804.19 | 0.37* | 25.05 | 8.66 | 26.25 | 9.35 | 0.25 * |
| BMI body mass index | 26.03 | 4.39 | 25.53 | 4.03 | 0.027 * | 25.96 | 4.40 | 25.61 | 3.93 | 0.46 * |
| Waist–hip ratio | 0.88 | 0.07 | 0.88 | 0.07 | 0.85 * | 0.86 | 0.08 | 0.86 | 0.07 | 0.20 * |
| Weight | 70.64 | 12.55 | 69.09 | 12.08 | 0.004 * | 71.74 | 12.53 | 70.81 | 11.74 | 0.20 * |
| Fat kg | 18.32 | 8.05 | 17.08 | 8.57 | 0.025 * | 19.96 | 8.52 | 19.13 | 8.41 | 0.10 * |
| Variable | Group | p Value |
|---|---|---|
| BMI_Diff | Gender | 0.080 |
| BMI_Diff | Age | 0.750 |
| Muscle_Mass_Diff | Gender | 0.185 |
| Muscle_Mass_Diff | Age | 0.643 |
| Fat_Mass_Diff | Gender | 0.212 |
| Fat_Mass_Diff | Age | 0.819 |
| Fat_Free_Mass_Diff | Gender | 0.089 |
| Fat_Free_Mass_Diff | Age | 0.680 |
| Visceral_Fat_Index_Diff | Gender | 0.240 |
| Visceral_Fat_Index_Diff | Age | 0.974 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maior, R.; Ruta, F.; Badea, M.-A.; Avram, C.; Bacârea, V. Gender and Age-Specific Responses to Non-Invasive Body-Contouring Interventions and Their Impact on Body Composition—Pilot Study. Nutrients 2025, 17, 2639. https://doi.org/10.3390/nu17162639
Maior R, Ruta F, Badea M-A, Avram C, Bacârea V. Gender and Age-Specific Responses to Non-Invasive Body-Contouring Interventions and Their Impact on Body Composition—Pilot Study. Nutrients. 2025; 17(16):2639. https://doi.org/10.3390/nu17162639
Chicago/Turabian StyleMaior, Raluca, Florina Ruta, Mihail-Alexandru Badea, Calin Avram, and Vladimir Bacârea. 2025. "Gender and Age-Specific Responses to Non-Invasive Body-Contouring Interventions and Their Impact on Body Composition—Pilot Study" Nutrients 17, no. 16: 2639. https://doi.org/10.3390/nu17162639
APA StyleMaior, R., Ruta, F., Badea, M.-A., Avram, C., & Bacârea, V. (2025). Gender and Age-Specific Responses to Non-Invasive Body-Contouring Interventions and Their Impact on Body Composition—Pilot Study. Nutrients, 17(16), 2639. https://doi.org/10.3390/nu17162639





