Skin Aging and Carotenoids: A Systematic Review of Their Multifaceted Protective Mechanisms
Abstract
1. Introduction
1.1. Objective
1.2. Skin Functions
- Intrinsic factors: genetics, hormonal changes, and metabolic processes
- Extrinsic factors: UV radiation, pollution, smoking, and poor nutrition
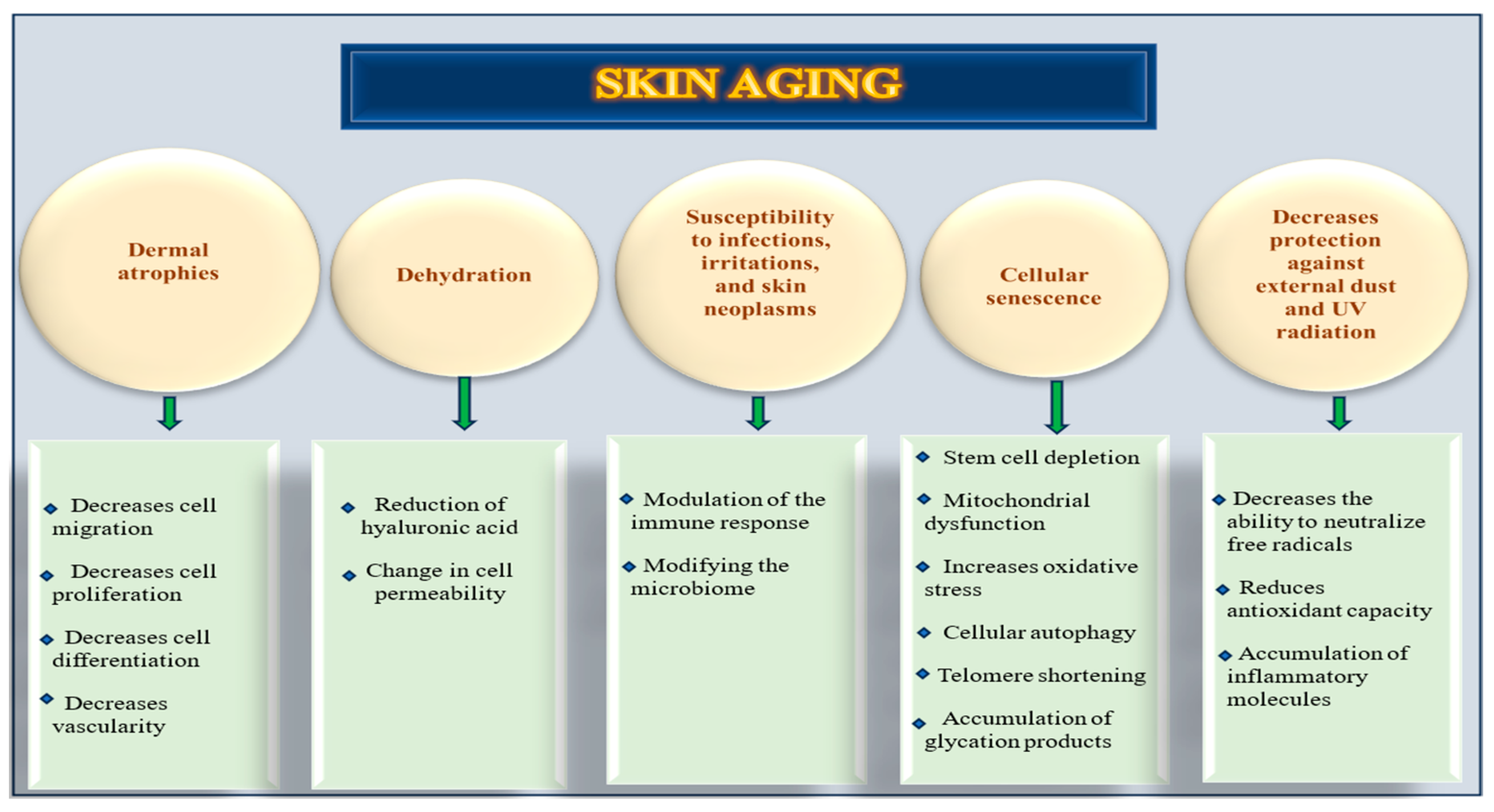
1.3. Skin Aging Mechanisms and Molecular Pathways
- Dermal thinning, loss of collagen and elastin, and decreased vascularity.
- Dryness, reduction in hyaluronic acid, and change in cell permeability.
- Pigmentation, xerosis, and elastosis.
- Increasing risk for non-melanomatous cutaneous carcinomas.
- Susceptibility to infections and irritations.
- Modifying the microbiome and immune response.
- Cellular senescence, stem cell depletion, mitochondrial dysfunction, cellular autophagy, telomere shortening, accumulation of glycation products, and increased oxidative stress.
- The consequence of these changes was the formation of wrinkles. This phenomenon is driven by a constellation of interrelated molecular and cellular mechanisms, including a decreased ability to neutralize free radicals, a reduction in anti-oxidant capacity, and chronic inflammation [8].
1.3.1. Oxidative Stress and Skin Aging
1.3.2. Aquaporins Dysregulation in Skin Aging
1.3.3. Melatonin in Skin Aging
1.3.4. Environmental Stressors in Skin Aging
1.4. Protective Roles of Carotenoids
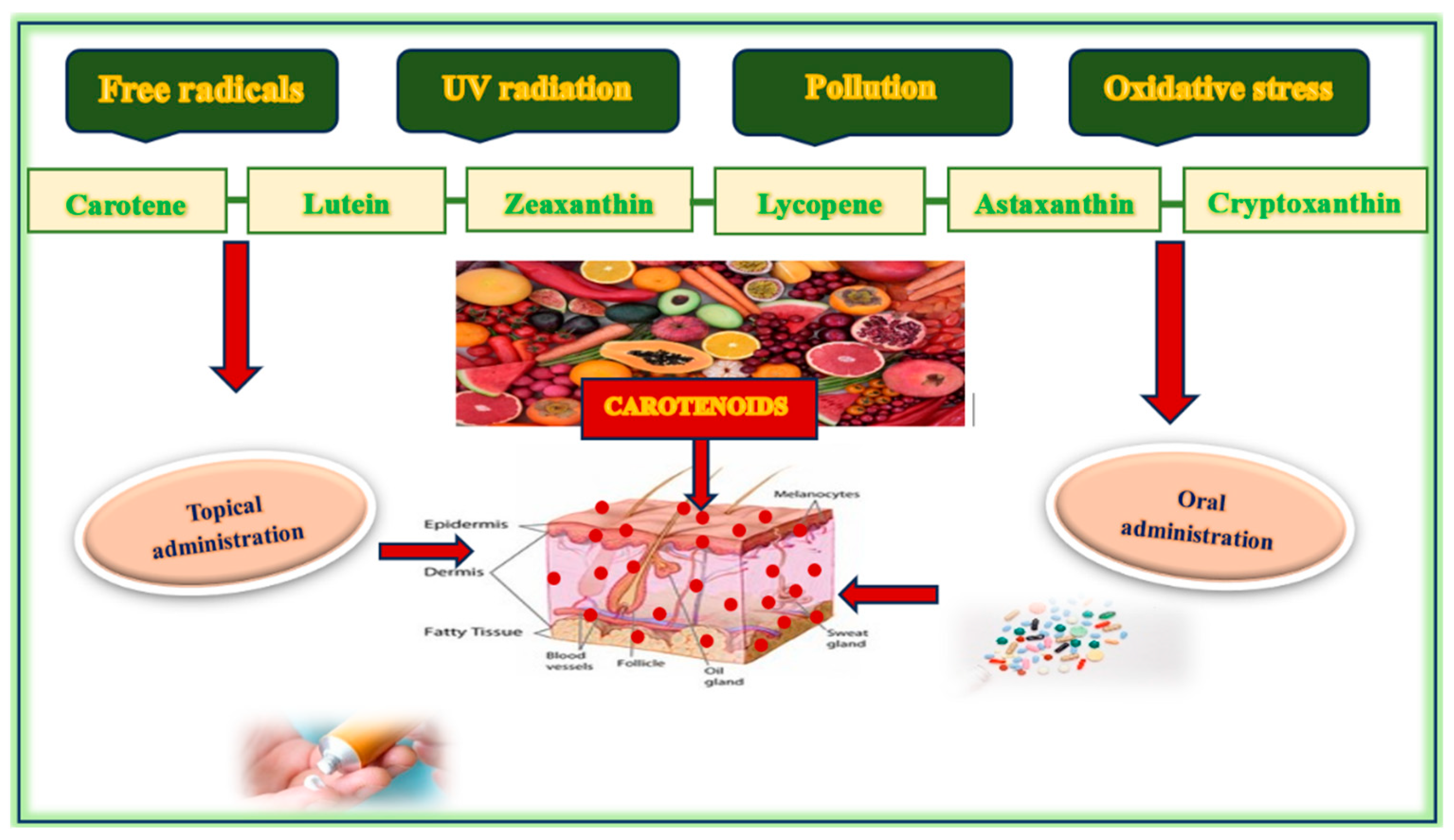
- Carotenes: These are hydrocarbon carotenoids composed solely of carbon and hydrogen atoms (e.g., β-carotene, lycopene).
- Xanthophylls: These are oxygenated carotenoids that contain oxygen atoms in addition to carbon and hydrogen (e.g., lutein, zeaxanthin, astaxanthin).
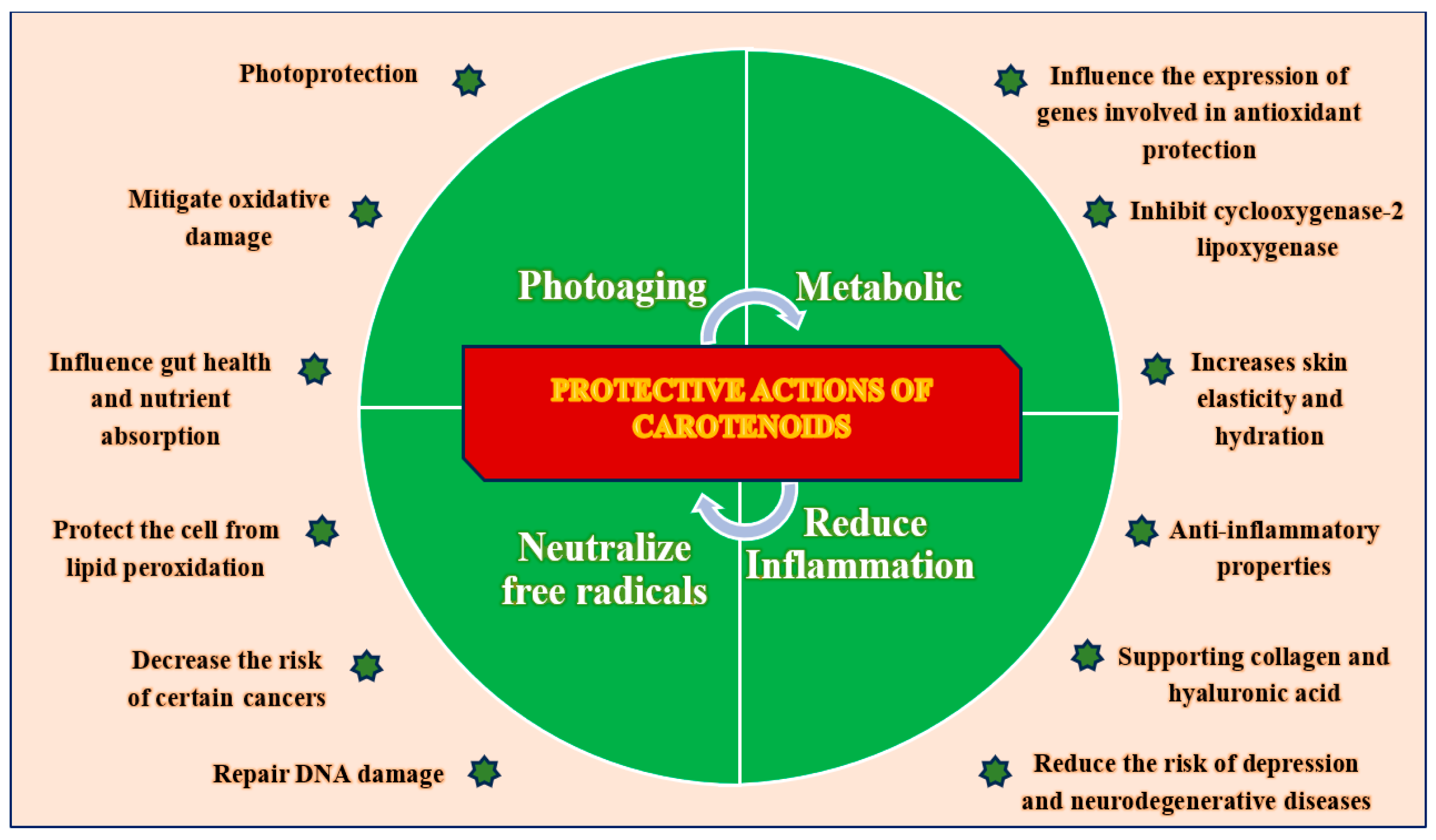
- Photoprotection: Carotenoids, including β-carotene, lutein, and lycopene, absorb specific wavelengths of light and mitigate the phototoxic effects of ultraviolet radiation. This process helps limit DNA photodamage, oxidative stress, and inflammation related to photoaging. Their accumulation in skin tissue offers intrinsic protection against UV-induced erythema and pigmentary changes [36,37,38,39].
- Anti-oxidant Activity: Carotenoids play a crucial role in maintaining cellular redox balance by scavenging reactive oxygen species (ROS), reducing lipid peroxidation, and enhancing the activity of endogenous anti-oxidant enzymes, such as superoxide dismutase and glutathione peroxidase. This function is especially important in the epidermal and dermal layers, which are often subjected to environmental stressors [11,40,41,42,43].
- Anti-inflammatory Properties: By suppressing NF-κB and MAPK signaling, carotenoids reduce the expression of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, while also inhibiting key enzymes, such as COX-2 and LOX. This action prevents chronic skin inflammation and the associated tissue degradation [44,45,46].
- DNA Protection and Repair: Carotenoids contribute to the maintenance of genomic stability by minimizing oxidative DNA damage and preserving mitochondrial integrity. Some carotenoids may also affect DNA repair pathways, although this requires further investigation in human skin models [47,48,49,50,51].
- Collagen and ECM Support: Carotenoids enhance fibroblast activity and promote collagen synthesis by activating TGF-β signaling while also inhibiting MMPs that break down structural proteins. This dual mechanism helps to preserve skin elasticity and dermal density, which are essential features of young skin [52,53,54,55,56].
- Nutrient Bioavailability and Metabolic Interactions: Carotenoids enhance the overall anti-oxidant capacity and demonstrate synergistic effects when combined with other micronutrients such as vitamins C and E, selenium, and polyphenols. These interactions amplify protective effects at multiple cellular checkpoints [69,70].
1.5. Food Sources of Carotenoids
1.6. Carotenoids in Skincare Products
- −
- −
- Improving skin elasticity and hydration through membrane stabilization and interaction with structural lipids [20,21,22,23,24].Matrix metalloproteinases (MMPs) and pro-inflammatory cytokines are downregulated, thereby preserving dermal collagen and inhibiting extracellular matrix degradation [44,45,53,54,56]. When applied topically, carotenoids penetrate the stratum corneum and accumulate within skin lipids, where they exert localized protection against photo-oxidative damage. Carotenoid concentrations in the skin vary depending on diet, sun exposure, and metabolism. A diet rich in fruits and vegetables significantly raises carotenoid levels, while stress factors, including illness and UV radiation, can cause a rapid decrease. Bioavailability is increased in the presence of dietary fats and can be influenced by the individual’s physiological state. Additionally, higher cardiovascular fitness and lower body fat can increase skin carotenoid levels, enhancing skin yellowness and contributing to a healthier appearance [83,84]. β-Carotene, predominantly located in the stratum corneum and dermis, functions as a precursor to retinoic acid. The concentration of this compound in skin tissue varies significantly, ranging from 0.2 to 1.0, with elevated levels observed in individuals who consume diets rich in carotenoids. Lycopene is predominantly localized in the deeper layers of the epidermis and demonstrates a significant capacity for quenching singlet oxygen. Its concentration typically ranges from 0.1 to 0.8 µg/g, which is often lower than that of β-carotene, yet it exhibits comparable anti-oxidant efficacy. Lutein and zeaxanthin, although present in lower concentrations, play a crucial role in protecting against blue light exposure and oxidative damage. Astaxanthin, although less prevalent, exhibits exceptional anti-oxidant activity and has been researched for its anti-aging properties. It is found in nanomolar concentrations but offers remarkable anti-aging benefits due to its potent anti-oxidant capabilities. Carotenoids are integral to skin defense mechanisms, and their concentrations serve as biomarkers of nutritional status and oxidative resilience [46,69]. Their presence has been associated with reduced wrinkle depth, improved surface smoothness, and enhanced dermal resilience, particularly in aged or photodamaged skin. To improve skin bioavailability and penetration, carotenoids are frequently incorporated into advanced delivery systems, including the following:
- Nanoemulsions and liposomes enhance solubility and skin permeation.
- Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) offer controlled release, photostability, and deep-tissue delivery.
- Hydrogel-based systems provide occlusion and prolong skin contact.
1.7. Mechanisms of Carotenoids
1.7.1. Anti-Oxidant Protection of Carotenoids
1.7.2. Photoprotection
1.7.3. Anti-Inflammatory Properties of Carotenoids
1.7.4. Supporting Collagen Production
- Upregulation of transforming growth factor-beta (TGF-β), a key growth factor that stimulates fibroblast proliferation and promotes expression of collagen genes.
- Matrix metalloproteinases (MMPs), particularly MMP-1 (collagenase) and MMP-9 (gelatinase), are responsible for extracellular matrix (ECM) degradation during both intrinsic and extrinsic aging processes.
- Stimulation of fibroblast activity, including increased migration, contractility, and secretion of collagen and elastin, enhances extracellular matrix remodeling and dermal regeneration.
1.7.5. Synthesis of Hyaluronic Acid
- −
- Cytokine expression, including the inhibition of pro-inflammatory mediators (e.g., IL-1β and TNF-α), is known to downregulate hyaluronan synthase (HAS) expression and accelerate HA degradation.
- −
- The activation of growth factors, especially transforming growth factor-beta (TGF-β), a known stimulator of HAS-2 and HAS-3, is the key isoenzyme responsible for HA synthesis in dermal fibroblasts.
- −
- Reduction in oxidative stress accelerates HA catabolism via ROS-mediated degradation and upregulation of hyaluronidases.
1.7.6. Moisturizing the Skin
1.8. Gut Health and Nutrient Absorption
1.8.1. Unsaturated Fatty Acids and Carotenoid Bioavailability
1.8.2. Oxidized Lipids and Anti-Oxidant Transport
- −
- Glutathione peroxidase 4 (GPX4): A selenoenzyme essential for reducing lipid hydroperoxides to non-toxic lipid alcohols, thereby protecting cellular membranes.
- −
- Ferroptosis suppressor protein 1 (FSP1): Functions independently of GPX4 by recycling coenzyme Q10 into its reduced anti-oxidant form (ubiquinol), which prevents nonenzymatic lipid peroxidation in neutral lipid domains.
- −
- Superoxide dismutase (SOD) and catalase (CAT): Reduce superoxide radicals and hydrogen peroxide, thereby mitigating the upstream drivers of lipid oxidation.
2. Materials and Methods
2.1. Eligibility Criteria
2.1.1. Inclusion Criteria
- −
- The original research, clinical trials, and reviews have focused on the role of carotenoids in skin physiology and aging.
- −
- Articles written in English and available in full-text format.
- −
- Studies that provided mechanistic insights, clinical outcomes, or interventional results related to dietary or topical carotenoid application.
2.1.2. Exclusion Criteria
- −
- Non-English articles;
- −
- Studies unrelated to skin or focusing solely on non-carotenoid agents;
- −
- Abstract-only publications or inaccessible sources.
2.2. Information Sources and Search Strategies
2.3. Selection Process
2.4. Data Collection Process and Data Items
2.5. Study Risk of Bias Assessment
2.6. Effect Measures and Statistical Analysis
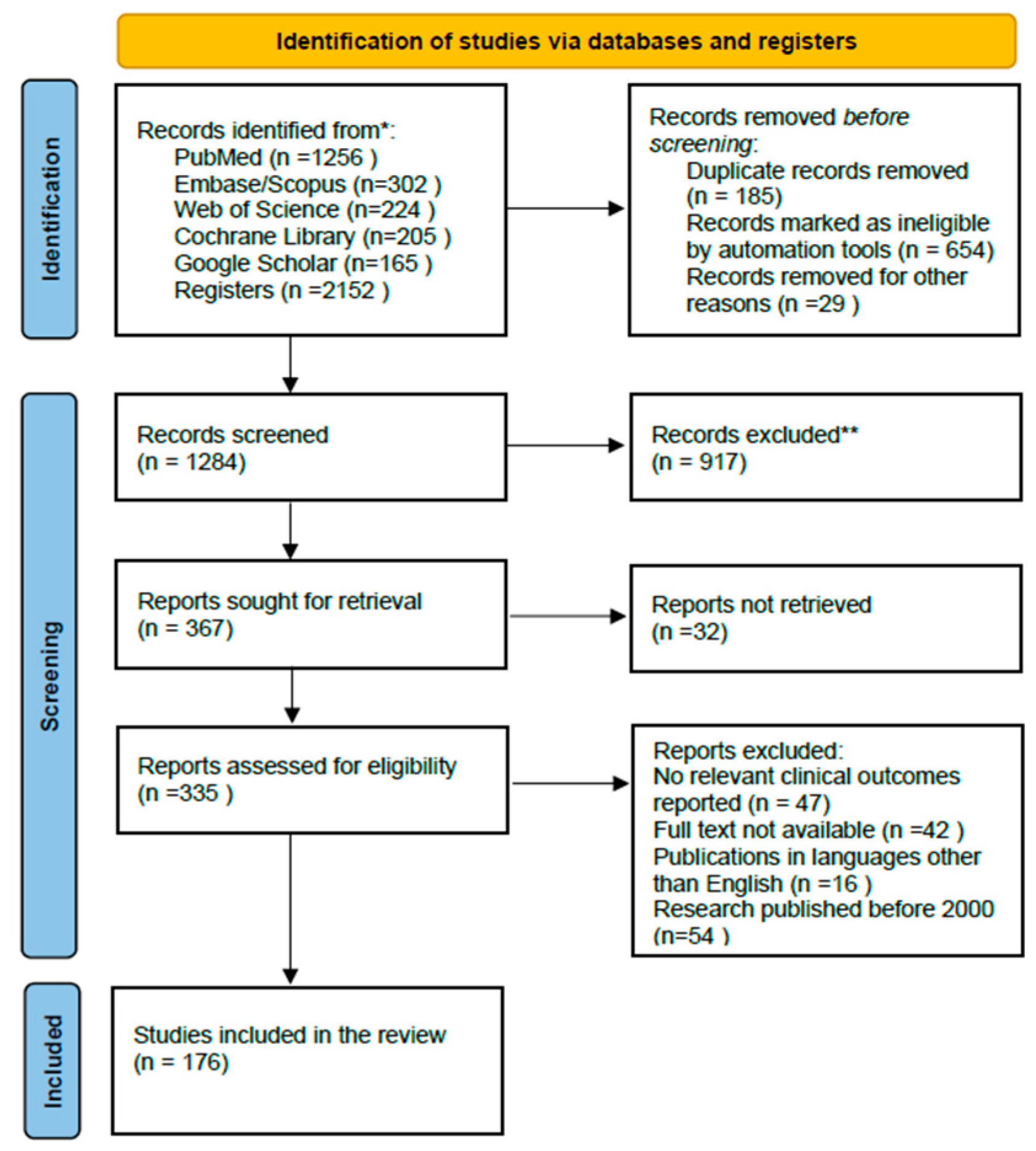
2.7. Study Selection
3. Results and Discussion
- Cardiovascular Health: Lycopene and other carotenoids have been associated with improved endothelial function, reduced oxidative LDL, and a decreased risk of atherosclerosis [162].
- Neurocognitive Support: Some studies suggest a link between high dietary intake of carotenoids and cognitive benefits and reduced risk of neurodegenerative disorders, including Alzheimer’s and Parkinson’s disease, potentially through anti-inflammatory and neuroprotective mechanisms. It is important to note that research in this area is ongoing, and results have yet to conclusively establish causal relationships [60,61].
- Metabolic Regulation: Carotenoids exert significant metabolic effects, including antilipidemic and antiglucemic properties. They enhance insulin sensitivity, reduce adiposity, and ameliorate markers of metabolic syndrome, thus indirectly benefiting skin health by improving systemic homeostasis [62].
- 1.
- Dietary Changes
- 2.
- Combination of Oral and Topical Carotenoids
- 3.
- Avoidance of tobacco and reduced exposure to UV radiation
- 4.
- Optimal Carotenoid Levels
- 5.
- Routine Monitoring and Supplementation
3.1. Controversies Surrounding Carotenoids in Skin Aging
3.2. Strengths and Limitations of the Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stamatas, G.N.; Roux, P.F.; Boireau-Adamezyk, E.; Lboukili, I.; Oddos, T. Skin maturation from birth to 10 years of age: Structure, function, composition, and microbiome. Exp. Dermatol. 2023, 32, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.H. Skin Barrier Function in Neonates and Infants. Allergy Asthma Immunol. Res. 2025, 17, 32–46. [Google Scholar] [CrossRef] [PubMed]
- de Szalay, S.; Wertz, P.W. Protective Barriers Provided by the Epidermis. Int. J. Mol. Sci. 2023, 24, 3145. [Google Scholar] [CrossRef]
- Dingwall, H.L.; Tomizawa, R.R.; Aharoni, A.; Hu, P.; Qiu, Q.; Kokalari, B.; Martinez, S.M.; Donahue, J.C.; Aldea, D.; Mendoza, M.; et al. Sweat gland development requires an eccrine dermal niche and couples two epidermal programs. Dev. Cell 2024, 59, 20–32. [Google Scholar] [CrossRef]
- Stevenson, S.; Thornton, J. Effect of estrogens on skin aging and the potential role of SERMs. Clin. Interv. Aging 2007, 2, 283–297. [Google Scholar] [CrossRef]
- Bravo, B.; Penedo, L.; Carvalho, R.; Dal Vesco, C.; Calomeni, M.; Gapanowicz, D.; Kemen, E.; Paes, R.; Renke, G. Dermatological Changes during Menopause and HRT: What to Expect? Cosmetics 2024, 11, 9. [Google Scholar] [CrossRef]
- Nedelec, B.; Forget, N.J.; Hurtubise, T.; Cimino, S.; de Muszka, F.; Legault, A.; Liu, W.L.; de Oliveira, A.C.M.T.G.; Calva, V.; Correa, J.A.A. Skin characteristics: Normative data for elasticity, erythema, melanin, and thickness at 16 different anatomical locations. Ski. Res. Technol. 2016, 22, 263–275. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; D’Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Quan, T. Oxidative Stress and Human Skin Connective Tissue Aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Wedel, S.; Martic, I.; Navarro, L.G.; Ploner, C.; Pierer, G.; Jansen-Dürr, P.; Cavinato, M. Depletion of growth differentiation factor 15 (GDF15) leads to mitochondrial dysfunction and premature senescence in human dermal fibroblasts. Aging Cell 2023, 22, e13752. [Google Scholar] [CrossRef]
- Li, P.; Lv, H.; Zhang, B.; Duan, R.; Zhang, X.; Lin, P.; Song, C.; Liu, Y. Growth Differentiation Factor 15 Protects SH-SY5Y Cells from Rotenone-Induced Toxicity by Suppressing Mitochondrial Apoptosis. Front. Aging Neurosci. 2022, 14, 869558. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Key players in skin aging? Dermato-Endocrinology 2012, 4, 259–270. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Tu, C.; Chen, X.; He, R. Advanced Glycation End Products in Disease Development and Potential Interventions. Antioxidants 2025, 14, 492. [Google Scholar] [CrossRef]
- Fournet, M.; Bonté, F.; Desmoulière, A. Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging. Aging Dis. 2018, 9, 880–900. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Zhang, J.-Q.; Li, L.; Guo, M.-M.; He, Y.-F.; Dong, Y.-M.; Meng, H.; Yi, F. Advanced Glycation End Products in the Skin: Molecular Mechanisms, Methods of Measurement, and Inhibitory Pathways. Front. Med. 2022, 9, 837222. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Ahmadi, V. Aquaporin Channels in Skin Physiology and Aging Pathophysiology: Investigating Their Role in Skin Function and the Hallmarks of Aging. Biology 2024, 13, 862. [Google Scholar] [CrossRef]
- Hara, M.; Ma, T.; Verkman, A.S. Selectively Reduced Glycerol in Skin of Aquaporin-3-deficient Mice May Account for Impaired Skin Hydration, Elasticity, and Barrier Recovery. J. Biol. Chem. 2002, 277, 46616–46621. [Google Scholar] [CrossRef] [PubMed]
- Tricarico, P.M.; Mentino, D.; De Marco, A.; Del Vecchio, C.; Garra, S.; Cazzato, G.; Foti, C.; Crovella, S.; Calamita, G. Aquaporins Are One of the Critical Factors in the Disruption of the Skin Barrier in Inflammatory Skin Diseases. Int. J. Mol. Sci. 2022, 23, 4020. [Google Scholar] [CrossRef]
- Vrettou, C.S.; Issaris, V.; Kokkoris, S.; Poupouzas, G.; Keskinidou, C.; Lotsios, N.S.; Kotanidou, A.; Orfanos, S.E.; Dimopoulou, I.; Vassiliou, A.G. Exploring Aquaporins in Human Studies: Mechanisms and Therapeutic Potential in Critical Illness. Life 2024, 14, 1688. [Google Scholar] [CrossRef]
- Meli, R.; Pirozzi, C.; Pelagalli, A. New Perspectives on the Potential Role of Aquaporins (AQPs) in the Physiology of Inflammation. Front. Physiol. 2018, 9, 101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.-L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Siquier-Dameto, G.; Boadas-Vaello, P.; Verdú, E. Intradermal Treatment with a Hyaluronic Acid Complex Supplemented with Amino Acids and Antioxidant Vitamins Improves Cutaneous Hydration and Viscoelasticity in Healthy Subjects. Antioxidants 2024, 13, 770. [Google Scholar] [CrossRef]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A Cutaneous Perspective on its Production, Metabolism, and Functions. J. Investig. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Kleszczynski, K.; Fischer, T.W. Melatonin and human skin aging. Dermato-Endocrinology 2012, 4, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.-K.; Böhm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczyński, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef]
- Rusanova, I.; Martínez-Ruiz, L.; Florido, J.; Rodríguez-Santana, C.; Guerra-Librero, A.; Acuña-Castroviejo, D.; Escames, G. Protective Effects of Melatonin on the Skin: Future Perspectives. Int. J. Mol. Sci. 2019, 20, 4948. [Google Scholar] [CrossRef]
- Farris, P.K.; Valacchi, G. Ultraviolet Light Protection: Is It Really Enough? Antioxidants 2022, 11, 1484. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davó, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Environmental Air Pollutants Affecting Skin Functions with Systemic Implications. Int. J. Mol. Sci. 2023, 24, 10502. [Google Scholar] [CrossRef]
- Drakaki, E.; Dessinioti, C.; Antoniou, C.V. Air pollution and the skin. Front. Environ. Sci. 2014, 2, 11. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, Y.; Xiao, X.; Cui, X.; Gu, X. Omics-Based Interaction Analysis Reveals Interplay of Chemical Pollutant (Ozone) and Photoradiation (UVSSR) Stressors in Skin Damage. Biology 2025, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, C.; Su, W.; Sun, Z.; Gao, S.; Xie, W.; Zhang, B.; Sui, L. Carotenoids in Skin Photoaging: Unveiling Protective Effects, Molecular Insights, and Safety and Bioavailability Frontiers. Antioxidants 2025, 14, 577. [Google Scholar] [CrossRef] [PubMed]
- Parrado, C.; Philips, N.; Gilaberte, Y.; Juarranz, A.; González, S. Oral Photoprotection: Effective Agents and Potential Candidates. Front. Med. 2018, 5, 188. [Google Scholar] [CrossRef]
- Flieger, J.; Raszewska-Famielec, M.; Radzikowska-Büchner, E.; Flieger, W. Skin Protection by Carotenoid Pigments. Int. J. Mol. Sci. 2024, 25, 1431. [Google Scholar] [CrossRef]
- Sullivan, M.; Gonzalez Obezo, C.; Lipsky, Z.; Panchal, A.; Jensen, J. Frontiers in Topical Photoprotection. Cosmetics 2025, 12, 96. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Miazek, K.; Beton, K.; Śliwińska, A.; Brożek-Płuska, B. The Effect of β-Carotene, Tocopherols and Ascorbic Acid as Anti-Oxidant Molecules on Human and Animal In Vitro/In Vivo Studies: A Review of Research Design and Analytical Techniques Used. Biomolecules 2022, 12, 1087. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Stromsnes, K.; Correas, A.G.; Lehmann, J.; Gambini, J.; Olaso-Gonzalez, G. Anti-Inflammatory Properties of Diet: Role in Healthy Aging. Biomedicines 2021, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Majdan, M.; Bobrowska-Korczak, B. Active Compounds in Fruits and Inflammation in the Body. Nutrients 2022, 14, 2496. [Google Scholar] [CrossRef]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging—From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R. Carotenoids and genomic stability. Mutat. Res. 2001, 475, 21–28. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Karwowski, B.T. Nutrition Can Help DNA Repair in the Case of Aging. Nutrients 2020, 12, 3364. [Google Scholar] [CrossRef]
- Brand, R.M.; Wipf, P.; Durham, A.; Epperly, M.W.; Greenberger, J.S.; Falo, L.D., Jr. Targeting Mitochondrial Oxidative Stress to Mitigate UV-Induced Skin Damage. Front. Pharmacol. 2018, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sampath, H. Mitochondrial DNA Integrity: Role in Health and Disease. Cells 2019, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Actin cytoskeleton assembly regulates collagen production via TGF-β type II receptor in human skin fibroblasts. J. Cell. Mol. Med. 2018, 22, 4085–4096. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef]
- Wang, K.; Wen, D.; Xu, X.; Zhao, R.; Jiang, F.; Yuan, S.; Zhang, Y.; Gao, Y.; Li, Q. Extracellular matrix stiffness-The central cue for skin fibrosis. Front. Mol. Biosci. 2023, 10, 1132353. [Google Scholar] [CrossRef]
- Yue, Z.; Shao, K. Visualization of the Relationship Between Hyaluronic Acid and Wound Healing: A Bibliometric Analysis. Ski. Res. Technol. 2025, 31, e70164. [Google Scholar] [CrossRef]
- Chylińska, N.; Maciejczyk, M. Hyaluronic Acid and Skin: Its Role in Aging and Wound-Healing Processes. Gels 2025, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Rasmus, P.; Kozłowska, E. Antioxidant and Anti-Inflammatory Effects of Carotenoids in Mood Disorders: An Overview. Antioxidants 2023, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Forma, A.; Flieger, W.; Flieger, M.; Gawlik, P.J.; Dzierżyński, E.; Maciejewski, R.; Teresiński, G.; Baj, J. Carotenoid Supplementation for Alleviating the Symptoms of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 8982. [Google Scholar] [CrossRef]
- Kalogerakou, T.; Antoniadou, M. The Role of Dietary Antioxidants, Food Supplements and Functional Foods for Energy Enhancement in Healthcare Professionals. Antioxidants 2024, 13, 1508. [Google Scholar] [CrossRef]
- Liu, F.; Bai, Q.; Tang, W.; Zhang, S.; Guo, Y.; Pan, S.; Ma, X.; Yang, Y.; Fan, H. Antioxidants in neuropsychiatric disorder prevention: Neuroprotection, synaptic regulation, microglia modulation, and neurotrophic effects. Front. Neurosci. 2024, 18, 1505153. [Google Scholar] [CrossRef] [PubMed]
- Tanprasertsuk, J.; Scott, T.M.; Barbey, A.K.; Barger, K.; Wang, X.-D.; Johnson, M.A.; Poon, L.W.; Vishwanathan, R.; Matthan, N.R.; Lichtenstein, A.H.; et al. Carotenoid-Rich Brain Nutrient Pattern Is Positively Correlated with Higher Cognition and Lower Depression in the Oldest Old with No Dementia. Front. Nutr. 2021, 8, 704691. [Google Scholar] [CrossRef] [PubMed]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Heidari, M.; Maleki Vareki, S.; Yaghobi, R.; Karimi, M.H. Microbiota activation and regulation of adaptive immunity. Front. Immunol. 2024, 15, 1429436. [Google Scholar] [CrossRef] [PubMed]
- Amimo, J.O.; Michael, H.; Chepngeno, J.; Raev, S.A.; Saif, L.J.; Vlasova, A.N. Immune Impairment Associated with Vitamin A Deficiency: Insights from Clinical Studies and Animal Model Research. Nutrients 2022, 14, 5038. [Google Scholar] [CrossRef]
- Bhutta, N.K.; Xu, X.; Jian, C.; Wang, Y.; Liu, Y.; Sun, J.; Han, B.; Wu, S.; Javeed, A. Gut microbiota mediated T cells regulation and autoimmune diseases. Front. Microbiol. 2024, 15, 1477187. [Google Scholar] [CrossRef]
- Shanaida, M.; Mykhailenko, O.; Lysiuk, R.; Hudz, N.; Balwierz, R.; Shulhai, A.; Shapovalova, N.; Shanaida, V.; Bjørklund, G. Carotenoids for Antiaging: Nutraceutical, Pharmaceutical, and Cosmeceutical Applications. Pharmaceuticals 2025, 18, 403. [Google Scholar] [CrossRef]
- Molteni, C.; La Motta, C.; Valoppi, F. Improving the Bioaccessibility and Bioavailability of Carotenoids by Means of Nanostructured Delivery Systems: A Comprehensive Review. Antioxidants 2022, 11, 1931. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Antioxidant Metabolism Pathways in Vitamins, Polyphenols, and Selenium: Parallels and Divergences. Int. J. Mol. Sci. 2024, 25, 2600. [Google Scholar] [CrossRef]
- Pradel, P.; Calisto, N.; Navarro, L.; Barriga, A.; Vera, N.; Aranda, C.; Simpfendorfer, R.; Valdés, N.; Corsini, G.; Tello, M.; et al. Carotenoid Cocktail Produced by An Antarctic Soil Flavobacterium with Biotechnological Potential. Microorganisms 2021, 9, 2419. [Google Scholar] [CrossRef]
- Conboy Stephenson, R.; Ross, R.P.; Stanton, C. Carotenoids in Milk and the Potential for Dairy Based Functional Foods. Foods 2021, 10, 1263. [Google Scholar] [CrossRef]
- Iddir, M.; Porras Yaruro, J.F.; Cocco, E.; Hardy, E.M.; Appenzeller, B.M.R.; Guignard, C.; Larondelle, Y.; Bohn, T. Impact of Protein-Enriched Plant Food Items on the Bioaccessibility and Cellular Uptake of Carotenoids. Antioxidants 2021, 10, 1005. [Google Scholar] [CrossRef]
- Etcheverry, P.; Grusak, M.A.; Fleige, L.E. Application of in vitro bioaccessibility and bioavailability methods for calcium, carotenoids, folate, iron, magnesium, polyphenols, zinc, and vitamins B6, B12, D, and E. Front. Physiol. 2012, 3, 317. [Google Scholar] [CrossRef]
- Casagrande, S.; Dell’omo, G.; Costantini, D.; Tagliavini, J.; Groothuis, T. Variation of a carotenoid-based trait in relation to oxidative stress and endocrine status during the breeding season in the Eurasian kestrel: A multi-factorial study. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 160, 16–26. [Google Scholar] [CrossRef]
- Ferrando, B.O.; Baenas, N.; Periago, M.J. Changes in Carotenoids and Quality Parameters of Sweet Paprika (Capsicum annuum) After an Accelerated Heat Treatment. Antioxidants 2024, 13, 1492. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cao, X.; Azam, M.; Wang, C.; Liu, C.; Qiao, Y.; Zhang, B. Metabolism of Carotenoids and β-Ionone Are Mediated by Carotenogenic Genes and PpCCD4 Under Ultraviolet B Irradiation and During Fruit Ripening. Front. Plant Sci. 2022, 13, 814677. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Fu, W.; Du, M.; Chen, Z.-X.; Lei, A.-P.; Wang, J.-X. Carotenoids Biosynthesis, Accumulation, and Applications of a Model Microalga Euglenagracilis. Mar. Drugs 2022, 20, 496. [Google Scholar] [CrossRef]
- Adamantidi, T.; Lafara, M.-P.; Venetikidou, M.; Likartsi, E.; Toganidou, I.; Tsoupras, A. Utilization and Bio-Efficacy of Carotenoids, Vitamin A and Its Vitaminoids in Nutricosmetics, Cosmeceuticals, and Cosmetics’ Applications with Skin-Health Promoting Properties. Appl. Sci. 2025, 15, 1657. [Google Scholar] [CrossRef]
- de Souza Guedes, L.; Martinez, R.M.; Bou-Chacra, N.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. An Overview on Topical Administration of Carotenoids and Coenzyme Q10 Loaded in Lipid Nanoparticles. Antioxidants 2021, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Pincemail, J.; Meziane, S. On the Potential Role of the Antioxidant Couple Vitamin E/Selenium Taken by the Oral Route in Skin and Hair Health. Antioxidants 2022, 11, 2270. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Cantrell, A.; McGarvey, D.J.; Truscott, T.G.; Rancan, F.; Böhm, F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch. Biochem. Biophys. 2003, 412, 47–54. [Google Scholar] [CrossRef]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Boehm, F.; Edge, R.; Truscott, T.G. Photochemical and Photophysical Properties of Carotenoids and Reactive Oxygen Species: Contradictions Relating to Skin and Vision. Oxygen 2023, 3, 322–335. [Google Scholar] [CrossRef]
- Chisté, R.C.; Freitas, M.; Mercadante, A.Z.; Fernandes, E. Carotenoids inhibit lipid peroxidation and hemoglobin oxidation, but not the depletion of glutathione induced by ROS in human erythrocytes. Life Sci. 2014, 99, 52–60. [Google Scholar] [CrossRef]
- Jafari, Z.; Bigham, A.; Sadeghi, S.; Dehdashti, S.M.; Rabiee, N.; Abedivash, A.; Bagherzadeh, M.; Nasseri, B.; Karimi-Maleh, H.; Sharifi, E.; et al. Nanotechnology-Abetted Astaxanthin Formulations in Multimodel Therapeutic and Biomedical Applications. J. Med. Chem. 2022, 65, 2–36. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Razis, A.F.A.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Genç, Y.; Bardakci, H.; Yücel, Ç.; Karatoprak, G.Ş.; Küpeli Akkol, E.; Hakan Barak, T.; Sobarzo-Sánchez, E. Oxidative Stress and Marine Carotenoids: Application by Using Nanoformulations. Mar. Drugs 2020, 18, 423. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppine, A. Photoaging: UV radiation-induced inflammation and immunosuppression accelerate the aging process in the skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Catanzaro, E.; Bishayee, A.; Fimognari, C. On a Beam of Light: Photoprotective Activities of the Marine Carotenoids Astaxanthin and Fucoxanthin in Suppression of Inflammation and Cancer. Mar. Drugs 2020, 18, 544. [Google Scholar] [CrossRef]
- Balić, A.; Mokos, M. Do We Utilize Our Knowledge of the Skin Protective Effects of Carotenoids Enough? Antioxidants 2019, 8, 259. [Google Scholar] [CrossRef]
- Anbualakan, K.; Tajul Urus, N.Q.; Makpol, S.; Jamil, A.; Mohd Ramli, E.S.; Md Pauzi, S.H.; Muhammad, N. A Scoping Review on the Effects of Carotenoids and Flavonoids on Skin Damage Due to Ultraviolet Radiation. Nutrients 2023, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Lademann, J.; von Hagen, J.; Lohan, S.B.; Kolmar, H.; Meinke, M.C.; Jung, S. Carotenoids in Human Skin In Vivo: Antioxidant and Photo-Protectant Role against External and Internal Stressors. Antioxidants 2022, 11, 1451. [Google Scholar] [CrossRef]
- Bono, M.R.; Tejon, G.; Flores-Santibañez, F.; Fernandez, D.; Rosemblatt, M.; Sauma, D. Retinoic Acid as a Modulator of T Cell Immunity. Nutrients 2016, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.M.; Teixeira, F.M.E.; Sato, M.N. Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases. Mediat. Inflamm. 2018, 2018, 3067126. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Brancewicz, J.; Wójcik, N.; Sarnowska, Z.; Robak, J.; Król, M. The Multifaceted Role of Macrophages in Biology and Diseases. Int. J. Mol. Sci. 2025, 26, 2107. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage Polarization and Its Role in Liver Disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef]
- Lis-López, L.; Bauset, C.; Seco-Cervera, M.; Cosín-Roger, J. Is the Macrophage Phenotype Determinant for Fibrosis Development? Biomedicines 2021, 9, 1747. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.X.; Xiong, F. Astaxanthin and its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef] [PubMed]
- Pružinská, K.; Chrastina, M.; Khademnematolahi, S.; Vyletelová, V.; Gajdošová, L.; Pastvová, L.; Dráfi, F.; Poništ, S.; Pašková, Ľ.; Kucharská, J.; et al. Astaxanthin, Compared to Other Carotenoids, Increases the Efficacy of Methotrexate in Rat Adjuvant Arthritis. Int. J. Mol. Sci. 2024, 25, 8710. [Google Scholar] [CrossRef]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Morales, A.; Medina-García, M.; Martínez-Peinado, P.; Pascual-García, S.; Pujalte-Satorre, C.; López-Jaén, A.B.; Martínez-Espinosa, R.M.; Sempere-Ortells, J.M. The Antitumour Mechanisms of Carotenoids: A Comprehensive Review. Antioxidants 2024, 13, 1060. [Google Scholar] [CrossRef]
- Joshi, M.; Hiremath, P.; John, J.; Ranadive, N.; Nandakumar, K.; Mudgal, J. Modulatory role of vitamins A, B3, C, D, and E on skin health, immunity, microbiome, and diseases. Pharmacol. Rep. 2023, 75, 1096–1114. [Google Scholar] [CrossRef]
- Camera, E.; Mastrofrancesco, A.; Fabbri, C.; Daubrawa, F.; Picardo, M.; Sies, H.; Stahl, W. Astaxanthin, canthaxanthin and beta-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Exp. Dermatol. 2009, 18, 222–231. [Google Scholar] [CrossRef]
- Park, S. Biochemical, structural and physical changes in aging human skin, and their relationship. Biogerontology 2022, 23, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Jadach, B.; Mielcarek, Z.; Osmałek, T. Use of Collagen in Cosmetic Products. Curr. Issues Mol. Biol. 2024, 46, 2043–2070. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Boraldi, F.; Lofaro, F.D.; Bonacorsi, S.; Mazzilli, A.; Garcia-Fernandez, M.; Quaglino, D. The Role of Fibroblasts in Skin Homeostasis and Repair. Biomedicines 2024, 12, 1586. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic Acid: A Powerful Biomolecule with Wide-Ranging Applications—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 10296. [Google Scholar] [CrossRef] [PubMed]
- Lierova, A.; Kasparova, J.; Filipova, A.; Cizkova, J.; Pekarova, L.; Korecka, L.; Mannova, N.; Bilkova, Z.; Sinkorova, Z. Hyaluronic Acid: Known for Almost a Century, but Still in Vogue. Pharmaceutics 2022, 14, 838. [Google Scholar] [CrossRef]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Zhang, H.; Ding, Z.; Han, J. The Application of Natural Carotenoids in Multiple Fields and Their Encapsulation Technology: A Review. Molecules 2024, 29, 967. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Muguet, V.; Christen-Zaech, S. Restoring Skin Hydration and Barrier Function: Mechanistic Insights Into Basic Emollients for Xerosis Cutis. Int. J. Dermatol. 2025, 64, 5–12. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Alexis, A.F.; Andriessen, A.; Barrios, O.L.F.; Bjerring, P.; Foley, P.; Gold, M.H.; Kaderbhai, H.; Zhang, C. A global perspective on the treatment and maintenance of mature skin using gentle cleansers and moisturizers. Int. J. Dermatol. 2024, 63, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Załęcki, P.; Rogowska, K.; Wąs, P.; Łuczak, K.; Wysocka, M.; Nowicka, D. Impact of Lifestyle on Differences in Skin Hydration of Selected Body Areas in Young Women. Cosmetics 2024, 11, 13. [Google Scholar] [CrossRef]
- Bakac, E.R.; Percin, E.; Gunes-Bayir, A.; Dadak, A. A Narrative Review: The Effect and Importance of Carotenoids on Aging and Aging-Related Diseases. Int. J. Mol. Sci. 2023, 24, 15199. [Google Scholar] [CrossRef]
- Bollag, W.B.; Aitkens, L.; White, J.; Hyndman, K.A. Aquaporin-3 in the epidermis: More than skin deep. Am. J. Physiol.-Cell Physiol. 2020, 318, C1144–C1153. [Google Scholar] [CrossRef]
- Qin, H.; Zheng, X.; Zhong, X.; Shetty, A.K.; Elias, P.M.; Bollag, W.B. Aquaporin-3 in keratinocytes and skin: Its role and interaction with phospholipase D2. Arch Biochem. Biophys. 2011, 508, 138–143. [Google Scholar] [CrossRef]
- Ren, Q.; Qu, L.; Yuan, Y.; Wang, F. Natural Modulators of Key Signaling Pathways in Skin Inflammageing. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2967–2988. [Google Scholar] [CrossRef]
- Donato, A.; Belluzzi, E.; Mattiuzzo, E.; Venerando, R.; Cadamuro, M.; Ruggieri, P.; Vindigni, V.; Brun, P. Anti-Inflammatory and Pro-Regenerative Effects of Hyaluronan-Chitlac Mixture in Human Dermal Fibroblasts: A Skin Ageing Perspective. Polymers 2022, 14, 1817. [Google Scholar] [CrossRef]
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the Skin Barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef]
- Singampalli, K.L.; Balaji, S.; Wang, X.; Parikh, U.M.; Kaul, A.; Gilley, J.; Birla, R.K.; Bollyky, P.L.; Keswani, S.G. The Role of an IL-10/Hyaluronan Axis in Dermal Wound Healing. Front. Cell Dev. Biol. 2020, 8, 636. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef]
- Gibbs, S.; Silva Pinto, A.N.; Murli, S.; Huber, M.; Hohl, D.; Ponec, M. Epidermal growth factor and keratinocyte growth factor differentially regulate epidermal migration, growth, and differentiation. Wound Repair Regen. 2000, 8, 192–203. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.P.; Singh, V.K.; Singh, P.R.; Jaiswal, J.; Kumari, N.; Upadhye, V.; Singh, S.C.; Sinha, R.P. Natural Sun-Screening Compounds and DNA-Repair Enzymes: Photoprotection and Photoaging. Catalysts 2023, 13, 745. [Google Scholar] [CrossRef]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Sanchez, M.; Celiberto, L.S.; Yang, H.; Sham, H.P.; Vallance, B.A. The gut-skin axis: A bi-directional, microbiota-driven relationship with therapeutic potential. Gut Microbes 2025, 17, 2473524. [Google Scholar] [CrossRef]
- Ma, Z.F.; Lee, Y.Y. The Role of the Gut Microbiota in Health, Diet, and Disease with a Focus on Obesity. Foods 2025, 14, 492. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef]
- Jiang, Z.; Mei, L.; Li, Y.; Guo, Y.; Yang, B.; Huang, Z.; Li, Y. Enzymatic Regulation of the Gut Microbiota: Mechanisms and Implications for Host Health. Biomolecules 2024, 14, 1638. [Google Scholar] [CrossRef]
- Boev, M.; Stănescu, C.; Turturică, M.; Cotârleţ, M.; Batîr-Marin, D.; Maftei, N.; Chiţescu, C.; Grigore-Gurgu, L.; Barbu, V.; Enachi, E.; et al. Bioactive Potential of Carrot-Based Products Enriched with Lactobacillus plantarum. Molecules 2024, 29, 917. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef]
- Widjaja-Adhi, M.A.K.; Golczak, M. The molecular aspects of absorption and metabolism of carotenoids and retinoids in vertebrates. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158571. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Absorption of vitamin A and carotenoids by the enterocyte: Focus on transport proteins. Nutrients 2013, 5, 3563–3581. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, A.; Al’Abri, I.S.; Kopec, R.E.; Crook, N.; Bohn, T. Carotenoids and Their Health Benefits as Derived via Their Interactions with Gut Microbiota. Adv. Nutr. 2023, 14, 238–255. [Google Scholar] [CrossRef]
- Randeni, N.; Bordiga, M.; Xu, B. A Comprehensive Review of the Triangular Relationship among Diet–Gut Microbiota–Inflammation. Int. J. Mol. Sci. 2024, 25, 9366. [Google Scholar] [CrossRef]
- Munteanu, C.; Schwartz, B. Interactions between Dietary Antioxidants, Dietary Fiber and the Gut Microbiome: Their Putative Role in Inflammation and Cancer. Int. J. Mol. Sci. 2024, 25, 8250. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, H.K.; Pereira, M.; Bosco, J.; George, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K.; Rajasekaran, J.J. Gut commensals and their metabolites in health and disease. Front. Microbiol. 2023, 14, 1244293. [Google Scholar] [CrossRef]
- Abrante-Pascual, S.; Nieva-Echevarría, B.; Goicoechea-Oses, E. Vegetable Oils and Their Use for Frying: A Review of Their Compositional Differences and Degradation. Foods 2024, 13, 4186. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Bogensberger, B.; Benčič, A.; Knüppel, S.; Boeing, H.; Hoffmann, G. Effects of oils and solid fats on blood lipids: A systematic review and network meta-analysis. J. Lipid Res. 2018, 59, 1771–1782. [Google Scholar] [CrossRef]
- Verge-Mèrida, G.; Barroeta, A.C.; Ferrer, C.; Serrano, T.; Guardiola, F.; Soler, M.D.; Sala, R. Olive Pomace and Soybean-Sunflower Acid Oils as Alternative Fat Sources in European Seabass (Dicentrarchus labrax) Diets: Effects on Performance, Digestibility and Flesh Fatty Acid Composition and Quality Parameters. Animals 2022, 12, 1198. [Google Scholar] [CrossRef]
- Rosqvist, F.; Niinistö, S. Fats and oils—A scoping review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2024, 68. [Google Scholar] [CrossRef] [PubMed]
- Spiteller, G. The relation of lipid peroxidation processes with atherogenesis: A new theory on atherogenesis. Mol. Nutr. Food Res. 2005, 49, 999–1013. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Li, X.M.; Gugiu, B.G.; Gu, X.; Qin, J.; Salomon, R.G.; Hazen, S.L. The lipid whisker model of the structure of oxidized cell membranes. J. Biol. Chem. 2008, 283, 2385–2396. [Google Scholar] [CrossRef]
- Duché, G.; Sanderson, J.M. The Chemical Reactivity of Membrane Lipids. Chem. Rev. 2024, 124, 3284–3330. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Valgimigli, L. Lipid Peroxidation and Antioxidant Protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- Kilicarslan You, D.; Fuwad, A.; Lee, K.H.; Kim, H.K.; Kang, L.; Kim, S.M.; Jeon, T.-J. Evaluation of the Protective Role of Vitamin E against ROS-Driven Lipid Oxidation in Model Cell Membranes. Antioxidants 2024, 13, 1135. [Google Scholar] [CrossRef]
- Salehi, B.; Azzini, E.; Zucca, P.; Maria Varoni, E.; Anil Kumar, N.V.; Dini, L.; Panzarini, E.; Rajkovic, J.; Valere Tsouh Fokou, P.; Peluso, I.; et al. Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef]
- Athanasopoulou, S.; Spanidi, E.; Panagiotidou, E.; Cavagnino, A.; Bobier, A.; Gardikis, K. An Advanced Combinatorial System from Vitis vinifera Leaves and Propolis Enhances Antioxidants’ Skin Delivery and Fibroblasts Functionality. Pharmaceuticals 2024, 17, 1610. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Riccioni, G.; D'Orazio, N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Mar. Drugs. 2015, 13, 6226–6246. [Google Scholar] [CrossRef] [PubMed]
- Sumalla-Cano, S.; Eguren-García, I.; Lasarte-García, Á.; Prola, T.A.; Martínez-Díaz, R.; Elío, I. Carotenoids Intake and Cardiovascular Prevention: A Systematic Review. Nutrients 2024, 16, 3859. [Google Scholar] [CrossRef]
- Tamas, C.; Hreniuc, I.M.J.; Tecuceanu, A.; Ciuntu, B.M.; Ibanescu, C.L.; Tamas, I.; Ianole, V.; Stanescu, C.; Pintilie, C.T.; Zamfir, C.L.; et al. Non-Melanoma Facial Skin Tumors—The Correspondence between Clinical and Histological Diagnosis. Appl. Sci. 2021, 11, 7543. [Google Scholar] [CrossRef]
- Anghel, L.; Boev, M.; Stanescu, C.; Mitincu Caramfil, S.; Luca, L.; Muşat, C.L.; Ciubara, A. Depression in the diabetic patient. BRAIN Broad Res. Artif. Intell. Neurosci. 2023, 14, 658–672. [Google Scholar] [CrossRef]
- Batir-Marin, D.; Ștefan, C.S.; Boev, M.; Gurău, G.; Popa, G.V.; Matei, M.N.; Ursu, M.; Nechita, A.; Maftei, N.-M. A Multidisciplinary Approach of Type 1 Diabetes: The Intersection of Technology, Immunotherapy, and Personalized Medicine. J. Clin. Med. 2025, 14, 2144. [Google Scholar] [CrossRef]
- Ohrnberger, J.; Sutton, M.; Fichera, E. The dynamics of physical and mental health in the older population. J. Econ. Ageing 2016, 9, 52–62. [Google Scholar] [CrossRef]
- Hernandez, R.; Boughton, S.W.; Schuette, S.A.; Moskowitz, J.T.; Bassett, S.M.; Shiu, E.W. Psychological Well-being and Physical Health: Associations, Mechanisms, and Future Directions. Emot. Rev. 2017, 10, 18–29. [Google Scholar] [CrossRef]
- Stanescu, C.; Anghel, L.; Tamas, C.; Ciubara, A. Education of Patients and Their Families to Manage Emotional Impact of Skin Scars. BRAIN Broad Res. Artif. Intell. Neurosci. 2025, 16, 324–341. [Google Scholar] [CrossRef]
- Lem, K.; McGilton, K.S.; Aelick, K.; Iaboni, A.; Babineau, J.; Colborne, D.H.; Edwards, C.; Bretzlaff, M.; Lender, D.; Gibson, J.-L.; et al. Social connection and physical health outcomes among long-term care home residents: A scoping review. BMC Geriatr. 2021, 21, 722. [Google Scholar] [CrossRef] [PubMed]
- Stanescu, C.; Boev, M.; Avram, O.A.; Ciubara, A. Investigating the Connection Between Skin Cancers and Mental Disorders: A Thorough Analysis. BRAIN Broad Res. Artif. Intell. Neurosci. 2024, 15, 118–131. [Google Scholar] [CrossRef]
- Anghel, L.; Ciubară, A.; Nechita, A.; Nechita, L.; Manole, C.; Baroiu, L.; Ciubară, A.B.; Mușat, C.L. Sleep Disorders Associated with Neurodegenerative Diseases. Diagnostics 2023, 13, 2898. [Google Scholar] [CrossRef] [PubMed]
- Knaggs, H.; Lephart, E.D. Enhancing Skin Anti-Aging through Healthy Lifestyle Factors. Cosmetics 2023, 10, 142. [Google Scholar] [CrossRef]
- Li, X.; Wang, S. Dose-response relationship between carotenoid intake and risk of depressive symptoms in postmenopausal women. Front. Psychiatry 2025, 16, 1525631. [Google Scholar] [CrossRef]
- Shin, J.; Song, M.-H.; Oh, J.-W.; Keum, Y.-S.; Saini, R.K. Pro-oxidant Actions of Carotenoids in Triggering Apoptosis of Cancer Cells: A Review of Emerging Evidence. Antioxidants 2020, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Nishino, H.; Masuda, M.; Takayasu, J.; Murakoshi, M.; Yano, M.; Tsuruta, J.; Tsuruta, J.; Okuda, M.; Khachik, F. Cancer prevention by natural carotenoids. BioFactors. 2000, 13, 89–94. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanescu, C.; Chiscop, I.; Mihalache, D.; Popa, F.; Tamas, C.; Stoleriu, G. Skin Aging and Carotenoids: A Systematic Review of Their Multifaceted Protective Mechanisms. Nutrients 2025, 17, 2596. https://doi.org/10.3390/nu17162596
Stanescu C, Chiscop I, Mihalache D, Popa F, Tamas C, Stoleriu G. Skin Aging and Carotenoids: A Systematic Review of Their Multifaceted Protective Mechanisms. Nutrients. 2025; 17(16):2596. https://doi.org/10.3390/nu17162596
Chicago/Turabian StyleStanescu, Cristina, Iulia Chiscop, Daniela Mihalache, Florina Popa, Camelia Tamas, and Gabriela Stoleriu. 2025. "Skin Aging and Carotenoids: A Systematic Review of Their Multifaceted Protective Mechanisms" Nutrients 17, no. 16: 2596. https://doi.org/10.3390/nu17162596
APA StyleStanescu, C., Chiscop, I., Mihalache, D., Popa, F., Tamas, C., & Stoleriu, G. (2025). Skin Aging and Carotenoids: A Systematic Review of Their Multifaceted Protective Mechanisms. Nutrients, 17(16), 2596. https://doi.org/10.3390/nu17162596





