1. Introduction
Oxaliplatin-induced peripheral neurotoxicity (OIPN) is a severe limiting side effect in the management of patients undergoing chemotherapy who are using this anticancer drug [
1]. Although oxaliplatin-induced peripheral neuropathy is not a life-threatening condition, it has a substantial impact on patients’ quality of life. It is often considered highly debilitating, as it can not only lead to dose reductions or discontinuation of chemotherapy, but also represent a major source of chronic pain in cancer survivors [
2]. The nervous damage induces neuropathic hypersensitivity characterized by hyperalgesia, paresthesia, allodynia, tingling, and shooting pain [
3].
Despite the abundance of available drugs [
4], OIPN remains inadequately alleviated by conventional analgesics [
5], given that its molecular mechanisms and pathogenesis are not yet fully understood. Consequently, research is progressively directing its attention towards innovative therapeutic alternatives, including the exploration of bioactive compounds found in medicinal plants [
6]. The terrestrial environment serves as a huge source, housing approximately 300,000 plant species, with only 15% of them having undergone a study of their biological effects. Notably, among various habitats, the Amazon rainforest stands out as one of the most prolific natural product reservoirs [
7].
In this regard,
Acmella oleracea (L.) R.K. Jansen (Asteraceae), commonly known as jambù, is a medicinal plant native to Brazil. It is also known as the toothache plant due to its traditional use in the treatment of oral pain. The phytochemical composition of the plant has been extensively investigated by several authors. The essential oil from the inflorescences of
Acmella oleracea, resulting in a rich content of monoterpene and sesquiterpene hydrocarbons, has shown antimicrobial and insecticidal activities [
8]. To date, the plant is best known for the molecule spilanthol, an alkylamide formed by a condensation reaction between a fatty acid and a decarboxylated amino acid, which is mainly responsible for the biological effects attributed to
Acmella oleracea. Spilanthol is found in the aerial parts of the plant, especially in the flowers, and its concentration in the different extracts depends on the type of cultivation and the extraction method [
9]. The plant also contains flavonoids and other phenolic compound previously identified in aerial parts of plants grown by conventional and hydroponic methods [
10] and in
in vitro seedlings [
11]. The roots contain a lower amount of alkylamides and approximately twice the total phenols in respect to the aerial parts [
11]. Minor secondary metabolites such as triterpenoids and phytosterols have also been reported [
12]. Phenolic compounds have been associated with the antioxidant properties of the plant [
13], but their possible role in enhancing or not enhancing the effects of spilanthol has not been well studied so far. Thus, the aim of the study was to evaluate the in vitro and in vivo effect of two
Acmella oleracea extracts, obtained from the aerial parts and roots of in vitro seedlings, mitigating the neurotoxic effects of oxaliplatin.
The extracts were chemically characterized to determine the different concentration of total phenols, total alkylamides, and spilanthol. SH-SY5Y, a human neuroblastoma cell line differentiated in neurons [
14,
15], was used for an initial comprehensive assessment of cellular and molecular responses to the neurotoxicity evoked by oxaliplatin. Subsequently, further assessments were performed in vivo on mice developing painful chemotherapy-induced neuropathy [
16].
2. Materials and Methods
2.1. Samples of Acmella Oleracea
Aerial parts and roots of
Acmella oleracea were obtained from in vitro seedlings. Five different organogenesis-derived regenerating lines were produced according to Maggini and colleagues [
17]. Extractions were performed on representative samples of the two tissues obtained by combining the five regenerating lines which showed similar content of secondary metabolites [
11]. Hydroalcoholic extracts were prepared following the procedure reported in [
11]. Briefly, a hydroalcoholic extraction (80%
v/
v) with ultrasounds (10 min, 60 °C) was carried out on dried leaves and roots (1:20
w/
v) in coarse powder, followed by a liquid/liquid extraction 1:2
v/v with hexane to remove some of the lipophilic molecules and chlorophylls. To obtain an easy-to-handle powder for the oral administration in the animal test, the defatted hydroalcoholic extracts of aerial parts and roots were combined with maltodextrin (1:1
w/
w) and lyophilized to give AP and R samples. The quantitative data were expressed on the dried weight of AP and R samples.
2.2. Chemical and Reagents
Maltodextrin (dextrose equivalent 4.0–6.0), D2O, and CDCl3, and all the other reagents and solvents of High-Performance Liquid Chromatography (HPLC) grade or analytical grade were purchased from Sigma-Aldrich (Steinheim, Germany). Ultrapure water for Mass Spectrometry (MS) analyses was from Milli-Q-system (Millipore, Molsheim, France). The standard sample of spilanthol was kindly provided by Indena S.p.A (Milan, Italy); the purity grade was of 47.64% w/w, evaluated by HPLC at 229 nm. The pure standards used for quantitation of phenolic acids and flavonoids were chlorogenic acid (purity ≥ 95%) and quercitrin (purity ≥ 97%), both purchased from Sigma Aldrich (Steinheim, Germany).
2.3. HPLC-DAD-ESI-MS Analyses
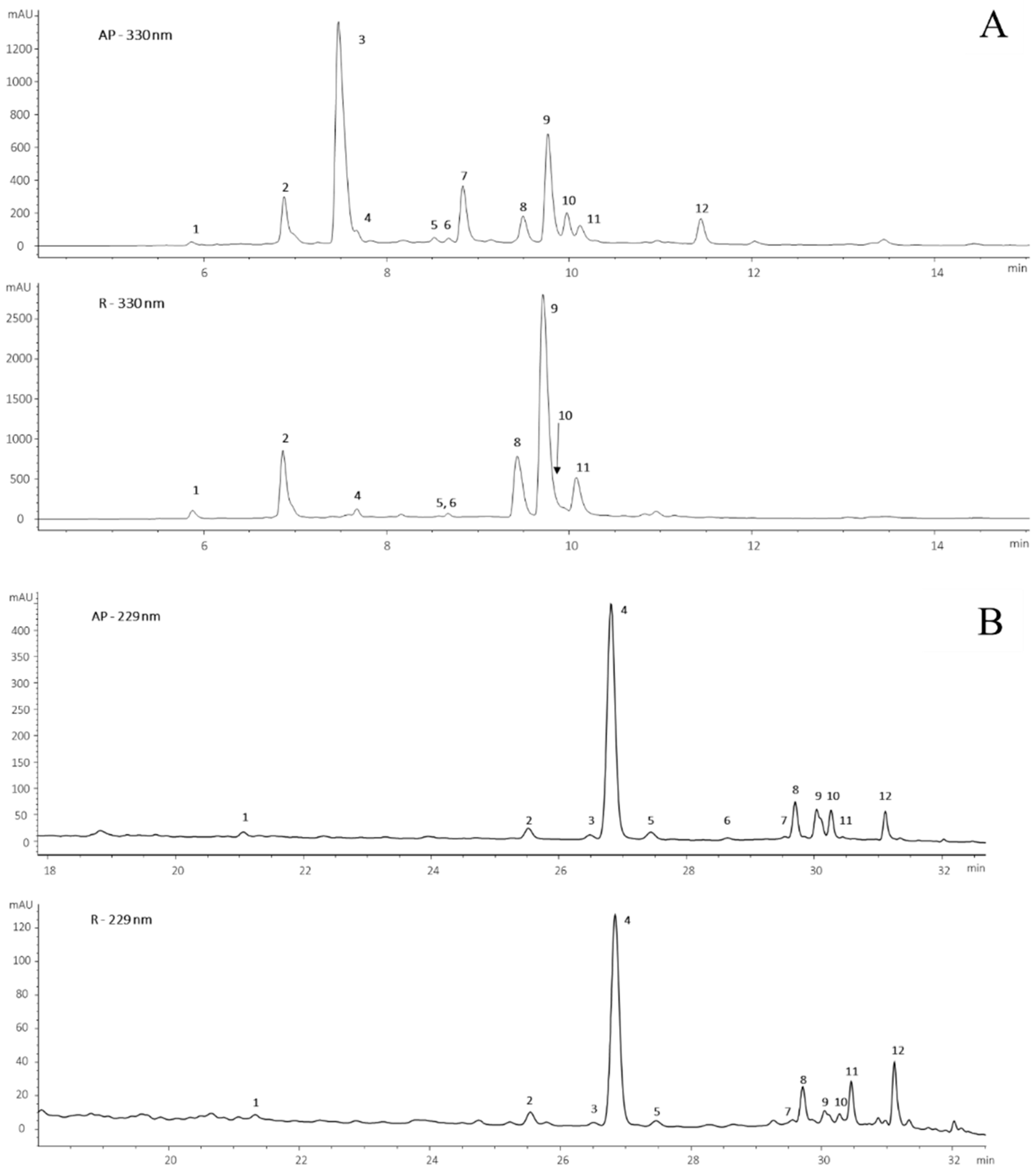
The dried extracts were analyzed by HPLC-DAD-ESI-MS (Agilent Technologies, Palo Alto, CA, USA) according to [
11]. Briefly, the instrument was an HP 1260 Infinity II liquid chromatograph equipped with a Diode Array Detector (DAD) detector and MS detector all from Agilent Technologies (Palo Alto, CA, USA). The column was a RaptorTM ARC-18 of 150 × 3 mm, 5 µm (Restek, Milan, Italy), and flow rate of 0.4 mL min
−1. Solvent A was water at pH 3.2 by formic acid, and solvent B acetonitrile; A varied from 5 to 25% in 5 min, from 25 to 50% in 20 min, from 50 to 100% in 5 min, with a final plateau of 5 min; total time of analysis 37 min.
A five-point calibration curve at λ 229 nm of spilanthol (purity grade by HPLC 47.6% w/w) was applied for spilanthol and alkylamides; the mother solution was 238 µg/mL, a linearity range of 0–3.57 μg and R2 0.9985. A five-point calibration line of chlorogenic acid (0.458 mg/mL) at λ 330 nm (linearity range 0–3.66 µg, R2 0.9998) was used for the phenolic acids. Flavonoids were determined using a five-point calibration curve of quercitrin (quercetin-3 L rhamnoside) evaluated at λ 350 nm (linearity range 0–2.45 µg; R2 0.9999).
Spilanthol was quantified by MS with a five-point calibration curve; mother solution 23.8 μg/mL, linearity range 0–0.0014 μg, R
2 0.9956. The applied method was previously validated [
11].
Flow injection analyses were conducted to optimize electrospray ionization (ESI) parameters for the generation of the spilanthol molecular ion [M + H]
+ 222.2 m/z. The acquisition was performed in a Selected Ion Monitoring (SIM) scan, positive ionization mode with fragmentor 120 V, and the following parameters: nitrogen flow rate 10.5 mL min
−1, drying gas temperature 300 °C, nebulizer pressure 1035 Torr, capillary voltage 3500 V. Polyphenols and alkylamides were identified by comparison with authentic pure standards, based on retention times, UV spectra, and mass spectral data; compounds without available standards were tentatively identified through retention times and characteristic MS fragmentation patterns, supported by comparison with the literature data [
10,
11].
2.4. 1H-NMR Analyses
Proton spectra were carried out on a Bruker instrument Advance 400 MHz (Bruker, Bremen, Germany). AP and R dried extracts were analyzed in D2O and in CDCl3 (50 mg/mL, while spilanthol was dissolved in CDCl3 (12 mg/mL); AP and R samples in both the deuterated solvents were only partially dissolved (saturated solutions). The acquisition of proton spectra was carried out by applying Bruker sequence “zg” with temperature 298 K; relaxation delay 12 s; pulse width 7 μs; and number of scans 12.
2.5. SH-SY5Y Cell Culture Protocol and Differentiation
SY-SY5Y cultures were set up according to the previously reported method, with minor modifications [
15]. Human neuroblastoma SH-SY5Y cell line was maintained in DMEM High Glucose/Ham’s F12 Mixture Medium (1:1) (Merck, Milan, Italy), supplemented with 10% FBS (Euroclone S.p.A., Milan, Italy), 2 mM l-Glutamine (Merck, Milan, Italy), 100 U/mL penicillin (Merck, Milan, Italy), 100 μg/mL streptomycin (Merck, Milan, Italy), at 37 °C in 5% CO
2 in a cell culture incubator. Once cells reached 70–80% confluence, cells were harvested and seeded into a multi-well plates at the desired density.
For differentiation, SH-SY5Y cells were pre-treated with Retinoic Acid (RA) 10 µM (Merck, Milan, Italy) in DMEM/Nutrient Mixture F-12 Ham (F12) 1% FBS. The medium was changed every 2 days for 6 days. Starting from day 6, the medium was changed to Neurobasal-A (NB) medium (Life Technologies, Rockford, IL, USA) supplemented with brain-derived neurotrophic factor (BDNF) 100 ng/mL (Sino Biological, Inc., Beijing, China), N2 supplement (1X) (Life Technologies, Rockford, IL, USA), and Amphotericin B (ATB, 2.5 µg/mL) (Merck, Milan, Italy) for 2 additional days. After the differentiation, cells were incubated with Acmella oleracea AP and R extracts (15, 50, and 150 µg/mL), alone and in combination with oxaliplatin (10 µM).
2.6. Pharmacological Treatments
Following differentiation, SH-SY5Y cells were incubated with oxaliplatin at a concentration of 10µM in Neurobasal medium for 24 h. SH-SY5Y cells were treated with hydroalcoholic extracts of Acmella oleracea from aerial parts (AP) and roots (R) at concentrations of 15–50–150 µg/mL. The extracts were obtained by solubilizing 50 mg/mL lyophilized samples in dimethyl sulfoxide (DMSO, Merck, Milan, Italy); the final dilution of DMSO was 1%.
2.7. Cell Viability Assay
Cell viability was assessed using the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Merck, Milan, Italy), which reflects the functional activity of the mitochondrial compartment. SH-SY5Y cells were plated at a density of 3 × 104 cells/well in 96-well plates (Corning, Tewksbury, MA, USA) and after differentiation, cells were incubated with oxaliplatin (10 µM) and Acmella oleracea extracts (R and AP) (15–150 µg/mL), for 24 h. At the end of the treatment, MTT was added to the medium at a final concentration of 1 mg/mL and then the colored formazan crystals were dissolved in 200 µL of dimethyl sulfoxide (DMSO, Merck, Milan, Italy). Absorbance was measured at 550 nm. Data are presented as mean ± S.E.M. of N = 3 independent experiments, each performed with six technical replicates
2.8. LDH Release Assay
Cytotoxicity was evaluated through the release of lactate dehydrogenase (LDH) in the culture medium of differentiated SH-SY5Y cell line.
SH-SY5Y cells were plated at a density of 1.5 × 104 cells/well in 24-well plates (Corning, Tewksbury, MA, USA), and after differentiation, cells were incubated with oxaliplatin (10 µM) and R HAE or AP HAE (15–150 µg/mL) for 24 h.
Following the treatments, the release of LDH was determined using the Cytotoxicity Detection Kit (Roche 11644793001, Roche Diagnostics GmbH Roche Applied Science Mannheim, Germany) following the manufacturer’s instructions. Protein concentration was quantified using the BCA assay (Merck, Milan, Italy). The amount of LDH released in each sample was normalized to protein concentration. Data are presented as mean ± S.E.M. of N = 3 independent experiments, each per-formed with six technical replicates
2.9. Superoxide Dismutase (SOD) Assay
Oxidative stress was evaluated by performing an SOD assay on differentiated SH-SY5Y. SH-SY5Y cells were plated at a density of 1.5 × 104 cells/well in 24-well plates (Corning, Tewksbury, MA, USA), and after differentiation, cells were incubated with oxaliplatin (10 µM) and AP or R (15–150 µg/mL) for 24 h. SOD activity was measured in the supernatant using the SOD Assay Kit (Merck, Milan, Italy), following the manufacturer’s instructions. Protein concentration was quantified using the bicinchoninic acid (BCA) assay (Merck, Milan, Italy). SOD activity in each sample was normalized to protein concentration. Data are presented as mean ± S.E.M. of N = 3 independent experiments, each per-formed with six technical replicates.
2.10. RNA Isolation, Reverse Transcription, and Real-Time Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from SH-SY5Y cell lines (5 × 105 cells/well in 6-well plate; Corning, Tewksbury, MA, USA). In total, 250 nanograms of RNA for SH-SY5Y were retrotranscribed using the PrimeScriptTM RT reagent Kit with gDNA eraser (Takara Bio, Kusatsu, Japan). RT-PCR was performed using SsoAdvanced Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA) following the thermal profile suggested by the kit. For SH-SY5Y, the following validated human primers for hERN1, hATF3, hNEFH, hACTB, and hGAPDH (qHsaCID0016338, qHsaCED0002279, qHsaCID0011672, qHsaCED0036269, and qHsaCED0038674) were purchased from Bio-Rad. The differential expression of transcripts was normalized on the expression level of housekeeping genes. Data are presented as mean ± S.E.M. of N = 3 independent experiments, each performed with six technical replicates
2.11. Animals
CD-1 adult male mice (20–25 g) used in the experiments were provided from Envigo RMS SRL (Varese, Italy). The animals were fed a standard laboratory diet and tap water ad libitum and housed in a room kept at 23 ± 1 °C with a 12 h light/dark cycle (light on at 7 a.m.). Environmental enrichment was provided to all animals throughout the study to promote natural behaviors and improve welfare. This included nesting materials, shelters, and objects for manipulation, which were regularly refreshed to maintain interest and stimulation. Animals were randomly assigned to the different experimental groups to reduce the risk of systematic bias. Group allocation was performed using a computer-generated randomization procedure (Microsoft Excel) to assign a random number to each subject by applying the =RAND() function. The list of animals was then sorted in ascending order based on these random values, and mice were allocated sequentially to the different experimental groups. Each experimental group consisted of eight animals (N = 8). All animal manipulations were carried out according to the Directive 2010/63/EU of the European Parliament and of the European Union council (22 September 2010) on the protection of animals used for scientific purposes and conformed to the International Association for the Study of Pain (IASP) guidelines on ethical standards for the investigation of experimental pain in animals. Given the objective of our study, to evaluate novel approaches for chemotherapy-induced neuropathic pain, analgesics were not administered, as they would interfere with the validity of the pain model and confound experimental outcomes. Although the presence of pain is inherent to the model, animals were monitored daily to assess their general health and well-being. Specific attention was paid to signs such as reduced activity or social interaction, pallor (indicative of anemia), grooming behavior, body condition, and progressive weight loss. Humane endpoints were predefined to ensure animal welfare. Criteria included severe and sustained weight loss (>20%), signs of systemic distress (e.g., dehydration, persistent lethargy, or lack of responsiveness), or other indicators of deteriorating general health. However, none of the animals enrolled in the study reached these predefined endpoints, and therefore, no animals were euthanized for humane reasons during the experimental timeline. The ethical policy of the University of Florence complies with the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health (NIH Publication No. 85–23, revised 1996; University of Florence assurance number: A5278-01). We performed an a priori power analysis using G*Power (version 3.1.9.2) to determine the appropriate sample size. The calculation was based on pilot data, assuming an effect size of 1.6, a significance level (α) of 0.05, and a statistical power (1–β) of 0.8.
As a result, a minimum of 8 animals per group was determined to be sufficient to detect statistically meaningful differences.
2.12. Oxaliplatin-Induced Neuropathic Pain Model and Pharmacological Treatments
Oxaliplatin (2.4 mg/kg) was dissolved in 5% glucose solution and intraperitoneally (i.p.) administered for 5 consecutive days every week for two weeks (10 injections) [
18,
19] (with minor modifications).
Control animals received an equivalent volume of 5% glucose saline solution intraperitoneally administered.
Regarding animal treatments with plant-derived products, hydroalcoholic extracts of
Acmella oleracea from roots and aerial parts, R and AP (200, 600, and 1200 mg/kg), were dissolved in 1% carboxymethylcellulose sodium salt (CMC; Sigma-Aldrich, Milan, Italy) and administered
per os; spilanthol (2.38 and 7.146 mg/kg) was dissolved in saline/DMSO/Tween20 (90%, 5%, 5%) and administered subcutaneously; all treatments were tested by acute administration when neuropathy was well established (day 14) [
20]. Control groups received vehicles.
2.13. Assessment of Thermal Allodynia (Cold Plate Test)
To minimize interaction between the operator and the animals, each mouse was gently removed from its home cage and placed on the surface of a cold plate apparatus (Ugo Basile, Varese, Italy) maintained at a constant temperature of 4 ± 1 °C. Animal movement was restricted using an open-top Plexiglas cylindrical chamber (10 cm in diameter, 15 cm in height). Pain-related behaviors (i.e., lifting and licking of the hind paw) were observed and the time (s) of the first sign was recorded. The cut-off for paw licking or licking was set at 30 s [
20].
2.14. Statistical Analysis
Chemical data were expressed as mean ± SD. One-way ANOVA followed by Tukey’s post hoc test was used to assess significant differences between AP and R. Statistical analysis was performed in RStudio (2024.09.0+375).
Biological results were expressed as mean ± S.E.M. and analysis of variance (one-way ANOVA) was performed. A Bonferroni significant difference procedure was used as post hoc comparison. Data were analyzed using the “Origin 9.1” software (OriginLab, Northampton, MA, USA). p-values less than 0.05 were considered significant; * p < 0.05; ** p < 0.01; *** p < 0.001 vs. control, and ^ p < 0.05; ^^ p < 0.01; ^^^ p < 0.001 vs. treatment. To reduce experimental bias, all in vivo procedures were carried out by researchers blinded to the treatment assignments.
4. Discussion
Oxaliplatin-induced neuropathy is an important dose-limiting toxic effect boosting the research of alternative chemotherapeutic treatments. Our previous work showed the therapeutic potential of
Echinacea purpurea extracts (i.e., an
n-hexane extract rich in alkylamides and a butanolic extract rich in polyphenols) in neuropathic pain treatment [
20]; thus, we investigated another medicinal plant,
Acmella oleracea, rich in the same secondary metabolites [
11]. To prevent low yield and high variability of active constituents, in vitro seedling cultures were obtained by a standardized cultivation method [
17].
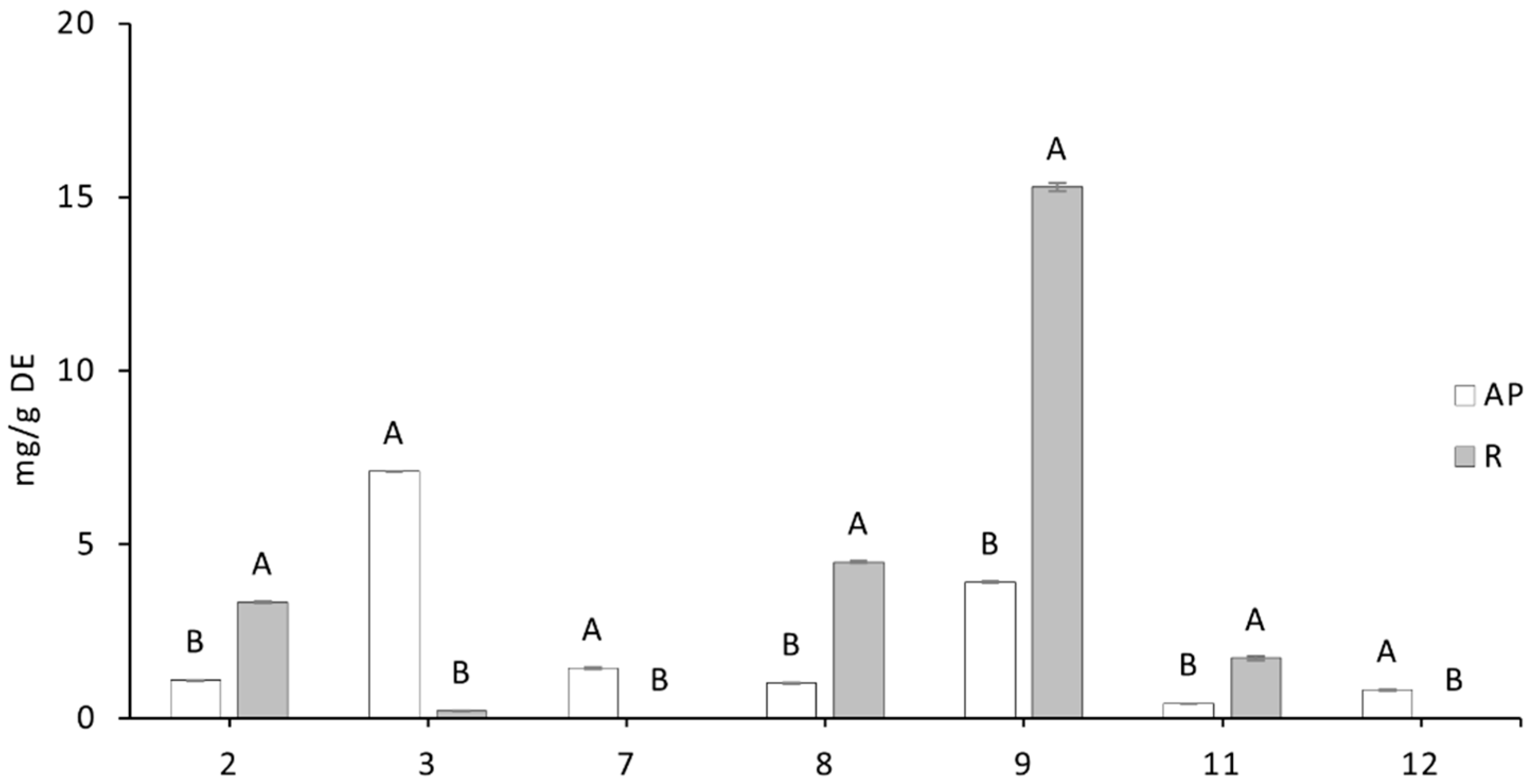
The extracts of Acmella oleracea selected for this study had different contents of secondary metabolites. The extract from the aerial part contained mainly phenolic compounds and about one third alkylamides, whereas the root extract was richer in phenols with negligible amounts of alkylamides like spilanthol. The different ratios between the two classes of metabolites in the extracts allowed for an investigation of the possible different activity of the different parts of the plant. This distinct phytochemical profile allowed us to examine the combined or individual effects of these two classes of compounds on neuroprotective mechanisms.
The biological data highlighted the anti-neuropathic properties of the plant. In particular, the two main phytochemical components, alkylamides and phenols, were active. In neuronal cells, both AP, the extract rich in spilanthol and phenols, and R, the extract with ten times lower spilanthol and twice the phenols, showed a similar protective effect against oxaliplatin-induced neurotoxicity. These extracts, depending on concentration, prevented the decrease in cell viability as evidenced by the MTT assay and the drug-induced cytotoxicity evaluated by LDH release.
The fact that both extracts exhibited similar neuroprotective effects, despite differences in spilanthol and phenol concentrations, suggests that phenolic compounds may play a central role in mediating the protective effect against neurotoxicity.
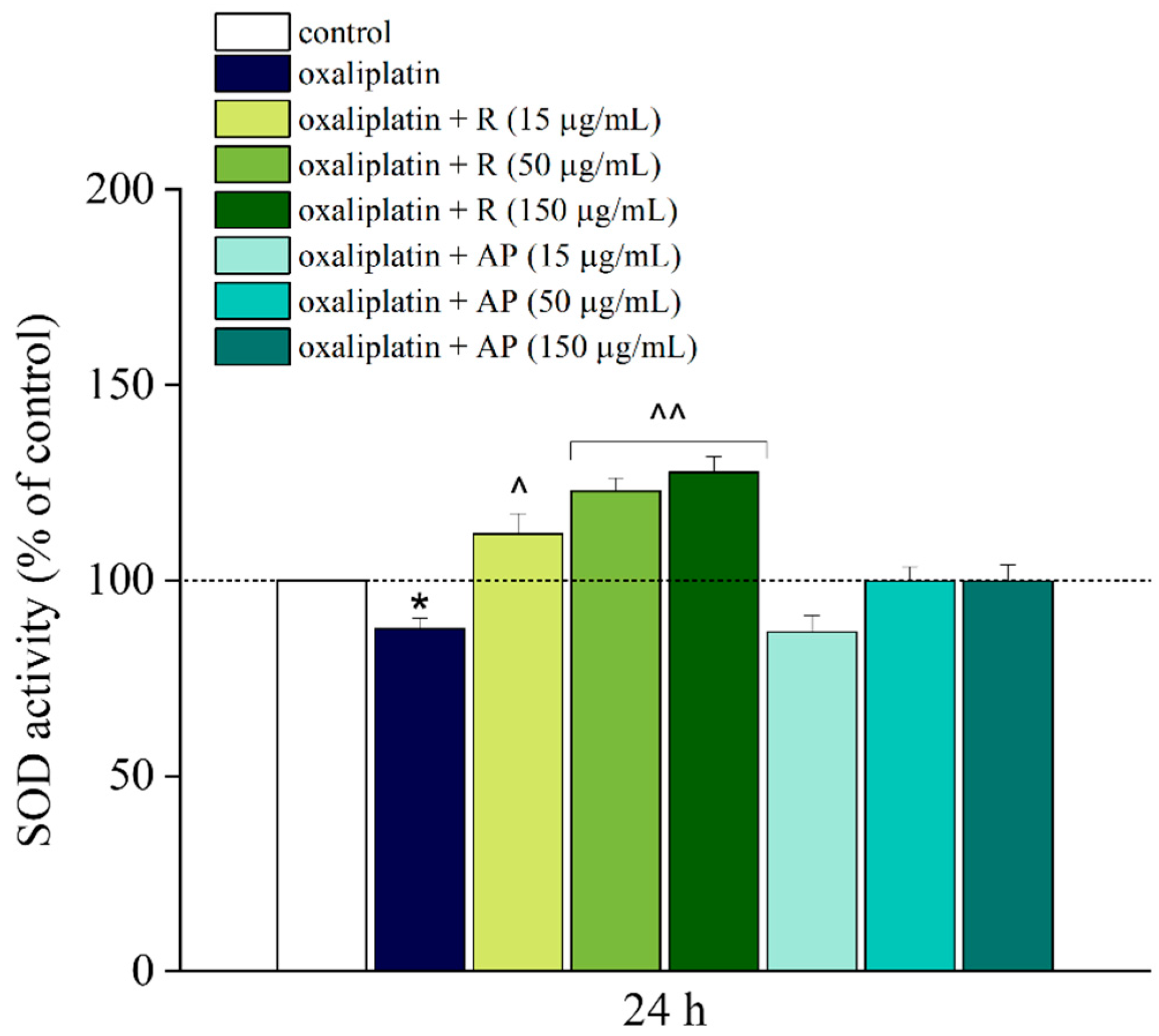
Additionally, an improvement in redox balance was observed. Treatment with oxaliplatin slightly reduced the activity of SOD, a crucial enzyme for protection against oxidative stress. Notably, platinum-based drugs cause an alteration of mitochondrial function followed by the disruption of the respiratory chain function and an increased production of reactive oxygen species (ROS) [
26]. The root extract R, rich in phenolic compounds, reversed this trend, significantly enhancing SOD activity across all tested concentrations. This result highlights the importance of the antioxidant properties of phenolic compounds in counteracting oxaliplatin-induced oxidative stress, a mechanism known to underline the neurotoxicity associated with this chemotherapy [
3]. The antioxidant properties of phenolic compounds, such as caffeoylquinic acids and ferulic acid derivatives, are well documented, and their potential in counteracting oxidative stress could explain part of the observed neuroprotective activity [
13].
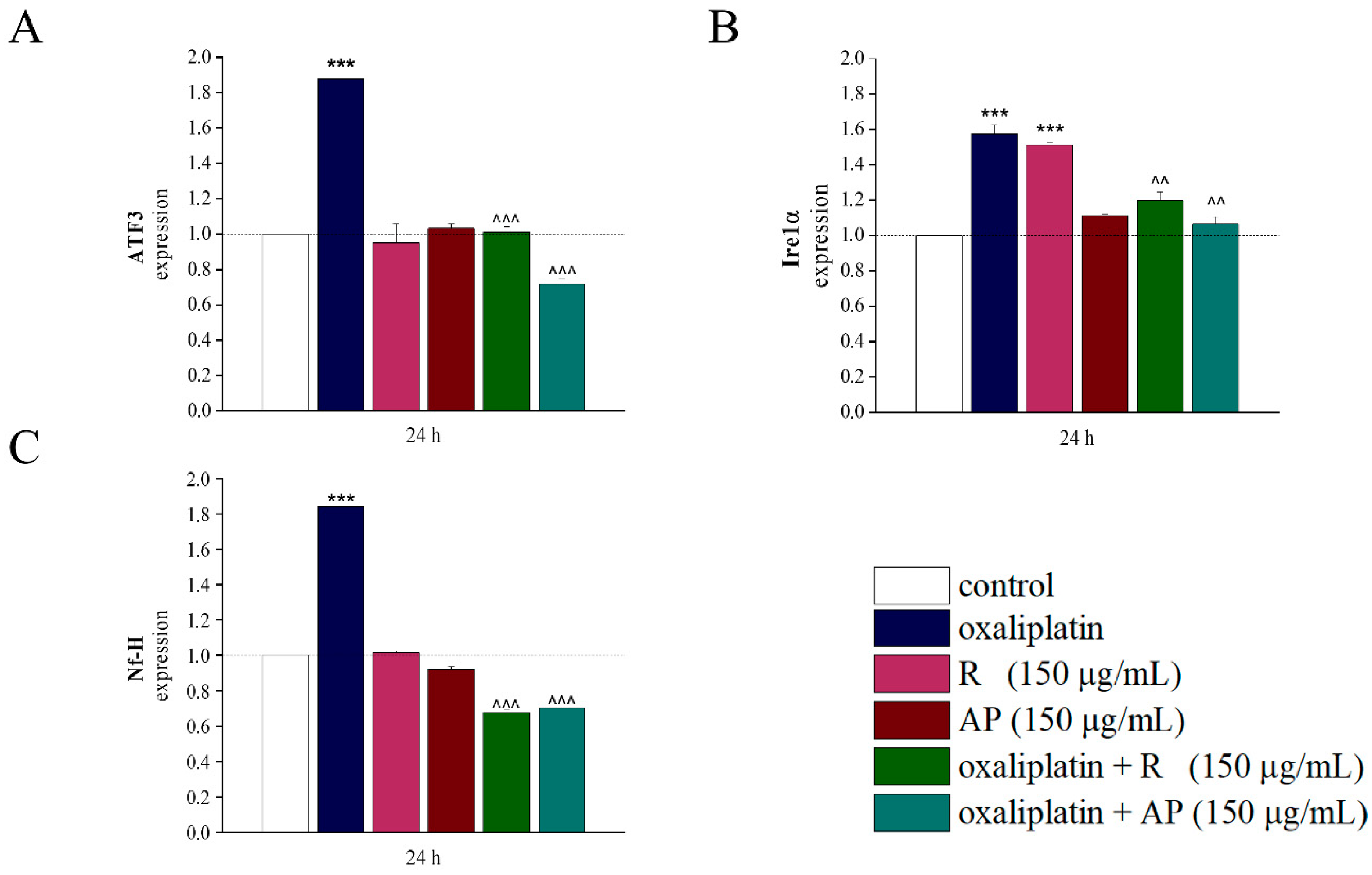
Interestingly,
Acmella oleracea extracts act up to the transcriptional level normalizing the expression of genes altered by oxaliplatin. The analysis of gene expression for markers associated with cellular stress (Ire1α was related to endoplasmic reticulum stress, [
27]; ATF3 with intracellular oxidative stress, mitochondrial dysfunction, cell apoptosis, [
28]) and neurotoxicity (Nf-H, [
29]) provided further evidence supporting the neuroprotective effect of the extracts. Oxaliplatin induced a significant increase in the expression of these genes, as expected in cases of neurotoxic damage. However, both extracts exhibited a modulatory effect, preventing the drug-induced upregulation of gene expression. This result points out that
Acmella oleracea acts not only at the cellular level but also at the transcriptional level, modulating key molecular pathways involved in the cellular stress response. This suggests that the neuroprotective activity of the extracts is not limited to the enhancement of redox potential but also involves gene regulation mechanisms associated with stress response and neuronal survival.
On this basis, the extracts were tested in vivo in a mice model of oxaliplatin-induced neuropathy. AP and R in the dose range 200–1200 mg/kg reduced neuropathic pain after a single injection in a dose-dependent manner. These data are in line with recent evidence in which
Acmella oleracea extract has been demonstrated to possess anti-allodynic activity in a preclinical model of spared nerve injury induced neuropathic pain [
30]. AP exhibited a more favorable trend in efficacy and duration (although the difference in effect was not statistically significant). These results suggest that the combination of spilanthol and phenols present in aerial parts and roots contributes to an additive anti-neuropathic effect. The response confirms the role of alkylamides (e.g., spilanthol) in pain relief but strongly highlights the relevance of phenols. In fact, R extract—despite containing a small amount of spilanthol (0.13 mg/kg in the dose of 600 mg DE/kg bwt)—exerts significant anti-neuropathic effects. This consideration is also supported by the finding that spilanthol standard, administered at a dose approximately twenty times higher (2.38 mg/kg), achieved a lower level of efficacy. This suggests that the pharmacological properties of extracts in vivo are not solely due to the alkylamides and spilanthol, but rather to the interaction between spilanthol and phenolic compounds, present in the dried extract. This point warrants further investigation to clarify the mechanism of action of the single phenols detected in root and aerial parts of
Acmella oleracea to identify the specific compounds responsible for the observed analgesic effect. The identification and quantification of phenols in these extracts, in addition to spilanthol, have proven crucial for the possible future development of novel therapeutic strategies for the management of neuropathic pain with
Acmella oleracea extracts.
5. Conclusions
This study highlights the neuroprotective and anti-neuropathic potential of Acmella oleracea extracts in oxaliplatin-induced toxicity models (both in vitro and in vivo). Both aerial part and root extracts, showed comparable efficacy in preserving cell viability, reducing oxidative stress, modulating stress-related gene expression, and alleviating pain. Although the root extract contained ten times less spilanthol, it was the richest in total phenolic suggesting a central role for phenolics, potentially acting synergistically with alkylamides.
However, some limitations must be acknowledged. The contribution of individual phenols remains unclear, and the mechanism of action has not yet been fully elucidated. Moreover, pharmacokinetic properties and long-term safety of the extracts were not assessed.
Future studies should focus on identifying the main active phenolic constituents, investigating their molecular targets, and evaluating the efficacy of the extracts in chronic treatment settings. This knowledge could support the development of Acmella oleracea-extract-based therapies as complementary strategies for managing chemotherapy-induced neuropathic pain and other similar debilitating conditions.