Dietary Acrylamide Exposure and Its Correlation with Nutrition and Exercise Behaviours Among Turkish Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection Tools and Data Collection
2.3. Dietary Questionnaires
2.4. Acrylamide Levels in Food
2.5. Dietary Acrylamide Exposure
2.6. Carcinogenic and Non-Carcinogenic Health Risk Assessment
2.7. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Adolescents
3.2. NEBS and Sub-Dimensions in Adolescents
3.3. Dietary Acrylamide Exposure and Health Risk Assessment
4. Discussion
4.1. Adolescents’ Nutrition and Exercise Behaviors
4.2. Dietary Acrylamide Exposure
4.3. Carcinogenic and Non-Carcinogenic Health Risk Assessment
4.4. Relationship Between Dietary Acrylamide Exposure, THQ and CR Values, and Nutritional and Exercise Behaviors and Their Sub-Dimensions
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| NEBS | Nutrition Exercise Behavior Scale |
| PAEB | Psychological/addictive eating behavior |
| HEEB | Healthy eating-exercise behavior |
| UEEB | Unhealthy eating-exercise behavior |
| MR | Meal regularity |
| CR | Carcinogenic Risk |
| THQ | Target Hazard Quotient |
References
- Canavan, C.R.; Fawzi, W.W. Addressing Knowledge Gaps in Adolescent Nutrition: Toward Advancing Public Health and Sustainable Development. Curr. Dev. Nutr. 2019, 3, nzz062. [Google Scholar] [CrossRef]
- Ayaz-Alkaya, S.; Kulakçı-Altıntaş, H. Nutrition-Exercise Behaviors, Health Literacy Level, and Related Factors in Adolescents in Turkey. J. Sch. Health 2021, 91, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Karageorgou, D.; Bakogianni, I.; Trichia, E.; Whitsel, L.P.; Story, M.; Peñalvo, J.L.; Mozaffarian, D. Effectiveness of school food environment policies on children’s dietary behaviors: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0194555. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines on Physical Activity and Sedentary Behaviour; WHO: Geneva, Switzerland, 2020; Available online: https://iris.who.int/bitstream/handle/10665/336656/9789240015128-eng.pdf?sequence=1 (accessed on 19 March 2025).
- World Health Organization. Adolescent and Young Adult Health. Erişim Tarihi. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/adolescents-health-risks-and-solutions (accessed on 27 March 2025).
- Banfield, E.C.; Liu, Y.; Davis, J.S.; Chang, S.; Frazier-Wood, A.C. Poor adherence to US dietary guidelines for children and adolescents in the national health and nutrition examination survey population. J. Acad. Nutr. Diet. 2016, 116, 21–27. [Google Scholar] [CrossRef]
- Winpenny, E.M.; Greenslade, S.; Corder, K.; van Sluijs, E.M.F. Diet quality through adolescence and early adulthood: Cross-sectional associations of the dietary approaches to stop hypertension diet index and component food groups with age. Nutrients 2018, 10, 1585. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, S.; De Cosmi, V.; Ciappolino, V.; Parazzini, F.; Brambilla, P.; Agostoni, C. Factors Influencing Children’s Eating Behaviours. Nutrients 2018, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Rehm, C.D.; Onopa, J.; Mozaffarian, D. Trends in diet quality among youth in the United States, 1999–2016. JAMA 2020, 323, 1161–1174. [Google Scholar] [CrossRef]
- Roberts, C.; Steer, T.; Maplethorpe, N.; Cox, L.; Meadows, S.; Nicholson, S.; Page, P.; Swan, G. National Diet and Nutrition Survey: Results from Years 7 and 8 (Combined) of the Rolling Programme (2014/2015–2015/2016); Public Health England: London, UK, 2018.
- Han, R.; Todd, A.; Wardak, S.; Partridge, S.R.; Raeside, R. Feasibility and Acceptability of Chatbots for Nutrition and Physical Activity Health Promotion Among Adolescents: Systematic Scoping Review With Adolescent Consultation. JMIR Hum. Factors 2023, 10, e43227. [Google Scholar] [CrossRef]
- Dunford, E.K.; Popkin, B.M. 37 Year snacking trends for US children 1977–2014. Pediatr. Obes. 2018, 13, 247–255. [Google Scholar] [CrossRef]
- Liberali, R.; Kupek, E.; Assis, M.A.A. Dietary patterns and childhood obesity risk: A systematic review. Child Obes. 2020, 16, 7085. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, A.; Murugan, R.; Jeraud, M.; Dakkumadugula, A.; Periyasamy, R.; Arjunan, S. Additives in Processed Foods as a Potential Source of Endocrine-Disrupting Chemicals: A Review. J. Xenobiot. 2024, 14, 1697–1710. [Google Scholar] [CrossRef]
- Martínez Steele, E.; Buckley, J.P.; Monteiro, C.A. Ultra-processed food consumption and exposure to acrylamide in a nationally representative sample of the US population aged 6 years and older. Prev. Med. 2023, 174, 107598. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Song, Z.; Xu, X.; Yang, X.; Wei, S.; Chen, F.; Dong, X.; Zhang, X.; Zhu, Y. The neurotoxicity of acrylamide in ultra-processed foods: Interventions of polysaccharides through the microbiota-gut-brain axis. Food Funct. 2025, 16, 10–23. [Google Scholar] [CrossRef]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Stadler, R.H.; Gökmen, V. Acrylamide formation mechanisms. In Acrylamide in Food; Academic Press: Cambridge, MA, USA, 2024; pp. 1–17. [Google Scholar] [CrossRef]
- Pesce, F.; Ponzo, V.; Mazzitelli, D.; Varetto, P.; Bo, S.; Saguy, I.S. Strategies to reduce acrylamide formation during food processing focusing on cereals, children and toddler consumption: A review. Food Rev. Int. 2024, 40, 185–211. [Google Scholar] [CrossRef]
- Esposito, F.; Velotto, S.; Rea, T.; Stasi, T.; Cirillo, T. Occurrence of acrylamide in Italian baked products and dietary exposure assessment. Molecules 2020, 25, 4156. [Google Scholar] [CrossRef]
- Tajner-Czopek, A.; Kita, A.; Rytel, E. Characteristics of french fries and potato chips in aspect of acrylamide content—Methods of reducing the toxic compound content in ready potato snacks. Appl. Sci. 2021, 11, 3943. [Google Scholar] [CrossRef]
- Schouten, M.A.; Tappi, S.; Glicerina, V.; Rocculi, P.; Angeloni, S.; Cortese, M.; Romani, S. Formation of acrylamide in biscuits during baking under different heat transfer conditions. LWT 2022, 153, 112541. [Google Scholar] [CrossRef]
- Basaran, B.; Abanoz, Y.Y.; Boyraz, A. Effects of different brewing conditions on acrylamide levels in Turkish black tea and health risk assessment. J. Food Compos. Anal. 2024, 133, 106420. [Google Scholar] [CrossRef]
- Yashwanth, B.S.; Premachandran, M.S.; Karkera, P.S.; Murthy, P.S. Acrylamide in coffee: Strategies, research and future perspectives. Food Control 2024, 163, 110484. [Google Scholar] [CrossRef]
- Adimas, M.A.; Abera, B.D.; Adimas, Z.T.; Woldemariam, H.W.; Delele, M.A. Traditional food processing and Acrylamide formation: A review. Heliyon 2024, 10, e30258. [Google Scholar] [CrossRef]
- Başaran, B.; Çuvalcı, B.; Kaban, G. Dietary acrylamide exposure and cancer risk: A systematic approach to human epidemiological studies. Foods 2023, 12, 346. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientific ppinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); Benford, D.; Bignami, M.; Chipman, J.K.; Ramos Bordajandi, L. Assessment of the genotoxicity of acrylamide. EFSA J. 2022, 20, e07293. [Google Scholar] [CrossRef]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives). Evaluation of Certain Contaminants in Food: Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series; WHO: Geneva, Switzerland, 2011; p. 959. [Google Scholar]
- European Commission. Commission Recommendation (EU) 2019/1888 of 7 November 2019 on the Monitoring of the Presence of Acrylamide in Certain Foods. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019H1888&from=EN (accessed on 10 March 2024).
- Hendekci, A.; Avcı, İ.A. Adölesanlarda internet bağımlılığı ile beslenme egzersiz davranışları arasındaki ilişki [The relationship between internet addiction and nutrition-exercise behaviors in adolescents]. Ank. Med. J. 2020, 20, 315–326. [Google Scholar] [CrossRef]
- Sarı, Ç.; Ceylan, Ç. Determination of Nutrition Exercise Behaviors of Adolescents and Young Adults in the COVID-19 Pandemic. Mersin Üniv. Tıp Fak. Lokman Hekim Tıp Tarihi Ve Folk. Tıp Derg. 2022, 12, 390–399. [Google Scholar] [CrossRef]
- Akdeniz Kudubes, A.; Ayar, D.; Bektas, İ.; Bektas, M. Predicting the effect of healthy lifestyle belief on attitude toward nutrition, exercise, physical activity, and weight-related self-efficacy in Turkish adolescents. Arch. Pediatr. 2022, 29, 44–50. [Google Scholar] [CrossRef]
- Biazzi Leal, D.; Altenburg de Assis, M.A.; Hinnig, P.F.; Schmitt, J.; Soares Lobo, A.; Bellisle, F.; Di Pietro, P.F.; Vieira, F.K.; de Moura Araujo, P.H.; de Andrade, D.F. Changes in Dietary Patterns from Childhood to Adolescence and Associated Body Adiposity Status. Nutrients 2017, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Child Growth Standards: Course Modules and Growth Records—Measuring a Child’s Growth. 2008. Available online: https://www.who.int/tools/child-growth-standards (accessed on 5 May 2025).
- World Health Organization. BMI-For-Age (5–19 years): Interpretation of Cut-Offs. 2025. Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age (accessed on 5 May 2025).
- Yurt, S.; Save, D.; Yıldız, A. Adolesanlar için beslenme egzersiz davranışlarını değerlendirme ölçüm aracının geliştirilmesi, geçerliliği ve güvenilirliği. Türkiye Klinikleri. J. Public Health Nu.-Spec. Top. 2016, 2, 19–25. [Google Scholar]
- Taber, K.S. The use of Cronbach’s alpha when developing and reporting research instruments in science education. Res. Sci. Educ. 2018, 48, 1273–1296. [Google Scholar] [CrossRef]
- Basaran, B.; Anlar, P.; Oral, Z.F.Y.; Polat, Z.; Kaban, G. Risk assessment of acrylamide and 5-hydroxymethyl-2-furfural (5-HMF) exposure from bread consumption: Turkey. J. Food Compos. Anal. 2022, 107, 104409. [Google Scholar] [CrossRef]
- Basaran, B.; Faiz, O. Determining the levels of acrylamide in some traditional foods unique to Turkey and risk assessment. J Pharm Res. 2022, 21, e123948. [Google Scholar] [CrossRef]
- Başaran, B.; Aydın, F.; Kaban, G. The determination of acrylamide content in brewed coffee samples marketed in Turkey. Food Addit. Contam. Part. A 2020, 37, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Basaran, B.; Sadighara, P. The level, human exposure, and health risk assessment of acrylamide in chips and breakfast cereals: A study from Türkiye. J. Food Compos. Anal. 2024, 134, 106584. [Google Scholar] [CrossRef]
- US EPA (United States Environmental Protection Agency). Guidance Manual for Assessing Human Health Risks from Chemically Contaminated, Fish and Shellfish [EPA-503/8-89-002]; U.S. Environmental Protection Agency: Washington, DC, USA, 1987.
- US EPA (United States Environmental Protection Agency). Acrylamide. 2010. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0286_summary.pdf#nameddest=rfd (accessed on 29 May 2025).
- US EPA (United States Environmental Protection Agency). Basic Information About the Integrated Risk Information System. 2021. Available online: https://www.epa.gov/iris/basic-information-about-integrated-risk-information-system (accessed on 29 May 2025).
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Crone, E.A.; van Duijvenvoorde, A.C.K. Multiple pathways of risk taking in adolescence. Dev. Rev. 2021, 62, 100996. [Google Scholar] [CrossRef]
- Rodríguez-Romo, G.; Acebes-Sánchez, J.; García-Merino, S.; Garrido-Muñoz, M.; Blanco-García, C.; Diez-Vega, I. Physical Activity and Mental Health in Undergraduate Students. Int. J. Environ. Res. Public Health 2022, 20, 195. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Vancampfort, D. Physical activity, exercise, and mental disorders: It is time to move on. Trends Psychiatry Psychother. 2021, 43, 177–184. [Google Scholar] [CrossRef]
- Sampasa-Kanyinga, H.; Colman, I.; Goldfield, G.S.; Janssen, I.; Wang, J.; Podinic, I.; Tremblay, M.S.; Saunders, T.J.; Sampson, M.; Chaput, J.P. Combinations of physical activity, sedentary time, and sleep duration and their associations with depressive symptoms and other mental health problems in children and adolescents: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 72. [Google Scholar] [CrossRef]
- Güler, S.; Yavaş Çelik, M.; Öztürk Çopur, E. Tip 1 diyabetli ergenlerin beslenme ve egzersiz davranışlarının değerlendirilmesi [Evaluation of nutrition and exercise behaviors in adolescents with type 1 diabetes]. J. Educ. Train. Res. (JETR) 2023, 10, 211–218. [Google Scholar]
- Yarar, H.; Karahan Yılmaz, S.; Eskici, G.; Köksal, B.; Ceylan, H.B.; Balıkcı, R.; Saraç, O.E. Adölesan sporcuların beslenme ve egzersiz davranışlarının incelenmesi [Investigation of nutrition and exercise behaviors of adolescent athletes]. CBÜ J. Phys. Educ. Sports Sci. 2023, 18, 350–361. [Google Scholar] [CrossRef]
- Aykut, T.; Avcı, P.; Kılınçarslan, G.; Bayrakdar, A. Lise öğrencilerinin beslenme ve egzersiz davranışlarının belirlenmesi [Determination of nutrition and exercise behaviors of high school students]. J. Phys. Educ. Sports Sci. 2021, 23, 33–45. [Google Scholar]
- Erdem, E.; Efe, Y.S.; Özbey, H. A predictor of emotional eating in adolescents: Social anxiety. Arch. Psychiatr. Nurs. 2023, 43, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Athanasian, C.E.; Lazarevic, B.; Kriegel, E.R.; Milanaik, R.L. Alternative diets among adolescents: Facts or fads? Curr. Opin. Pediatr. 2021, 33, 252–259. [Google Scholar] [CrossRef]
- Dell’Osbel, R.S.; Donatti, T.; Henn, R.L.; Cremonese, C.; Capp, E.; Gregoletto, M.L.O. Parental practices, body dissatisfaction and weight control practices in female adolescents from public schools in southern Brazil. Br. J. Nutr. 2021, 126, 470–480. [Google Scholar] [CrossRef]
- Hallal, P.C.; Andersen, L.B.; Bull, F.C.; Guthold, R.; Haskell, W.; Ekelund, U.; Lancet Physical Activity Series Working Group. Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 2012, 380, 247–257. [Google Scholar] [CrossRef]
- Keleş, B.; Göbel, P. Adölesanların Akdeniz Diyetine Uyumu ile Duygusal Yeme ve Stres Durumları Arasındaki İlişkinin Belirlenmesi. Beslenme Ve Diyet Derg. 2023, 51, 16–25. [Google Scholar] [CrossRef]
- Mojska, H.; Gielecińska, I.; Szponar, L.; Ołtarzewski, M. Estimation of the dietary acrylamide exposure of the Polish population. Food Chem. Toxicol. 2010, 48, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Sirot, V.; Hommet, F.; Tard, A.; Leblanc, J.C. Dietary acrylamide exposure of the French population: Results of the second French Total Diet Study. Food Chem. Toxicol. 2012, 50, 889–894. [Google Scholar] [CrossRef]
- Cieslik, I.; Cieslik, E.; Topolska, K.; Surma, M. Dietary acrylamide exposure from traditional food products in Lesser Poland and associated risk assessment. Ann. Agric. Environ. Med. 2020, 27, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Sobue, T.; Kitamura, T.; Kitamura, Y.; Ishihara, J.; Kotemori, A.; Liu, R.; Ikeda, S.; Sawada, N.; Iwasaki, M.; et al. Dietary acrylamide intake and the risk of liver cancer: The Japan public health center-based prospective study. Nutrients 2020, 12, 2503. [Google Scholar] [CrossRef]
- Kito, K.; Ishihara, J.; Kotemori, A.; Zha, L.; Liu, R.; Sawada, N.; Iwasaki, M.; Sobue, T.; Tsugane, S. Dietary acrylamide intake and the risk of pancreatic cancer: The Japan public health center-based prospective study. Nutrients 2020, 12, 3584. [Google Scholar] [CrossRef]
- Basaran, B.; Abanoz, Y.Y.; Şenol, N.D.; Oral, Z.F.Y.; Öztürk, K.; Kaban, G. The levels of heavy metal, acrylamide, nitrate, nitrite, N-nitrosamine compounds in brewed black tea and health risk assessment: Türkiye. J. Food Compos. Anal. 2023, 120, 105285. [Google Scholar] [CrossRef]
- Tardiff, R.G.; Gargas, M.L.; Kirman, C.R.; Carson, M.L.; Sweeney, L.M. Estimation of safe dietary intake levels of acrylamide for humans. Food Chem. Toxicol. 2010, 48, 658–667. [Google Scholar] [CrossRef]
- Freisling, H.; Moskal, A.; Ferrari, P.; Nicolas, G.; Knaze, V.; Clavel-Chapelon, F.; Slimani, N. Dietary acrylamide intake of adults in the European Prospective Investigation into Cancer and Nutrition differs greatly according to geographical region. Eur. J. Nutr. 2013, 52, 1369–1380. [Google Scholar] [CrossRef]
- Basaran, B. Estimation of the Dietary Acrylamide Exposure of the Turkish Population: An Emerging Threat for Human Health. Nutrients 2024, 16, 3088. [Google Scholar] [CrossRef]
- The Türkiye Nutrition and Health Survey. 2019. Available online: https://hsgm.saglik.gov.tr/depo/birimler/saglikli-beslenme-ve-hareketli-hayat-db/Dokumanlar/Ingilizce_Yayinlar/TBSA_RAPOR_KITAP_2017_ENG_.pdf (accessed on 15 March 2025).
- De Boni, A.; Pasqualone, A.; Roma, R.; Acciani, C. Traditions, health and environment as bread purchase drivers: A choice experiment on high-quality artisanal Italian bread. J. Clean. Prod. 2019, 221, 249–260. [Google Scholar] [CrossRef]
- McCullough, M.L.; Hodge, R.A.; Um, C.Y.; Gapstur, S.M. Dietary acrylamide is not associated with renal cell cancer risk in the CPS-II nutrition cohort. Cancer Epidemiol. Biomark. Prev. 2019, 28, 616–619. [Google Scholar] [CrossRef]
- Jeong, H.; Hwang, S.; Kwon, H. Survey for acrylamide in processed foods from Korean market and individual exposure estimation using a non-parametric probabilistic model. Food Addit. Contam. Part A 2020, 37, 916–930. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, T.; Kontto, J.; Jestoi, M.; Valsta, L.; Peltonen, K.; Pietinen, P.; Virtamo, J. Dietary acrylamide intake and the risk of cancer among Finnish male smokers. Cancer Causes Control 2010, 21, 2223–2229. [Google Scholar] [CrossRef]
- Eslamizad, S.; Kobarfard, F.; Tsitsimpikou, C.; Tsatsakis, A.; Tabib, K.; Yazdanpanah, H. Health risk assessment of acrylamide in bread in Iran using LC-MS/MS. Food Chem. Toxicol. 2019, 126, 162–168. [Google Scholar] [CrossRef]
- Oroian, M.; Amariei, S.; Gutt, G. Acrylamide in Romanian food using HPLC-UV and a health risk assessment. Food Addit. Contam. Part B 2015, 8, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi, A.; Kamankesh, M.; Hosseini, H.; Ghasemi, J.; Hosseini-Esfahani, F.; Mohammadi, A.; Mousavi Khaneghah, A. Acrylamide content of collected food products from Tehran’s market: A risk assessment study. Environ. Sci. Pollut. Res. 2020, 27, 30558–30570. [Google Scholar] [CrossRef]
- Rakha, A.; Mehak, F.; Shabbir, M.A.; Arslan, M.; Ranjha, M.M.A.N.; Ahmed, W.; Aadil, R.M. Insights into the constellating drivers of satiety impacting dietary patterns and lifestyle. Front. Nutr. 2022, 9, 1002619. [Google Scholar] [CrossRef] [PubMed]
- Hyldelund, N.B.; Frederiksen, C.; Byrne, D.V.; Andersen, B.V. Is stress taking the pleasure out of food?—A characterization of the food pleasure profiles, appetite, and eating behaviors of people with chronic stress. Foods 2022, 11, 1980. [Google Scholar] [CrossRef]
- Lytvynenko, O.; König, L.M. Investigation of Ukrainian refugees’ eating behavior, food intake, and psychological distress: Study protocol and baseline data. Appl. Psychol. Health Well-Being 2024, 16, 923–943. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Gallego, G.; Dalmau-Torres, J.M.; Jiménez-Boraita, R.; Ortuño-Sierra, J.; Gargallo-Ibort, E. Adherence to the Mediterranean diet in Spanish university students: Association with lifestyle habits, mental and emotional well-being. Nutrients 2025, 17, 698. [Google Scholar] [CrossRef]
- Arsalandeh, F.; Shemirani, F.; Nazari, M.A.; Mirmiran, P.; Golzarand, M. Effect of low-carbohydrate diets on quality of life, mental health, and speed of memory in adults: A systematic review and meta-analysis of randomised controlled trials. Int. J. Food Sci. Nutr. 2025, 76, 4–19. [Google Scholar] [CrossRef]
- Thornley, S.; Russell, B.; Kydd, R. Carbohydrate reward and psychosis: An explanation for neuroleptic induced weight gain and path to improved mental health? Curr. Neuropharmacol. 2011, 9, 370–375. [Google Scholar] [CrossRef]
- Lennerz, B.; Lennerz, J.K. Food addiction, high-glycemic-index carbohydrates, and obesity. Clin. Chem. 2018, 64, 64–71. [Google Scholar] [CrossRef]
- Unwin, J.; Delon, C.; Giæver, H.; Kennedy, C.; Painschab, M.; Sandin, F.; Wiss, D.A. Low carbohydrate and psychoeducational programs show promise for the treatment of ultra-processed food addiction. Front. Psychiatry 2022, 13, 1005523. [Google Scholar] [CrossRef]
- Fitrikasari, A.; Wardani, N.D.; Sumekar, T.A.; Saktini, F.; Asikin, H.G.; Sulchan, M. The role of psychosocial stressors, carbohydrate and protein intake on serum serotonin and cortisol levels in patients with depression: A preliminary evaluation. Bali Med. J. 2021, 10, 137–141. [Google Scholar] [CrossRef]
- Wu, Y.K.; Pacchioni, T.G.; Gehi, A.K.; Fitzgerald, K.E.; Tailor, D.V. Emotional eating and cardiovascular risk factors in the police force: The Carolina blue project. Int. J. Environ. Res. Public Health 2024, 21, 332. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Mielgo-Ayuso, J.; Martín-Rodríguez, A.; Ramos-Campo, D.J.; Redondo-Flórez, L.; Tornero-Aguilera, J.F. The burden of carbohydrates in health and disease. Nutrients 2022, 14, 3809. [Google Scholar] [CrossRef]
- Basaran, A.G.; Ozbek, Y.D. A study of the relationship between university students’ food neophobia and their tendencies towards orthorexia nervosa. Behav. Sci. 2023, 13, 958. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.; Arendt, S.W. Understanding healthy eating behaviors at casual dining restaurants using the extended theory of planned behavior. Int. J. Hosp. Manag. 2016, 53, 106–115. [Google Scholar] [CrossRef]
- Martínez-Vargas, L.; Vermandere, H.; Bautista-Arredondo, S.; Colchero, M.A. The role of social determinants on unhealthy eating habits in an urban area in Mexico: A qualitative study in low-income mothers with a young child at home. Appetite 2022, 169, 105852. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, F.; Luo, P.; Zhou, P. Levels of acrylamide in food products from a Chinese market and their risk assessment. J. Food Compos. Anal. 2025, 144, 107664. [Google Scholar] [CrossRef]
- Rafacz, S.D. Healthy eating: Approaching the selection, preparation, and consumption of healthy food as choice behavior. Perspect. Behav. Sci. 2019, 42, 647–674. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.A.; German, C.; Imboden, M.; Ozemek, C.; Peterman, J.E.; Brubaker, P.H. The importance of healthy lifestyle behaviors in the prevention of cardiovascular disease. Prog. Cardiovasc. Dis. 2022, 70, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.; Dastmalchi, L.N.; Gulati, M.; Michos, E.D. A heart-healthy diet for cardiovascular disease prevention: Where are we now? Vasc. Health Risk Manag. 2023, 19, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, T.; Jung, H. The relationships between food literacy, health promotion literacy and healthy eating habits among young adults in South Korea. Foods 2022, 11, 2467. [Google Scholar] [CrossRef]
- Ljubičić, M.; Sarić, M.M.; Rumbak, I.; Barić, I.C.; Sarić, A.; Komes, D.; Guiné, R.P. Is better knowledge about health benefits of dietary fiber related to food labels reading habits? A Croatian overview. Foods 2022, 11, 2347. [Google Scholar] [CrossRef]
- Leech, R.M.; Worsley, A.; Timperio, A.; McNaughton, S.A. Understanding meal patterns: Definitions, methodology and impact on nutrient intake and diet quality. Nutr. Res. Rev. 2015, 28, 1–21. [Google Scholar] [CrossRef]
- Aljuraiban, G.S.; Chan, Q.; Oude Griep, L.M.; Brown, I.J.; Daviglus, M.L.; Stamler, J.; INTERMAP Research Group. The impact of eating frequency and time of intake on nutrient quality and body mass index: The INTERMAP study, a population-based study. J. Acad. Nutr. Diet. 2015, 115, 528–536. [Google Scholar] [CrossRef]
- Al-Sowayan, N.S.; Almeneay, B.; Al Othaim, T. Effect of Low and High Glycemic Index Meals on Hunger and Satiety. Adv. Biosci. Biotechnol. 2023, 14, 409–418. [Google Scholar] [CrossRef]
- Oliveira, G.A.L.; Santos Gonçalves, V.S.; Nakano, E.Y.; Toral, N. Consumption of ultra-processed foods and low dietary diversity are associated with sedentary and unhealthy eating behaviors: A nationwide study with Brazilian Schoolchildren. PLoS ONE 2024, 19, e0294871. [Google Scholar] [CrossRef]
- Kang, Y.; Kang, M.; Lim, H. Age-specific association between meal-skipping patterns and the risk of hyperglycemia in Korean adults: A national cross-sectional study using the KNHANES data. BMC Public Health 2024, 24, 1697. [Google Scholar] [CrossRef]
- Galdino-Silva, M.B.; Almeida, K.M.M.; Oliveira, A.D.S.D.; Santos, J.V.L.D.; Macena, M.D.L.; Silva, D.R.; Bueno, N.B. A Meal with Ultra-Processed Foods Leads to a Faster Rate of Intake and to a Lesser Decrease in the Capacity to Eat When Compared to a Similar, Matched Meal Without Ultra-Processed Foods. Nutrients 2024, 16, 4398. [Google Scholar] [CrossRef] [PubMed]
- Ducrot, P.; Méjean, C.; Aroumougame, V.; Ibanez, G.; Allès, B.; Kesse-Guyot, E.; Péneau, S. Meal planning is associated with food variety, diet quality and body weight status in a large sample of French adults. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.M.; Livingstone, K.M.; Worsley, A.; Timperio, A.; McNaughton, S.A. Meal frequency but not snack frequency is associated with micronutrient intakes and overall diet quality in Australian men and women. J. Nutr. 2016, 146, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Almoraie, N.M.; Alothmani, N.M.; Alomari, W.D.; Al-Amoudi, A.H. Addressing nutritional issues and eating behaviours among university students: A narrative review. Nutr. Res. Rev. 2025, 38, 53–68. [Google Scholar] [CrossRef] [PubMed]
| Food Groups | Types of Food | Numbers of Sample | Mean Acrylamide Level | References |
|---|---|---|---|---|
| Breads | Multi-grain bread | 12 | 79.2 µg/kg | [40,41] |
| Whole-meal bread | 10 | 83.5 µg/kg | ||
| Whole wheat bread | 12 | 76.8 µg/kg | ||
| Rye bread | 8 | 82.6 µg/kg | ||
| White bread | 10 | 87.4 µg/kg | ||
| Rize simit * | 6 | 0.75 µg/1 portion (60 g) | ||
| Sesame simit * | 6 | 6.35 µg/1 portion (60 g) | ||
| Desserts | Baklava (pistachio) | 10 | 6.49 µg/1 portion (160 g) | [41] |
| Künefe | 6 | 5.47 µg/1 portion (175 g) | ||
| Halka dessert | 6 | 37.0 µg/1 portion (125 g) | ||
| Lokma dessert | 6 | 11.3 µg/1 portion (100 g) | ||
| Tulumba dessert | 6 | 12.8 µg/1 portion (100 g) | ||
| Beverages | Black tea | 20 | 3.2 µg/1 cup (80 mL) | [24,42] |
| Turkish coffee | 8 | 1.3 µg/1 cup (70 mL) | ||
| 3-in-1 (instant coffee) | 12 | 9.6 µg/1 cup (200 mL) | ||
| 2-in-1 (instant coffee) | 8 | 9.1 µg/1 cup (200 mL) | ||
| Latte (instant coffee) | 8 | 6.8 µg/1 cup (200 mL) | ||
| Mocha (instant coffee) | 4 | 14.6 µg/1 cup (200 mL) | ||
| Americano (ready-to-drink) | 8 | 3.9 µg/1 cup (200 mL) | ||
| Filter coffee (ready-to-drink) | 8 | 3.3 µg/1 cup (200 mL) | ||
| Espresso (ready-to-drink) | 8 | 0.8 µg/1 cup (200 mL) |
| Demographic Characteristics | Number of People (n = 370) | Percentage (%) | Consumption Amounts (Mean ± SD) | ||
|---|---|---|---|---|---|
| Bread (g/day) | Desserts (g/day) | Beverage (mL/day) | |||
| Gender | |||||
| Girls | 198 | 53.5 | 65 ± 82 b | 4 ± 17 b | 119 ± 109 a |
| Boys | 172 | 46.5 | 134 ± 114 a | 11 ± 27 a | 123 ± 99 a |
| BMI | |||||
| Underweight | 68 | 18.4 | 106 ± 113 a | 7 ± 24 b | 120 ± 104 b |
| Normal | 247 | 66.8 | 93 ± 103 b | 6 ± 21 b | 123 ± 104 b |
| Overweight/Obese | 55 | 14.8 | 117 ± 99 a | 11 ± 27 a | 138 ± 97 a |
| Demographic Characteristics | NEBS | PAEB | HEEB | UEEB | MR | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Min-Max. | Mean ± SD | Min-Max. | Mean ± SD | Min-Max. | Mean ± SD | Min-Max. | Mean±SD | Min-Max. | |
| Gender | ||||||||||
| Girls | 129 ± 17.6 b | 77–179 | 29.4 ± 8.75 a | 11–53 | 40.6 ± 8.81 b | 18–64 | 39.0 ± 7.13 a | 19–62 | 20.4 ± 5.04 b | 6–30 |
| Boys | 137 ± 22.2 a | 90–202 | 29.3 ± 9.98 a | 11–55 | 46.5 ± 9.93 a | 18–69 | 38.7 ± 8.19 a | 21–62 | 22.6 ± 5.15 a | 8–30 |
| BMI | ||||||||||
| Underweight | 132 ± 19.5 a | 96–184 | 30.7 ± 10.2 a | 11–54 | 41.0 ± 8.46 a | 26–60 | 39.2 ± 6.63 a | 26–55 | 21.0 ± 4.95 a | 6–30 |
| Normal | 133 ± 19.7 a | 77–202 | 28.7 ± 8.81 a | 12–51 | 43.6 ± 9.91 a | 18–69 | 38.6 ± 7.65 a | 19–62 | 21.6 ± 5.24 a | 6–30 |
| Overweight/Obese | 136 ± 23.6 a | 94–198 | 30.5 ± 10.3 a | 16–55 | 44.8 ± 10.4 a | 18–64 | 39.8 ± 8.72 a | 25–62 | 21.0 ± 5.35 a | 8–30 |
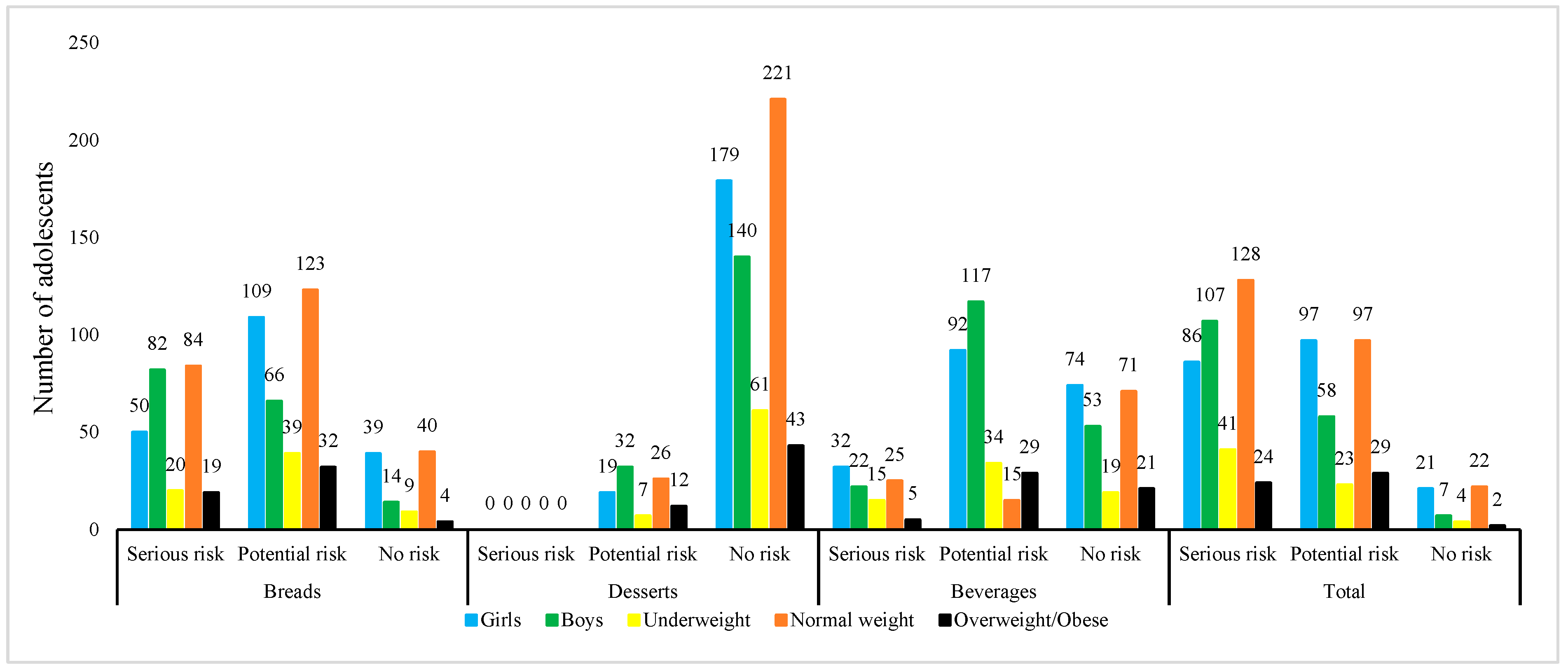
| Demographic Characteristics | DE (µg/Day) | BWE (µg/kg/Day) | THQ | CR | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (Min–Max) | Mean ± SD | Median (Min–Max) | Mean ± SD | Median (Min–Max) | Mean ± SD | Median (Min–Max) | |
| Bread | ||||||||
| Gender | ||||||||
| Girls | 5.65 ± 7.02 | 2.62 (0–37.2) b | 0.11 ± 0.14 | 0.05 (0–0.70) b | 0.05 ± 0.07 | 0.02 (0–0.35) b | 5.52 10−5 ± 7.00 10−5 (Potential risk) | 2.00 10−5 (0–3.50 10−4) b |
| Boys | 13.8 ± 11.9 | 13.1 (0–50.0) a | 0.23 ± 0.21 | 0.18 (0–0.95) a | 0.11 ± 0.11 | 0.09 (0–0.48) a | 1.13 10−4 ± 1.05 10−4 (Serious risk) | 2.30 10−5 (0–4.76 10−4) a |
| BMI | ||||||||
| Underweight | 10.1 ± 10.8 | 3.50 (0–41.9) a | 0.22 ± 0.23 | 0.08 (0–0.95) a | 0.11 ± 0.12 | 0.04 (0–0.48) a | 1.12 10−4 ± 1.22 10−4 (Serious risk) | 4.07 10−5 (0–4.76 10−4) a |
| Normal | 8.84 ± 10.3 | 3.50 (0–50.0) a | 0.15 ± 0.17 | 0.06 (0–0.82) a | 0.08 ± 0.09 | 0.03 (0–0.41) a | 1.13 10−4 ± 1.06 10−4 (Serious risk) | 8.89 10−5 (0–4.76 10−4) a |
| Overweight/Obese | 11.4 ± 10.9 | 8.24 (0–44.5) a | 0.15 ± 0.14 | 0.10 (0–0.54) a | 0.07 ± 0.07 | 0.05 (0–0.27) a | 7.43 10−5 ± 7.00 10−5 (Potential risk) | 4.90 10−5 (0–2.68 10−4) a |
| Desserts | ||||||||
| Gender | ||||||||
| Girls | 0.21 ± 0.77 | 0 (0–4.06) b | 0.00 ± 0.02 | 0 (0–0.09) b | 0 ± 0.01 | 0 (0–0.04) b | 1.94 10−6 ± 7.44 10−6 (Potential risk) | 0 (0–4.32 10−5) b |
| Boys | 0.51 ± 1.23 | 0 (0–7.01) a | 0.01 ± 0.02 | 0 (0–0.09) a | 0 ± 0.01 | 0 (0–0.05) a | 4.00 10−6 ± 9.89 10−6 (Potential risk) | 0 (0–4.51 10−5) a |
| BMI | ||||||||
| Underweight | 0.34 ± 1.04 | 0 (0–4.06) a | 0.01 ± 0.02 | 0 (0–0.09) a | 0 ± 0.01 | 0 (0–0.05) a | 3.65 10−6 ± 1.13 10−5 (Potential risk) | 0 (0–4.51 10−5) a |
| Normal | 0.27 ± 0.87 | 0 (0–4.06) a | 0.00 ± 0.02 | 0 (0–0.08) a | 0 ± 0.01 | 0 (0–0.04) a | 4.04 10−6 ± 9.94 10−6 (Potential risk) | 0 (0–4.51 10−5) a |
| Overweight/Obese | 0.67 ± 1.48 | 0 (0–7.01) a | 0.01 ± 0.02 | 0 (0–0.09) a | 0 ± 0.01 | 0 (0–0.04) a | 4.25 10−6 ± 9.60 10−6 (Potential risk) | 0 (0–4.44 10−5) a |
| Beverage | ||||||||
| Gender | ||||||||
| Girls | 5.36 ± 5.59 | 5 (0–23.7) a | 0.10 ± 0.11 | 0.09 (0–0.40) a | 0.05 ± 0.05 | 0.04 (0–0.24) a | 5.09 10−5 ± 5.31 10−5 (Potential risk) | 4.39 10−5 (0–2.36 10−4) a |
| Boys | 5.69 ± 5.21 | 5 (0–22.1) a | 0.09 ± 0.09 | 0.07 (0–0.34) a | 0.05 ± 0.04 | 0.04 (0–0.17) a | 4.69 10−5 ± 4.39 10−5 (Potential risk) | 3.52 10−5 (0–1.68 10−4) a |
| BMI | ||||||||
| Underweight | 5.25 ± 4.84 | 5 (0–18.1) a | 0.12 ± 0.11 | 0.11 (0–0.43) a | 0.06 ± 0.06 | 0.06 (0–0.22) a | 6.00 10−5 ± 5.66 10−5 (Potential risk) | 5.56 10−5 (0–2.15 10−4) a |
| Normal | 5.59 ± 5.37 | 5 (0–23.7) a | 0.10 ± 0.09 | 0.08 (0–0.47) ab | 0.05 ± 0.05 | 0.04 (0–0.24) ab | 4.74 10−5 ± 4.39 10−5 (Potential risk) | 3.57 10−5 (0–1.68 10−4) ab |
| Overweight/Obese | 5.49 ± 6.29 | 5 (0–22.1) a | 0.07 ± 0.09 | 0.06 (0–0.36) b | 0.04 ± 0.05 | 0.03 (0–0.18) b | 3.71 10−5 ± 4.46 10−5 (Potential risk) | 3.17 10−5 (0–1.82 10−4) b |
| Total | ||||||||
| Gender | ||||||||
| Girls | 11.2 ± 9.49 | 9.98 (0–45.8) b | 0.21 ± 0.19 | 0.18 (0–0.86) b | 0.11 ± 0.09 | 0.09 (0–0.43)b | 1.08 10−4 ± 9.28 10−5 (Serious risk) | 8.88 10−5 (0–4.32 10−4) b |
| Boys | 20.0 ± 14.1 | 18.1 (0–70.7) a | 0.33 ± 0.25 | 0.29 (0–1.35) a | 0.16 ± 0.13 | 0.14 (0–0.67) a | 1.64 10−4 ± 1.26 10−4 (Serious risk) | 1.43 10−4 (0–6.74 10−4) a |
| BMI | ||||||||
| Underweight | 15.7 ± 12.8 | 13.1 (0–60.7) a | 0.35 ± 0.29 | 0.31 (0–1.35) a | 0.18 ± 0.15 | 0.15 (0–0.67) a | 1.76 10−4 ± 1.47 10−5 (Serious risk) | 1.54 10−4 (0–6.74 10−4) a |
| Normal | 14.7 ± 12.2 | 12.1 (0–61.6) a | 0.25 ± 0.21 | 0.21 (0–0.86) b | 0.13 ± 0.10 | 0.11 (0–0.43) b | 1.65 10−4 ± 1.26 10−4 (Serious risk) | 1.44 10−4 (0–6.74 10−4) b |
| Overweight/Obese | 17.6 ± 14.17 | 15.1 (0–70.7) a | 0.23 ± 0.19 | 0.18 (0–0.85) b | 0.12 ± 0.09 | 0.09 (0–0.43) b | 1.16 10−4 ± 9.39 10−5 (Serious risk) | 8.91 10−5 (0–4.26 10−4) b |
| Parameters | Bread | Desserts | Beverage | Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DE (µg/Day) | BWE (µg/kg/Day) | THQ | CR | DE (µg/Day) | BWE (µg/kg/Day) | THQ | CR | DE (µg/Day) | BWE (µg/kg/Day) | THQ | CR | DE (µg/Day) | BWE (µg/kg/Day) | THQ | CR | ||
| NEBS | r | 0.184 | 0.168 | 0.168 | 0.168 | 0.118 | 0.114 | 0.114 | 0.114 | 0.111 | 0.093 | 0.093 | 0.093 | 0.170 | 0.152 | 0.152 | 0.152 |
| p | 0.000 *** | 0.001 ** | 0.001 ** | 0.001 ** | 0.023 * | 0.029 * | 0.029 * | 0.029 * | 0.033 * | 0.075 | 0.075 | 0.075 | 0.001 ** | 0.003 ** | 0.003 ** | 0.003 ** | |
| PAEB | r | 0.162 | 0.166 | 0.166 | 0.166 | 0.122 | 0.120 | 0.120 | 0.120 | 0.020 | 0.024 | 0.024 | 0.024 | 0.129 | 0.138 | 0.138 | 0.138 |
| p | 0.002 ** | 0.001 ** | 0.001 ** | 0.001 ** | 0.019 * | 0.021 * | 0.021 * | 0.021 * | 0.708 | 0.644 | 0.644 | 0.644 | 0.013 * | 0.008 ** | 0.008 ** | 0.008 ** | |
| HEEB | r | 0.100 | 0.068 | 0.068 | 0.068 | 0.061 | 0.055 | 0.055 | 0.055 | 0.074 | 0.033 | 0.033 | 0.033 | 0.095 | 0.054 | 0.054 | 0.054 |
| p | 0.055 | 0.193 | 0.193 | 0.193 | 0.244 | 0.292 | 0.292 | 0.292 | 0.157 | 0.521 | 0.521 | 0.521 | 0.067 | 0.298 | 0.298 | 0.298 | |
| UEEB | r | 0.103 | 0.103 | 0.103 | 0.103 | 0.169 | 0.167 | 0.167 | 0.167 | 0.126 | 0.122 | 0.122 | 0.122 | 0.144 | 0.143 | 0.143 | 0.143 |
| p | 0.048 * | 0.047 * | 0.047 * | 0.047 * | 0.001 ** | 0.001 ** | 0.001 ** | 0.001 ** | 0.015 * | 0.019 * | 0.019 * | 0.019 * | 0.006 ** | 0.006 ** | 0.006 ** | 0.006 ** | |
| MR | r | 0.061 | 0.050 | 0.050 | 0.050 | −0.064 | −0.064 | −0.064 | −0.064 | −0.017 | −0.021 | −0.021 | −0.021 | −0.001 | −0.010 | −0.010 | −0.010 |
| p | 0.239 | 0.338 | 0.338 | 0.338 | 0.221 | 0.221 | 0.221 | 0.221 | 0.743 | 0.687 | 0.687 | 0.687 | 0.987 | 0.855 | 0.855 | 0.855 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metin Karaaslan, M.; Basaran, B. Dietary Acrylamide Exposure and Its Correlation with Nutrition and Exercise Behaviours Among Turkish Adolescents. Nutrients 2025, 17, 2534. https://doi.org/10.3390/nu17152534
Metin Karaaslan M, Basaran B. Dietary Acrylamide Exposure and Its Correlation with Nutrition and Exercise Behaviours Among Turkish Adolescents. Nutrients. 2025; 17(15):2534. https://doi.org/10.3390/nu17152534
Chicago/Turabian StyleMetin Karaaslan, Mehtap, and Burhan Basaran. 2025. "Dietary Acrylamide Exposure and Its Correlation with Nutrition and Exercise Behaviours Among Turkish Adolescents" Nutrients 17, no. 15: 2534. https://doi.org/10.3390/nu17152534
APA StyleMetin Karaaslan, M., & Basaran, B. (2025). Dietary Acrylamide Exposure and Its Correlation with Nutrition and Exercise Behaviours Among Turkish Adolescents. Nutrients, 17(15), 2534. https://doi.org/10.3390/nu17152534





