Influence of Matcha and Tea Catechins on the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)—A Review of Patient Trials and Animal Studies
Abstract
1. Introduction
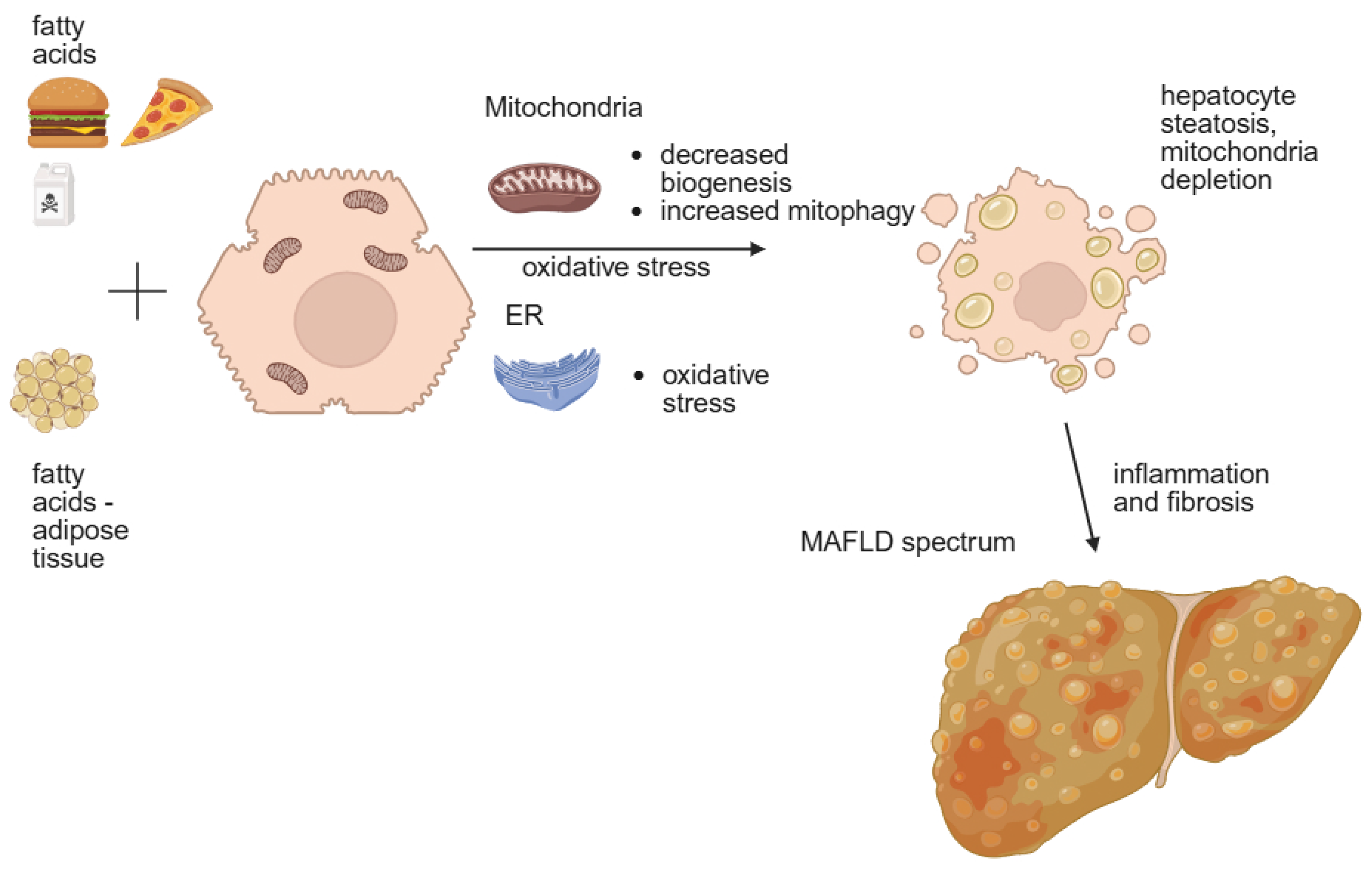
2. Factors Leading to MAFLD Development and Progression
2.1. Genetic Factors
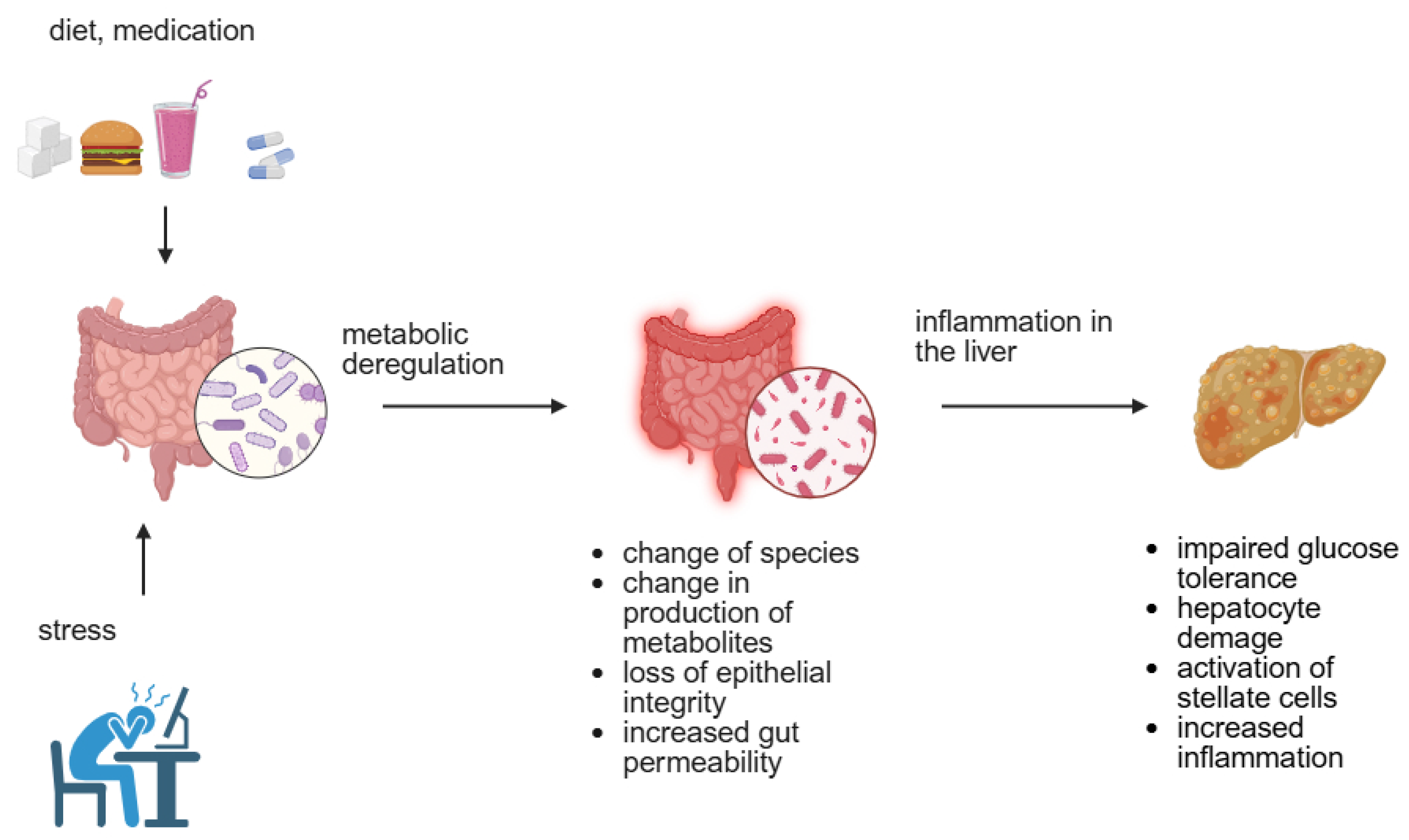
2.2. Environmental Factors
3. Health-Promoting Effects of Matcha Tea
3.1. The Impact of Matcha and Green Tea on Human Metabolism
3.2. Animal Studies with Matcha and EGCG
| Animal | Duration | Treatment | Results | Reference |
|---|---|---|---|---|
| Otsuka Long-Evans Tokushima Fatty (OLETF) rats with T2DM | 16 weeks | Matcha 50, 100, 200 mg/kg b.w./day | ≈Tg, ↓glucose, ↓TC in serum and liver, ↑SREBP-2 | [51] |
| C57BL/6J mice, M, 5 weeks old | 6 weeks | HFD (60% fat), 0.1% 0.5% 1% matcha blend with chow | ↓b.w., ↓liver w., ↓adipose tissue, ↓size of adipocytes, ↓glucose ↓Tg, ↓TC, ↓LDL/HDL ratio, ↓AST, ↓ALT- all dose dependent manner, in liver: ↓steatosis, ↓inflammatory foci, ↓Il-6, ↓ IL-1β, ↓TNF-α | [52] |
| C57BL/6J mice, M, 8 weeks old | 15 weeks oral gavage | HFD (60% fat), 200 mg/kg b.w. Matcha, daily | ↓b.w., ↓liver w. ↓adipose tissue, ≈ALT, AST (in liver), ≈TC, Tg, LDL (in serum) | [31] |
| C57BL/6J mice, M, 8 weeks old | 8 weeks | HFD (45% fat), matcha 1% in chaw | ↓b.w., ↓lipid deposits in adipose tissue, and liver, ↓cell rupture, ↓Tg, ↓LDL, ↓ALT, ↓AST | [53] |
| C57BL/6J mice, M, 8 weeks old | 8 weeks oral gavage | HFD (60% fat), Matcha 150 mg/kg b.w. /day | ↓lipid deposits in adipose, ↓inflammation in liver, ↓IL-6, ↓TNF-α, ↓TC, ↓Tg, ↓LDL, ↑HDL, ↑ microbiota richness and diversity | [54] |
| C57BL/6J mice, M, 6 weeks old | 11 weeks | 6 weeks HFD (60% fat) + 5 weeks [HFD + Matcha (1g/kg b.w. solution)] | ↓b.w., ↓adipose tissue, ↓size of adipocytes,-vs. HFD ↓glucose ↓Tg,-vs. HFD ↓TC, ↓LDL, ↑HDL-vs. C, ≈Tg-vs. C and HFD, reverse of unfavorable changes in microbiota caused by HFD | [29] |
| rats M, 12 weeks old | 4 weeks oral gavage | HFD + matcha 1.5 g/kg b.w. | ≈Tg, ≈HDL in plasma, ↓LDL, ↓fat accumulation in liver, ↓foci with Kupffer cells in liver | [55] |
| Animal | Duration | Treatment | Results | Reference |
|---|---|---|---|---|
| Wistar rats, F, (125–135 g b.w.) | 4 weeks | HFD + EGCG (0.2 g, 0.4 g, 0.7 g/kg b.w./day) | ≈b.w., ≈FFA, ≈Tg, ↓TC, ↓non-HDL-c,↓liver TC, | [58] |
| C57BL/6J mice, M, 5–6 weeks old | 16 weeks | HFD + 3.2 g/kg b.m. in diet | ↓b.w. ↓b.Fat, and visceral fat, ↓liver weight, ↓liver Tg, ↓plasma ALT, ↓lipid accumulation in liver | [59] |
| Wistar rats, M, 7 weeks old | 17 days | 50 mg/kg b.w./daily before bile duct injury | ↓AST, ↓ALT, ↑antioxidative processes, fibrotic markers: ↓FGF-α1, ↓α-SMA, ↓mRNA for AP-1, TIMP-1 | [60] |
| e ICR mice, F, 10 weeks old | 4 weeks | 0.1% EGCG in chow | ≈b.w., with caffeine: ↓intraperitoneal fat, ↓FAS | [61] |
| Beagle dogs, M, 13–14 mo. Old | 12 weeks | HFD + 0.25 g or 0.5 g/kg b.w. polyphenols | ↓b.w., ↓liver w. ↓LDL, ≈TC,↑HDL, ↓COX-2, ↓iNOs in liver, ↓TNF-α, ↓IL-1β, ↓IL-6, ↓fat droplets, ↓adipocyte size | [30] |
| Sprague-Dowley rats, M, 4 weeks old | 8 weeks | HFD (60%fat) + EGCG in nano-capsules or 100 mg/kg b.w. in 1 mL oral gavage | ↓lipids droplets, ↓size of adipocytes, ↓MDA, ↓SOD, ↓CAT, change in microbiota species | [62] |
| Balb/c mice, 6–8 weeks old | 10 days | 10 mg, 25 mg, 50 mg/kg b.w./day EGCG before LPS administration | ↓inflammation and necrosis in liver, ↓ALT, ↓AST (in plasma), ↑survival rate after LPS administration | [63] |
| C57BL/6J mice, sex and age unknown | 8 weeks | Polyphenols (70 mg/kg b.w./day) + chemical liver injury | ≈b.w, ↓lipids in liver, ↓fibrosis, ↓mitochondrial swelling | [64] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| ALT | alanine transaminase |
| AST | aspartate transaminase |
| b.w. | body weight |
| BMI | body mass index |
| CAT | catalase |
| COX-2 | cyclooxygenase type 2 |
| ER | endoplasmic reticulum |
| F | female |
| FFAs | free fatty acids |
| GCKR | glucokinase regulator gene |
| GT | green tea |
| HbA1c | glycolyzed hemoglobin |
| HCC | hepatocellular carcinoma |
| HDL | high density lipoprotein |
| HFD | High fat diet |
| HOMA-IR index | homeostasis model of insulin resistance index |
| HSD17B13 | hydroxysteroid 17β-dehydrogenase 13 |
| iNOs | inducible Nitric Oxide synthase |
| LBM | lean body mass |
| LDL-c | low density lipoprotein-cholesterol |
| M | male |
| MBOAT7 | membrane-bound O-acyltransferase 7 |
| MDA | malondialdehyde |
| MeS | metabolic syndrome |
| NAFLD | Non-alcoholic fatty liver disease |
| PNPLA3 | patatin-like phospholipase domain-containing protein 3 |
| SOD | superoxide dismutase |
| SREBP-2 | Sterol regulatory element-binding protein 2 |
| T2DM | type 2 diabetes mellitus |
| TC | total cholesterol |
| Tg | triglycerides |
| TM6SF2 | transmembrane 6 superfamily member 2 |
| WC | waist circumference |
References
- Machado, M.V. Aerobic Exercise in the Management of Metabolic Dysfunction Associated Fatty Liver Disease. Diabetes Metab. Syndr. Obes. 2021, 14, 3627–3645. [Google Scholar] [CrossRef]
- Marques, P.; Francisco, V.; Martínez-Arenas, L.; Carvalho-Gomes, Â.; Domingo, E.; Piqueras, L.; Berenguer, M.; Sanz, M.J. Overview of cellular and soluble mediators in systemic inflammation associated with non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2023, 24, 2313. [Google Scholar] [CrossRef]
- Pan, Z.; Khatry, M.A.; Yu, M.L.; Choudhury, A.; Sebastiani, G.; Alqahtani, S.A.; Eslam, M. MAFLD: An ideal framework for understanding disease phenotype in individuals of normal weight. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188241252543. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Schattenberg, J.M. NAFLD in the elderly. Clin. Interv. Aging. 2021, 16, 1633–1649. [Google Scholar] [CrossRef]
- Lin, Y.; Feng, X.; Cao, X.; Miao, R.; Sun, Y.; Li, R.; Ye, J.; Zhong, B. Age patterns of nonalcoholic fatty liver disease incidence: Heterogeneous associations with metabolic changes. Diabetol. Metab. Syndr. 2022, 14, 181. [Google Scholar] [CrossRef]
- Riazi, K.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. Race and ethnicity in non-alcoholic fatty liver disease (NAFLD): A narrative review. Nutrients 2022, 14, 4556. [Google Scholar] [CrossRef]
- Pal, P.; Palui, R.; Ray, S. Heterogeneity of non-alcoholic fatty liver disease: Implications for clinical practice and research activity. World J. Hepatol. 2021, 13, 1584–1610. [Google Scholar] [CrossRef]
- Kubiliun, M.J.; Cohen, J.C.; Hobbs, H.H.; Kozlitina, J. Contribution of a genetic risk score to ethnic differences in fatty liver disease. Liver Int. 2022, 42, 2227–2236. [Google Scholar] [CrossRef]
- Pirola, C.J.; Fernández Gianotti, T.; Castaño, G.O.; Mallardi, P.; San Martino, J.; Mora Gonzalez Lopez Ledesma, M.; Flichman, D.; Mirshahi, F.; Sanyal, A.J.; Sookoian, S. Circulating microRNA signature in non-alcoholic fatty liver disease: From serum non-coding RNAs to liver histology and disease pathogenesis. Gut 2015, 64, 800–812. [Google Scholar] [CrossRef]
- Hardy, T.; Zeybel, M.; Day, C.P.; Dipper, C.; Masson, S.; McPherson, S.; Henderson, E.; Tiniakos, D.; White, S.; French, J.; et al. Plasma DNA methylation: A potential biomarker for stratification of liver fibrosis in non-alcoholic fatty liver disease. Gut 2017, 66, 1321–1328. [Google Scholar] [CrossRef]
- Darci-Maher, N.; Alvarez, M.; Arasu, U.T.; Selvarajan, I.; Lee, S.H.T.; Pan, D.Z.; Miao, Z.; Das, S.S.; Kaminska, D.; Örd, T.; et al. Cross-tissue omics analysis discovers ten adipose genes encoding secreted proteins in obesity-related non-alcoholic fatty liver disease. EBioMedicine 2023, 92, 104620. [Google Scholar] [CrossRef]
- Della Torre, S. Non-alcoholic Fatty Liver Disease as a Canonical Example of Metabolic Inflammatory-Based Liver Disease Showing a Sex-Specific Prevalence: Relevance of Estrogen Signaling. Front. Endocrinol. 2020, 11, 572490. [Google Scholar] [CrossRef]
- Associazione Italiana per lo Studio del Fegato (AISF); Società Italiana di Diabetologia (SID); Società Italiana dell’Obesità (SIO). Non-alcoholic fatty liver disease in adults 2021: A clinical practice guideline of the Italian Association for the Study of the Liver (AISF), the Italian Society of Diabetology (SID) and the Italian Society of Obesity (SIO). Eat. Weight. Disord. 2022, 27, 1603–1619, Erratum in Eat. Weight. Disord. 2023, 28, 27. [Google Scholar] [CrossRef]
- Kim, H.K.; Bae, S.J.; Lee, M.J.; Kim, E.H.; Park, H.; Kim, H.S.; Cho, Y.K.; Jung, C.H.; Lee, W.J.; Choe, J. Association of visceral fat obesity, sarcopenia, and myosteatosis with non-alcoholic fatty liver disease without obesity. Clin. Mol. Hepatol. 2023, 29, 987–1001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lan, Y.; Lu, Y.; Li, J.; Hu, S.; Chen, S.; Wang, Y.; Yuan, X.; Liu, H.; Wang, X.; Wu, S.; et al. Outcomes of subjects who are lean, overweight or obese with nonalcoholic fatty liver disease: A cohort study in China. Hepatol. Commun. 2022, 6, 3393–3405. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, Y.; Zhou, K.; Zheng, Y.; Chen, Y.; Li, Q.; Zhu, C.; Xia, F.; Gu, T.; Guo, Y.; et al. Comparison of abdominal obesity and fatty liver and their association with insulin resistance and metabolic syndrome in Chinese adults. Obesity 2019, 27, 707–715. [Google Scholar] [CrossRef]
- Bahitham, W.; Alghamdi, S.; Omer, I.; Alsudais, A.; Hakeem, I.; Alghamdi, A.; Abualnaja, R.; Sanai, F.M.; Rosado, A.S.; Sergi, C.M. Double trouble: How microbiome dysbiosis and mitochondrial dysfunction drive non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Biomedicines 2024, 12, 550. [Google Scholar] [CrossRef]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the role of the gut microbiome and microbial metabolites in non-alcoholic fatty liver disease: Current evidence and perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017, 25, 1054–1062.e5, Erratum in Cell Metab. 2019, 30, 607. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Q.; Li, D.; Chen, S.; Chen, J.; Zhu, X.; Bai, F. Gut microbiome composition and metabolic activity in metabolic-associated fatty liver disease. Virulence 2025, 16, 2482158. [Google Scholar] [CrossRef]
- Kim, J.M.; Heo, H.J. The roles of catechins in regulation of systemic inflammation. Food Sci. Biotechnol. 2022, 31, 957–970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sokary, S.; Zakaria, Z.; Bawadi, H.; Al-Asmakh, M. Testing the anticancer effect of matcha using zebrafish as an animal model. Nutrients 2023, 15, 2369. [Google Scholar] [CrossRef]
- Liu, S.; Fan, B.; Li, X.; Sun, G. Global hotspots and trends in tea anti-obesity research: A bibliometric analysis from 2004 to 2024. Front. Nutr. 2024, 11, 1496582. [Google Scholar] [CrossRef]
- Sae-tan, S.; Grove, K.A.; Lambert, J.D. Weight control and prevention of metabolic syndrome by green tea. Pharmacol. Res. 2011, 64, 146–154. [Google Scholar] [CrossRef]
- Rha, C.S.; Jung, Y.S.; Lee, J.D.; Jang, D.; Kim, M.S.; Lee, M.S.; Hong, Y.D.; Kim, D.O. Chemometric analysis of extracts and fractions from green, oxidized, and microbial fermented teas and their correlation to potential antioxidant and anticancer effects. Antioxidants 2020, 9, 1015. [Google Scholar] [CrossRef]
- Rusak, G.; Šola, I.; Vujčić Bok, V. Matcha and Sencha green tea extracts with regard to their phenolics pattern and antioxidant and antidiabetic activity during in vitro digestion. J. Food Sci. Technol. 2021, 58, 3568–3578. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef]
- Luo, Y.; Wen, Y.; Huang, J.; Chen, B.; Lv, S.; Qiu, H.; Li, S.; Liu, S.; Yang, Q.; He, L.; et al. Matcha alleviates obesity by modulating gut microbiota and its metabolites. Curr. Res. Food Sci. 2024, 9, 100823. [Google Scholar] [CrossRef]
- Rahman, S.U.; Huang, Y.; Zhu, L.; Chu, X.; Junejo, S.A.; Zhang, Y.; Khan, I.M.; Li, Y.; Feng, S.; Wu, J.; et al. Tea polyphenols attenuate liver inflammation by modulating obesity-related genes and down-regulating COX-2 and iNOS expression in high fat-fed dogs. BMC Vet. Res. 2020, 16, 234. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Mao, Q.-Q.; Li, B.-Y.; Saimaiti, A.; Huang, S.-Y.; Xiong, R.-G.; Shang, A.; Luo, M.; Li, H.-Y.; Gan, R.-Y.; et al. Effects of different green teas on obesity and non-alcoholic fatty liver disease induced by a high-fat diet in mice. Front. Nutr. 2022, 9, 929210. [Google Scholar] [CrossRef]
- James, A.; Wang, K.; Wang, Y. Therapeutic activity of green tea epigallocatechin-3-gallate on metabolic diseases and non-alcoholic fatty liver diseases: The current updates. Nutrients 2023, 15, 3022. [Google Scholar] [CrossRef]
- Capasso, L.; De Masi, L.; Sirignano, C.; Maresca, V.; Basile, A.; Nebbioso, A.; Rigano, D.; Bontempo, P. Epigallocatechin gallate (EGCG): Pharmacological properties, biological activities and therapeutic potential. Molecules 2025, 30, 654. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Szymczykowska, K.; Kika, J.; Janda-Milczarek, K.; Palma, J.; Melkis, K.; Alshekh, R.; Maciejewska-Markiewicz, D. Exploring the influence of origin, harvest time, and cultivation method on antioxidant capacity and bioactive compounds of matcha teas. Foods 2024, 13, 1270. [Google Scholar] [CrossRef]
- Najman, K.; Sadowska, A.; Wolińska, M.; Starczewska, K.; Buczak, K. The content of bioactive compounds and technological properties of matcha green tea and its application in the design of functional beverages. Molecules 2023, 28, 7018. [Google Scholar] [CrossRef]
- Sokary, S.; Al-Asmakh, M.; Zakaria, Z.; Bawadi, H. The therapeutic potential of matcha tea: A critical review on human and animal studies. Curr. Res. Food Sci. 2022, 6, 100396. [Google Scholar] [CrossRef]
- Meyer, B.R.; White, H.M.; McCormack, J.D.; Niemeyer, E.D. Catechin Composition, Phenolic Content, and Antioxidant Properties of Commercially-Available Bagged, Gunpowder, and Matcha Green Teas. Plant Foods Hum. Nutr. 2023, 78, 662–669. [Google Scholar] [CrossRef]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef]
- Hsu, C.H.; Liao, Y.L.; Lin, S.C.; Tsai, T.H.; Huang, C.J.; Chou, P. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebo-controlled clinical trial. Altern. Med. Rev. 2011, 16, 157–163. [Google Scholar] [PubMed]
- Yang, H.Y.; Yang, S.C.; Chao, J.C.; Chen, J.R. Beneficial effects of catechin-rich green tea and inulin on the body composition of overweight adults. Br. J. Nutr. 2012, 107, 749–754. [Google Scholar] [CrossRef]
- Cardoso, G.A.; Salgado, J.M.; Cesar Mde, C.; Donado-Pestana, C.M. The effects of green tea consumption and resistance training on body composition and resting metabolic rate in overweight or obese women. J. Med. Food 2013, 16, 120–127. [Google Scholar] [CrossRef]
- Wang, X.; Tian, J.; Jiang, J.; Li, L.; Ying, X.; Tian, H.; Nie, M. Effects of green tea or green tea extract on insulin sensitivity and glycaemic control in populations at risk of type 2 diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. J. Hum. Nutr. Diet. 2014, 27, 501–512. [Google Scholar] [CrossRef]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef]
- Alves Ferreira, M.; Oliveira Gomes, A.P.; Guimarães de Moraes, A.P.; Ferreira Stringhini, M.L.; Mota, J.F.; Siqueira Guedes Coelho, A.; Borges Botelho, P. Green tea extract outperforms metformin in lipid profile and glycaemic control in overweight women: A double-blind, placebo-controlled, randomized trial. Clin. Nutr. ESPEN 2017, 22, 1–6. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Şahin, M.A.; Cook, M.D. Matcha green tea drinks enhance fat oxidation during brisk walking in females. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 536–541. [Google Scholar] [CrossRef]
- Huang, L.H.; Liu, C.Y.; Wang, L.Y.; Huang, C.J.; Hsu, C.H. Effects of green tea extract on overweight and obese women with high levels of low density-lipoprotein-cholesterol (LDL-C): A randomised, double-blind, and cross-over placebo-controlled clinical trial. BMC Complement. Altern. Med. 2018, 18, 294. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Fry, H.L.; Belding, M.A.; Kaviani, M. Three weeks daily intake of matcha green tea powder affects substrate oxidation during moderate-intensity exercise in females. J. Diet. Suppl. 2021, 18, 566. [Google Scholar] [CrossRef]
- Roberts, J.D.; Willmott, A.G.B.; Beasley, L.; Boal, M.; Davies, R.; Martin, L.; Chichger, H.; Gautam, L.; Del Coso, J. The impact of decaffeinated green tea extract on fat oxidation, body composition and cardio-metabolic health in overweight, recreationally active individuals. Nutrients 2021, 13, 764. [Google Scholar] [CrossRef]
- El-Elimat, T.; Qasem, W.M.; Al-Sawalha, N.A.; AbuAlSamen, M.M.; Munaiem, R.T.; Al-Qiam, R.; Al Sharie, A.H. A prospective non-randomized open-label comparative study of the effects of matcha tea on overweight and obese individuals: A pilot observational study. Plant Foods Hum. Nutr. 2022, 77, 447–454. [Google Scholar] [CrossRef]
- Morishima, S.; Kawada, Y.; Fukushima, Y.; Takagi, T.; Naito, Y.; Inoue, R. A randomized, double-blinded study evaluating effect of matcha green tea on human fecal microbiota. J. Clin. Biochem. Nutr. 2023, 72, 165–170. [Google Scholar] [CrossRef]
- Yamabe, N.; Kang, K.S.; Hur, J.M.; Yokozawa, T. Matcha, a powdered green tea, ameliorates the progression of renal and hepatic damage in type 2 diabetic OLETF rats. J. Med. Food. 2009, 12, 714–721. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, Y.; Ding, L.; Xu, P.; Wang, Y. Matcha green tea alleviates non-alcoholic fatty liver disease in high-fat diet-induced obese mice by regulating lipid metabolism and inflammatory responses. Nutrients 2021, 13, 1950. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Ding, L.; Xu, P.; Zhou, J. Matcha green tea targets the gut-liver axis to alleviate obesity and metabolic disorders induced by a high-fat diet. Front. Nutr. 2022, 9, 931060. [Google Scholar] [CrossRef]
- Wang, J.; Dong, L.; Hu, J.Q.; Wang, Y.Y.; Li, A.; Peng, B.; Zhang, B.W.; Liu, J.M.; Wang, S. Differential regulation and preventive mechanisms of green tea powder with different quality attributes on high-fat diet-induced obesity in mice. Front. Nutr. 2022, 9, 992815. [Google Scholar] [CrossRef]
- Mohammed, S.H.; Shakor, J.K.; Salih, M.; Khafar, K.; Ali, H.M.; Baqi, H.R.; Karim, D.H.; Muhammed, S.J.; Khdhir, C.J.; Raouf, C. A comparative effect of different herbal products on lipid metabolism and hepatic tissue: An experimental study on a rat model. Cureus 2024, 16, e73799. [Google Scholar] [CrossRef]
- Pezeshki, A.; Safi, S.; Feizi, A.; Askari, G.; Karami, F. The Effect of green tea extract supplementation on liver enzymes in patients with non-alcoholic fatty liver disease. Int. J. Prev. Med. 2016, 7, 28. [Google Scholar] [CrossRef]
- Torres, L.F.; Cogliati, B.; Otton, R. Green tea prevents NAFLD by modulation of mir-34a and mir-194 expression in a high-fat diet mouse model. Oxid. Med. Cell Longev. 2019, 2019, 4168380. [Google Scholar] [CrossRef]
- Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003, 14, 326–332. [Google Scholar] [CrossRef]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tanaka, Y.; Asagiri, K.; Asakawa, T.; Tanikawa, K.; Kage, M.; Yagi, M. The antioxidant effect of green tea catechin ameliorates experimental liver injury. Phytomedicine 2010, 17, 197–202. [Google Scholar] [CrossRef]
- Sugiura, C.; Nishimatsu, S.; Moriyama, T.; Ozasa, S.; Kawada, T.; Sayama, K. Catechins and caffeine inhibit fat accumulation in mice through the improvement of hepatic lipid metabolism. J. Obes. 2012, 2012, 520510. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, B.; Gong, Z.; Huang, H.; Gong, Y.; Xiao, W. Metagenomics approach to the intestinal microbiome structure and abundance in high-fat-diet-induced hyperlipidemic rat fed with (-)-epigallocatechin-3-gallate nanoparticles. Molecules 2022, 27, 4894. [Google Scholar] [CrossRef]
- Xu, T.; Liu, R.; Zhu, H.; Zhou, Y.; Pei, T.; Yang, Z. The inhibition of lps-induced oxidative stress and inflammatory responses is associated with the protective effect of (-)-epigallocatechin-3-gallate on bovine hepatocytes and murine liver. Antioxidants 2022, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhao, X.H.; Peng, Q.; Zhou, X.L.; Liu, S.S.; Sun, C.C.; Cao, Q.Y.; Zhu, S.P.; Sun, S.Y. Green tea polyphenols alleviate di-(2-ethylhexyl) phthalate-induced liver injury in mice. World J. Gastroenterol. 2023, 29, 5054–5074. [Google Scholar] [CrossRef] [PubMed]
| Participants (Number) | Duration | Type of Tea Used in the Study | Results | Other | Reference |
|---|---|---|---|---|---|
| 35, obese, MeS | 8 weeks | Green tea cups or capsules of EGCG | ↓b.w. BMI, lipid peroxidation (↓MDA) | [38] | |
| 68, obese T2DM | 16 weeks | 1.5 g decaffeinated green tea (EGCG) | ↓HbA1c, ↓WC, ↓HOMA-IR index,↓ insulin | [39] | |
| 30 | 6 weeks | Green tea + inulin | ↓b.w., ↓fat mass, ↓WC | [40] | |
| 36 overweight | 8 weeks | Green tea + exercise | ↓WC (green tea), | GT + exercise: ↓fat, ↓tg, ↑LBM | [41] |
| 510 risk of T2DM | -- | Green tea/GT extracts | ≈plasma fasting glucose, insulin HbA1c, HOMA-IR index | Review of 7 randomized control trials | [42] |
| 102, central obesity | 12 weeks | EGCG (Green tea) | ↓BMI, WC, LDL-c, tend to decrease: TC | [43] | |
| 32, overweight non-diabetic | 12 weeks | 1 g /daily extract GT | ↓fasting glucose, ↓TC, ↓LDL-c | [44] | |
| 13 females | Twice: 1 day before and 2 h before exercise | 1g matcha | ↓respiratory exchange ratio, ↑ fat oxidation | Exercise: 30 min brisk walk | [45] |
| 73 overweight or obese | 14 weeks (6 + 2 + 6) | GT extracts (EGCG) | ≈TC, Tg, HDL, b.w., BMI, WC, ↓LDL-c, ↑leptin | Switch between GT and C groups -each group was C and GT | [46] |
| 12 | 3 weeks | 1 g matcha capsules | ↓respiratory exchange ratio, ↑fat oxidation | +moderate walking | [47] |
| 27 healthy overweight | 8 weeks | decaffeinated green tea +EGCG or + quercitin | ↑maximal fat oxidation, adiponectin, ↓ALT, ≈AST, ALP | [48] | |
| 34 | 12 weeks | Matcha (2 g in beverage daily) | ↓HbA1c, fasting glucose, ↑IL-10 | With low calorie diet | [49] |
| 33 young, BMI in norm | 2 weeks | 1.5 g matcha in capsules (beverage twice a day) | ↑ diversity of microbiota, ↑ Caprococcus spp., ↓Fusobacterium spp. | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosik-Bogacka, D.I.; Piotrowska, K. Influence of Matcha and Tea Catechins on the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)—A Review of Patient Trials and Animal Studies. Nutrients 2025, 17, 2532. https://doi.org/10.3390/nu17152532
Kosik-Bogacka DI, Piotrowska K. Influence of Matcha and Tea Catechins on the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)—A Review of Patient Trials and Animal Studies. Nutrients. 2025; 17(15):2532. https://doi.org/10.3390/nu17152532
Chicago/Turabian StyleKosik-Bogacka, Danuta I., and Katarzyna Piotrowska. 2025. "Influence of Matcha and Tea Catechins on the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)—A Review of Patient Trials and Animal Studies" Nutrients 17, no. 15: 2532. https://doi.org/10.3390/nu17152532
APA StyleKosik-Bogacka, D. I., & Piotrowska, K. (2025). Influence of Matcha and Tea Catechins on the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)—A Review of Patient Trials and Animal Studies. Nutrients, 17(15), 2532. https://doi.org/10.3390/nu17152532







