Three Major Deficiency Diseases Harming Mankind (Protein, Retinoid, Iron) Operate Under Tryptophan Dependency
Abstract
1. Introductory Steps
2. Historical Background
3. The Discovery of LBM, TTR, and RBP in Healthy Subjects
4. Energy and Protein Requirements in Healthy Subjects
5. LBM and W in Inflammatory Disorders
5.1. The Primary LBM Domain
5.2. The Second LBM Domain
5.3. Contributory Roles Played by Major APRs
5.4. Fe-Depleted Patients Reveal Declining Plasma W Values Correlated to Iron Indices
5.5. A Scoring Formula Comprising the Main APRs Allows US to Tackle Stress Responses
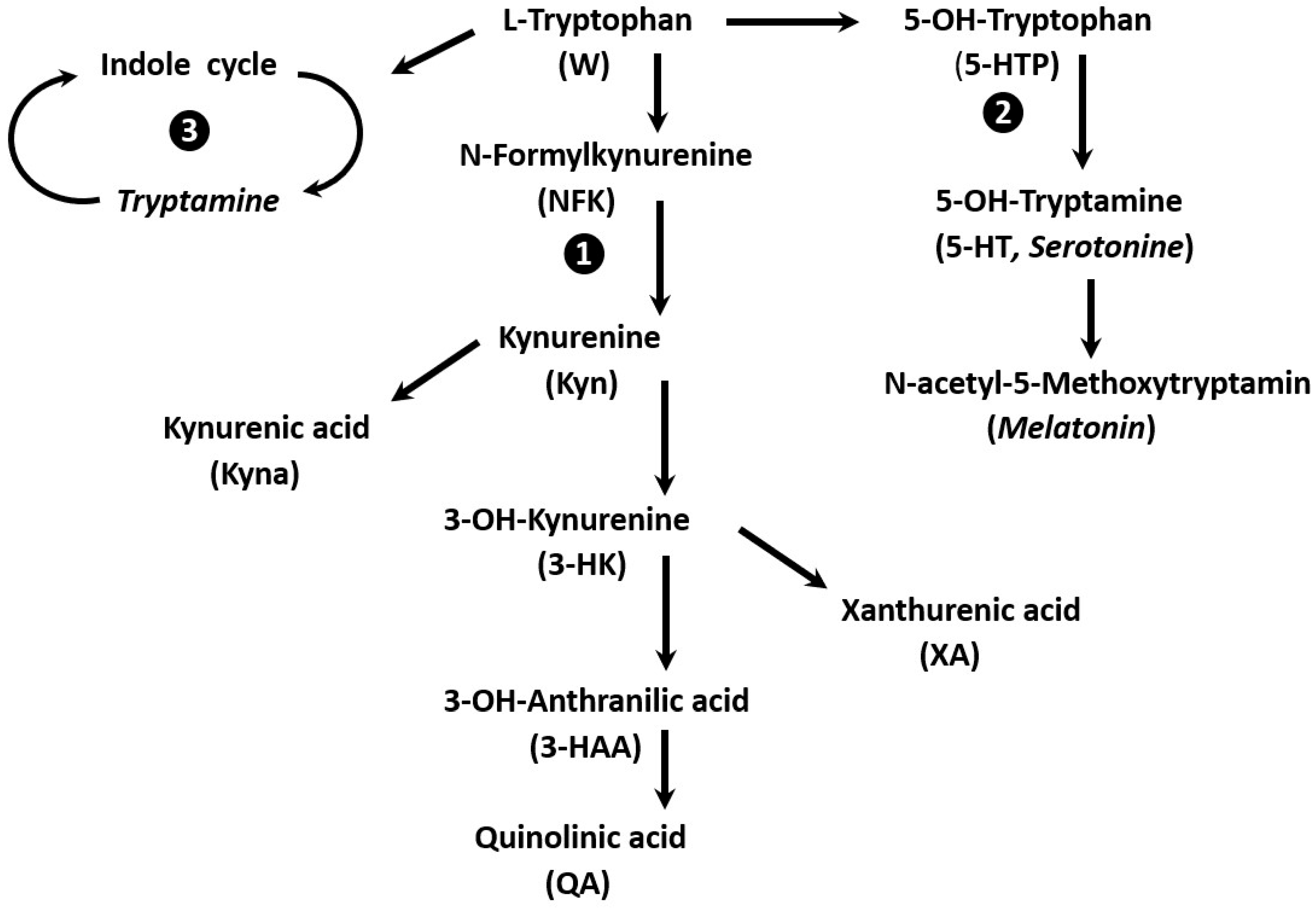
6. W Catabolism: Disparity of Immune Responses and Specific Therapeutic Approaches
6.1. Specificities of W Metabolism in Developing Countries
6.2. Specificities of W Metabolism in Brain Disorders
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan metabolism in health and disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Török, N.; Tanaka, M.; Vécsei, L. Searching for peripheral biomarkers in neurodegenerative diseases: The tryptophan-kynurenine metabolic pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef] [PubMed]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CSN: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Turner, E.H.; Loftis, J.M.; Blackwell, A.D. Serotonine à la carte: Supplementation with the serotonine precursor 5-hydroxytryptophan. Pharmacol. Ther. 2006, 109, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Kearns, R. Gut-brain axis and neuroinflammation: The role of gut permeability and the kynurenine pathway in neurological disorders. Cell Mol. Neurobiol. 2024, 44, 64. [Google Scholar] [CrossRef]
- Hestad, K.; Alexander, J.; Rootwel, H.; Aaseth, J.O. The role of tryptophan dysmetabolism and quinolinic acid in depressive and neurodegenerative diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef]
- Duan, K.M.; Ma, J.H.; Wang, S.Y.; Huang, S.Y.; Zhou, Y.Y.; Yu, H. The role of tryptophan metabolism in postpartum depression. Metab. Brain Dis. 2018, 33, 647–660. [Google Scholar] [CrossRef]
- Mugge, L.; Mansour, T.R.; Crippen, M.; Alam, M.; Schroeder, J. Depression and glioblastoma, complicated concomitant diseases: A systemic review of published literature. Neurosurg. Rev. 2020, 43, 497–511. [Google Scholar] [CrossRef]
- Fathi, M.; Vakili, K.; Yaghoobpoor, S.; Tavasol, A.; Jazi, K.; Hajibeygi, R.; Shool, S.; Sodeifian, F.; Klegeris, A.; McElhinney, A.; et al. Dynamic changes in metabolites of the kynurenine pathway in Alzheimer’s disease, Parkinson’s disease, and Huntington’s diseases: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 997240. [Google Scholar] [CrossRef]
- Almulla, A.F.; Thipakorn, Y.; Zhou, B.; Vojdani, A.; Paunov, R.; Maes, M. The tryptophan catabolite or kynurenine pathway in long COVID disease: A systematic review and meta-analysis. Neuroscience 2024, 563, 268–277. [Google Scholar] [CrossRef]
- Williams, C.D. A nutritional disease of childhood associated with a maize diet. Arch. Dis. Child. 1933, 48, 423–433. [Google Scholar] [CrossRef]
- Brock, J.F.; Autret, M. Kwashiorkor in Africa. Bull. World Health Organ. 1952, 5, 1–71. [Google Scholar] [PubMed]
- Anderson, C.G.; Altmann, A. The electrophoretic serum-protein pattern in malignant malnutrition. Lancet 1951, 257, 203–204. [Google Scholar] [CrossRef]
- Bistrian, B.R.; Blackburn, G.; Vitale, J.; Cochran, D.; Naylor, J. Prevalence of malnutrition in general medical patients. JAMA 1976, 235, 1567–1570. [Google Scholar] [CrossRef]
- Hill, G.L.; Blackett, R.L.; Pickford, I.; Burkinshaw, L.; Young, G.A.; Warren, J.V.; Schorah, C.J.; Morgan, D.B. Malnutrition in surgical patients. An unrecognized problem. Lancet 1977, 309, 689–692. [Google Scholar] [CrossRef]
- Ingbar, S.H. Prealbumin: A thyroxine binding protein of human plasma. Endocrinology 1958, 63, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y.; De Visscher, M.; De Nayer, P. Measurement of prealbumin as index of protein-calorie malnutrition. Lancet 1972, 300, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Schultze, H.E.; Schönenberger, M.; Schwick, G. Uber eine präalbumin des menschlishen serums. Biochem. Z. 1956, 328, 267–284. [Google Scholar]
- Bleiberg-Daniel, F.; Le Moullac, B.; Maire, J.C.; Wade, S. Failure of tryptophan deficiency to reduce specifically serum levels of transthyretin or albumin in rats. J. Nutr. 1990, 120, 1610–1616. [Google Scholar] [CrossRef]
- Kanda, Y.; Goodman, D.S.; Canfield, R.E.; Morgan, F.J. The amino acid sequence of human plasma prealbumin. J. Biol. Chem. 1974, 249, 6796–6805. [Google Scholar] [CrossRef]
- Kanai, M.; Raz, A.; Goodman, D.S. Retinol-binding protein: The transport protein for vitamin A in human plasma. J. Clin. Investig. 1968, 47, 2025–2044. [Google Scholar] [CrossRef] [PubMed]
- Goodman, D.S.; Peters, T.; Robbins, J.; Schwick, G. Prealbumin becomes transthyretin. Nomenclature Committee-IUB and JCBN Newsletter. J. Biol. Chem. 1981, 256, 571–577. [Google Scholar]
- Monaco, H.L. The transthyretin-retinol binding protein complex. In Recent Advances in Transthyretin, Structure and Biological Functions; Richardson, S.J., Cody, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 123–143. [Google Scholar]
- Rose, W. Protein Requirements and their Fulfillment in Practice. In Proceedings of a Conference in Princetown, USA, 1955; Waterlow, J.C., Stephen, J.M.L., Eds.; John Wright & Sons: Bristol, UK, 1957. [Google Scholar]
- Straus, D.S.; Marten, N.W.; Hayden, J.M.; Burke, E.J. Protein restriction specifically decreases the abundance of serum albumin and transthyretin nuclear transcripts in rat liver. J. Nutr. 1994, 124, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Forbes, G.B. Human Body Composition, Growth, Aging, Nutrition, and Activity; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Cohn, S.H.; Vartsky, D.; Yasumura, S.; Vaswani, A.N.; Ellis, K.J. Indexes of body cell mass: Nitrogen versus potassium. Am. J. Physiol. 1983, 27, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, J.; Jeppson, J.O.; Ingenbleek, Y. Transthyretin & Retinol-binding protein. In Serum Proteins in Clinical Medicine; Ritchie, R.F., Navolotskaia, O., Eds.; Foundation for Blood Research: Scarborough, ME, USA, 1996. [Google Scholar]
- Ingenbleek, Y. Plasma transthyretin is a nutritional biomarker in human morbidities. Front. Med. 2022, 16, 540–550. [Google Scholar] [CrossRef]
- Sergi, G.; Coin, A.; Enzi, G.; Volpato, S.; Inelmen, E.M.; Buttarello, M.; Peloso, M.; Mulone, S.; Marin, S.; Bonometto, P. Role of visceral proteins in detecting malnutrition in the elderly. Eur. J. Clin. Nutr. 2006, 60, 203–209. [Google Scholar] [CrossRef]
- Takeda, H.; Ishihama, K.; Fukui, T.; Fujishima, S.; Orii, T.; Nakazama, Y.; Shu, H.J.; Kawata, S. Significance of rapid turnover proteins in protein-losing gastroenteropathy. Hepatogastroenterology 2003, 50, 1963–1965. [Google Scholar]
- Beetham, R.; Dawnay, A.; Ghany, C.; Dubrey, S.; Miles, J. A radioimmunoassay for human urinary prealbumin. Ann. Clin. Biochem. 1993, 30, 377–382. [Google Scholar] [CrossRef]
- World Health Organization. Serum Retinol Concentrations for Determining the Prevalence of Vitamin a Deficiency in Populations; World Health Organization: Geneva, Switzerland, 2011; pp. 1–5. [Google Scholar]
- Steinhoff, J.S.; Lass, A.; Schupp, M. Retinoid homeostasis and beyond: How retinol binding protein 4 contributes to health and disease. Nutrients 2022, 14, 1236. [Google Scholar] [CrossRef]
- Stephensen, C.B.; Alvarez, J.O.; Kohatsu, J.; Hardmeier, R.; Kennedy, J.J., Jr.; Gammon, R.B., Jr. Vitamin A is excreted in the urine during acute infection. Am. J. Clin. Nutr. 1994, 60, 388–392. [Google Scholar] [CrossRef]
- Gradel, K.O. Interpretations of the role of plasma albumin in prognostic indices: A literature review. J. Clin. Med. 2023, 12, 6132. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Müller, M.J.; Bosy-Westphal, A.; Thomas, D.; Shen, W. Human brain mass: Similar body composition associations as observed across mammals. Am. J. Hum. Biol. 2012, 24, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Nakshabendi, I.M.; McKee, R.; Downie, S.; Russell, R.I.; Rennie, M.J. Rates of small intestinal mucosal protein synthesis in human jejunum and ileum. Am. J. Physiol. 1999, 277, E1028–E1031. [Google Scholar] [CrossRef]
- McNurlan, M.A.; Sandgren, A.; Hunter, K.; Essén, P.; Garlick, P.J.; Wernerman, J. Protein synthesis rates of skeletal muscle, lymphocytes and albumin with stress hormone infusion in healthy man. Metabolism 1996, 45, 1388–1394. [Google Scholar] [CrossRef]
- Lasztity, N.; Biro, L.; Nemeth, E.; Pap, A.; Antal, M. Protein status in pancreatitis. Transthyretin is a sensitive biomarker of malnutrition in acute and chronic pancreatitis. Clin. Chem. Lab. Med. 2002, 40, 1320–1324. [Google Scholar] [CrossRef]
- Elhasid, R.; Laor, A.; Lischinsky, S.; Postovsky, S.; Weyl Ben Arush, M. Nutritional status of children with solid tumors. Cancer 1999, 86, 119–125. [Google Scholar] [CrossRef]
- McMillan, S.A.; Dickey, W.; Douglas, J.P.; Hughes, D.F. Transthyretin values correlate with mucosal recovery in patients with coeliac disease taking a gluten free diet. J. Clin. Pathol. 2001, 54, 783–786. [Google Scholar] [CrossRef]
- Doi, N.; Kakukawa, K.; Oishi, Y.; Yanagawa, H. High solubility of random-sequence proteins consisting of five kinds of primitive amino acids. Protein Eng. Des. Sel. 2005, 18, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.M.; Tenorio, C.A.; Kumru, O.S.; Middaugh, R.; Blaber, M. A single aromatic core mutation converts a designed “primitive” protein from halophile to mesophile folding. Protein Sci. 2015, 24, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Baumann, U.; Oro, J. Three stages in the evolution of the genetic code. Biosystems 1993, 29, 133–141. [Google Scholar] [CrossRef]
- Weber, A.L.; Miller, R.H. Reason for the occurrence of the twenty coded protein amino acids. J. Mol. Evol. 1981, 17, 273–284. [Google Scholar] [CrossRef]
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef]
- Li, C.H.; Dixon, J.S.; Liu, W.K. Human pituitary growth hormone. XIX. The primary structure of the hormone. J. Am. Chem. Soc. 1966, 88, 2050–2051. [Google Scholar] [CrossRef]
- Sanger, F. Chemistry of insulin: Determination of the structure of insulin opens the way to greater understanding of life processes. Science 1959, 129, 1340–1344. [Google Scholar] [CrossRef]
- Rinderknecht, E.; Humbel, R.E. The amino acid sequence of human insulin-like growth factor 1 and its structural homology with proinsulin. J. Biol. Chem. 1978, 253, 2769–2776. [Google Scholar] [CrossRef]
- Bromer, W.W.; Staub, A.; Sinn, L.G.; Behrens, O.K. The amino acid sequence of glucagon. Diabetes 1957, 6, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.M.; Seakins, A. Fasting pancreatic glucagon in Jamaican children during malnutrition and subsequent recovery. Pediatr. Res. 1982, 16, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.K.; Park, H.J.; Macleod, M.; Chandler, P.; Munn, D.H.; Mellor, A.L. Tryptophan deprivation sensitizes activated cells to apoptosis prior to cell division. Immunology 2002, 107, 452–460. [Google Scholar] [CrossRef]
- Summers, B.S.; Broome, S.T.; Pang, T.W.R.; Mundell, H.D.; Belic, N.K.; Tom, N.C.; Yap, M.; Sen, M.K.; Sedaghat, S.; Weible, M.W.; et al. A review of the evidence for tryptophan and the kynurenine pathway as a regulator of stem cell niches in health and disease. Int. J. Tryptophan Res. 2024, 17, 1–27. [Google Scholar] [CrossRef]
- Ingenbleek, Y. The retinol circulating complex releases hormonal ligands during acute stress disorders. Front. Endocrinol. 2018, 9, 487. [Google Scholar] [CrossRef]
- Mendel, C.M. The free hormone hypothesis: A physiologically based mathematical model. Endocr. Rev. 1989, 10, 2329–2374. [Google Scholar] [CrossRef] [PubMed]
- Gregerman, R.I.; Solomon, N. Acceleration of thyroxine and triodothyronine turnover during bacterial pulmonary infections and fever: Implications for the functional state of the thyroid during stress and senescence. J. Clin. Endocrinol. Metab. 1967, 27, 93–105. [Google Scholar] [CrossRef]
- Xiao, K.; Su, L.; Yan, P.; Han, B.; Li, J.; Wang, H.; Jia, Y.; Li, X.; Xie, L. α-1-acid glycoprotein as a biomarker for the early diagnosis and monitoring the prognosis of sepsis. J. Crit. Care 2015, 30, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, J.; Kane, M.; Moise, A.R. Modulation of retinoid signaling: Therapeutic opportunities in organ fibrosis and repair. Pharmacol. Ther. 2020, 205, 107415. [Google Scholar] [CrossRef]
- Pervaiz, S.; Brew, K. Homology and structure function correlations between alpha1-acid glycoprotein and serum retinol- binding protein and its relatives. FASEB J. 1987, 3, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Meloun, B.; Morávek, L.; Kostka, V. Complete amino acid sequence of human serum albumin. FEBS Lett. 1975, 58, 134–137. [Google Scholar] [CrossRef]
- Qian, S.Z.; Jin, D.; Chen, Z.B.; Ye, Y.C.; Xiang, W.W.; Ye, L.M.; Pan, J.Y. Hypoalbuminemia, a novel prognostic factor for prediction of long-term outcomes in critically ill patients with septic shock. Int. J. Clin. Exp. Med. 2019, 12, 7401–7409. [Google Scholar]
- Anraku, M.; Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Redox properties of serum albumin. Biochim. Biophys. Acta 2013, 1830, 5465–5472. [Google Scholar] [CrossRef]
- Chanut, E.; Zini, R.; Trouvin, J.H.; Riant, P.; Tillement, J.P.; Jacquot, C. Albumin binding and brain uptake of 6-fluoro-DL-tryptophan: Competition with L-tryptophan. Biochem. Pharmacol. 1992, 44, 2082–2085. [Google Scholar] [CrossRef]
- Charlton, M.R.; Adey, D.B.; Nair, K.S. Evidence for a catabolic role of glucagon during an amino acid load. J. Clin. Investig. 1996, 98, 90–99. [Google Scholar] [CrossRef]
- Boden, G.; Rezvani, I.; Owen, O.E. Effects of glucagon on plasma amino acids. J. Clin. Investig. 1984, 73, 785–793. [Google Scholar] [CrossRef]
- Flakoll, P.J.; Borel, M.J.; Wentzel, L.S.; Williams, P.E.; Lacy, D.B.; Abumrad, N.N. The role of glucagon in the control of protein and amino acid metabolism in vivo. Metabolism 1994, 43, 1509–1516. [Google Scholar] [CrossRef]
- Bteich, M. An overview of albumin and alpha-1-acid glycoprotein main characteristics: Highlighting the roles of amino acid binding kinetics and molecular interactions. Heliyon 2019, 5, e02879. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.B.; Gotschlich, E.C.; Liu, T.Y. Primary structure of human C-reactive protein. Proc. Natl. Acad. Sci. USA 1977, 74, 3148–3151. [Google Scholar] [CrossRef] [PubMed]
- Koningsberg, W.; Hill, R.J. The structure of human hemoglobin. V. The digestion of the α chain of human hemoglobin with pepsin. J. Biol. Chem. 1962, 237, 3157–3162. [Google Scholar]
- MacGillivray, R.T.A.; Mendez, E.; Simba, S.K.; Sutton, M.R.; Lineback-Zins, J.; Brew, K. The complete amino acid sequence of human serum transferrin. Proc. Natl. Acad. Sci. USA 1982, 79, 2504–2508. [Google Scholar] [CrossRef]
- Luo, Z.; Lei, H.; Sun, Y.; Liu, X.; Su, D.F. Orosomucoid, an acute response protein with multiple modulating activities. J. Physiol. Biochem. 2015, 71, 329–340. [Google Scholar] [CrossRef]
- Black, S.; Kushner, I.; Samols, D. C-reactive protein. J. Biol. Chem. 2004, 279, 4848–4849. [Google Scholar] [CrossRef]
- Semba, R.D.; Shardell, M.; Sakr Ashour, F.A.; Moaddel, R.; Trehan, I.; Maleta, K.M.; Ordiz, M.I.; Kraemer, K.; Khadeer, M.A.; Ferrucci, L.; et al. Child stunting is associated with low circulating essential amino acids. EBioMedicine 2016, 6, 246–252. [Google Scholar] [CrossRef]
- Ingenbleek, Y.; Van den Schrieck, H.G.; De Nayer, P.; De Visscher, M. Albumin, transferrin, and the thyroxine-binding prealbumin/retinol binding protein (TBPA-RBP) complex in assessment of malnutrition. Clin. Chim. Acta 1975, 63, 61–67. [Google Scholar] [CrossRef]
- Kohgo, Y.; Nishisato, T.; Kondo, H.; Tsushima, N.; Niitsu, Y.; Urushizaki, I. Circulating transferrin receptor in human serum. Br. J. Haematol. 1986, 64, 277–281. [Google Scholar] [CrossRef]
- Wennninger, J.; Meinitzer, A.; Holasek, S.; Schnedl, W.; Zelzer, S.; Mangge, H.; Herrmann, M.; Enko, D. Associations between tryptophan and iron metabolism observed in individuals with and without iron deficiency. Sci. Rep. 2019, 9, 14548. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Maestri, R.; Boselli, M.; Achilli, M.P.; Arrigoni, N.; Bruni, M.; Dossena, M.; Verri, M.; Buonocore, D.; Pasini, E.; et al. The relationship between plasma amino acid and circulating albumin and hemoglobin in postabsorptive stroke patients. PLoS ONE 2019, 14, e0219756. [Google Scholar] [CrossRef]
- Bipath, P.; Levay, P.F.; Viljoen, M. Tryptophan depletion in context of the inflammatory and general nutritional status of a low-income South Africa HIV-infected population. J. Health Popul. Nutr. 2016, 35, 5. [Google Scholar] [CrossRef]
- Makarska-Bialokoz, M. Interactions of hemin with bovine serum albumin and human hemoglobin: A fluorescence quenching study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 93, 23–32. [Google Scholar] [CrossRef]
- Guo, D.; Liu, R. Spectroscopic investigation of the effects of aqueous-phase prepared CdTe quantum dots on protein hemoglobin at the molecular level. J. Biochem. Mol. Toxicol. 2017, 31, e21953. [Google Scholar] [CrossRef]
- Ingenbleek, Y.; Carpentier, Y.A. A prognostic inflammatory and nutritional index scoring critically ill patients. Int. J. Vitam. Nutr. Res. 1985, 55, 91–101. [Google Scholar] [PubMed]
- World Health Organization. C-Reactive Protein Concentrations as a Marker of Inflammation or Infection for Interpreting Biomarkers of Micronutrient Status; World Health Organization: Geneva, Switzerland, 2014; pp. 1–4. [Google Scholar]
- Pressac, M.; Vignoli, L.; Aymard, P.; Ingenbleek, Y. Usefulness of a prognostic inflammatory and nutritional index in pediatric clinical practice. Clin. Chim. Acta 1990, 188, 129–136. [Google Scholar] [CrossRef]
- Kudlácková, M.; Andĕl, M.; Hájková, H.; Nováková, J. Acute phase and prognostic inflammatory and nutritional index (PINI) in moderately burned children aged up to 3 years. Burns 1990, 16, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Vehe, K.L.; Brown, R.O.; Kuhl, D.A.; Boucher, B.A.; Luther, R.W.; Kudsk, K.A. The prognostic inflammatory and nutritional index in traumatized patients receiving enteral nutrition support. J. Am. Coll. Nutr. 1991, 10, 355–363. [Google Scholar] [CrossRef]
- Terrier, N.; Sénécal, L.; Dupuy, A.M.; Jaussent, I.; Delcourt, C.; Leray, H.; Rafaelsen, S.; Bosc, J.Y.; Maurice, F.; Canaud, B.; et al. Association between novel indices of malnutrition-inflammation complex syndrome and cardiovascular disease in hemodialysis patients. Hemodial. Int. 2005, 2, 159–168. [Google Scholar] [CrossRef]
- Schlossmacher, P.; Hasselmann, M.; Meyer, N.; Kara, F.; Delabranche, X.; Kummerlen, C.; Ingenbleek, Y. The prognostic value of nutritional and inflammatory indices in critically ill patients with acute respiratory failure. J. Clin. Chem. Lab. Med. 2002, 40, 1339–1343. [Google Scholar] [CrossRef]
- Reynolds, T.M.; Stokes, A.; Russell, L. Assessment of a prognostic biochemical indicator of nutrition and inflammation for identification of pressure ulcer risk. J. Clin. Pathol. 2006, 59, 308–310. [Google Scholar] [CrossRef]
- Dessi, M.; Noce, A.; Agnoli, A.; De Angelis, S.; Fuiano, L.; Tozzo, C.; Taccone-Gallucci, M.; Fuiano, G.; Federici, G. The usefulness of the prognostic and nutritional index (PINI) in a haemodialysis population. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 811–815. [Google Scholar] [CrossRef]
- Wiese, D.; Lashner, B.; Seidner, D. Measurement of nutrition status in Crohn’s disease patients receiving Infliximab therapy. Nutr. Clin. Pract. 2008, 23, 551–556. [Google Scholar] [CrossRef]
- Walsh, D.; Mahmoud, F.; Barna, B. Assessment of nutritional status in advanced cancer: Interleukin-6, C-reactive protein, and the prognostic and inflammatory nutritional index. Support. Care Cancer 2003, 11, 60–62. [Google Scholar] [CrossRef]
- Dupire, S.; Wemeau, M.; Debarri, H.; Pascal, L.; Hivert, B.; Willekens, C.; Boyle, E.; Manier, S.; Thielemans, B.; Onraed, B.; et al. Prognostic value of PINI index in patients with multiple myeloma. Eur. J. Haematol. 2012, 88, 306–313. [Google Scholar] [CrossRef] [PubMed]
- de Souza Costa, M.D.; Vieira de Melo, C.Y.S.; Ribeiro de Amorim, A.C.; Cipriano Torres, D.O.; Dos Santos, A.C.O. Association between nutritional status, inflammatory condition, and prognostic indexes with postoperative complications and clinical outcome of patients with gastrointestinal neoplasia. Nutr. Cancer 2016, 68, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoy, M.; Ayzac, L.; Ingenbleek, Y.; Kostka, T.; Boisson, R.C.; Bienvenu, J. Usefulness of the prognostic inflammatory and nutritional index (PINI) in hospitalized elderly patients. Int. J. VItam Nutr. Res. 1998, 68, 189–195. [Google Scholar]
- Monnet, D.; Ahouty, C.P.; Malan, K.A.; Houenou, A.Y.; Tebi, A.; Yapo, A.E. Protein profile in malnutrition states of the Ivory Coast child. Bull. Soc. Pathol. Exot. 1995, 88, 50–53. [Google Scholar] [PubMed]
- Allico Mousso, J.M.; Agbo Adouko, E.; Séri Kipré, L.; Boyvin, L.; Kouamé, C.; Yapi Houphouèt, F.; Djaman Allico, J. Impact of dietary diversification on the prognostic inflammatory and nutritional index in school-age children in the Nawa region (Côte d’Ivoire). Int. J. Child. Health Nutr. 2021, 10, 154–161. [Google Scholar]
- Mikolélé-Bilombo, C.; Atipo-Ibara Ollandzobo, L.C.; Missambou Mandilou, S.V.; Gokaba Lethso, T.O.; Louokdom, J.S.; Mikolélé-Bilombo, A.; Mabiala-Babela, J.R.; Ibara, J.R. Undernutrition and mortality risk of children hospitalized at Brazzaville University Hospital: Comparison of MUAC and Z-weight-for-height Z-score vs PINI. Int. J. Health Sci. Res. 2021, 11, 105–111. [Google Scholar]
- Cordos, M.; Martu, M.A.; Vlad, C.E.; Toma, V.; Ciubotaru, A.D.; Badescu, M.C.; Goriuc, A.; Foia, L. Early detection of inflammation and malnutrition and prediction of acute events in hemodialysis patients through PINI (Prognostic Inflammatory and Nutritional Index). Diagnostics 2024, 14, 1273. [Google Scholar] [CrossRef]
- Tessema, M.; Gunaratna, N.S.; Brouwer, I.D.; Donato, K.; Cohen, J.L.; McConnell, M.; Belachew, T.; Belayneh, D.; De Groote, H. Associations among high-quality protein and energy intake, serum transthyretin, serum amino acids and linear growth of children in Ethiopia. Nutrients 2018, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Antener, I.; Tonney, G.; Verwilghen, A.M.; Mauron, J. Biochemical study of malnutrition. Part IV. Determination of amino acids in the serum, erythrocytes, urine and stool ultrafiltrates. Int. J. Vitam. Nutr. Res. 1981, 51, 64–78. [Google Scholar]
- Schoonees, A.; Lombard, M.J.; Musewika, A.; Nel, E.; Volmink, J. Ready to use therapeutic food (RUFT) for home-based nutritional rehabilitation of severe malnutrition in children from six months to five year of age. Cochrane Database Syst. Rev. 2019, 5, CD009000. [Google Scholar] [CrossRef] [PubMed]
- Imdad, A.; Rogner, J.I.; Francois, M.; Ahmed, S.; Smith, A.; Tsistinas, O.J.; Tanner-Smith, T.; Das, J.K.; Chen, F.F.; Bhutta, Z.A. Increased vs. standard dose of iron in ready-to-use therapeutic food for the treatment of severe acute malnutrition in a community setting: A systemic review and meta-analysis. Nutrients 2002, 3116, e000100. [Google Scholar] [CrossRef]
- Sigh, S.; Roos, N.; Chhoun, C.; Wieringa, F.T. Ready-to-use therapeutic foods fail to improve vitamin A and iron status meaningfully during treatment for severe acute malnutrition in 6–59-month-old Cambodian children. Nutrients 2023, 15, 905. [Google Scholar] [CrossRef]
- Ingenbleek, Y.; Van den Schrieck, H.G.; De Nayer, P.; De Visscher, M. The role of retinol-binding protein in protein-calorie malnutrition. Metabolism 1975, 24, 633–641. [Google Scholar] [CrossRef]
- Anderson, M.; Braegger, C.P. The role of iodine for thyroid function in lactating women and infants. Endocr. Rev. 2022, 43, 469–506. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Kennedy, P.T.; Dahal, L.N. Investigating the role of indoleamine 2,3-dioxygenase in acute myeloid leukemia: A systematic review. Front. Immunol. 2021, 12, 651687. [Google Scholar] [CrossRef]
- Choe, J.Y.; Yun, J.Y.; Jeon, Y.K.; Kim, S.H.; Park, G.; Huh, J.R.; Oh, S.; Kim, J.E. Indoleamine 2,3-dioxygenase (IDO) is frequently expressed in stroma cells of Hodgkin lymphoma and is associated with adverse clinical features: A retrospective cohort study. BMC Cancer 2014, 14, 335. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Gilot, D.; Ahn, S.B.; Lam, V.; Shin, J.S.; Guillemin, G.K.; Heng, B. Involvement of kynurenine pathway in hepatocellular carcinoma. Cancers 2021, 13, 5180. [Google Scholar] [CrossRef] [PubMed]
- Levina, V.; Su, Y.; Gorelik, E. Immunological and nonimmunological effects of indoleamine 2,3-dioxygenase on breast tumor growth and spontaneous metastasis formation. Clin. Dev. Immunol. 2012, 2012, 173029. [Google Scholar] [CrossRef]
- Tang, D.; Yue, L.; Yao, R.; Zhou, L.; Yang, Y.; Lu, L.; Gao, W. P53 prevent tumor invasion and metastasis by down-regulating IDO in lung cancer. Oncotarget 2017, 8, 54548–54557. [Google Scholar] [CrossRef]
- Ala, M. Tryptophan metabolites modulate inflammatory bowel disease and colorectal cancer by affecting immune system. Int. Rev. Immunol. 2022, 41, 326–345. [Google Scholar] [CrossRef]
- Huang, J.Y.; Butler, L.M.; Midttun, Ø.; Ulvik, A.; Wang, R.; Jin, A.; Gao, Y.T.; Ueland, P.M.; Koh, W.P.; Yuan, J.M. A prospective evaluation of serum kynurenine metabolites and risk of pancreatic cancer. PLoS ONE 2018, 13, e0196465. [Google Scholar] [CrossRef] [PubMed]
- Dickson, P.W.; Aldred, A.L.; Marley, P.D.; Bannister, D.; Schreiber, G. Rat choroid plexus specializes in the synthesis and the secretion of transthyretin (prealbumin). Regulation of transthyretin synthesis in choroid plexus is independent from that of liver. J. Biol. Chem. 1986, 261, 3475–3478. [Google Scholar] [CrossRef] [PubMed]
- Laferla, F.M.; Green, K.N.; Oddo, S. Intracellular amyloid-beta in Alzheimer’s disease. Nat. Rev. Neurosci. 2007, 8, 499–509. [Google Scholar] [CrossRef]
- Schwarzman, A.L.; Gregori, L.; Vitek, M.P.; Lyubski, S.; Strittmatter, W.J.; Enghilde, J.J.; Bhasin, R.; Silverman, J.; Weisgraber, K.H.; Coyle, P.K. Transthyretin sequesters amyloid beta protein and prevents amyloid formation. Proc. Natl. Acad. Sci. USA 1994, 91, 8368–8372. [Google Scholar] [CrossRef]
- Ghadami, S.A.; Chia, S.; Ruggeri, F.S.; Meisl, G.; Bemporad, F.; Habchi, J.; Cascella, R.; Dobson, C.M.; Vendruscolo, M.; Knowles, T.P.J.; et al. Transthyretin inhibits primary and secondary nucleation of amyloid-ß peptides aggregation and reduces the toxicity of its oligomers. Biomacromolecules 2020, 21, 1112–1125. [Google Scholar] [CrossRef]
- Costa, R.; Gonçalves, A.; Saraiva, M.J.; Cardoso, I. Transthyretin binding to A-Beta peptide. Impact on A-Beta fibrillogenesis and toxicity. FEBS Lett. 2008, 582, 936–942. [Google Scholar] [CrossRef]
- Chatterjee, P.; Zetterberg, H.; Goozee, K.; Lim, C.K.; Jacobs, K.R.; Ashton, N.J.; Hye, A.; Pedrini, S.; Sohrabi, H.R.; Shah, T.; et al. Plasma neurofilament light chain and amyloid-ß are associated with the kynurenine pathway metabolites in preclinical Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 186. [Google Scholar] [CrossRef]
- Leuzy, A.; Cullen, N.C.; Mattsson-Carlgren, N.; Hanson, O. Current advances in plasma and cerebrospinal fluid biomarkers in Alzheimer’s disease. Curr. Opin. Neurol. 2021, 34, 266–274. [Google Scholar] [CrossRef]
- Bakker, L.; Kölher, S.; Eussen, S.J.P.M.; Choe, K.; van den Hove, D.L.A.; Kenis, G.; Rutten, B.P.F.; Ulvik, A.; Ueland, P.M.; Verhey, F.R.J.; et al. Correlations between kynurenines in plasma and CSF, and their relation to markers of Alzheimer’s disease pathology. Brain Behav. Immun. 2023, 111, 312–319. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Dzięgielewska, K.M.; Møllgård, K.; Whish, S.C.; Noor, N.M.; Wheaton, B.J.; Gehwolf, R.; Wagner, A.; Traweger, A.; Bauer, H.; et al. Cellular specificity of the blood-CSF barrier for albumin transfer across the choroid plexus epithelium. PLoS ONE 2014, 9, e106592. [Google Scholar] [CrossRef]
- Jung, S.M.; Lee, K.; Lee, J.W.; Namkoong, H.; Kim, H.K.; Kim, S.; Na, H.R.; Ha, S.A.; Kim, J.R.; Ko, J.; et al. Both plasma retinol-binding protein and haptoglobin precursor allele 1 in CSF: Candidate biomarkers for the progression of normal to mild cognitive impairment to Alzheimer’s disease. Neurosci. Lett. 2008, 436, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Vrethem, M.; Ohman, S.; von Schenck, H.; Forsberg, P.; Olsson, J.E. Comparison of concentration of orosomucoid in serum and cerebrospinal fluid in different neurological diseases. Acta Neurol. Scand. 1987, 75, 328–331. [Google Scholar] [CrossRef]
- Coccaro, E.F.; Lee, R.; Breen, E.C.; Irwin, M.R. Plasma and cerebrospinal fluid inflammatory markers and human aggression. Neuropsychopharmacology 2023, 48, 1060–1066. [Google Scholar] [CrossRef]
- Chen, C.P.C.; Chen, R.L.; Preston, J.E. The influence of ageing in the cerebrospinal fluid concentrations of proteins that are derived from the choroid plexus, brain, and plasma. Exp. Gerontol. 2012, 47, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y.; Bernstein, L.H. Downsizing of lean body mass is a key determinant of Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 44, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y. Implications of protein malnutrition and inflammatory disorders in the pathophysiology of Alzheimer’s disease. Asia Pac. J. Clin. Nutr. 2020, 29, 450–461. [Google Scholar] [PubMed]
| Denomination | AA mol. | AA | Trp | Ratio | Ref. |
|---|---|---|---|---|---|
| Mass (kDa) | Residues | Residues | W/AA | ||
| HGH | 21 | 191 | 1 (W25) | 0.52 | [48] |
| IGF-1 | 7.6 | 70 | 0 | 0 | [50] |
| Insulin | 5.8 | 51 | 0 | 0 | [49] |
| Glucagon | 3.4 | 29 | 1 (W25) | 3.45 | [51] |
| Biomarker | Conformation | Molecular | AA Sequence | Trp Residues | Ratio W/AA | Biological (T1/2) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Mass (kDa) | ||||||||
| NUTRITION BIOMARKERS | TTR | Tetrameric (4 subunits) | 55 | 508 (4 × 127) | ⑧ 4 × W41,79 | 1.57 | 2 days | [20] |
| RBP | Monopeptide | 21 | 182 | ④ W24,67,91,105 | 2.19 | 14 h | [21] | |
| ALB | Polypeptide | 66.5 | 585 | ① W214 | 0.17 | 19 days | [61] | |
| ACUTE- PHASE REACTANTS | AGP | Peptide~58% & glycosides~42% | 41–43 | 183 | ③ W25,122,160 | 1.64 | 3–5 days | [68] |
| CRP | Pentameric monopeptide | 21.5 | 187 | ⑤ W81,91,142,168,186 | 2.67 | variable | [69] | |
| IRON STATUS ANALYTES | HGB | Tetrameric (2 α chains + 2 ß chains) | 64 | 574 (α: 282, ß: 292) | ⑥ α: W14, ß: W15,37 | 1.04 | 20 days | [70] |
| TF | Monopeptide (2 glycan chains) | 79.5 | 678 | ⑧ W8,128,264,344 358,441,460,549 | 1.18 | 8–10 days | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingenbleek, Y. Three Major Deficiency Diseases Harming Mankind (Protein, Retinoid, Iron) Operate Under Tryptophan Dependency. Nutrients 2025, 17, 2505. https://doi.org/10.3390/nu17152505
Ingenbleek Y. Three Major Deficiency Diseases Harming Mankind (Protein, Retinoid, Iron) Operate Under Tryptophan Dependency. Nutrients. 2025; 17(15):2505. https://doi.org/10.3390/nu17152505
Chicago/Turabian StyleIngenbleek, Yves. 2025. "Three Major Deficiency Diseases Harming Mankind (Protein, Retinoid, Iron) Operate Under Tryptophan Dependency" Nutrients 17, no. 15: 2505. https://doi.org/10.3390/nu17152505
APA StyleIngenbleek, Y. (2025). Three Major Deficiency Diseases Harming Mankind (Protein, Retinoid, Iron) Operate Under Tryptophan Dependency. Nutrients, 17(15), 2505. https://doi.org/10.3390/nu17152505






