Egg Yolk Immunoglobulins (IgY) Purification, Activity Enhancement, and Potential Benefits for Human Health
Abstract
1. Introduction
2. Structure and Functions of IgY
2.1. Biological Structure
2.2. Biochemical Functions
3. Characteristics and Action Mechanisms of IgY
3.1. Biological Characteristics
3.2. Immune Mechanisms
4. Extraction and Activity Enhancement of IgY
4.1. Methods for IgY Isolation and Purification
4.2. Enhancement of IgY Activity
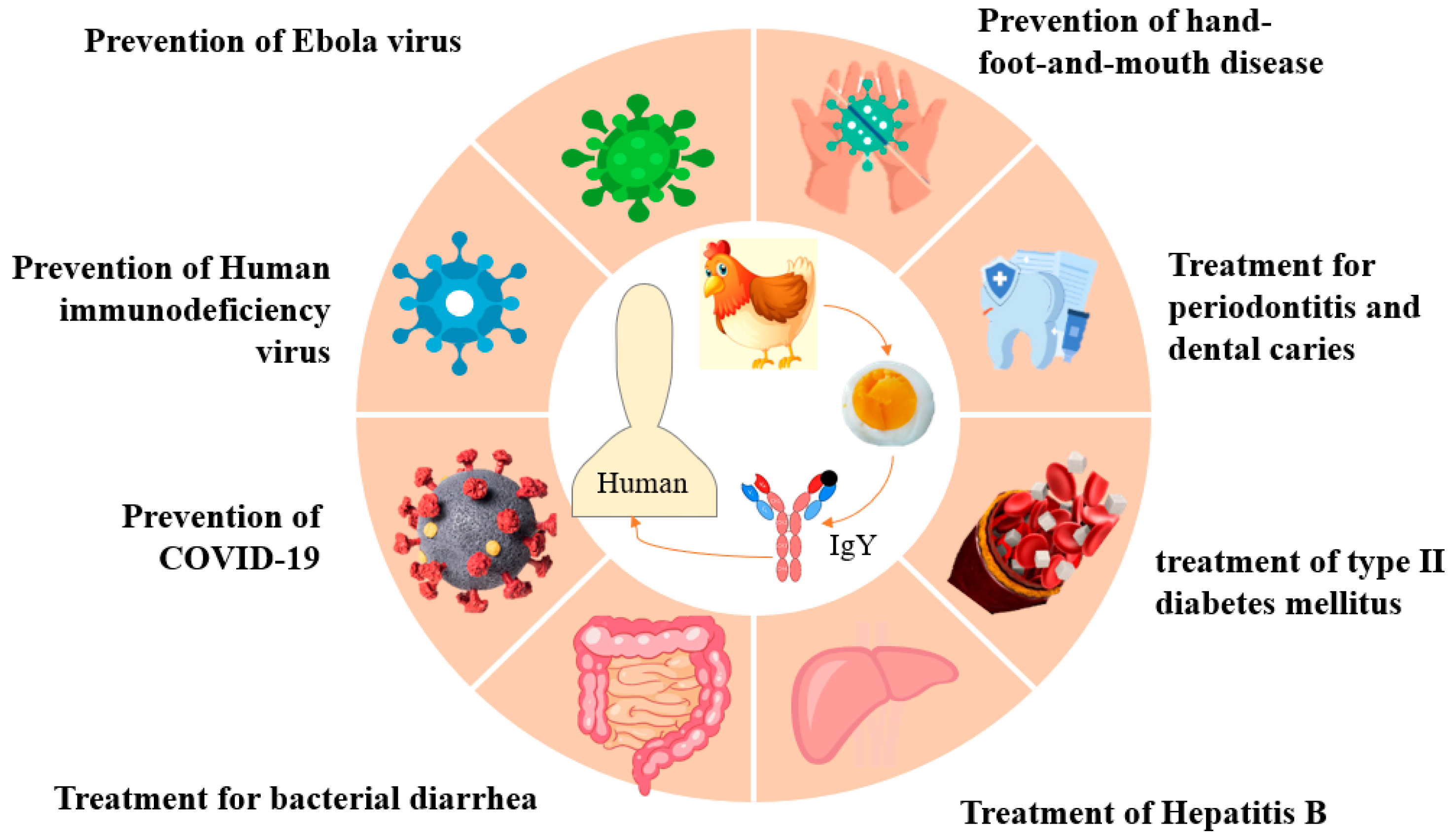
5. IgY Applications in Human Health
5.1. The Mechanism of IgY Treatment and the Prevention of Human Diseases
5.2. Potential Human Health Benefits of IgY
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metheenukul, P.; Surachetpong, W.; Prasertsincharoen, N.; Arreesrisom, P.; Thengchaisri, N. Comparison of immunoglobulin Y antibody production in new and spent laying hens. Vet. World 2024, 17, 2177–2184. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, Q.; Lin, J. Egg Yolk Antibody for Passive Immunization: Status, Challenges, and Prospects. J. Agric. Food Chem. 2023, 71, 5053–5061. [Google Scholar] [CrossRef]
- Grzywa, R.; Łupicka-Słowik, A.; Sieńczyk, M. IgYs: On her majesty’s secret service. Front. Immunol. 2023, 14, 1199427. [Google Scholar] [CrossRef]
- Yakhkeshi, S.; Isah, M.B.; Sadeghi-Abandansari, H.; Zhang, X. Advances in IgY antibody dosage form design and delivery strategies: Current status and future perspective. Int. J. Biol. Macromol. 2025, 300, 140291. [Google Scholar] [CrossRef]
- Zhang, X.; Calvert, R.A.; Sutton, B.J.; Doré, K.A. IgY: A key isotype in antibody evolution. Biol. Rev. Camb. Philos. Soc. 2017, 92, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Syed-Atif, A.; Tan, S.C.; Leow, C.H. Insights into the chicken IgY with emphasis on the generation and applications of chicken recombinant monoclonal antibodies. J. Immunol. Methods 2017, 447, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Samardzic, K.; Wallach, M.; Frumkin, L.R.; Mochly-Rosen, D. Immunoglobulin Y for potential diagnostic and therapeutic applications in infectious diseases. Front. Immunol. 2021, 12, 696003. [Google Scholar] [CrossRef]
- Okamoto, M.; Sasaki, R.; Ikeda, K. FcRY is a key molecule controlling maternal blood IgY transfer to yolks during egg development in avian species. Front. Immunol. 2024, 15, 1305587. [Google Scholar] [CrossRef]
- Ge, S.K.; Yang, Y.L.; Brindha, C.; Antonysamy, M.; Zhong, F.G.; Zhang, X.Y. Evaluation of Different IgY Preparation Methods and Storage Stability as Potential Animal Feed Supplement. Pak. J. Zool. 2020, 52, 2027–2426. [Google Scholar] [CrossRef]
- Dávalos-Pantoja, L.; Ortega-Vinuesa, J.L.; Bastos-González, D.; Hidalgo-Alvarez, R. A comparative study between the adsorption of IgY and IgG on latex particles. J. Biomater. Sci. Polym. Ed. 2000, 11, 657–673. [Google Scholar] [CrossRef]
- Gandhi, S.; Alshehri, S.M. Molecular stability of the rabbit and chicken egg yolk immunoglobulins. Front. Biosci. 2021, 13, 185–194. [Google Scholar] [CrossRef]
- Liew, C.Y.; Yen, C.C.; Chen, J.L. Structural identification of N-glycan isomers using logically derived sequence tandem mass spectrometry. Commun. Chem. 2021, 4, 92. [Google Scholar] [CrossRef]
- Zhou, Q.; Qiu, H. The mechanistic impact of N-glycosylation on stability, pharmacokinetics, and immunogenicity of therapeutic proteins. J. Pharm. Sci. 2019, 108, 1366–1377. [Google Scholar] [CrossRef]
- Yang, Y.E.; Wen, J.; Zhao, S.; Zhang, K. Prophylaxis and therapy of pandemic H1N1 virus infection using egg yolk antibody. J. Virol. Methods 2014, 206, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Schubert, A.; Zajac, J.; Dyck, T.; Oelkrug, C. IgY antibodies in human nutrition for disease prevention. Nutr. J. 2015, 14, 109. [Google Scholar] [CrossRef]
- Wang, L.Y. Study on the Isolation and Purification, mPEG Modification and Stability of Chicken Egg Yolk Immunoglobulin. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2012. (In Chinese). [Google Scholar] [CrossRef]
- Zhang, X.; Wu, R.; Chelliappan, B. Proteomic investigation and understanding on IgY purification and product development. Poult. Sci. 2023, 102, 102843. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Ahn, D.U.; Cai, Z. An easy and simple separation method for Fc and Fab fragments from chicken immunoglobulin Y (IgY). J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2020, 1141, 122011. [Google Scholar] [CrossRef] [PubMed]
- Redwan, E.M.; Aljadawi, A.A.; Uversky, V.N. Simple and efficient protocol for immunoglobulin Y purification from chicken egg yolk. Poult. Sci. 2021, 100, 100956. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D.; Chacana, P.A.; Calzado, E.G.; Brembs, B.; Schade, R. IgY technology: Extraction of chicken antibodies from egg yolk by polyethylene glycol (PEG) precipitation. J. Vis. Exp. 2011, 51, 3084. [Google Scholar] [CrossRef]
- Madera-Contreras, A.M.; Solano-Texta, R.; Cisneros-Sarabia, A. Optimized method for the extraction of contaminant-free IgY antibodies from egg yolk using PEG 6000. Methods X 2022, 9, 101874. [Google Scholar] [CrossRef]
- Ren, H.; Yang, W.; Thirumalai, D.; Zhang, X.; Schade, R. A comparative evaluation of six principal IgY antibody extraction methods. Altern. Lab. Anim. 2016, 44, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ren, H.; Schade, R.; Zhang, X. A novel and efficient immunoglobulin Y extraction method using poloxamer-polyethylene glycol. Prep. Biochem. Biotechnol. 2017, 47, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Nakai, S. Simple separation of immunoglobulin from egg yolk by ultrafiltration. J. Food Sci. 1998, 63, 485–490. [Google Scholar] [CrossRef]
- Jiang, X.; Diraviyam, T.; Zhang, X. Affinity purification of egg yolk immunoglobulins (IgY) using a human mycoplasma protein. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2016, 1012–1013, 37–41. [Google Scholar] [CrossRef]
- Cassedy, A.; O’Kennedy, R. Antibody Purification Using Affinity Chromatography. Methods Mol. Biol. 2022, 2466, 3–22. [Google Scholar] [CrossRef]
- Hatta, H.; Kim, M.; Yamamoto, T. A novel isolation method for hen egg yolk antibody, IgY. Agric. Biol. Chem. 1990, 54, 2531–2535. [Google Scholar] [CrossRef]
- Tong, C.; Geng, F.; He, Z.; Cai, Z.; Ma, M. A simple method for isolating chicken egg yolk immunoglobulin using effective delipidation solution and ammonium sulfate. Poult. Sci. 2015, 94, 104–110. [Google Scholar] [CrossRef]
- Akita, E.M.; Nakai, S. Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic E. coli strain. J. Immunol. Methods 1993, 160, 207–214. [Google Scholar] [CrossRef]
- Nolden, C.; Grisham, A.; Schaefer, D.; Akins, M.; Cook, M. PSVIII-2 Antibody yield for polyclonal egg yolk antibody (IgY) production in laying hens. J. Anim. Sci. 2020, 98, 252–253. [Google Scholar] [CrossRef]
- Oostindie, S.C.; Lazar, G.A.; Schuurman, J.; Parren, P.W. Avidity in antibody effector functions and biotherapeutic drug design. Nat. Rev. Drug Discov. 2022, 21, 715–735. [Google Scholar] [CrossRef]
- Shimizu, M.; Miwa, Y.; Hashimoto, K.; Goto, A. Encapsulation of chicken egg yolk immunoglobulin G (IgY) by liposomes. Biosci. Biotechnol. Biochem. 1993, 57, 1445–1449. [Google Scholar] [CrossRef]
- Chang, H.M.; Lee, Y.C.; Chen, C.C. Microencapsulation protects immunoglobulin in yolk (IgY) specific against Helicobacter pylori urease. J. Food Sci. 2002, 67, 15–20. [Google Scholar] [CrossRef]
- Li, X.Y.; Jin, L.J.; McAllister, T.A. Chitosan-alginate microcapsules for oral delivery of egg yolk immunoglobulin (IgY). J. Agric. Food Chem. 2007, 55, 2911–2917. [Google Scholar] [CrossRef]
- Luping, G.; McClements, D.J.; Jiaying, L.; Yujie, S. Formulation of alginate/carrageenan microgels to encapsulate, protect and release immunoglobulins: Egg Yolk IgY. Food Hydrocoll. 2021, 112, 106349. [Google Scholar] [CrossRef]
- Kamee, N.A.; Shuib, A.S.; Tayyab, S. Effect of Various Polyols on the Acid-Denatured States of Champedak Galactose-Binding Lectin. Protein Pept. Lett. 2018, 25, 314–324. [Google Scholar] [CrossRef]
- Lee, K.A.; Chang, S.K.; Lee, Y.J.; Lee, J.H.; Koo, N.S. Acid stability of anti-Helicobacter pyroli IgY in aqueous polyol solution. J. Biochem. Mol. Biol. 2002, 35, 488–493. [Google Scholar] [CrossRef]
- Ehsani, A.; Naghibi, S.S.; Aminzare, M.; Keykhosravi, K.; Hashemi, M. Extraction of specific egg yolk antibodies and application in chitosan coating: Effect on microbial and sensory properties of rainbow trout fillet during chilled storage. J. Sci. Food Agric. 2019, 99, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Wada, R.; Matsui, M.; Kawasaki, N. Influence of N-glycosylation on effector functions and thermal stability of glycoengineered IgG1 monoclonal antibody with homogeneous glycoforms. MAbs 2019, 11, 350–372. [Google Scholar] [CrossRef]
- Duran-Romaña, R.; Houben, B.; De Vleeschouwer, M. N-glycosylation as a eukaryotic protective mechanism against protein aggregation. Sci. Adv. 2024, 10, eadk8173. [Google Scholar] [CrossRef]
- Tabll, A.A.; Shahein, Y.E.; Omran, M.M. Monoclonal IgY antibodies: Advancements and limitations for immunodiagnosis and immunotherapy applications. Ther. Adv. Vaccines Immunother. 2024, 12, 25151355241264520. [Google Scholar] [CrossRef]
- Leiva, C.L.; Gallardo, M.J.; Casanova, N.; Terzolo, H.; Chacana, P. IgY-technology (egg yolk antibodies) in human medicine: A review of patents and clinical trials. Int. Immunopharmacol. 2020, 81, 106269. [Google Scholar] [CrossRef]
- Cunningham, O.; Scott, M.; Zhou, Z.S.; Finlay, W.J. Polyreactivity and polyspecificity in therapeutic antibody development: Risk factors for failure in preclinical and clinical development campaigns. MAbs 2021, 13, 1999195. [Google Scholar] [CrossRef]
- Schön, J.; Aebischer, A.; Halwe, N.J. Evaluation of SARS-CoV-2-Specific IgY antibodies: Production, reactivity, and neutralizing capability against virus Variants. Int. J. Mol. Sci. 2024, 25, 7976. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Zhang, Z.; Huang, J.; Yao, M.; Huang, G.; Ge, Y.; Zhang, P.; Huang, H.; Wang, Y.; et al. Generation of chicken IgY against SARS-CoV-2 spike protein and epitope mapping. J. Immunol. Res. 2020, 2020, 9465398. [Google Scholar] [CrossRef]
- Capotă, R.; Ciaușu-Sliwa, D.; Bostănaru-Iliescu, A.C.; Năstasă, V. Latest Findings in Immunoglobulin Y Technologies and Applications. Int. J. Mol. Sci. 2025, 26, 6380. [Google Scholar] [CrossRef]
- Lisicka, W.; Earley, Z.M.; Sifakis, J.J.; Erickson, S.A.; Mattingly, J.R.; Wu-Woods, N.J.; Krishnamurthy, S.R.; Belkaid, Y.; Ismagilov, R.F.; Cyster, J.G.; et al. Immunoglobulin a controls intestinal virus colonization to preserve immune homeostasis. Cell Host Microbe 2025, 33, 498–511.e10. [Google Scholar] [CrossRef]
- Pacheco, B.L.; Nogueira, C.P.; Venancio, E.J. IgY antibodies from birds: A review on affinity and avidity. Animals 2023, 13, 3130. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-X.; Zhou, W.-Y.; Zhu, X.-L.; Wen, Z.; Wu, L.-H.; Wu, X.-M.; Wei, H.-P.; Wang, W.-D.; He, D.; Xiang, Q.; et al. Anti-interleukin-1 Beta/Tumor necrosis factor-alpha IgY antibodies reduce pathological allergic responses in guinea pigs with allergic rhinitis. Mediat. Inflamm. 2016, 2016, 3128182. [Google Scholar] [CrossRef]
- O’Donnell, K.L.; Meberg, B.; Schiltz, J.; Nilles, M.L. Zika virus-specific IgY results are therapeutic following a lethal zika virus challenge without inducing antibody-dependent enhancement. Viruses 2019, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Wang, D.; Feng, K.; Ge, Z.L.; Zhou, H.F.; Shao, M. Preparation of egg yolk antibodies against human isomaltase and determination of their biological activities. J. Med. Postgrad. 2018, 31, 137–141. [Google Scholar] [CrossRef]
- Vaillant, A.; Mcfarlane-Anderson, N.; Akpaka, P.; Akpaka, P.E.; Smikle, M.P.; Ramirez, N.; Cadiz, A. Use of dot blots analysis in the separation of anti-HIV antibodies in animals. J. Chromat Sep. Techniq. 2013, 4, 5. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Chen, J.C.; Savitha, S.; Lin, F.H.; Yang, Y.Y.; Lee, C.H. A real time detection of the ovarian tumor associated antigen 1 (OVTA 1) in human serum by quartz crystal microbalance immobilized with anti-OVTA 1 polyclonal chicken IgY antibodies. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 2073–2078. [Google Scholar] [CrossRef]
- Li, X.; He, P.; Yu, L. Production and characteristics of a novel chicken egg yolk antibody (IgY) against periodontitis-associated pathogens. J. Oral Microbiol. 2020, 12, 1831374. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wei, K.; Zhao, M.; Li, X.; Zhang, Y. Research Note: A novel method for preparation of egg yolk immunoglobulin Y against Porphyromonas gingivalis. Poult. Sci. 2023, 102, 102863. [Google Scholar] [CrossRef] [PubMed]
- Bachtiar, E.W.; Soejoedono, R.D.; Bachtiar, B.M.; Henrietta, A. Effects of soybean milk, chitosan, and anti-Streptococcus mutans IgY in malnourished rats’ dental biofilm and the IgY persistency in saliva. Interv. Med. Appl. Sci. 2015, 7, 118–123. [Google Scholar] [CrossRef]
- Hatta, H.; Tsuda, K.; Ozeki, M. Passive immunization against dental plaque formation in humans: Effect of a mouth rinse containing egg yolk antibodies (IgY) specific to Streptococcus mutans. Caries Res. 1997, 31, 268–274. [Google Scholar] [CrossRef]
- Du, P. Isolation of anti-HBV Immunoglobulin of Egg Yolk (IgY). Master’s Thesis, Tianjin University, Tianjin, China, 2012. (In Chinese). [Google Scholar] [CrossRef]
- Mulvey, G.L.; Dingle, T.C.; Fang, L.; Strecker, J.; Armstrong, G.D. Therapeutic potential of egg yolk antibodies for treating Clostridium difficile infection. J. Med. Microbiol. 2011, 60, 1181–1187. [Google Scholar] [CrossRef]
- Gao, E.; Wu, S.; Xu, Q. Enterovirus type 71-immunized chicken egg yolk immunoglobulin has cross antiviral activity against coxsackievirus A16 in vitro. Exp. Ther. Med. 2019, 18, 332–341. [Google Scholar] [CrossRef]
- Shen, H.; Cai, Y.; Zhang, H. Anti-SARS-CoV-2 IgY isolated from egg yolks of hens immunized with inactivated SARS-CoV-2 for immunoprophylaxis of COVID-19. Virol. Sin. 2021, 36, 1080–1082. [Google Scholar] [CrossRef]
- Frumkin, L.R.; Lucas, M.; Scribner, C.L. Egg-derived anti-SARS-CoV-2 immunoglobulin Y (IgY) with broad variant activity as intranasal prophylaxis against COVID-19. Front. Immunol. 2022, 13, 899617. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Y.; Li, Y. IgY antibodies against Ebola virus possess post-exposure protection in a murine pseudovirus challenge model and excellent thermostability. PLoS Neglected Trop. Dis. 2021, 15, e0008403. [Google Scholar] [CrossRef] [PubMed]
- Bentes, G.A.; Lanzarini, N.M.; Guimarães, J.R.; Heinemann, M.B.; Volotão, E.d.M.; Silva, A.d.S.d.; Heneine, L.G.D.; de Oliveira, J.M.; Pinto, M.A. Production and evaluation of chicken egg yolk immunoglobulin (IgY) against human and simian rotaviruses. Viruses 2022, 14, 1995. [Google Scholar] [CrossRef] [PubMed]
| Methods | Reagents or Devices | Steps or Principles | Purity | Recovery | Results | Reference |
|---|---|---|---|---|---|---|
| Inorganic precipitation | Ammonium sulfate | Hydrolysed with papain for 6 h; 45% saturated ammonium sulfate to remove low molecular weight peptides. | Fab 88.7% and Fc 90.1%. | No | Papain digest of IgY over 96% | [18] |
| Organic solvent precipitation | Octanoic acid | Ultrafiltration at pH 9.0 after addition of 2% caprylic acid in two batches. | 97.9% | 78.9% | 99% activity | [19] |
| Polymer organic polymer precipitation | PEG precipitates | Isolation and purification of IgY at 6% PEG concentration; | 81% | 72.63% | Retention of activity 83.31% | [16] |
| PBS was mixed with egg yolk and then 3.5% PEG, 8.5% PEG, 12% PEG were added. | 80% | No | Average 60 mg IgY per egg | [20,21] | ||
| PEG and ammonium sulfate union | 3.5% PEG, 15% ammonium sulfate added to filtrate. | 85% | No | Protein yield 6.8 mg/mL | [22] | |
| Poloxamer-PEG method | Adding skimming solution, then add PEG-6000 and mix with shaking. | 92.71% | No | Total protein yield 30 mg/mL | [23] | |
| Ultrafiltration | Ultrafiltration centrifuge | IgY forms dimers in 1.5 mol/L NaCl. | 74–99% | 80–85% | Ultrafiltration was more practical for industrial application | [24] |
| Chromatography | Ion exchange chromatography | PBS conditions with more net charge on the protein surface resulted in a more complete separation. | 95% | 94% | Retention of activity 73.77% | [16] |
| Affinity chromatography | Epichlorohydrin and cyanuric chloride methods;highly stable affinity ligand named as ligand 8–6 purification by ligand. | 92.1% | 78.2% | Found 125 times increase in effective IgY | [25,26] | |
| Xanthan and carrageenan gums | Acidic natural gums remove lipoproteins. | 98% | 86% | Yield was 70 to 100 mg per egg | [27] | |
| Pectin | Dilution of egg yolks with 0.1% pectin in a 6-fold defatted solution. | 83.3% | No | Protein yield 8.36 mg/mL | [28] | |
| Water dilution | Proteins and lipids were separated by water dilution, and IgY was purified by sedimentation and ultrafiltration. | 94% | No | Protein yield 9.8 mg/mL | [29] | |
| Type of Diseases | Preparation of Specific IgY Antibodies | IgY Types | Potential Role | Reference |
|---|---|---|---|---|
| Diabetes mellitus | Human isomaltase (HISO) recombinant protein, emulsified with Freund’s adjuvant, was used to immunize laying hens for specific IgY. Antibodies were extracted via water dilution and sodium sulfate extraction. | Anti-HISO IgY | Targets HISO and inhibits alpha-glucosidase activity for the possible treatment of type II diabetes mellitus. | [51] |
| Human immunodeficiency virus (HIV) | Keyhole limpet hemocynin (KLH) by the glutaraldehyde method and chicken immunization with KLH-gp120 HIV fragment; antibodies were extracted by PEG precipitation. | Anti-HIV-gp120 IgY | Produced in chickens for diagnosis or treatment of patients with HIV. | [52] |
| Ovarian cancer | Chicken immunization with ovarian tumor-associated antigen 1 (OVTA 1) and generation of anti-OVTA 1 polyclonal IgY antibodies. | Anti-OVTA 1 polyclonal IgY | Ovarian cancer can be diagnosed using this method, which is also effective for the early screening of ovarian cancer. | [53] |
| Periodontitis | Porphyromonas gingivalis (Pg) and Actinobacillus actinomycetemcomitans (Aa) from dental plaque were used as antigens to immunize chickens for the preparation of anti-periodontitis-causing bacterial complex IgY, and antibodies were extracted by PEG precipitation. | Anti-periodontitis-causing bacterial complex IgY | Specific complex IgY inhibits the formation of Pg and Aa bacterial biofilms and exerts an antibacterial effect, which can be used for the targeted treatment of periodontitis. | [54,55] |
| Dental caries | Streptococcus sobrinus as the antigen, Freund’s complete adjuvant fully emulsified to immunize laying hens, IgY antibody purified by anion-exchange chromatography. | Anti S. sobrinus IgY | IgY inhibits the adhesion and acid production of S. sobrinus, reduces the abundance of oral Streptococcus, and effectively prevents dental caries. | [56,57] |
| Viral hepatitis type B(HBV) | Preparation of anti-HBV IgY for specific immunotherapy in laying hens immunized with hepatitis B vaccine (HepB). | Anti-HBV IgY | Showed strong antigen-specific binding activity as determined by ELISA. | [58] |
| Diarrhea | Clostridium difficile (CD) was detoxified with formaldehyde and mixed 1:1 with Freund’s complete adjuvant (FCA), and anti-CD IgY was prepared by immunizing laying hens, and the antibody was purified by ammonium sulfate precipitation. | Anti-CD IgY | Treating acute and recurring Clostridium difficile infection (CDI) in humans. | [59] |
| Hand-foot-and-mouth disease, (HFMD) | Anti-EV71 IgY was prepared by mixing EV71 strain as antigen with Freund’s incomplete adjuvant, 1:1 emulsion, immunizing laying hens and purifying the antibody by ammonium sulfate precipitation. | Anti-EV71 IgY | Recognizes the envelope proteins of EV71 and effectively inhibits viral infections. | [60] |
| COVID-19 | Anti-SARS-CoV-2 IgY was prepared by immunizing laying hens with a mixture of formaldehyde-inactivated SARS-CoV-2 and Freund’s incomplete adjuvant emulsified in water, and antibodies were extracted by water dilution. | Anti-SARS-CoV-2 IgY | Strong activity in the upper respiratory tract and inhibition of SARS-CoV-2 infection with good safety and drug resistance. | [61,62] |
| Ebola virus (EBOV) | A thermostable therapeutic antibody against EBOV was developed modeled on the IgY; encoding the EBOV glycoprotein could enhance antibodies against EBOV. | Anti-EBOV IgY | Exhibits excellent thermostability and protective efficacy. | [63] |
| Human and simian rotaviruses | Inoculated Rhodia laying chickens with two or three doses of RVA combined with adjuvants or only adjuvants, prepared anti-RVA IgY. | Anti-RVA IgY | Latex beads performed concordant results with 75% ELISA and PCR-positive and 87.5% negative samples. | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, H.; Jin, X.; Zhang, X.; Chen, K.; Wang, L.; Huang, J. Egg Yolk Immunoglobulins (IgY) Purification, Activity Enhancement, and Potential Benefits for Human Health. Nutrients 2025, 17, 2890. https://doi.org/10.3390/nu17172890
Qiu H, Jin X, Zhang X, Chen K, Wang L, Huang J. Egg Yolk Immunoglobulins (IgY) Purification, Activity Enhancement, and Potential Benefits for Human Health. Nutrients. 2025; 17(17):2890. https://doi.org/10.3390/nu17172890
Chicago/Turabian StyleQiu, Huilong, Xiaomin Jin, Xiaomei Zhang, Ke Chen, Lianshun Wang, and Jiaqiang Huang. 2025. "Egg Yolk Immunoglobulins (IgY) Purification, Activity Enhancement, and Potential Benefits for Human Health" Nutrients 17, no. 17: 2890. https://doi.org/10.3390/nu17172890
APA StyleQiu, H., Jin, X., Zhang, X., Chen, K., Wang, L., & Huang, J. (2025). Egg Yolk Immunoglobulins (IgY) Purification, Activity Enhancement, and Potential Benefits for Human Health. Nutrients, 17(17), 2890. https://doi.org/10.3390/nu17172890







