Randomised Trial Shows Readymade Oral Nutritional Supplements in Older Malnourished People in the Community Improve Total Nutrient Intakes and Meet More Dietary Reference Values Without Reducing Intake from the Diet
Abstract
1. Introduction
2. Materials and Methods
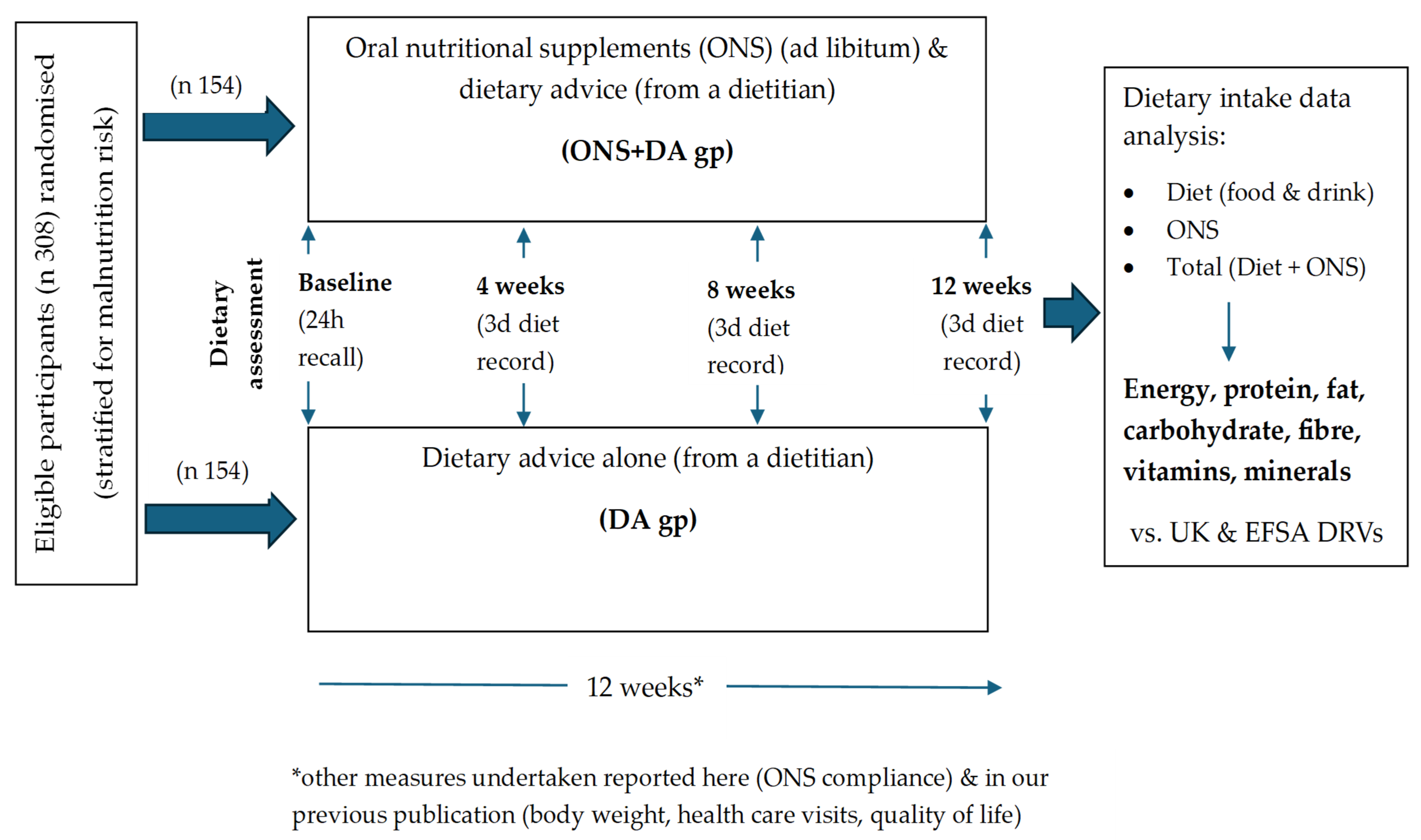
2.1. Overview
2.2. Participants Selection and Ethics
2.3. Types of Intervention
2.4. Data Collection and Outcomes
2.5. Dietary Intake Assessment
2.6. Compliance
2.7. Dietary Intake Analysis
2.8. Dietary Intake Analysis: Equations and Modelling
- (i)
- Equations to assess the relationship between ONS intake and percent of ONS nutrients that add to the diet
- (ii)
- Dose-response modelling to assess the relationship between ONS intake and percent of ONS nutrients that add to the diet:
- the two groups (ONS + DA and DA) had the same nutrient intake at baseline. (In the present study, the post-intervention results were adjusted for baseline nutrient intake);
- ingestion of ONS by the ONS + DA group (group A) during the intervention did not affect the composition of the diet, even when intake is suppressed (or stimulated)
- there was no ONS intake in the DA group. However, if the observed data from the randomized trial involved prescription of a small amount of ONS in the DA group for clinical reasons, the extra ONS (ONSe) consumed by the ONS + DA group (ONSe = ONSA − ONSB) was used in the above equations (instead of ONS intakeA), as an approximation to the model.
2.9. Statistics and Data Analysis
3. Results
3.1. Population Characteristics
3.2. Nutritional Intake at Baseline (ITT Analysis)
3.3. Nutritional Intake (Average) from the Diet During the Intervention Period (ITT Analysis)
3.4. Nutritional Intake (Average) from ONS During the Intervention Period (ITT Analysis)
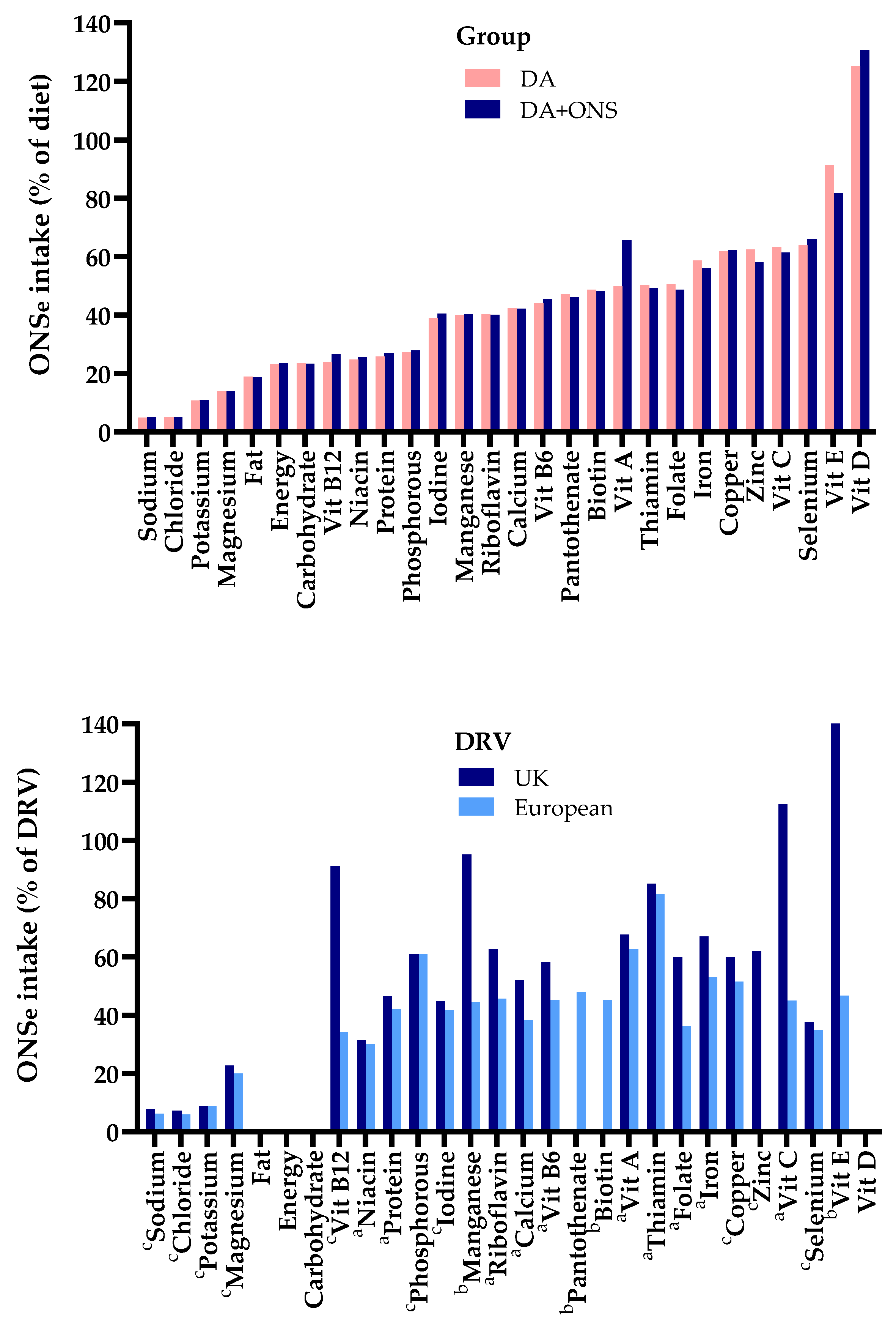
3.4.1. Contribution of the Nutrients from ONS to Total Dietary Intake
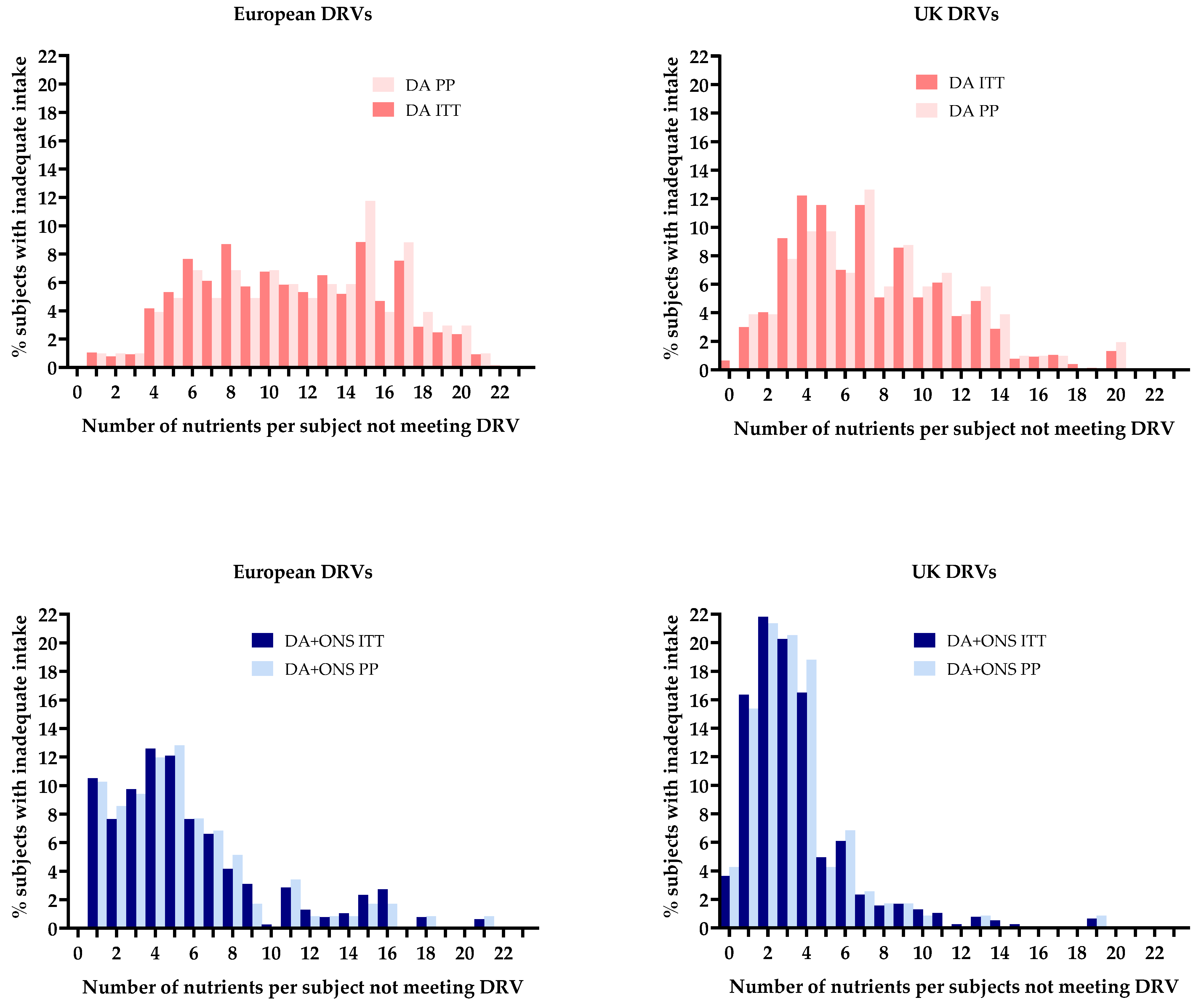
3.4.2. Contribution of the Nutrients from ONS to Meeting Dietary Reference Values
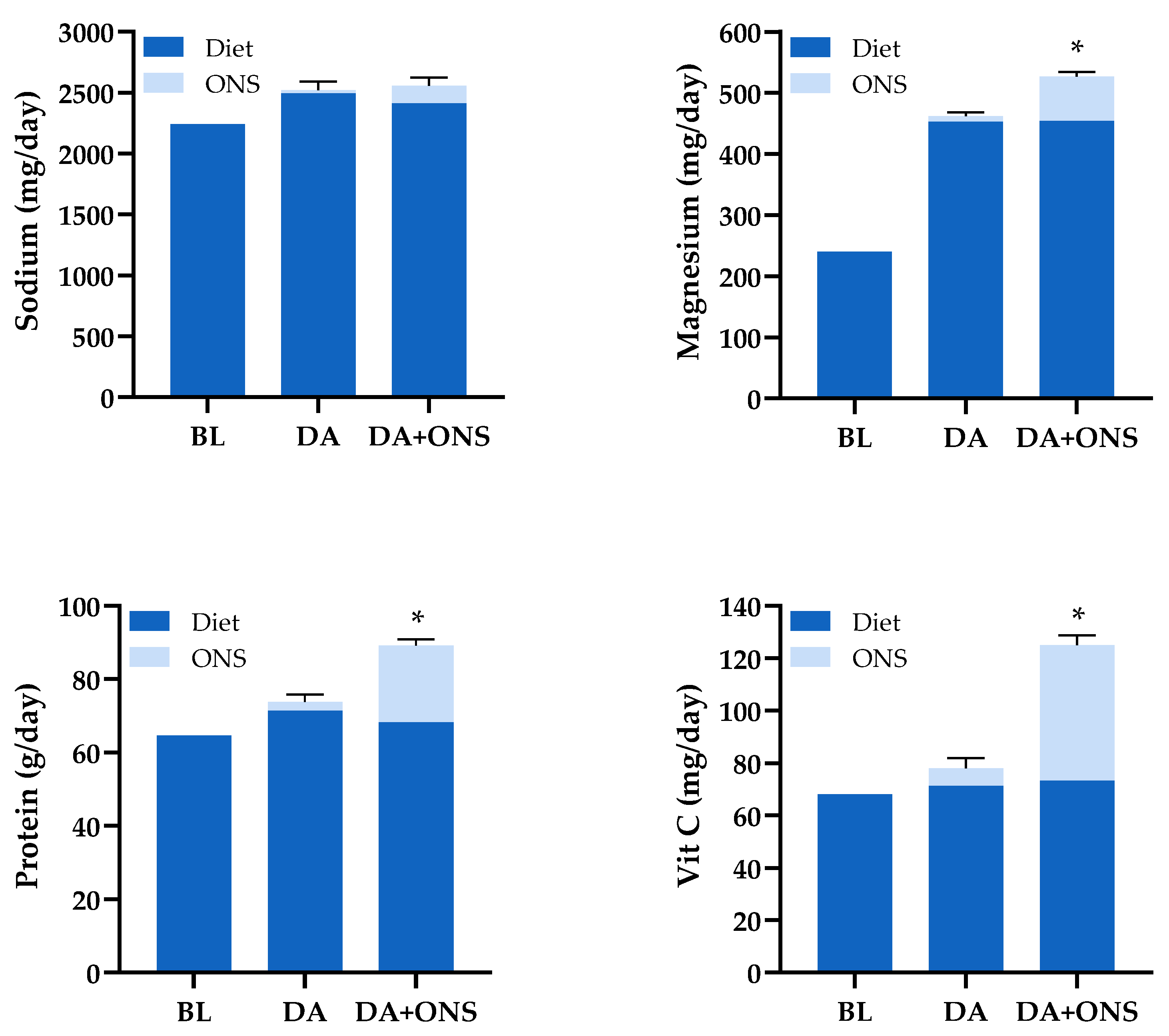
3.5. Total Nutritional Intake (ONS and Diet) (Average) During the Intervention Period (ITT Analysis)
3.5.1. Comparison of the Total Nutrient Intakes Between the ONS + DA and DA Groups
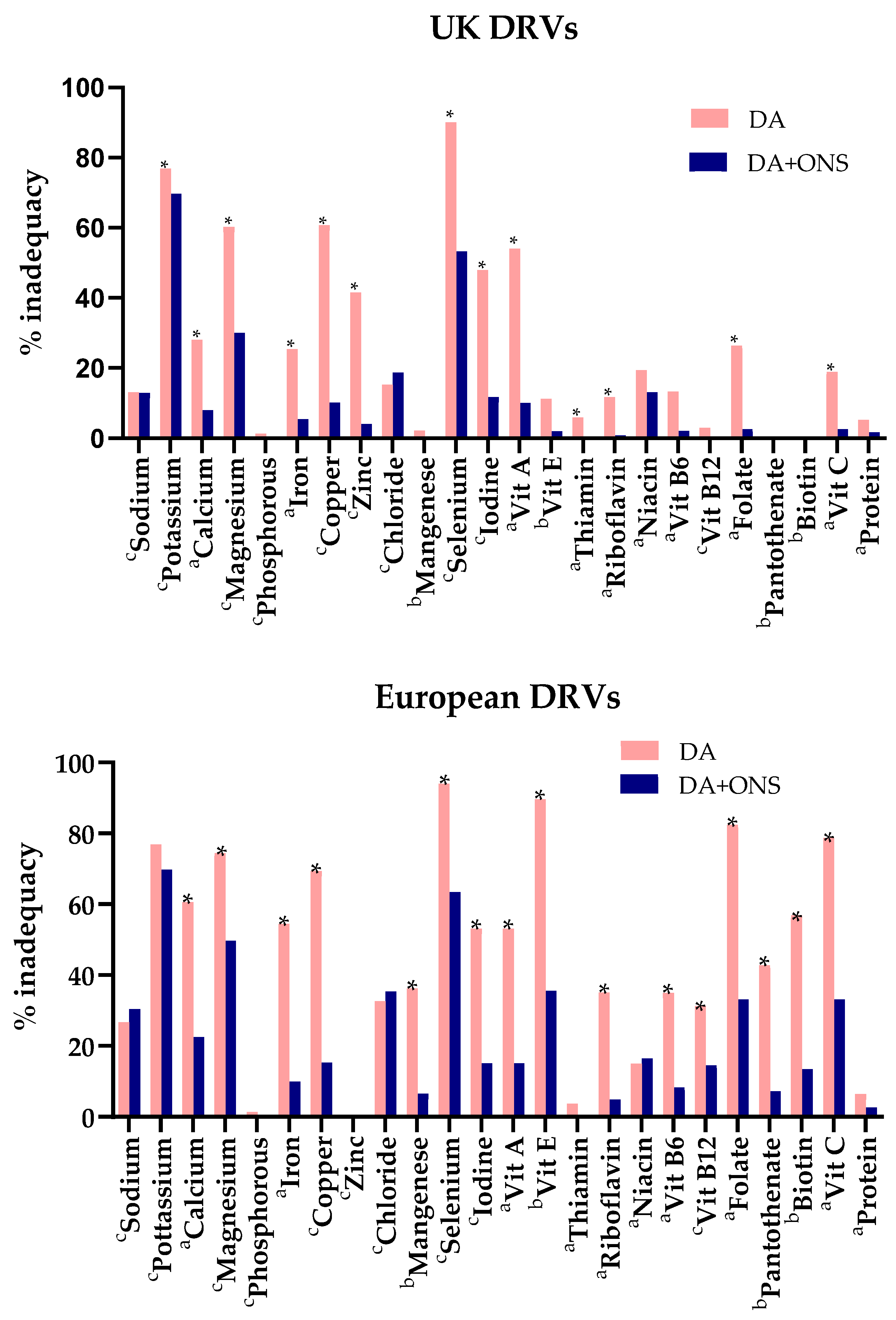
3.5.2. Comparison of Total Nutritional Intakes with Dietary Reference Values
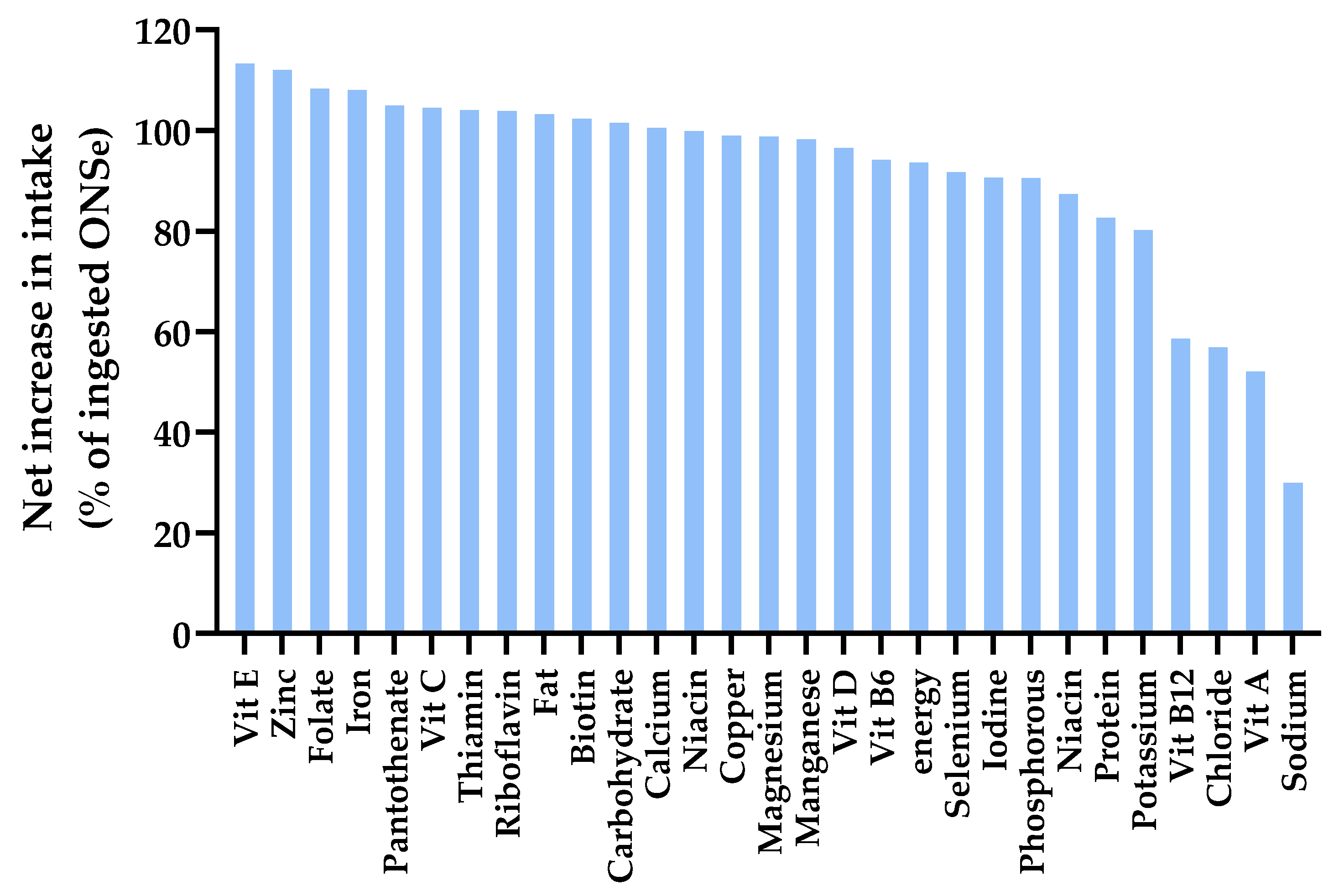
3.5.3. Net Increase in Intake with Supplementation (% ONS That Adds to Dietary Intake)
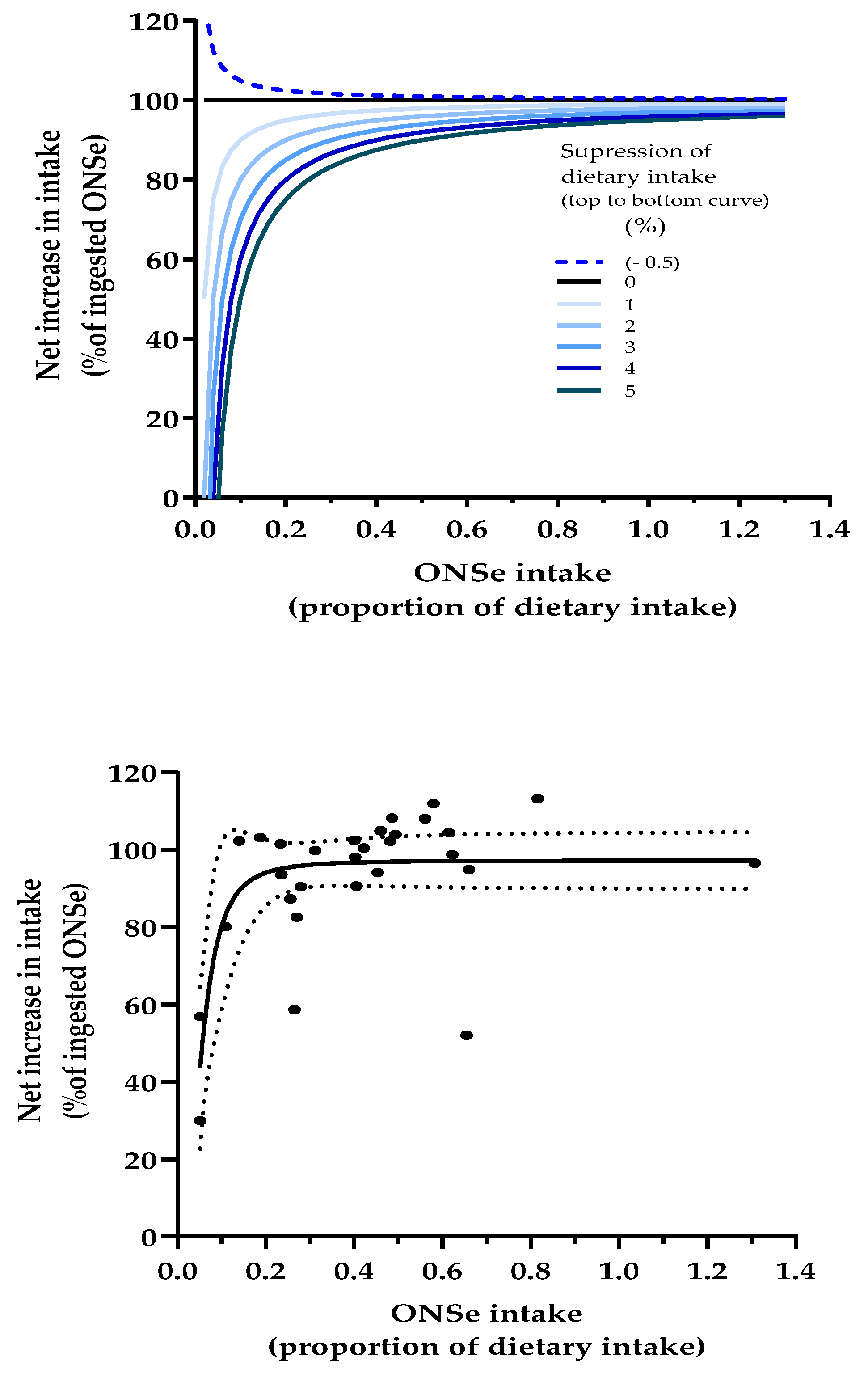
3.5.4. Dose-Response Modelling to Assess the Relationship Between ONS Intake and Percent of ONS Nutrients That Add to the Diet
3.6. Nutritional Intake (Average) from the Diet: Per Protocol (PP) and Intention to Treat (ITT) Analyses Compared
3.7. Nutritional Intake (Average) from ONS: Per Protocol (PP) and Intention to Treat (ITT) Analyses Compared
3.8. Total Nutritional Intake (ONS and Diet) (Average): Per Protocol (PP) and Intention to Treat (ITT) Analyses Compared
3.8.1. Comparison with Dietary Reference Values
3.8.2. Dose-Response Modelling to Assess the Relationship Between ONS Intake and Percent of ONS Nutrients That Add to the Diet
4. Discussion
4.1. Impact of ONS on Total Nutrient Intake and Meeting DRVs
4.2. Benchmarking the Total Intake of Our Study Population Against the UK and European
4.3. Energy/Nutrient Displacement from the Diet and % ONS That Adds to Dietary Intake
4.4. Limitations of Study
4.5. Clinical Applications for the Management of Malnutrition in Older People
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cawood, A.L.; Elia, M.; Stratton, R.J. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing. Res. Rev. 2012, 11, 278–296. [Google Scholar] [CrossRef]
- Stratton, R.J.; Hebuterne, X.; Elia, M. A systematic review and meta-analysis of the impact of oral nutritional supplements on hospital readmissions. Ageing. Res. Rev. 2013, 12, 884–897. [Google Scholar] [CrossRef] [PubMed]
- Elia, M.; Normand, C.; Norman, K.; Laviano, A. A systematic review of the cost and cost-effectiveness of standard oral nutritional supplements in community and care home settings. Clin. Nutr. 2016, 35, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Elia, M.; Normand, C.; Norman, K.; Laviano, A. A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in the hospital setting. Clin. Nutr. 2016, 35, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, J.H.; Duckworth, A.D.; Clement, N.D. Effect of oral nutritional supplementation on outcomes in older adults with hip fractures and factors influencing compliance. Bone Jt. J. 2023, 105–B, 1149–1158. [Google Scholar] [CrossRef]
- Stratton, R.J.; Green, C.J.; Elia, M. Disease-related malnutrition. An Evidence-Based Approach to Treatment; CABI Publishing (CAB International): Oxford, UK, 2003. [Google Scholar]
- Dent, E.; Wright, O.R.L.; Woo, J.; Hoogendijk, E.O. Malnutrition in older adults. Lancet 2023, 401, 951–966. [Google Scholar] [CrossRef]
- Stratton, R.J. Managing malnutrition and multimorbidity in primary care: Dietary approaches to reduce treatment burden. Proc. Nutr. Soc. 2024, 16, 1–9. [Google Scholar] [CrossRef]
- Hubbard, G.P.; Elia, M.; Holdoway, A.; Stratton, R.J. A systematic review of compliance to oral nutritional supplements. Clin. Nutr. 2012, 31, 293–312. [Google Scholar] [CrossRef]
- Lester, S.; Kleijn, M.; Cornacchia, L.; Hewson, L.; Taylor, M.A.; Fisk, I. Factors Affecting Adherence, Intake, and Perceived Palatability of Oral Nutritional Supplements: A Literature Review. J. Nutr. Health Aging. 2022, 26, 663–674. [Google Scholar] [CrossRef]
- Wang, Q.C.; Yuan, H.; Chen, Z.M.; Wang, J.; Xue, H.; Zhang, X.Y. Barriers and Facilitators of Adherence to Oral Nutritional Supplements Among People Living with Cancer: A Systematic Review. Clin. Nurs. Res. 2023, 32, 209–220. [Google Scholar] [CrossRef]
- Manders, M.; de Groot, C.P.; Blauw, Y.H.; Dhonukshe-Rutten, R.A.M.; van Hoeckel-Prüst, L.; Bindels, J.G.; Siebelink, E.; van Staveren, W.A. Effect of a nutrient-enriched drink on dietary intake and nutritional status in institutionalised elderly. Eur. J. Clin. Nutr. 2009, 63, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.S.; Yousif, L.; Miles, A.; Braakhuis, A. A Comparison of Dietary Intake and Nutritional Status between Aged Care Residents Consuming Texture-Modified Diets with and without Oral Nutritional Supplements. Nutrients 2022, 14, 669. [Google Scholar] [CrossRef] [PubMed]
- Fiatarone Singh, M.A.; Bernstein, M.A.; Ryan, A.D.; O’Neill, E.F.; Clements, K.M.; Evans, W.J. The effect of oral nutritional supplements on habitual dietary quality and quantity in frail elders. J. Nutr. Health Aging. 2000, 4, 5–12. [Google Scholar] [PubMed]
- Parsons, E.L.; Stratton, R.J.; Cawood, A.L.; Smith, T.R.; Elia, M. Oral nutritional supplements in a randomised trial are more effective than dietary advice at improving quality of life in malnourished care home residents. Clin. Nutr. 2017, 36, 134–142. [Google Scholar] [CrossRef]
- Ferreira, I.B.; Lima, E.; Canto, P.P.L.; Gontijo, C.A.; Maia, Y.C.P.; Pena, G.D.G. Oral Nutritional Supplementation Affects the Dietary Intake and Body Weight of Head and Neck Cancer Patients during (Chemo) Radiotherapy. Nutrients 2020, 12, 2516. [Google Scholar] [CrossRef]
- Loman, B.R.; Luo, M.; Baggs, G.E.; Mitchell, D.C.; Nelson, J.L.; Ziegler, T.R.; Deutz, N.E.; Matarese, L.E.; NOURISH Study Group. Specialized High-Protein Oral Nutrition Supplement Improves Home Nutrient Intake of Malnourished Older Adults Without Decreasing Usual Food Intake. JPEN J. Parenter. Enteral. Nutr. 2019, 43, 794–802. [Google Scholar] [CrossRef]
- Smith, T.R.; Cawood, A.L.; Walters, E.R.; Guildford, N.; Stratton, R.J. Ready-Made Oral Nutritional Supplements Improve Nutritional Outcomes and Reduce Health Care Use-A Randomised Trial in Older Malnourished People in Primary Care. Nutrients 2020, 12, 517. [Google Scholar] [CrossRef]
- McCance, R.A.; Widdowson, E.M.; Great Britain Food Standards Agency. McCance and Widdowson’s The Composition of Foods, 6th ed; Royal Society of Chemistry: Cambridge, UK, 2002. [Google Scholar]
- Department of Health. Dietary Reference Values for Food, Energy and Nutrients for the United Kingdom; HMSO: London, UK, 1991; ISBN 0 11 321397 2. [Google Scholar]
- European Food Safety Authority (EFSA). Dietary Reference Values for Nutrients, Summary Report; EFSA Supporting Publication: Parma, Italy, 2017; pp. 1–98. [Google Scholar] [CrossRef]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons, Inc: Chichester, UK, 1987. [Google Scholar]
- Almiron-Roig, E.; Palla, L.; Guest, K.; Ricchiuti, C.; Vint, N.; A Jebb, S.; Drewnowski, A. Factors that determine energy compensation: A systematic review of preload studies. Nutr. Rev. 2013, 71, 458–473. [Google Scholar] [CrossRef]
- Fatima, S.; Gerasimidis, K.; Wright, C.; Malkova, D. Impact of high energy oral nutritional supplements consumed in the late afternoon on appetite, energy intake and cardio-metabolic risk factors in females with lower BMI. Eur. J. Clin. Nutr. 2022, 76, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Gerasimidis, K.; Wright, C.; Tsiountsioura, M.; Arvanitidou, E.I.; Malkova, D. Response of appetite and potential appetite regulators following intake of high energy nutritional supplements. Appetite 2015, 95, 36–43. [Google Scholar] [CrossRef]
- Miquel-Kergoat, S.; Azais-Braesco, V.; Burton-Freeman, B.; Hetherington, M.M. Effects of chewing on appetite, food intake and gut hormones: A systematic review and meta-analysis. Physiol. Behav. 2015, 151, 88–96. [Google Scholar] [CrossRef]
- Ye, Z.; Arumugam, V.; Haugabrooks, E.; Williamson, P.; Hendrich, S. Soluble dietary fiber (Fibersol-2) decreased hunger and increased satiety hormones in humans when ingested with a meal. Nutr. Res. 2015, 35, 393–400. [Google Scholar] [CrossRef]
- Krishnasamy, S.; Lomer, M.C.E.; Marciani, L.; Hoad, C.L.; Pritchard, S.E.; Paul, J.; Gowland, P.A.; Spiller, R.C. Processing Apples to Puree or Juice Speeds Gastric Emptying and Reduces Postprandial Intestinal Volumes and Satiety in Healthy Adults. J. Nutr. 2020, 150, 2890–2899. [Google Scholar] [CrossRef] [PubMed]
- Sturm, K.; MacIntosh, C.G.; Parker, B.A.; Wishart, J.; Horowitz, M.; Chapman, I.M. Appetite, food intake, and plasma concentrations of cholecystokinin, ghrelin, and other gastrointestinal hormones in undernourished older women and well-nourished young and older women. J. Clin. Endocrinol. Metab. 2003, 88, 3747–3755. [Google Scholar] [CrossRef] [PubMed]
- Boudville, A.; Bruce, D.G. Lack of meal intake compensation following nutritional supplements in hospitalised elderly women. Br. J. Nutr. 2005, 93, 879–884. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, Y.; Zhang, J.; Ma, Z.; Amoah, A.N.; Cao, Y.; Wang, X.; Fu, P.; Lyu, Q. The effect of oral nutritional supplements on the nutritional status of community elderly people with malnutrition or risk of malnutrition. Asia Pac. J. Clin. Nutr. 2021, 30, 415–423. [Google Scholar] [PubMed]
- Dingemans, A.-M.; van Walree, N.; Schramel, F.; Soud, M.Y.-E.; Baltruškevičienė, E.; Lybaert, W.; Veldhorst, M.; van der Berg, C.A.; Kaasa, S. High Protein Oral Nutritional Supplements Enable the Majority of Cancer Patients to Meet Protein Intake Recommendations during Systemic Anti-Cancer Treatment: A Randomised Controlled Parallel-Group Study. Nutrients 2023, 15, 5030. [Google Scholar] [CrossRef]
- Khaldilkar, A.; Dhongade, A.; Agrawal, N. Impact of Oral Nutritional Supplement on Growth, Nutrient Intake and Frequency of Illness in Children aged 4–6 Years: A Randomised Controlled Study. J. Clin. Diagn. Res. 2021, 15, 11. [Google Scholar]
- Victoria, C.-B.; Casilda, O.; Nuria, P.; José, A.-F.; María, G.-O.; José, S.-T.F.; Victoria, G.M.; Ignacio, R.-G.; Gabriel, O. Oral Nutritional Supplements in Adults with Cystic Fibrosis: Effects on Intake, Levels of Fat-Soluble Vitamins, and Bone Remodeling Biomarkers. Nutrients 2021, 13, 669. [Google Scholar] [CrossRef]
- Beaton, G.H.; Milner, J.; McGuire, V.; Feather, T.E.; Little, J.A. Source of variance in 24-hour dietary recall data: Implications for nutrition study design and interpretation. Carbohydrate sources, vitamins, and minerals. Am. J. Clin. Nutr. 1983, 37, 986–995. [Google Scholar] [CrossRef]
- Bingham, S. The dietary assessment of individuals; methods, accuracy, new techniques and recommendations. Nutr. Abstr. Rev. 1987, 57, 705–742. [Google Scholar]
| ONS + DA | DA | ALL | p-Value a | |
|---|---|---|---|---|
| n = 154 b | n = 152 b | n = 306 b | ||
| Energy (kcal) | 1759 ± 46 | 1711 ± 46 | 1735 ± 32 | 0.465 |
| Protein (g) | 65.4 ± 1.7 | 64 ± 1.8 | 64.7 ± 1.2 | 0.579 |
| Carbohydrate (g) | 207 ± 6 | 206 ± 6 | 207 ± 4 | 0.918 |
| Fat (g) | 76.1 ± 2.7 | 71.1 ± 2.6 | 73.6 ± 1.9 | 0.182 |
| Fibre (g) | 15.2 ± 0.6 | 15.1 ± 0.6 | 15.1 ± 0.4 | 0.951 |
| Sodium(mg/d) | 2228 ± 78 | 2246 ± 106 | 2237 ± 65 | 0.893 |
| Potassium (mg/d) | 2726 ± 74 | 2654 ± 69 | 2690 ± 51 | 0.478 |
| Calcium (mg/d) | 820 ± 26 | 816 ± 31 | 818 ± 20 | 0.918 |
| Magnesium (mg/d) | 241 ± 7 | 239 ± 7 | 240 ± 5 | 0.852 |
| Phosphorous (mg/d) | 1152 ± 30 | 1144 ± 32 | 1148 ± 22 | 0.856 |
| Iron (mg/d) | 9.45 ± 0.32 | 10.02 ± 0.38 | 9.73 ± 0.25 | 0.254 |
| Copper (mg/d) | 1.05 ± 0.05 | 1.15 ± 0.14 | 1.1 ± 0.07 | 0.474 |
| Zinc (mg/d) | 7.56 ± 0.22 | 7.66 ± 0.27 | 7.61 ± 0.17 | 0.783 |
| Chloride (mg/d) | 3303 ± 113 | 3318 ± 151 | 3311 ± 94 | 0.934 |
| Manganese (mg/d) | 3.28 ± 0.12 | 3.27 ± 0.11 | 3.27 ± 0.08 | 0.957 |
| Selenium (mcg/d) | 37.2 ± 1.86 | 34 ± 1.6 | 35.6 ± 1.2 | 0.194 |
| Iodine (mcg/d) | 143 ± 8 | 137 ± 8 | 140 ± 6 | 0.599 |
| Vitamin A (mcg/d) | 489 ± 49 | 758 ± 154 | 623 ± 81 | 0.095 |
| Vitamin D (mcg/d) | 2.57 ± 0.28 | 2.29 ± 0.24 | 2.43 ± 0.18 | 0.448 |
| Vitamin E (mg/d) | 6.77 ± 0.36 | 6.25 ± 0.34 | 6.51 ± 0.25 | 0.292 |
| Thiamin (mg/d) | 1.37 ± 0.04 | 1.39 ± 0.06 | 1.38 ± 0.04 | 0.781 |
| Riboflavin (mg/d) | 1.67 ± 0.06 | 1.76 ± 0.06 | 1.72 ± 0.04 | 0.294 |
| Niacin (mg/d) | 15.9 ± 0.7 | 16.6 ± 0.8 | 16.3 ± 0.5 | 0.472 |
| Potential niacin (mg/d) | 13.4 ± 0.4 | 13.0 ± 0.4 | 13.2 ± 0.3 | 0.485 |
| Vitamin B6 (mg/d) | 1.63 ± 0.05 | 1.61 ± 0.06 | 1.62 ± 0.04 | 0.82 |
| Vitamin B12 (mcg/d) | 4.43 ± 0.25 | 4.86 ± 0.38 | 4.64 ± 0.23 | 0.337 |
| Folate (mcg/d) | 224 ± 7 | 235 ± 8 | 230 ± 5 | 0.283 |
| Pantothenic acid (mg/d) | 4.89 ± 0.16 | 4.98 ± 0.18 | 4.93 ± 0.12 | 0.696 |
| Biotin (mcg/d) | 35.9 ± 1.3 | 36.5 ± 1.3 | 36.2 ± 0.9 | 0.774 |
| Vitamin C (mg/d) | 72.5 ± 4.5 | 63.4 ± 4.2 | 68 ± 3.1 | 0.142 |
| Baseline | ONS + DA Group (n = 154) | DA Group (n = 154) | |||||
|---|---|---|---|---|---|---|---|
| Diet | ONS Only | Diet Only | Diet + ONS | ONS Only | Diet Only | Diet + ONS | |
| Energy (kcal/d) | 1735 | 480 ± 12 b | 1820 ± 41 | 2300 ± 43 b | 51 ± 13 | 1848 ± 43 | 1899 ± 45 |
| Protein (g/d) | 64.7 | 21.0 ± 0.6 b | 68.2 ± 1.6 | 89.2 ± 1.7 b | 2.5 ± 0.7 | 71.4 ± 1.7 | 74.0 ± 1.9 |
| Carbohydrate (g/d) | 207 | 60 ± 2 b | 218 ± 5 | 278 ± 5 b | 9 ± 2 | 217 ± 5 | 226 ± 6 |
| Fat (g/d) | 73.7 | 16.9 ± 0.6 b | 76.3 ± 2.1 | 93.2 ± 2.1 b | 2.6 ± 0.6 | 75.8 ± 2.4 | 78.4 ± 2.4 |
| Fibre (g/d) | 15.18 | 0.02 ± 0.01 | 15.38 ± 0.43 | 15.39 ± 0.43 | 0.01 ± 0.01 | 15.94 ± 0.46 | 15.95 ± 0.47 |
| Sodium (mg/d) | 2243 | 149 ± 5 b | 2410 ± 63 | 2559 ± 64 | 25 ± 6 | 2497 ± 69 | 2521 ± 70 |
| Potassium (mg/d) | 2693 | 365 ± 14 b | 2831 ± 58 | 3196 ± 6 a | 58 ± 14 | 2892 ± 60 | 2950 ± 63 |
| Calcium (mg/d) | 819 | 417 ± 15 b | 862 ± 25 | 1279 ± 28 b | 54 ± 15 | 860 ± 27 | 914 ± 30 |
| Magnesium (mg/d) | 240 | 73 ± 2 b | 255 ± 6 | 328 ± 7 b | 9 ± 3 | 256 ± 6 | 265 ± 6 |
| Phosphorous (mg/d) | 1148 | 383 ± 14 b | 1203 ± 26 | 1586 ± 29 b | 48 ± 14 | 1234 ± 27 | 1282 ± 30 |
| Iron (mg/d) | 9.7 | 6.8 ± 0.2 b | 10.4 ± 0.3 | 17.2 ± 0.3 b | 1 ± 0.2 | 9.9 ± 0.3 | 10.9 ± 0.3 |
| Copper (mg/d) | 1.10 | 0.83 ± 0.02 b | 1.16 ± 0.06 | 1.99 ± 0.06 b | 0.11 ± 0.02 | 1.17 ± 0.06 | 1.28 ± 0.07 |
| Zinc (mg/d) | 7.61 | 5.6 ± 0.15 b | 8.36 ± 0.23 | 13.97 ± 0.27 b | 0.75 ± 0.16 | 7.78 ± 0.23 | 8.53 ± 0.27 |
| Chloride (mg/d) | 3314 | 216 ± 11 b | 3532 ± 86 | 3748 ± 88 | 36 ± 12 | 3610 ± 108 | 3645 ± 110 |
| Manganese (mg/d) | 3.28 | 1.54 ± 0.04 b | 3.32 ± 0.1 | 4.86 ± 0.1 b | 0.2 ± 0.04 | 3.34 ± 0.11 | 3.55 ± 0.12 |
| Selenium (mcg/d) | 35.8 | 28.3 ± 0.8 b | 37.0 ± 1.2 | 65.3 ± 1.4 b | 3.9 ± 0.8 | 39.1 ± 1.5 | 42.9 ± 1.6 |
| Iodine (mcg/d) | 140 | 73 ± 2 b | 155 ± 7 | 227 ± 7 b | 10 ± 2 | 160 ± 7 | 170 ± 8 |
| Vitamin A (mcg/d) | 629 | 498 ± 14 b | 654 ± 74 | 1152 ± 74 a | 70 ± 14 | 859 ± 77 | 929 ± 77 |
| Vitamin D (mcg/d) | 2.45 | 3.79 ± 0.11 b | 2.48 ± 0.17 | 6.26 ± 0.19 b | 0.55 ± 0.11 | 2.59 ± 0.23 | 3.14 ± 0.24 |
| Vitamin E (mg/d) | 6.53 | 6.47 ± 0.2 b | 6.67 ± 0.34 | 13.14 ± 0.33 b | 1.03 ± 0.2 | 5.95 ± 0.36 | 6.98 ± 0.37 |
| Thiamin (mg/d) | 1.37 | 0.84 ± 0.03 b | 1.44 ± 0.04 | 2.28 ± 0.05 b | 0.13 ± 0.03 | 1.41 ± 0.05 | 1.54 ± 0.05 |
| Riboflavin (mg/d) | 1.71 | 0.86 ± 0.03 b | 1.82 ± 0.05 | 2.68 ± 0.06 b | 0.13 ± 0.03 | 1.81 ± 0.06 | 1.94 ± 0.06 |
| Niacin (mg/d) | 16.28 | 5.3 ± 0.2 b | 16.4 ± 0.5 | 21.7 ± 0.5 b | 1.1 ± 0.3 | 17.0 ± 0.06 | 18.0 ± 0.6 |
| Potential niacin (mg/day) | 13.2 | 5.4 ± 0.3 b | 13.8 ± 0.4 | 19.2 ± 0.4 b | 1.1 ± 0.3 | 13.8 ± 0.4 | 14.9 ± 0.4 |
| Vitamin B6 (mg/d) | 1.62 | 0.91 ± 0.03 b | 1.63 ± 0.04 | 2.54 ± 0.05 b | 0.17 ± 0.03 | 1.67 ± 0.05 | 1.84 ± 0.05 |
| VitaminB12 (mcg/d) | 4.65 | 1.61 ± 0.05 b | 5.15 ± 0.28 | 6.77 ± 0.28 | 0.25 ± 0.05 | 5.72 ± 0.31 | 5.96 ± 0.31 |
| Folate (mcg/d) | 230 | 138 ± 4 b | 246 ± 7 | 384 ± 8 b | 18 ± 4 | 236 ± 7 | 255 ± 8 |
| Pantothenic acid (mg/d) | 4.93 | 2.77 ± 0.08 b | 5.23 ± 0.13 | 7.99 ± 0.15 b | 0.36 ± 0.08 | 5.11 ± 0.17 | 5.47 ± 0.19 |
| Biotin (mcg/d) | 36.2 | 20.6 ± 0.5 b | 37.5 ± 1.1 | 58.1 ± 1.2 b | 2.6 ± 0.6 | 37.1 ±1.3 | 39.7 ± 1.4 |
| Vitamin C (mg/d) | 68.1 | 51.7 ± 1.4 b | 73.3 ± 3.5 | 124.9 ± 3.7 b | 6.7 ± 1.4 | 71.3 ± 3.8 | 77.9 ± 4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elia, M.; Smith, T.R.; Cawood, A.L.; Walters, E.R.; Stratton, R.J. Randomised Trial Shows Readymade Oral Nutritional Supplements in Older Malnourished People in the Community Improve Total Nutrient Intakes and Meet More Dietary Reference Values Without Reducing Intake from the Diet. Nutrients 2025, 17, 2474. https://doi.org/10.3390/nu17152474
Elia M, Smith TR, Cawood AL, Walters ER, Stratton RJ. Randomised Trial Shows Readymade Oral Nutritional Supplements in Older Malnourished People in the Community Improve Total Nutrient Intakes and Meet More Dietary Reference Values Without Reducing Intake from the Diet. Nutrients. 2025; 17(15):2474. https://doi.org/10.3390/nu17152474
Chicago/Turabian StyleElia, Marinos, Trevor R. Smith, Abbie L. Cawood, Emily R. Walters, and Rebecca J. Stratton. 2025. "Randomised Trial Shows Readymade Oral Nutritional Supplements in Older Malnourished People in the Community Improve Total Nutrient Intakes and Meet More Dietary Reference Values Without Reducing Intake from the Diet" Nutrients 17, no. 15: 2474. https://doi.org/10.3390/nu17152474
APA StyleElia, M., Smith, T. R., Cawood, A. L., Walters, E. R., & Stratton, R. J. (2025). Randomised Trial Shows Readymade Oral Nutritional Supplements in Older Malnourished People in the Community Improve Total Nutrient Intakes and Meet More Dietary Reference Values Without Reducing Intake from the Diet. Nutrients, 17(15), 2474. https://doi.org/10.3390/nu17152474







