Anthocyanin-Rich Purple Plant Foods: Bioavailability, Antioxidant Mechanisms, and Functional Roles in Redox Regulation and Exercise Recovery
Abstract
1. Introduction
2. Oxidant–Antioxidant Balance and Mechanisms of Redox Regulation
3. Exercise-Induced ROS Production and Physiological Adaptation
4. Sources, Composition, and Structure of Anthocyanins in Purple Plant Foods
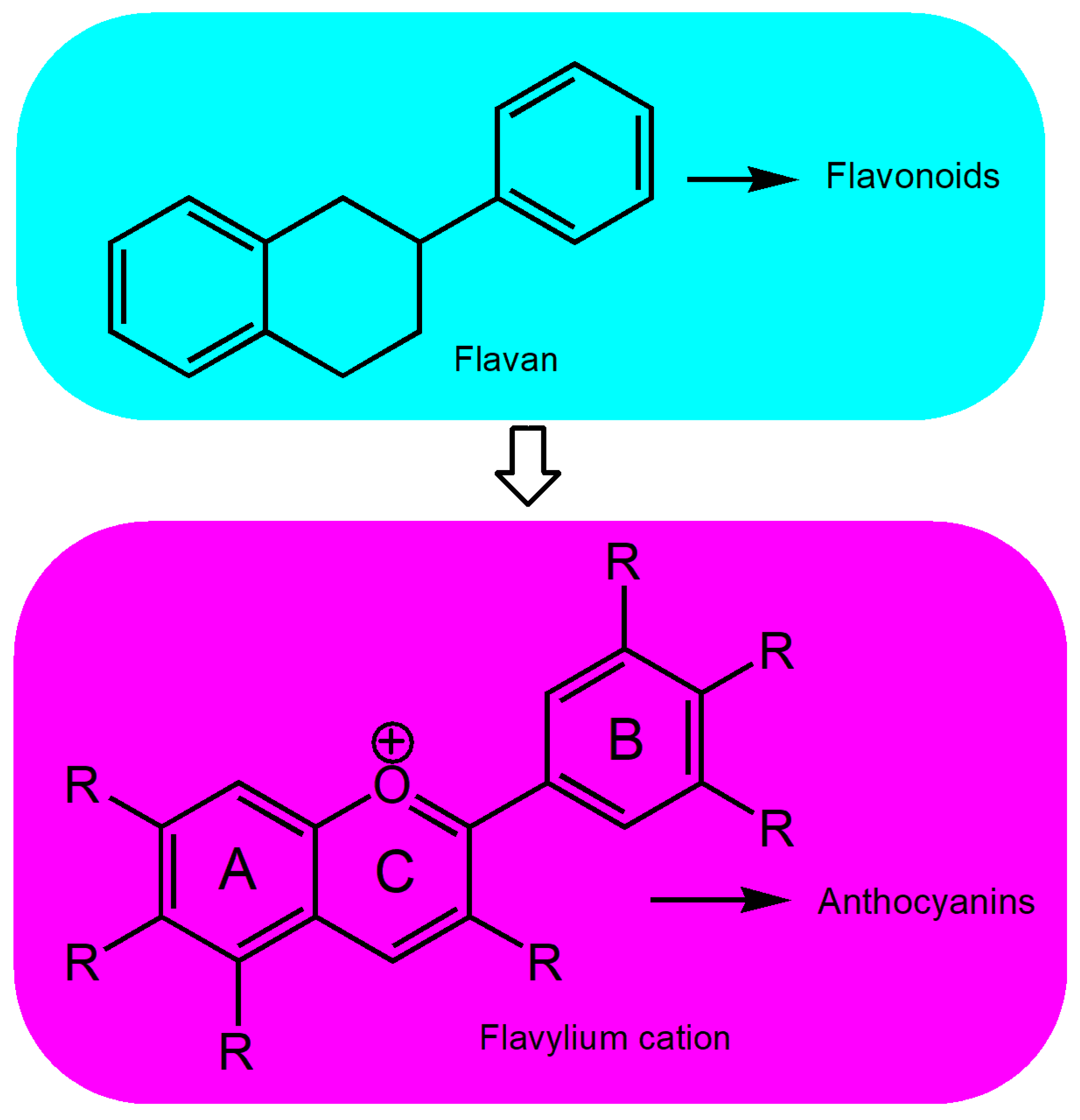
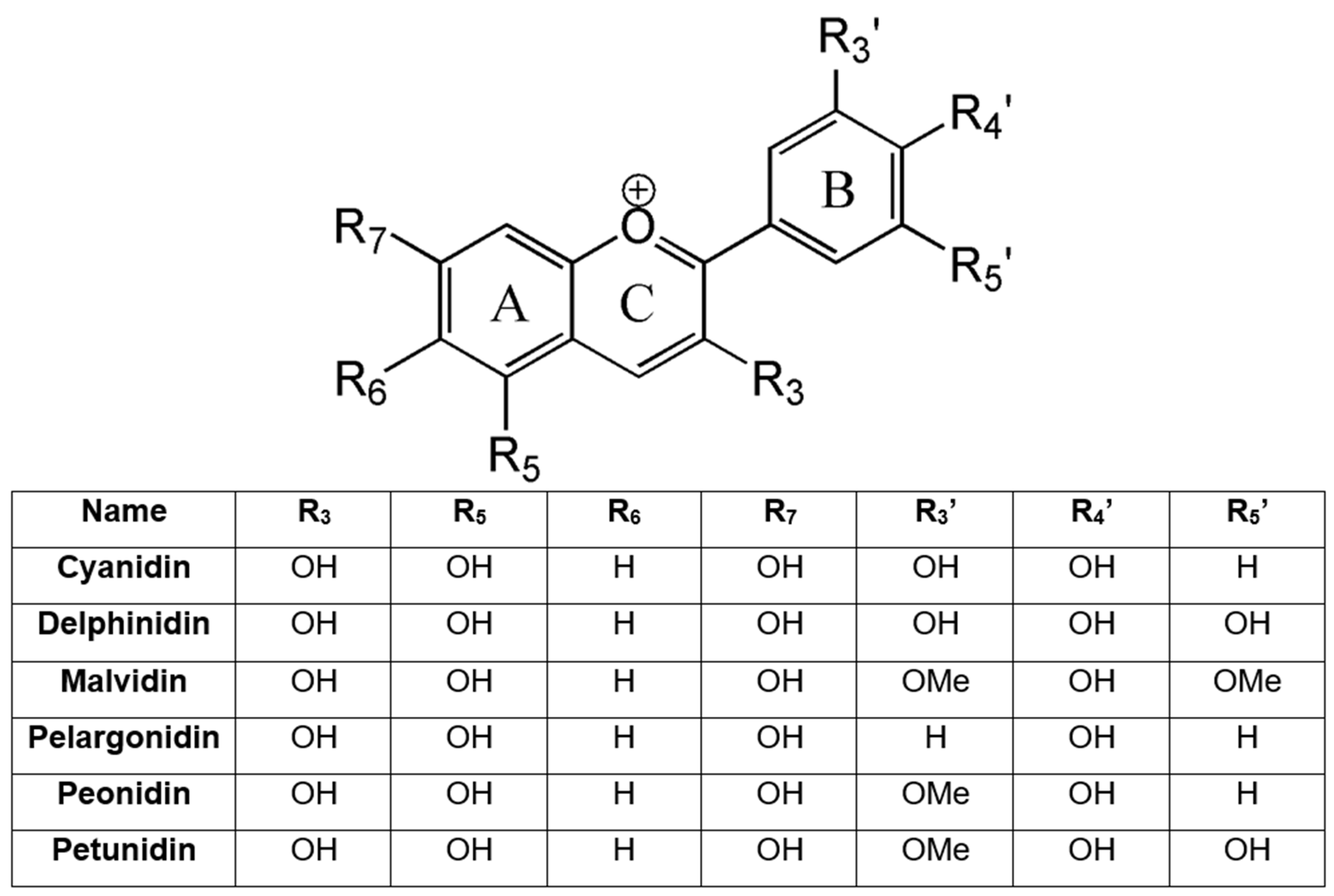
4.1. Chemical Structure and Biosynthesis of Anthocyanins
4.2. Botanical Sources of Purple Anthocyanins
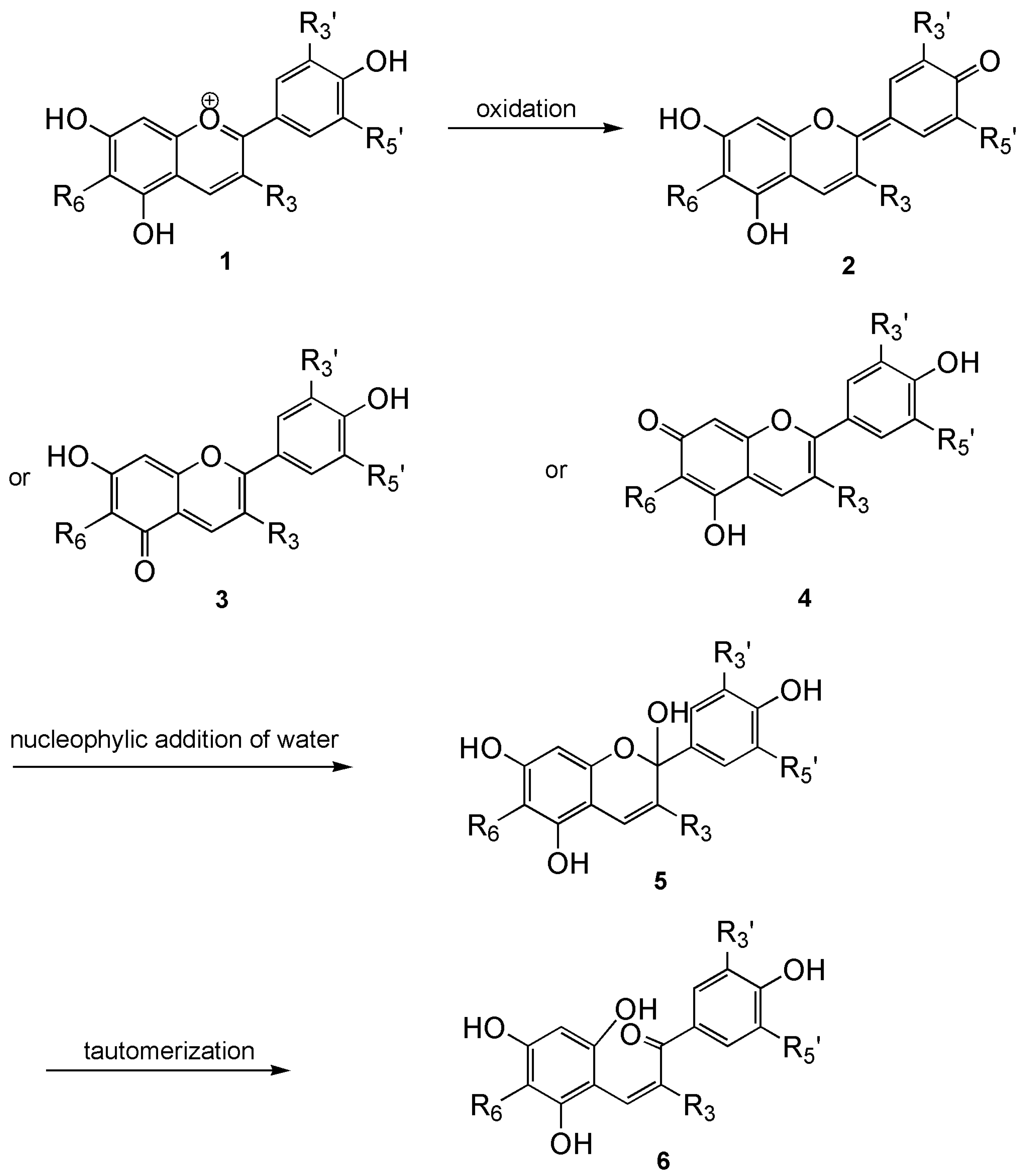
4.3. Stability, pH Effects, and Copigmentation Mechanisms
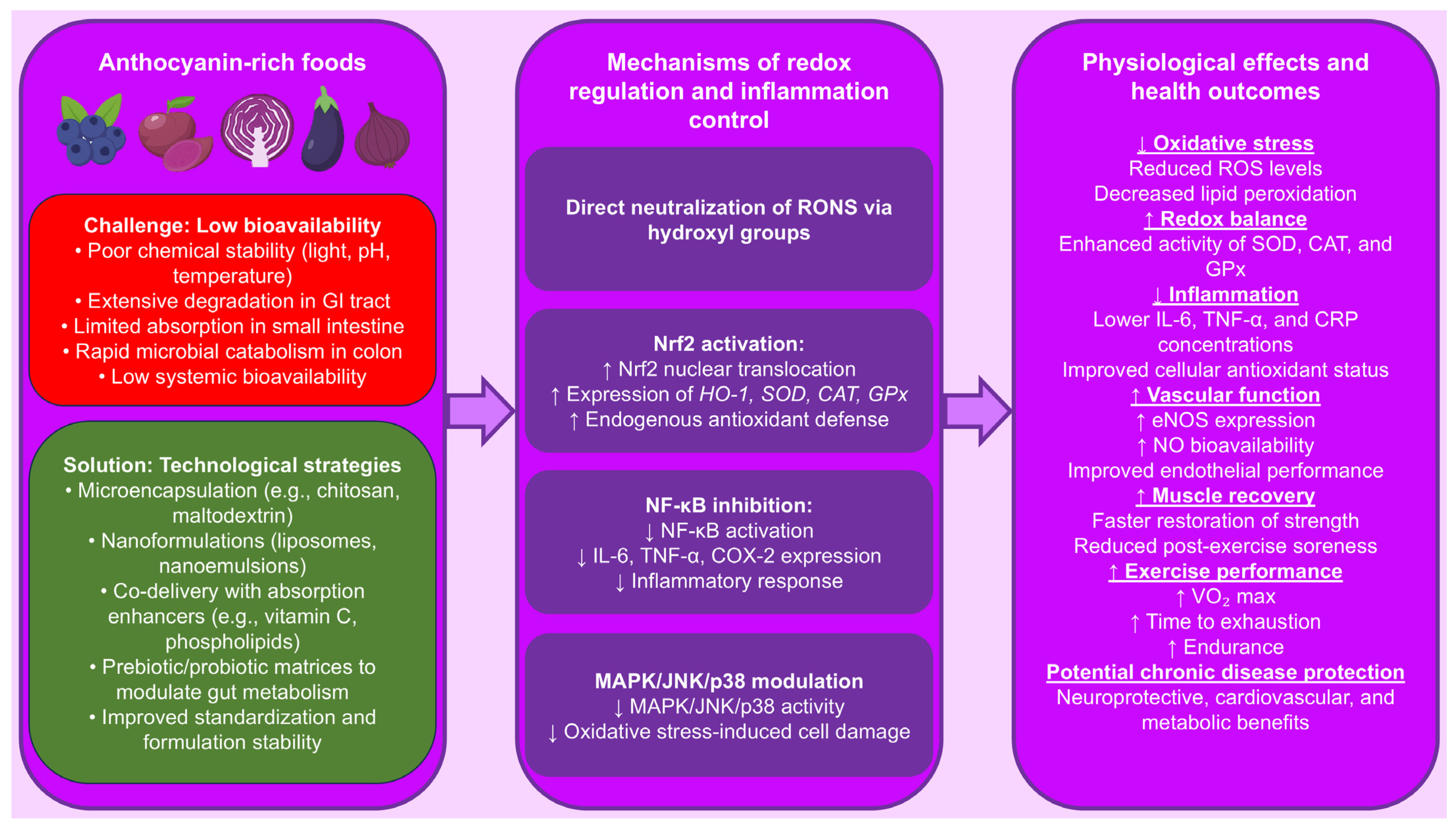
4.4. Applications, Intake, and Bioavailability Considerations
5. The Effects of Anthocyanin-Rich Foods on Oxidative Stress and Exercise Recovery
6. Dietary Recommendations and Research Perspectives
6.1. Anthocyanin Content, Structure, and Dietary Exposure
6.2. Intake Recommendations, Efficacy, and Applications in Exercise Nutrition
6.3. Delivery Innovations and Translational Challenges
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Williams, C.A.; Grayer, R.J. Anthocyanins and Other Flavonoids. Nat. Prod. Rep. 2004, 21, 539. [Google Scholar] [CrossRef]
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and Biological Activities of Anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between Color and Antioxidant Capacity of Fruits and Vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Frond, A.D.; Iuhas, C.I.; Stirbu, I.; Leopold, L.; Socaci, S.; Andreea, S.; Ayvaz, H.; Andreea, S.; Mihai, S.; Diaconeasa, Z.; et al. Phytochemical Characterization of Five Edible Purple-Reddish Vegetables: Anthocyanins, Flavonoids, and Phenolic Acid Derivatives. Molecules 2019, 24, 1536. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M. Characterization and Inheritance of the Anthocyanin Fruit (Aft) Tomato. J. Hered. 2003, 94, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Katragunta, K.; Osman, A.G.; Ali, Z.; John Adams, S.; Chittiboyina, A.G.; Khan, I.A. Advances in the Chemistry, Analysis and Adulteration of Anthocyanin Rich-Berries and Fruits: 2000–2022. Molecules 2023, 28, 560. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, B.; Cieślicka, M.; Kujawski, S.; Piskorska, E.; Kowalik, T.; Korycka, J.; Skarpańska-Stejnborn, A. Effects of Antioxidant Supplementation on Oxidative Stress Balance in Young Footballers- a Randomized Double-Blind Trial. J. Int. Soc. Sports Nutr. 2021, 18, 44. [Google Scholar] [CrossRef]
- Caturano, A.; Rocco, M.; Tagliaferri, G.; Piacevole, A.; Nilo, D.; Di Lorenzo, G.; Iadicicco, I.; Donnarumma, M.; Galiero, R.; Acierno, C.; et al. Oxidative Stress and Cardiovascular Complications in Type 2 Diabetes: From Pathophysiology to Lifestyle Modifications. Antioxidants 2025, 14, 72. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox Metabolism: ROS as Specific Molecular Regulators of Cell Signaling and Function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Dama, A.; Shpati, K.; Daliu, P.; Dumur, S.; Gorica, E.; Santini, A. Targeting Metabolic Diseases: The Role of Nutraceuticals in Modulating Oxidative Stress and Inflammation. Nutrients 2024, 16, 507. [Google Scholar] [CrossRef]
- Bellanti, F.; Coda, A.R.D.; Trecca, M.I.; Lo Buglio, A.; Serviddio, G.; Vendemiale, G. Redox Imbalance in Inflammation: The Interplay of Oxidative and Reductive Stress. Antioxidants 2025, 14, 656. [Google Scholar] [CrossRef]
- Barba-Ostria, C.; Gonzalez-Pastor, R.; Castillo-Solís, F.; Carrera-Pacheco, S.E.; Lopez, O.; Zúñiga-Miranda, J.; Debut, A.; Guamán, L.P. Bioactive Properties of Microencapsulated Anthocyanins from Vaccinium floribundum and Rubus glaucus. Molecules 2024, 29, 5504. [Google Scholar] [CrossRef]
- Costa, T.J.; Barros, P.R.; Arce, C.; Santos, J.D.; da Silva-Neto, J.; Egea, G.; Dantas, A.P.; Tostes, R.C.; Jiménez-Altayó, F. The Homeostatic Role of Hydrogen Peroxide, Superoxide Anion and Nitric Oxide in the Vasculature. Free Radic. Biol. Med. 2021, 162, 615–635. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F.; Bravo-Díaz, C. Biochemistry of Antioxidants: Mechanisms and Pharmaceutical Applications. Biomedicines 2022, 10, 3051. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Polidori, M.C.; Mecocci, P. Modeling the Dynamics of Energy Imbalance: The Free Radical Theory of Aging and Frailty Revisited. Free Radic. Biol. Med. 2022, 181, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Nuszkiewicz, J.; Sutkowy, P.; Wróblewski, M.; Pawłowska, M.; Wesołowski, R.; Wróblewska, J.; Woźniak, A. Links between Vitamin K, Ferroptosis and SARS-CoV-2 Infection. Antioxidants 2023, 12, 733. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid Peroxidation as Measured by Chromatographic Determination of Malondialdehyde. Human Plasma Reference Values in Health and Disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research Progress of Glutathione Peroxidase Family (GPX) in Redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural Antioxidants as Food and Feed Additives to Promote Health Benefits and Quality of Meat Products: A Review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Zaric, B.L.; Macvanin, M.T.; Isenovic, E.R. Free Radicals: Relationship to Human Diseases and Potential Therapeutic Applications. Int. J. Biochem. Cell Biol. 2023, 154, 106346. [Google Scholar] [CrossRef]
- García-Giménez, J.L.; Cánovas-Cervera, I.; Pallardó, F.V. Oxidative Stress and Metabolism Meet Epigenetic Modulation in Physical Exercise. Free Radic. Biol. Med. 2024, 213, 123–137. [Google Scholar] [CrossRef]
- El Assar, M.; Álvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodríguez-Mañas, L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8713. [Google Scholar] [CrossRef]
- Thirupathi, A.; Pinho, R.A. Effects of Reactive Oxygen Species and Interplay of Antioxidants during Physical Exercise in Skeletal Muscles. J. Physiol. Biochem. 2018, 74, 359–367. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, P.; Eckl, P. Impact of Oxidative Stress on Exercising Skeletal Muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, C.; Björnsdottir, H.; Sundqvist, M.; Christenson, K.; Bylund, J. Measurement of Respiratory Burst Products, Released or Retained, During Activation of Professional Phagocytes. In Methods in Molecular Biology; Springer Nature: Clifton, NJ, USA, 2020; Volume 2087, pp. 301–324. [Google Scholar]
- Pingitore, A.; Lima, G.P.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and Oxidative Stress: Potential Effects of Antioxidant Dietary Strategies in Sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Spirlandeli, A.; Deminice, R.; Jordao, A. Plasma Malondialdehyde as Biomarker of Lipid Peroxidation: Effects of Acute Exercise. Int. J. Sports Med. 2013, 35, 14–18. [Google Scholar] [CrossRef]
- Thomas, H.J.; Ang, T.; Morrison, D.J.; Keske, M.A.; Parker, L. Acute Exercise and High-Glucose Ingestion Elicit Dynamic and Individualized Responses in Systemic Markers of Redox Homeostasis. Front. Immunol. 2023, 14, 1127088. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Chung, Y.-S.; Yoon, H.-K.; Roh, H.-T. Impact of Exercise Intensity on Systemic Oxidative Stress, Inflammatory Responses, and Sirtuin Levels in Healthy Male Volunteers. Int. J. Environ. Res. Public Health 2022, 19, 11292. [Google Scholar] [CrossRef]
- Muñoz Marín, D.; Barrientos, G.; Alves, J.; Grijota, F.J.; Robles, M.C.; Maynar, M. Oxidative Stress, Lipid Peroxidation Indexes and Antioxidant Vitamins in Long and Middle Distance Athletes during a Sport Season. J. Sports Med. Phys. Fitness 2018, 58, 1713–1719. [Google Scholar] [CrossRef]
- Amiri, E.; Sheikholeslami-Vatani, D. The Role of Resistance Training and Creatine Supplementation on Oxidative Stress, Antioxidant Defense, Muscle Strength, and Quality of Life in Older Adults. Front. Public Health 2023, 11, 1062832. [Google Scholar] [CrossRef]
- Sielski, Ł.; Sutkowy, P.; Skopowska, A.; Pawlak-Osińska, K.; Augustyńska, Z.; Hewelt, K.; Drapała, R.; Woźniak, A. The Oxidant–Antioxidant Equilibrium and Inflammatory Process Indicators after an Exercise Test on the AlterG Antigravity Treadmill in Young Amateur Female Athletes. Oxid. Med. Cell. Longev. 2018, 2018, 3484159. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Fernández-García, B.; Lehmann, H.I.; Li, G.; Kroemer, G.; López-Otín, C.; Xiao, J. Exercise Sustains the Hallmarks of Health. J. Sport Health Sci. 2023, 12, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Kang, C.; Zhang, Y. Exercise-Induced Hormesis and Skeletal Muscle Health. Free Radic. Biol. Med. 2016, 98, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Napolitano, G.; Venditti, P. Mediators of Physical Activity Protection against ROS-Linked Skeletal Muscle Damage. Int. J. Mol. Sci. 2019, 20, 3024. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Kim, N.S.; Eun, P.Y.; Kim, S.-J.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.-Y.; Kim, J.K.; Park, S.U. Metabolic Profiling of Pale Green and Purple Kohlrabi (Brassica oleracea Var. gongylodes). Appl. Biol. Chem. 2017, 60, 249–257. [Google Scholar] [CrossRef]
- Li, J.; Jiang, S.; Yang, G.; Xu, Y.; Li, L.; Yang, F. RNA-Sequencing Analysis Reveals Novel Genes Involved in the Different Peel Color Formation in Eggplant. Hortic. Res. 2023, 10, uhad181. [Google Scholar] [CrossRef]
- Iorizzo, M.; Curaba, J.; Pottorff, M.; Ferruzzi, M.G.; Simon, P.; Cavagnaro, P.F. Carrot Anthocyanins Genetics and Genomics: Status and Perspectives to Improve Its Application for the Food Colorant Industry. Genes 2020, 11, 906. [Google Scholar] [CrossRef]
- Kodama, M.; Brinch-Pedersen, H.; Sharma, S.; Holme, I.B.; Joernsgaard, B.; Dzhanfezova, T.; Amby, D.B.; Vieira, F.G.; Liu, S.; Gilbert, M.T.P. Identification of Transcription Factor Genes Involved in Anthocyanin Biosynthesis in Carrot (Daucus carota L.) Using RNA-Seq. BMC Genom. 2018, 19, 811. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, Z.; Zhang, Y.; Li, Y.; Zhou, S.; Chen, G. A Putative Functional MYB Transcription Factor Induced by Low Temperature Regulates Anthocyanin Biosynthesis in Purple Kale (Brassica oleracea var. acephala f. tricolor). Plant Cell Rep. 2012, 31, 281–289. [Google Scholar] [CrossRef]
- Liu, C.; Yao, X.; Li, G.; Huang, L.; Liu, C.; Xie, Z. Development of Novel Markers and Creation of Non-Anthocyanin and Anthocyanin-Rich Broccoli (Brassica oleracea var. italica). Cultivars. Appl. Sci. 2022, 12, 6267. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Zhang, Y.; Cui, B.; Yin, W.; Yu, X.; Zhu, Z.; Hu, Z. Anthocyanin Composition and Expression Analysis of Anthocyanin Biosynthetic Genes in Kidney Bean Pod. Plant Physiol. Biochem. 2015, 97, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Falginella, L.; Castellarin, S.D.; Testolin, R.; Gambetta, G.A.; Morgante, M.; Di Gaspero, G. Expansion and Subfunctionalisation of Flavonoid 3′,5′-Hydroxylases in the Grapevine Lineage. BMC Genom. 2010, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Hu, Z.; Zhang, Y.; Tian, S.; Wang, Z.; Zhao, Z.; Yang, Y.; Chen, G. Accumulation and Molecular Regulation of Anthocyanin in Purple Tumorous Stem Mustard (Brassica juncea var. tumida Tsen et Lee). J. Agric. Food Chem. 2014, 62, 7813–7821. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, K.Y.; Kim, M.; Hong, M.; Deepa, P.; Kim, S. A Review of the Biological Properties of Purple Corn (Zea mays L.). Sci. Pharm. 2023, 91, 6. [Google Scholar] [CrossRef]
- Sampaio, S.L.; Lonchamp, J.; Dias, M.I.; Liddle, C.; Petropoulos, S.A.; Glamočlija, J.; Alexopoulos, A.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Barros, L. Anthocyanin-Rich Extracts from Purple and Red Potatoes as Natural Colourants: Bioactive Properties, Application in a Soft Drink Formulation and Sensory Analysis. Food Chem. 2021, 342, 128526. [Google Scholar] [CrossRef]
- Gebhardt, C. The Historical Role of Species from the Solanaceae Plant Family in Genetic Research. Theor. Appl. Genet. 2016, 129, 2281–2294. [Google Scholar] [CrossRef]
- Rasheed, H.; Shehzad, M.; Rabail, R.; Kowalczewski, P.Ł.; Kidoń, M.; Jeżowski, P.; Ranjha, M.M.A.N.; Rakha, A.; Din, A.; Aadil, R.M. Delving into the Nutraceutical Benefits of Purple Carrot against Metabolic Syndrome and Cancer: A Review. Appl. Sci. 2022, 12, 3170. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Vaccinium Myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications—A Review. Curr. Pharm. Des. 2020, 26, 1917–1928. [Google Scholar] [CrossRef]
- Salehi, B.; Vlaisavljevic, S.; Adetunji, C.O.; Adetunji, J.B.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Uprety, Y.; Mileski, K.S.; Devkota, H.P.; et al. Plants of the Genus Vitis: Phenolic Compounds, Anticancer Properties and Clinical Relevance. Trends Food Sci. Technol. 2019, 91, 362–379. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberič, R.; Štampar, F. European Elderberry (Sambucus nigra L.) and American Elderberry (Sambucus canadensis L.): Botanical, Chemical and Health Properties of Flowers, Berries and Their Products. In Berries: Properties, Consumption and Nutrition; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; pp. 127–144. [Google Scholar]
- Lozoya-Gloria, E.; Cuéllar-González, F.; Ochoa-Alejo, N. Anthocyanin Metabolic Engineering of Euphorbia Pulcherrima: Advances and Perspectives. Front. Plant Sci. 2023, 14, 1176701. [Google Scholar] [CrossRef]
- Cunha, R.; Trigueiro, P.; Orta Cuevas, M.d.M.; Medina-Carrasco, S.; Duarte, T.M.; Honório, L.M.d.C.; Damacena, D.H.L.; Fonseca, M.G.; da Silva-Filho, E.C.; Osajima, J.A. The Stability of Anthocyanins and Their Derivatives through Clay Minerals: Revising the Current Literature. Minerals 2023, 13, 268. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Blanco, I.; Romani, S.; Tylewicz, U.; Dalla Rosa, M. Gas Permeability and Thermal Behavior of Polypropylene Films Used for Packaging Minimally Processed Fresh-Cut Potatoes: A Case Study. J. Food Sci. 2012, 77, E264–E272. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.A.; Pérez-Balibrea, S.; Ferreres, F.; Gil-Izquierdo, Á.; García-Viguera, C. Acylated Anthocyanins in Broccoli Sprouts. Food Chem. 2010, 123, 358–363. [Google Scholar] [CrossRef]
- Zhu, P.; Tian, Z.; Pan, Z.; Feng, X. Identification and Quantification of Anthocyanins in Different Coloured Cultivars of Ornamental Kale (Brassica oleracea L. var. acephala DC). J. Hortic. Sci. Biotechnol. 2018, 93, 466–473. [Google Scholar] [CrossRef]
- Chen, D.; Yang, Y.; Niu, G.; Shan, X.; Zhang, X.; Jiang, H.; Liu, L.; Wen, Z.; Ge, X.; Zhao, Q.; et al. Metabolic and RNA Sequencing Analysis of Cauliflower Curds with Different Types of Pigmentation. AoB Plants 2022, 14, plac001. [Google Scholar] [CrossRef]
- Tong, T.; Niu, Y.-H.; Yue, Y.; Wu, S.; Ding, H. Beneficial Effects of Anthocyanins from Red Cabbage (Brassica oleracea L. var. capitata L.) Administration to Prevent Irinotecan-Induced Mucositis. J. Funct. Foods 2017, 32, 9–17. [Google Scholar] [CrossRef]
- Park, W.T.; Kim, J.K.; Park, S.; Lee, S.-W.; Li, X.; Kim, Y.B.; Uddin, M.R.; Park, N.I.; Kim, S.-J.; Park, S.U. Metabolic Profiling of Glucosinolates, Anthocyanins, Carotenoids, and Other Secondary Metabolites in Kohlrabi (Brassica oleracea var. gongylodes). J. Agric. Food Chem. 2012, 60, 8111–8116. [Google Scholar] [CrossRef]
- Bendokas, V.; Stanys, V.; Mažeikienė, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the Field to the Antioxidants in the Body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Identification and Characterization of Anthocyanins by High-Performance Liquid Chromatography−Electrospray Ionization−Tandem Mass Spectrometry in Common Foods in the United States: Vegetables, Nuts, and Grains. J. Agric. Food Chem. 2005, 53, 3101–3113. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, H.; Fan, Y.; Yan, L. Anthocyanins Accumulation Analysis of Correlated Genes by Metabolome and Transcriptome in Green and Purple Peppers (Capsicum annuum). BMC Plant Biol. 2022, 22, 358. [Google Scholar] [CrossRef]
- Aza-González, C.; Núñez-Palenius, H.G.; Ochoa-Alejo, N. Molecular Biology of Chili Pepper Anthocyanin Biosynthesis. J. Mex. Chem. Soc. 2017, 56, 93–98. [Google Scholar] [CrossRef]
- Maeda-Yamamoto, M.; Honmou, O.; Sasaki, M.; Haseda, A.; Kagami-Katsuyama, H.; Shoji, T.; Namioka, A.; Namioka, T.; Magota, H.; Oka, S.; et al. The Impact of Purple-Flesh Potato (Solanum tuberosum L.) Cv. “Shadow Queen” on Minor Health Complaints in Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2022, 14, 2446. [Google Scholar] [CrossRef]
- Cuevas, E.; Silke, M.; Peter, H. Anthocyanins in Purple Sweet Potato (Ipomoea batatas L.) Varieties. Fruit Veg. Cereal Sci. Biotechnol. 2011, 235, 19–24. [Google Scholar]
- Fang, J. Classification of Fruits Based on Anthocyanin Types and Relevance to Their Health Effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Vangdal, E.; Brede, C. Analysis of Phenolic Compounds in Six Norwegian Plum Cultivars (Prunus domestica L.). J. Agric. Food Chem. 2009, 57, 11370–11375. [Google Scholar] [CrossRef] [PubMed]
- Taheri, R.; Connolly, B.A.; Brand, M.H.; Bolling, B.W. Underutilized Chokeberry (Aronia melanocarpa, Aronia arbutifolia, Aronia prunifolia) Accessions Are Rich Sources of Anthocyanins, Flavonoids, Hydroxycinnamic Acids, and Proanthocyanidins. J. Agric. Food Chem. 2013, 61, 8581–8588. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Persike, M.; Carle, R.; Schieber, A. Characterization and Quantification of Anthocyanins in Selected Artichoke (Cynara scolymus L.) Cultivars by HPLC–DAD–ESI–MS N. Anal. Bioanal. Chem. 2006, 384, 1511–1517. [Google Scholar] [CrossRef]
- EFSA. EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific Opinion on the Re-evaluation of Anthocyanins (E 163) as a Food Additive. EFSA J. 2013, 11, 3145. [Google Scholar] [CrossRef]
- da Silva, L.P.; Pereira, E.; Prieto, M.A.; Simal-Gandara, J.; Pires, T.C.S.P.; Alves, M.J.; Calhelha, R.; Barros, L.; Ferreira, I.C.F.R. Rubus Ulmifolius Schott as a Novel Source of Food Colorant: Extraction Optimization of Coloring Pigments and Incorporation in a Bakery Product. Molecules 2019, 24, 2181. [Google Scholar] [CrossRef]
- Gamel, T.H.; Saeed, S.M.G.; Ali, R.; Abdel-Aal, E.-S.M. Purple Wheat: Food Development, Anthocyanin Stability, and Potential Health Benefits. Foods 2023, 12, 1358. [Google Scholar] [CrossRef] [PubMed]
- Jhin, C.; Hwang, K. Prediction of Radical Scavenging Activities of Anthocyanins Applying Adaptive Neuro-Fuzzy Inference System (ANFIS) with Quantum Chemical Descriptors. Int. J. Mol. Sci. 2014, 15, 14715–14727. [Google Scholar] [CrossRef] [PubMed]
- Kurata, R.; Kobayashi, T. Effect of Cultivation Temperature on Yield and Anthocyanin Content of Purple Sweet Potato (Ipomoea batatas L.). Hortic. J. 2023, 92, 290–298. [Google Scholar] [CrossRef]
- Yang, W.; Guo, Y.; Liu, M.; Chen, X.; Xiao, X.; Wang, S.; Gong, P.; Ma, Y.; Chen, F. Structure and Function of Blueberry Anthocyanins: A Review of Recent Advances. J. Funct. Foods 2022, 88, 104864. [Google Scholar] [CrossRef]
- Lin, Y.; Li, C.; Shi, L.; Wang, L. Anthocyanins: Modified New Technologies and Challenges. Foods 2023, 12, 1368. [Google Scholar] [CrossRef]
- Dewangga, M.W.; Dimyati, D.; Irianto, D.P. Antioxidant Effect of Purple Sweet Potato (Ipomoea batatas var. Antin 3) for the Prevention of Oxidative Stress after High-Intensity Physical Exercise in Rat. Eurasian Chem. Commun. 2022, 4, 921–929. [Google Scholar] [CrossRef]
- Chang, W.-H.; Chen, C.-M.; Hu, S.-P.; Kan, N.-W.; Chiu, C.-C.; Liu, J.-F. Effect of Purple Sweet Potato Leaf Consumption on the Modulation of the Antioxidative Status in Basketball Players during Training. Asia Pac. J. Clin. Nutr. 2007, 16, 455–461. [Google Scholar]
- Chen, C.-M.; Lin, Y.-L.; Chen, C.-Y.O.; Hsu, C.-Y.; Shieh, M.-J.; Liu, J.-F. Consumption of Purple Sweet Potato Leaves Decreases Lipid Peroxidation and DNA Damage in Humans. Asia Pac. J. Clin. Nutr. 2008, 17, 408–414. [Google Scholar]
- Petrovic, S.; Arsic, A.; Glibetic, M.; Cikiriz, N.; Jakovljevic, V.; Vucic, V. The Effects of Polyphenol-Rich Chokeberry Juice on Fatty Acid Profiles and Lipid Peroxidation of Active Handball Players: Results from a Randomized, Double-Blind, Placebo-Controlled Study. Can. J. Physiol. Pharmacol. 2016, 94, 1058–1063. [Google Scholar] [CrossRef]
- Cikiriz, N.; Milosavljevic, I.; Jakovljevic, B.; Bolevich, S.; Jeremic, J.; Nikolic Turnic, T.; Mitrovic, M.; Srejovic, I.; Bolevich, S.; Jakovljevic, V. The Influences of Chokeberry Extract Supplementation on Redox Status and Body Composition in Handball Players during Competition Phase. Can. J. Physiol. Pharmacol. 2021, 99, 42–47. [Google Scholar] [CrossRef]
- Morillas-Ruiz, J.M.; Villegas García, J.A.; López, F.J.; Vidal-Guevara, M.L.; Zafrilla, P. Effects of Polyphenolic Antioxidants on Exercise-Induced Oxidative Stress. Clin. Nutr. 2006, 25, 444–453. [Google Scholar] [CrossRef]
- Pilaczynska-Szczesniak, L.; Skarpanska-Steinborn, A.; Deskur, E.; Basta, P.; Horoszkiewicz-Hassan, M. The Influence of Chokeberry Juice Supplementation on the Reduction of Oxidative Stress Resulting from an Incremental Rowing Ergometer Exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 48–58. [Google Scholar] [CrossRef]
- McLeay, Y.; Barnes, M.J.; Mundel, T.; Hurst, S.M.; Hurst, R.D.; Stannard, S.R. Effect of New Zealand Blueberry Consumption on Recovery from Eccentric Exercise-Induced Muscle Damage. J. Int. Soc. Sports Nutr. 2012, 9, 19. [Google Scholar] [CrossRef]
- Park, C.H.; Kwak, Y.S.; Seo, H.K.; Kim, H.Y. Assessing the Values of Blueberries Intake on Exercise Performance, TAS, and Inflammatory Factors. Iran. J. Public Health 2018, 47, 27–32. [Google Scholar] [PubMed]
- Wei, P.; Jin, H.-m. Blueberries Extract Supplementation Improves Physical Performance and Decreases Oxidative Stress in Mice. African J. Biotechnol. 2011, 10, 12999–13003. [Google Scholar] [CrossRef]
- Toscano, L.T.; Tavares, R.L.; Toscano, L.T.; Silva, C.S.O.d.; Almeida, A.E.M.d.; Biasoto, A.C.T.; Gonçalves, M.d.C.R.; Silva, A.S. Potential Ergogenic Activity of Grape Juice in Runners. Appl. Physiol. Nutr. Metab. 2015, 40, 899–906. [Google Scholar] [CrossRef] [PubMed]
- de Lima Tavares Toscano, L.; Silva, A.S.; de França, A.C.L.; de Sousa, B.R.V.; de Almeida Filho, E.J.B.; da Silveira Costa, M.; Marques, A.T.B.; da Silva, D.F.; de Farias Sena, K.; Cerqueira, G.S.; et al. A Single Dose of Purple Grape Juice Improves Physical Performance and Antioxidant Activity in Runners: A Randomized, Crossover, Double-Blind, Placebo Study. Eur. J. Nutr. 2020, 59, 2997–3007. [Google Scholar] [CrossRef]
- Skarpańska-Stejnborn, A.; Basta, P.; Pilaczyńska-Szcześniak, Ł.; Horoszkiewicz-Hassan, M. Black Grape Extract Supplementation Attenuates Blood Oxidative Stress in Response to Acute Exercise. Biol. Sport 2010, 27, 41–46. [Google Scholar] [CrossRef]
- Bloedon, T.K.; Braithwaite, R.E.; Carson, I.A.; Klimis-Zacas, D.; Lehnhard, R.A. Impact of Anthocyanin-Rich Whole Fruit Consumption on Exercise-Induced Oxidative Stress and Inflammation: A Systematic Review and Meta-Analysis. Nutr. Rev. 2019, 77, 630–645. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Somerville, V.X.; Hurst, R.D. The Effect of New Zealand Blackcurrant on Sport Performance and Related Biomarkers: A Systematic Review and Meta-Analysis. J. Int. Soc. Sports Nutr. 2020, 17, 25. [Google Scholar] [CrossRef]
- Xue, H.; Sang, Y.; Gao, Y.; Zeng, Y.; Liao, J.; Tan, J. Research Progress on Absorption, Metabolism, and Biological Activities of Anthocyanins in Berries: A Review. Antioxidants 2022, 12, 3. [Google Scholar] [CrossRef]
- Araki, R.; Yada, A.; Ueda, H.; Tominaga, K.; Isoda, H. Differences in the Effects of Anthocyanin Supplementation on Glucose and Lipid Metabolism According to the Structure of the Main Anthocyanin: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 2003. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Stoner, G.D.; Sardo, C.; Apseloff, G.; Mullet, D.; Wargo, W.; Pound, V.; Singh, A.; Sanders, J.; Aziz, R.; Casto, B.; et al. Pharmacokinetics of Anthocyanins and Ellagic Acid in Healthy Volunteers Fed Freeze-Dried Black Raspberries Daily for 7 Days. J. Clin. Pharmacol. 2005, 45, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Wilmsen, P.K.; Spada, D.S.; Salvador, M. Antioxidant Activity of the Flavonoid Hesperidin in Chemical and Biological Systems. J. Agric. Food Chem. 2005, 53, 4757–4761. [Google Scholar] [CrossRef]
- Bińkowska, W.; Szpicer, A.; Stelmasiak, A.; Wojtasik-Kalinowska, I.; Półtorak, A. Microencapsulation of Polyphenols and Their Application in Food Technology. Appl. Sci. 2024, 14, 11954. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Asghar, W.; Akhtar, A.; Ayub, H.; Aslam, I.; Khalid, N.; Al-Mssallem, M.Q.; Alessa, F.M.; Ghazzawy, H.S.; Attimarad, M. Anthocyanin Delivery Systems: A Critical Review of Recent Research Findings. Appl. Sci. 2022, 12, 12347. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, J.; Li, L.; Ren, J.; Lu, J.; Luo, F. Advances in Embedding Techniques of Anthocyanins: Improving Stability, Bioactivity and Bioavailability. Food Chem. X 2023, 20, 100983. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Tang, J.; Cao, L.; Fan, M.; Lin, X.; Liu, G.; Liang, L.; Liu, X.; Zhang, J.; Li, Y.; et al. Strategic Approaches for Co-Encapsulation of Bioactive Compounds: Technological Advances and Mechanistic Insight. Foods 2025, 14, 2024. [Google Scholar] [CrossRef]
- Rezagholizade-shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive Compound Encapsulation: Characteristics, Applications in Food Systems, and Implications for Human Health. Food Chem. X 2024, 24, 101953. [Google Scholar] [CrossRef]
- Ćujić, N.; Kardum, N.; Šavikin, K.; Zdunić, G.; Janković, T.; Menković, N. Potential of Chokeberry (Aronia melanocarpa L.) as a Therapeutic Food. In Therapeutic Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 209–237. [Google Scholar]
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Dębski, H. Anthocyanins of Fruits and Vegetables—Their Occurrence, Analysis and Role in Human Nutrition. J. Fruit Ornam. Plant Res. 2008, 68, 5–22. [Google Scholar] [CrossRef]
- Sror, H.M.; Rizk, E.; Azouz, A.; Hareedy, L.A.M. Evaluation of Red Cabbage Anthocyanin Pigments and Its Potential Uses as Antioxidant and Natural Food Colorants. Arab Univ. J. Agric. Sci. 2009, 17, 361–372. [Google Scholar] [CrossRef]
- Pérez, M.B.; Carvajal, S.; Beretta, V.; Bannoud, F.; Fangio, M.F.; Berli, F.; Fontana, A.; Salomón, M.V.; Gonzalez, R.; Valerga, L.; et al. Characterization of Purple Carrot Germplasm for Antioxidant Capacity and Root Concentration of Anthocyanins, Phenolics, and Carotenoids. Plants 2023, 12, 1796. [Google Scholar] [CrossRef]
| Source | Main Anthocyanins | References | ||
|---|---|---|---|---|
| Family | Botanical Name | Common Name | ||
| Brassicaceae | Brassica oleracea, variety italica | Purple sprouting broccoli, broccoli sprouts | cyanidin-3-O-diglucoside-5-O-glucoside in acylated forms: [cyanidin-3-O-(p-coumaroyl)(sinapoyl)diglucoside-5-O-glucoside, cyanidin-3-O-(sinapoyl)(feruloyl)diglucoside-5-O-glucoside, cyanidin-3-O-(sinapoyl)(sinapoyl)diglucoside-5-O-glucoside] | [65,66] |
| Brassica oleracea L. var. acephala DC. | Ornamental purple kale | cyanidin-3-(sinapoyl)(feruloyl)-diglucoside-5-glucoside | [67] | |
| Brassica juncea | Purple mustards | cyanidin-3-O-diglucoside-5-O-glucoside in acylated forms: [cyanidin-3-O-(feruloyl)(sinapoyl)sophoroside-5-O-(malonyl)glucoside, cyanidin-3-O-(diferuloyl)sophoroside-5-O-(malonyl)glucoside] | [54] | |
| Brassica oleracea var. botrytis | Cauliflower, purple cape, purple of Sicily cauliflower | cyanidin-3-O-galactoside cyanidin-3-O-glucoside pelargonidin-3-O-glucoside delphinidin-3-O-glucoside petunidin-3-O-glucoside | [68] | |
| Brassica oleracea L. var. capitata | Red cabbage (with purplish colored leaves) | cyanidin-3-O-diglucoside-5-O-glucoside in acylated forms: [cyanidin-3-O-(p-coumaroyl)(sinapoyl) triglucoside-5-O-glucoside, cyanidin-3-O-(feruloyl)(sinapoyl)triglucoside-5-O-glucoside, cyanidin-3-O-(sinapoyl)(sinapoyl) diglucoside-5-O-glucoside] | [69] | |
| Brassica oleracea, variety gongylodes | Purple kohlrabi | cyanidin-3-O-(feruloyl)-diglucoside-5-O-glucoside cyanidin-3-O-(feruloyl)(sinapoyl) diglucoside-5-O-glucoside cyanidin-3-O-(sinapoyl)(sinapoyl) diglucoside-5-O-glucoside | [46,70] | |
| Solanaceae | Solanum melongena L. | Eggplants, aubergine | delphinidin-3-O-(coumaroyl)rutinoside-5-O-glucoside (nasunin) delphinidin-3-O-rutinoside-5-O-galactoside delphinidin-3-O-glucoside delphinidin-3-O-rutinoside | [47,71,72] |
| Capsicum annuum | Purple pepper (sample varieties: purple bell pepper, jalapeno pepper, purple reaper) | delphinidin-3-O-glucoside delphinidin-3-O-rutinoside delphinidin-3-O-(coumaroyl)rutinoside-5-O-glucoside delphinidin-3-O-(coumaroyl)ruti–noside-5-O-glucoside | [73,74] | |
| Solanum tuberosum L. Solanum tuberosum L. var. Purple Majesty | Purple flesh potato | cyanidin-3-O-rutinoside petunidin-3-O-(coumaryl)rutinoside-5-O-glucoside peonidine-3-O-(coumaroyl)rutinoside-5-O-glucoside peonidin-3-O-(caffeoyl-p-hydroxybenzoyl)sophoroside-5-O-glucoside | [4,5,75] | |
| Solanum scabrum | Garden huckleberry | petunidin-3-O-(coumaryl)rutinoside-5-O-glucoside | [5] | |
| Solanum lycopersicum L. cv. Del/Ros1 | Transgenic purple tomato | petunidin-3-O-(coumaryl) rutinoside-5-O-glucoside malvidin-3-O-(coumaryl)rutinoside-5-O-glucoside peonidin-3-O-(coumaryl)rutinoside-5-O-glucoside | [5] | |
| Physallis ixocarpa | Tomatillo, husk tomato, purple tamarillo Tomatillos small purple-tinged fruits | petunidin-3-O-(coumaryl)rutinoside-5-O-glucoside | [5] | |
| Convolvulaceae | Ipomoea batatas L. | Purple sweet potato | cyanidin-3-O-(caffeoyl)sophoroside-5-O-glucoside peonidin-3-O-(caffeoyl)sophoroside-5-O-glucoside | [76] |
| Apiaceae | Daucus carota spp. sativus var. atrorubens | Purple carrot | cyanidin-3-O-(xylosyl)(feruloyl)(glucosyl)-galactoside, cyanidin-3-O-(xylosyl)(glucosyl)galactoside | [48,58] |
| Alliaceae | Allium cepa | Red purplette onion | cyanidin-3-O-(malonoyl)glucoside-5-O-glucoside cyanidin-3-O-(acetoyl)glucoside cyanidin-3-O-(malonoyl)(acetoyl)glucoside | [72] |
| Fabaceae | Phaseolus vulgaris | Purple beans | malvidin-3,5-O-diglucoside | [52] |
| Poaceae | Zea mays L. var. rugosa | Purple corn, sweet corn | cyanidin-3-O-glucoside | [55] |
| Triticum aestivum L. | Purple wheat | cyanidin-3-O-glucoside | [71] | |
| Rosaceae | Rubus idaeus L. Amethyst (purple) | Purple and purple–black raspberries | cyanidin-3-O-glucoside cyanidin-3-O-rutinoside cyanidin-3-O-sophoroside cyanidin-3-O-(coumaroyl)glucoside | [6,64] |
| Rubus fruticosus L. | Blackberries | cyanidin-3-O-glucoside cyanidin-3-O-rutinoside cyanidin-3-O-(dioxaloyl)glucoside | [6,77] | |
| Prunus domestica L. | Plum | cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, peonidin-3-O-glucoside, peonidin-3-O-rutinoside | [6,78] | |
| Aronia prunifolia L. | Purple chokeberry | cyanidin-3-O-galactoside, cyanidin-3-O-glucoside, cyanidin-3-O-arabinoside, cyanidin-3-O-xyloside | [79] | |
| Ericaceae | Vaccinium myrtillus L. | Bilberries | cyanidin-3-O-glucoside cyanidin-3-O-galactoside cyanidin-3-O-arabinoside delphinidin-3-O-galactoside delphinidin-3-O-glucoside delphinidin-3-O-arabinoside malvidin-3-O-galactoside malvidin-3-O-glucoside malvidin-3-O-arabinoside petunidin-3-O-galactoside petunidin-3-O-glucoside petunidin-3-O-arabinoside | [6,59,64,71] |
| Vaccinium corymbosum L. | Blueberry (highbush and low bush) | cyanidin-3-O-galactoside, cyanidin-3-O-glucoside, malvidin-3-O-glucoside, malvidin-3-O-galactoside, delphinidin-3-O-galactoside, delphinidin-3-O-glucoside, petunidin-3-O-galactoside, petunidin-3-O-glucoside, peonidin-3-O-galactoside, peonidin-3-O-glucoside, | [6,64] | |
| Empetrum nigrum | Crowberry (black) | cyanidin-3-O-glucoside, cyanidin-3-O-arabinoside delphinidin-3-O-glucoside delphinidin-3-O-arabinoside malvidin-3-O-glucoside, malvidin-3-O-arabinoside peonidin-3-O-glucoside, peonidin-3-O-arabinoside petunidin-3-O-glucoside, petunidin-3-O-arabinoside | [6] | |
| Viburnaecea | Sambucus spp. | Elderberries | cyanidin-3-O-glucoside, cyanidin-3-O-sambubioside, cyanidin-3-O-sambubioside-5-O-glucoside | [61,71,77] |
| Vitaceae | Vitis vinifer L. Concord | Purple grapes | cyanidin-3-O-glucoside, delphinidin-3-O-glucoside, malvidin-3-O-glucoside, malvidin-3-O-(acetyl)glucoside, malvidin-3-O-(coumaroyl)glucoside, peonidin-3-O-glucoside, petunidin-3-O-glucoside | [6] |
| Caprifoliaceae | Lonicera caerulea | Honeyberries (haskaps, honeysuckle) | cyanidin-3-O-glucoside cyanidin-3-O-rutinoside, peonidin-3-O-glucoside, cyanidin-3,5-O-diglucoside | [77] |
| Grossulariaceae | Ribes nigrum L. | Black currant | cyanidin-3-O-glucoside cyanidin-3-O-rutinoside delphinidin-3-O-glucoside delphinidin-3-O-rutinoside | [6,64] |
| Asteraceae | Cynara scolymus | Artichoke | Cyanidin-3-O-(malonyl)glucoside | [80] |
| Population | Anthocyanin Source | Dose and Duration | Outcome Measures | Key Findings | References |
|---|---|---|---|---|---|
| Elite basketball players | Purple sweet potato leaves | 200 g/day, 2 weeks | 8-OHdG, LDL oxidation | ↑ Antioxidant status, ↓ DNA oxidation | [90] |
| Male soccer players | Chokeberry juice | 200 mL/day, 7 weeks | TBARS, 8-OHdG | No significant effect | [7] |
| Handball players | Chokeberry extract | 30 mL/day, 12 weeks | TBARS, HDL, RBC | ↓ Lipid peroxidation, ↑ HDL, RBC count | [92] |
| Healthy women | Blueberry smoothie | 96.6 mg pre/post-exercise | Muscle strength, TAS | ↑ Recovery, ↑ Antioxidant capacity | [95] |
| Trained runners | Blueberry-based beverage with 15% aronia | 140 g fruit pulp per day, for 4 weeks | IL-6, CRP, VO2 max | ↓ Inflammation, ↑ Performance | [96] |
| Recreational runners | Purple grape juice | 300 mL/day, 28 days | TAC, MDA | ↑ Antioxidant capacity, no differences in lipid peroxidation | [98] |
| Recreational runners | Purple grape juice | Single dose (10 mL/kg) | Plasma antioxidant activity, MDA | ↑ Antioxidant activity, no effect on MDA | [99] |
| PE students | Red grape extract | 3 capsules/day, 6 weeks | SOD, CAT, TAS, CK | No change in enzymes, possible effect on CK | [100] |
| Plant Source | Approx. Anthocyanin Content (mg/100 g Fresh Weight) | References |
|---|---|---|
| Purple sweet potato | 286.6 | [88] |
| Chokeberry | 500–1000 | [94,113] |
| Blueberry | 82.5–530 | [95,114] |
| Grape | 8–750 | [99,114] |
| Red cabbage | 25–90.5 | [114,115] |
| Purple carrot | 0.5–191 | [116] |
| Blackcurrant | 130–400 | [6,114] |
| Eggplant | 750 | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuszkiewicz, J.; Wróblewska, J.; Wróblewski, M.; Woźniak, A. Anthocyanin-Rich Purple Plant Foods: Bioavailability, Antioxidant Mechanisms, and Functional Roles in Redox Regulation and Exercise Recovery. Nutrients 2025, 17, 2453. https://doi.org/10.3390/nu17152453
Nuszkiewicz J, Wróblewska J, Wróblewski M, Woźniak A. Anthocyanin-Rich Purple Plant Foods: Bioavailability, Antioxidant Mechanisms, and Functional Roles in Redox Regulation and Exercise Recovery. Nutrients. 2025; 17(15):2453. https://doi.org/10.3390/nu17152453
Chicago/Turabian StyleNuszkiewicz, Jarosław, Joanna Wróblewska, Marcin Wróblewski, and Alina Woźniak. 2025. "Anthocyanin-Rich Purple Plant Foods: Bioavailability, Antioxidant Mechanisms, and Functional Roles in Redox Regulation and Exercise Recovery" Nutrients 17, no. 15: 2453. https://doi.org/10.3390/nu17152453
APA StyleNuszkiewicz, J., Wróblewska, J., Wróblewski, M., & Woźniak, A. (2025). Anthocyanin-Rich Purple Plant Foods: Bioavailability, Antioxidant Mechanisms, and Functional Roles in Redox Regulation and Exercise Recovery. Nutrients, 17(15), 2453. https://doi.org/10.3390/nu17152453






