Association Between Maternal Dietary Fatty Acid Intake and Fatty Acid Composition of Placental Phospholipids
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Assessment of Maternal Dietary Intake
2.3. Placenta PL Fatty Acids
2.4. Statistical Analysis
3. Results
3.1. Maternal Dietary Intake
3.2. Placental Fatty Acid Association with Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALA | α-linolenic acid |
| ARA | Arachidonic acid |
| BMI | Body mass index |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| FA | Fatty acid |
| FFQ | Food frequency questionnaire |
| GDM | Gestational diabetes mellitus |
| GWG | Gestational weight gain |
| LA | Linoleic acid |
| LC-PUFA | Long-chain polyunsaturated fatty acids |
| MUFA | Monounsaturated fatty acid |
| PL | Phospholipids |
| SFA | Saturated fatty acid |
References
- Sinclair, K.D.; Lea, R.G.; Rees, W.D.; Young, L.E. The developmental origins of health and disease: Current theories and epigenetic mechanisms. Soc. Reprod. Fertil. Suppl. 2007, 64, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, P.D.; Buss, C.; Entringer, S.; Swanson, J.M. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2009, 27, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Sánchez, M.T.; Ruiz-Palacios, M.; Blanco-Carnero, J.E.; Pagan, A.; Hellmuth, C.; Uhl, O.; Peissner, W.; Ruiz-Alcaraz, A.J.; Parrilla, J.J.; Koletzko, B.; et al. Placental MFSD2a transporter is related to decreased DHA in cord blood of women with treated gestational diabetes. Clin. Nutr. 2017, 36, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Gázquez, A.; Prieto-Sánchez, M.T.; Blanco-Carnero, J.E.; van Harskamp, D.; Perazzolo, S.; Oosterink, J.E.; Demmelmair, H.; Schierbeek, H.; Sengers, B.G.; Lewis, R.M.; et al. In vivo kinetic study of materno-fetal fatty acid transfer in obese and normal weight pregnant women. J. Physiol. 2019, 597, 4959–4973. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Campillo, M.; Ruiz-Palacios, M.; Ruiz-Alcaraz, A.J.; Prieto-Sánchez, M.T.; Blanco-Carnero, J.E.; Zornoza, M.; Ruiz-Pastor, M.J.; Demmelmair, H.; Sánchez-Solís, M.; Koletzko, B.; et al. Child Head Circumference and Placental MFSD2a Expression Are Associated to the Level of MFSD2a in Maternal Blood During Pregnancy. Front. Endocrinol. 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.F.; Østerdal, M.L.; Salvig, J.D.; Mortensen, L.M.; Rytter, D.; Secher, N.J.; Henriksen, T.B. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am. J. Clin. Nutr. 2008, 88, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Harvey, N.C.; Robinson, S.M.; Ntani, G.; Davies, J.H.; Inskip, H.M.; Godfrey, K.M.; Dennison, E.M.; Calder, P.C.; Cooper, C.; et al. Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J. Clin. Endocrinol. Metab. 2013, 98, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Carlson, S.E.; Burden, C.; da Fonseca, E.B.; di Renzo, G.C.; Hadjipanayis, A.; Harris, W.S.; Kumar, K.R.; Olsen, S.F.; Mader, S.; et al. Omega-3 fatty acid supply in pregnancy for risk reduction of preterm and early preterm birth. Am. J. Obstet. Gynecol. MFM 2024, 6, 101251. [Google Scholar] [CrossRef] [PubMed]
- Broś-Konopielko, M.; Białek, A.; Johne, M.; Czajkowski, K. Increased LC PUFA Levels in the Serum of Pregnant Women and Their Children as a Result of Dietary Supplementation with ‘Omega’ Fatty Acids. Nutrients 2023, 15, 231. [Google Scholar] [CrossRef] [PubMed]
- Donahue, S.M.A.; Rifas-Shiman, S.L.; Gold, D.R.; Jouni, Z.E.; Gillman, M.W.; Oken, E. Prenatal fatty acid status and child adiposity at age 3 y: Results from a US pregnancy cohort. Am. J. Clin. Nutr. 2011, 93, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.L.; Innis, S.M. Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length. Am. J. Clin. Nutr. 2001, 73, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Niinivirta, K.; Isolauri, E.; Laakso, P.; Linderborg, K.; Laitinen, K. Dietary counseling to improve fat quality during pregnancy alters maternal fat intake and infant essential fatty acid status. J. Nutr. 2011, 141, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Bitsanis, D.; Crawford, M.A.; Moodley, T.; Holmsen, H.; Ghebremeskel, K.; Djahanbakhch, O. Arachidonic acid predominates in the membrane phosphoglycerides of the early and term human placenta. J. Nutr. 2005, 135, 2566–2571. [Google Scholar] [CrossRef] [PubMed]
- Powell, T.L.; Ferchaud-Roucher, V.; Madi, L.; Uhlson, C.; Zemski-Berry, K.; Kramer, A.C.; Erickson, K.; Palmer, C.; Chassen, S.S.; Castillo-Castrejon, M. Synthesis of phospholipids in human placenta. Placenta 2024, 147, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Watkins, O.C.; Selvam, P.; Appukuttan Pillai, R.; Cracknell-Hazra, V.K.B.; Yong, H.E.J.; Sharma, N.; Cazenave-Gassiot, A.; Bendt, A.K.; Godfrey, K.M.; Lewis, R.M.; et al. Placental (13)C-DHA metabolism and relationship with maternal BMI, glycemia and birthweight. Mol. Med. 2021, 27, 84. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L.; Mark, P.J.; Waddell, B.J. Maternal dietary omega-3 fatty acids and placental function. Reproduction 2014, 147, R143–R152. [Google Scholar] [CrossRef] [PubMed]
- Berglund, S.K.; García-Valdés, L.; Torres-Espinola, F.J.; Segura, M.T.; Martínez-Zaldívar, C.; Aguilar, M.J.; Agil, A.; Lorente, J.A.; Florido, J.; Padilla, C.; et al. Maternal, fetal and perinatal alterations associated with obesity, overweight and gestational diabetes: An observational cohort study (PREOBE). BMC Public Health 2016, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef] [PubMed]
- Franke, C.; Verwied-Jorky, S.; Campoy, C.; Trak-Fellermeier, M.; Decsi, T.; Dolz, V.; Koletzko, B. Dietary intake of natural sources of docosahexaenoic acid and folate in pregnant women of three European cohorts. Ann. Nutr. Metab. 2008, 53, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.T.; Demmelmair, H.; Krauss-Etschmann, S.; Nathan, P.; Dehmel, S.; Padilla, M.C.; Rueda, R.; Koletzko, B.; Campoy, C. Maternal BMI and gestational diabetes alter placental lipid transporters and fatty acid composition. Placenta 2017, 57, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Bitsanis, D.; Ghebremeskel, K.; Moodley, T.; Crawford, M.A.; Djahanbakhch, O. Gestational diabetes mellitus enhances arachidonic and docosahexaenoic acids in placental phospholipids. Lipids 2006, 41, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Calabuig-Navarro, V.; Puchowicz, M.; Glazebrook, P.; Haghiac, M.; Minium, J.; Catalano, P.; Demouzon, S.H.; O’Tierney-Ginn, P. Effect of ω-3 supplementation on placental lipid metabolism in overweight and obese women. Am. J. Clin. Nutr. 2016, 103, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; Simpson, J.A.; Gibson, R.A.; Sinclair, A.J.; Makrides, M.; O’Dea, K.; English, D.R.; Giles, G.G. Plasma phospholipid fatty acid composition as a biomarker of habitual dietary fat intake in an ethnically diverse cohort. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.S.; Sharp, S.J.; Jansen, E.; Luben, R.N.; Khaw, K.T.; Wareham, N.J.; Forouhi, N.G. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: A pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am. J. Clin. Nutr. 2010, 92, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, L.C.; Hellerstein, M.; Seidman, C.; Neese, R.; Diakun, J.; Hirsch, J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J. Clin. Investig. 1996, 97, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Lattka, E.; Koletzko, B.; Zeilinger, S.; Hibbeln, J.R.; Klopp, N.; Ring, S.M.; Steer, C.D. Umbilical cord PUFA are determined by maternal and child fatty acid desaturase (FADS) genetic variants in the Avon Longitudinal Study of Parents and Children (ALSPAC). Br. J. Nutr. 2013, 109, 1196–1210. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, L.; Gohlke, H.; Müller, M.; Heid, I.M.; Palmer, L.J.; Kompauer, I.; Demmelmair, H.; Illig, T.; Koletzko, B.; Heinrich, J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet. 2006, 15, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Lakin, V.; Haggarty, P.; Abramovich, D.R.; Ashton, J.; Moffat, C.F.; McNeill, G.; Danielian, P.; Grubb, D. Dietary intake and tissue concentration of fatty acids in omnivore, vegetarian and diabetic pregnancy. Prostaglandins Leukot. Essent. Fat. Acids 1998, 59, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Aumeistere, L.; Ciproviča, I.; Zavadska, D.; Volkovs, V. Fish intake reflects on DHA level in breast milk among lactating women in Latvia. Int. Breastfeed. J. 2018, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Simon Sarkadi, L.; Zhang, M.; Muránszky, G.; Vass, R.A.; Matsyura, O.; Benes, E.; Vari, S.G. Fatty Acid Composition of Milk from Mothers with Normal Weight, Obesity, or Gestational Diabetes. Life 2022, 12, 1093. [Google Scholar] [CrossRef] [PubMed]
- Baylin, A.; Kabagambe, E.K.; Siles, X.; Campos, H. Adipose tissue biomarkers of fatty acid intake. Am. J. Clin. Nutr. 2002, 76, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, J.A.; Mori, T.A.; Barden, A.; Beilin, L.J.; Holt, P.G.; Calder, P.C.; Prescott, S.L. Effects of n-3 polyunsaturated fatty acid supplementation in pregnancy on maternal and fetal erythrocyte fatty acid composition. Eur. J. Clin. Nutr. 2004, 58, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Goyens, P.L.L.; Spilker, M.E.; Zock, P.L.; Katan, M.B.; Mensink, R.P. Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am. J. Clin. Nutr. 2006, 84, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sanchez, A.; Larque, E.; Demmelmair, H.; Acien, M.I.; Faber, F.L.; Parrilla, J.J.; Koletzko, B. Maternal-fetal in vivo transfer of [C-13]docosahexaenoic and other fatty acids across the human placenta 12 h after maternal oral intake. Am. J. Clin. Nutr. 2010, 92, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Escolano-Margarit, M.V.; Campoy, C.; Ramirez-Tortosa, M.C.; Demmelmair, H.; Miranda, M.T.; Gil, A.; Decsi, T.; Koletzko, B.V. Effects of fish oil supplementation on the fatty acid profile in erythrocyte membrane and plasma phospholipids of pregnant women and their offspring: A randomised controlled trial. Br. J. Nutr. 2013, 109, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Matorras, R.; Perteagudo, L.; Sanjurjo, P.; Ruiz, J.I. Intake of long chain w3 polyunsaturated fatty acids during pregnancy and the influence of levels in the mother on newborn levels. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 83, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Haghiac, M.; Yang, X.H.; Presley, L.; Smith, S.; Dettelback, S.; Minium, J.; Belury, M.A.; Catalano, P.M.; Mouzon, S.H.-D.; Norata, G.D. Dietary Omega-3 Fatty Acid Supplementation Reduces Inflammation in Obese Pregnant Women: A Randomized Double-Blind Controlled Clinical Trial. PLoS ONE 2015, 10, e0137309. [Google Scholar] [CrossRef] [PubMed]
- Haggarty, P. Placental regulation of fatty acid delivery and its effect on fetal growth—A review. Placenta 2002, 23 (Suppl. A), S28–S38. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.A.; Neumann, M.A.; Makrides, M. Effect of increasing breast milk docosahexaenoic acid on plasma and erythrocyte phospholipid fatty acids and neural indices of exclusively breast fed infants. Eur. J. Clin. Nutr. 1997, 51, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, X.; Zhou, B.; Jiang, A.C.; Chai, L. An updated review of worldwide levels of docosahexaenoic and arachidonic acid in human breast milk by region. Public Health Nutr. 2016, 19, 2675–2687. [Google Scholar] [CrossRef] [PubMed]
- Al, D.M.; Van Houwelingen, A.C.; Kester, A.; Hasaart, T.; De Jong, A.; Hornstra, G. Maternal essential fatty acid patterns during normal pregnancy and their relationships with the neonatal essential fatty acid status. Br. J. Nutr. 1995, 74, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Al, M.D.M.; Badart-Smook, A.; van Houwelingen, A.C. Fat intake of women during normal pregnancy: Relationship with maternal and neonatal essential fatty acid status. J. Am. Coll. Nutr. 1996, 15, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Al, D.M.; van Houwelingen, A.C.; Hornstra, G. Long-chain polyunsaturated fatty acids, pregnancy, and pregnancy outcome. Am. J. Clin. Nutr. 2000, 71, 2855–2915. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Mallick, R.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Maternal supply of both arachidonic and docosahexaenoic acids is required for optimal neurodevelopment. Nutrients 2021, 13, 2061. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Mean ± SD * | Median (Percentile 25th, 75th) ** | n (%) *** |
|---|---|---|---|
| Maternal age (years) * | 32.0 ± 4.0 | 33 (29, 35) | |
| Pregestational BMI (kg/m2) ** | 24.5 ± 4.0 | 23.8 (21.6, 26.5) | |
| GWG (kg) * | 10.9 ± 5.8 | 11.0 (8.0–14.0) | |
| GDM | 15.12 (28) | ||
| Placenta weight (g) * | 507 ± 143 | 500 (400, 600) | |
| Birth weight (g) * | 3331 ± 461 | 3320 (3040, 3610) | |
| Infant’s sex | |||
| Male | 25 (46.3%) | ||
| Female | 29 (53.7%) | ||
| Physical activity + | |||
| Low active | 20 (39.2%) | ||
| Active | 25 (49.0%) | ||
| Very active | 6 (11.8%) | ||
| Smoking before pregnancy + | |||
| Yes | 14 (27.5%) | ||
| No | 37 (72.5%) |
| Nutrient | g/Day | % Energy | % Fat |
|---|---|---|---|
| Energy (kcal/d) | 2019 ± 527 (1020–4080) | ||
| Carbohydrates | 221 ± 59 (96.5–404.0) | 44.3 ± 7.6 (29.93–65.87) | |
| Protein | 85 ± 22 (44.7–162.0) | 17.0 ± 2.6 (23.03–25.23) | |
| Fat | 87 ± 35 (30.6–236.0) | 38.6 ± 7.7 (19.89–56.70) | |
| Saturated fatty acids | 32.8 ± 17.5 (14.8–103.0) | 14.0 ± 3.9 (7.45–24.73) | 35.9 ± 5.9 (23.91–48.37) |
| Myristic acid (C14:0) | 3.1 ± 2.2 (0.95–13.1) | 1.3 ± 0.62 (0.54–3.07) | 3.3 ± 1.3 (1.53–6.86) |
| Palmitic acid (C16:0) | 16.2 ± 7.9 (6.4–48.1) | 7.0 ± 1.87 (3.34–12.21) | 18.2 ± 3.0 (10.34–23.20) |
| Stearic acid (C18:0) | 6.5 ± 3.2 (2.3–18.2) | 2.86 ± 0.85 (1.36–5.42) | 7.4 ± 1.5 (3.88–11.64) |
| Monounsaturated fatty acids | 36.2 ± 14.3 (9.2–84.1) | 15.8 ± 4.2 (5.98–28.28) | 41.08 ± 5.9 (30.07–54.60) |
| Palmitoleic acid (C16:1) | 1.4 ± 0.63 (0.68–4.2) | 0.65 ± 0.16 (0.32–1.01) | 1.7 ± 0.35 (1.09–2.63) |
| Oleic acid (C18:1n-9) | 32.5 ± 12.7 (7.5–74.2) | 14.4 ± 4.17 (4.88–25.52) | 37.1 ± 6.1 (24.51–52.34) |
| Polyunsaturated fatty acids | 12.5 ± 5.2 (3.3–32.7) | 5.4 ± 1.3 (2.15–9.97) | 14.2 ± 3.0 (8.33–22.71) |
| Linoleic acid (C18:2n-6) | 10.1 ± 4.3 (2.8–28.3) | 4.4 ± 1.2 (1.82–8.85) | 11.7 ± 2.8 (6.09–18.70) |
| α-Linolenic acid (C18:3n-3) | 1.20 ± 0.48 (0.48–2.5) | 0.55 ± 0.15 (0.29–0.98) | 1.4 ± 0.4 (0.82–3.06) |
| Arachidonic acid (C20:4n-6) | 0.14 ± 0.10 (0.008–0.71) | 0.06 ± 0.03 (0.01–0.18) | 0.17 ± 0.09 (0.03–0.45) |
| Eicosapentaenoic acid (C20:5n-3) | 0.12 ± 0.11 (0.0–0.53) | 0.06 ± 0.05 (0.0–0.19) | 0.15 ± 0.13 (0.00–0.47)) |
| Docosahexaenoic acid (C22:6n-3) | 0.26 ± 0.20 (0.0–0.88) | 0.12 ± 0.09 (0.0–0.40) | 0.32 ± 0.25 (0.00–1.22) |
| Fatty Acid | Mean | SD |
|---|---|---|
| C14:0 | 0.37 | 0.09 |
| C16:0 | 26.31 | 1.04 |
| C17:0 | 0.32 | 0.05 |
| C18:0 | 11.88 | 0.92 |
| C20:0 | 0.30 | 0.09 |
| C22:0 | 1.27 | 0.29 |
| C24:0 | 1.43 | 0.44 |
| C14:1 | 0.01 | 0.01 |
| C15:1 | 0.05 | 0.01 |
| C16:1n-7 | 0.42 | 0.10 |
| C17:1 | 0.08 | 0.22 |
| C18:1n-9 | 9.18 | 1.13 |
| C18:1n-7 | 1.65 | 0.18 |
| C20:1n-9 | 0.19 | 0.06 |
| C22:1n-9 | 0.21 | 0.14 |
| C24:1n-9 | 1.45 | 0.46 |
| C14:1t | 0.02 | 0.02 |
| C16:1T | 0.24 | 0.11 |
| C18:1t | 0.07 | 0.05 |
| C22:1t | 0.03 | 0.04 |
| C18:2tt | 0.05 | 0.02 |
| C20:3n-9 | 0.13 | 0.05 |
| C18:2n-6 | 9.32 | 1.04 |
| C18:3n-6 | 0.12 | 0.04 |
| C20:2n-6 | 0.43 | 0.09 |
| C20:3n-6 | 4.59 | 0.87 |
| C20:4n-6 | 21.64 | 1.54 |
| C22:2n-6 | 0.24 | 0.05 |
| C22:4n-6 | 1.56 | 0.26 |
| C22:5n-6 | 1.07 | 0.44 |
| C18:3n-3 | 0.02 | 0.01 |
| C18:4n-3 | 0.05 | 0.06 |
| C20:3n-3 | 0.11 | 0.03 |
| C20:5n-3 | 0.08 | 0.07 |
| C22:5n-3 | 0.46 | 0.33 |
| C22:6n-3 | 4.64 | 0.76 |
| Fatty Acids in Placental Phospholipids | FA Intake (g/Day) r Values | Energy (%) r Values | Fat (%) r Values |
|---|---|---|---|
| Total saturated fatty acid | 0.097 | 0.154 | 0.050 |
| Myristic acid (C14:0) | 0.276 * | 0.259 | 0.188 |
| Palmitic acid (C16:0) | −0.091 | 0.033 | 0.059 |
| Stearic acid (C18:0) | 0.278 * | 0.227 | 0.161 |
| Palmitoleic acid (C16:1) | 0.015 | −0.093 | −0.107 |
| Oleic acid (C18:1n-9) | 0.243 | 0.331 * | 0.315 * |
| Linoleic acid (C18:2n-6) | 0.139 | 0.110 | 0.216 |
| α-Linolenic acid (C18:3n-3) | 0.100 | 0.066 | 0.286 * |
| Arachidonic acid (C20:4n-6) | 0.142 | 0.208 | 0.197 |
| Eicosapentaenoic acid (C20:5n-3) | 0.002 | −0.043 | −0.057 |
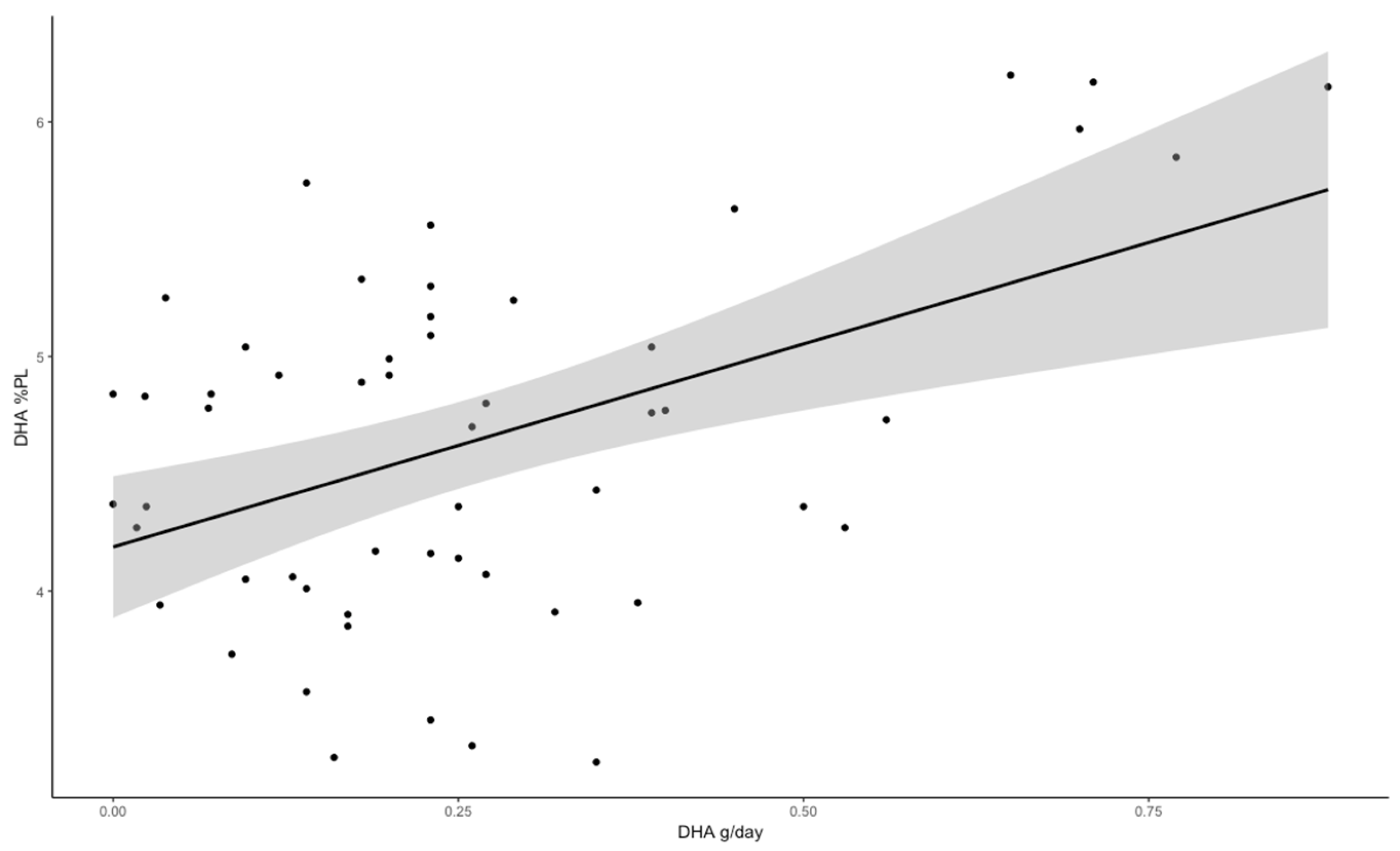
| Docosahexaenoic acid (C22:6n-3) | 0.469 *** | 0.435 ** | 0.429 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladino, L.; Demmelmair, H.; Segura, M.T.; Escudero-Marin, M.; Grote, V.; Koletzko, B.; Campoy, C. Association Between Maternal Dietary Fatty Acid Intake and Fatty Acid Composition of Placental Phospholipids. Nutrients 2025, 17, 2394. https://doi.org/10.3390/nu17152394
Ladino L, Demmelmair H, Segura MT, Escudero-Marin M, Grote V, Koletzko B, Campoy C. Association Between Maternal Dietary Fatty Acid Intake and Fatty Acid Composition of Placental Phospholipids. Nutrients. 2025; 17(15):2394. https://doi.org/10.3390/nu17152394
Chicago/Turabian StyleLadino, Liliana, Hans Demmelmair, María Teresa Segura, Mireia Escudero-Marin, Veit Grote, Berthold Koletzko, and Cristina Campoy. 2025. "Association Between Maternal Dietary Fatty Acid Intake and Fatty Acid Composition of Placental Phospholipids" Nutrients 17, no. 15: 2394. https://doi.org/10.3390/nu17152394
APA StyleLadino, L., Demmelmair, H., Segura, M. T., Escudero-Marin, M., Grote, V., Koletzko, B., & Campoy, C. (2025). Association Between Maternal Dietary Fatty Acid Intake and Fatty Acid Composition of Placental Phospholipids. Nutrients, 17(15), 2394. https://doi.org/10.3390/nu17152394






