Beverage-Specific Modulation of Urinary Inflammatory Biomarkers After Endurance Running in Trained Males
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocols
- Pre-exercise measurements and baseline urine collection
- Standardized warm-up (10 min of light jogging and dynamic stretching)
- 6000 m pace running at approximately 70–75% of maximum heart rate
- Immediate post-exercise measurements and urine collection
- Consumption of the designated test beverage (500 mL) within 5 min post-exercise
- 60 min seated recovery period in a temperature-controlled environment (21–23 °C, 45–55% relative humidity)
- 1000 m time trial performance test
- Post-time trial measurements and final urine collection
2.3. Beverage Interventions
2.4. Measurements
2.4.1. Physiological and Performance Measurements
2.4.2. Urine Sample Collection and Processing
2.4.3. Urine Biochemical Analysis
2.4.4. Enzyme-Linked Immunosorbent Assays (ELISAs)
2.5. Statistical Analysis
3. Results
3.1. Performance and Physiological Measures
3.2. Basic Urinary Parameters
3.2.1. Urinary Protein and Albumin
3.2.2. Urinary Glucose
3.2.3. Urinary pH, Specific Gravity, and Osmolarity
3.3. Renal Function Markers
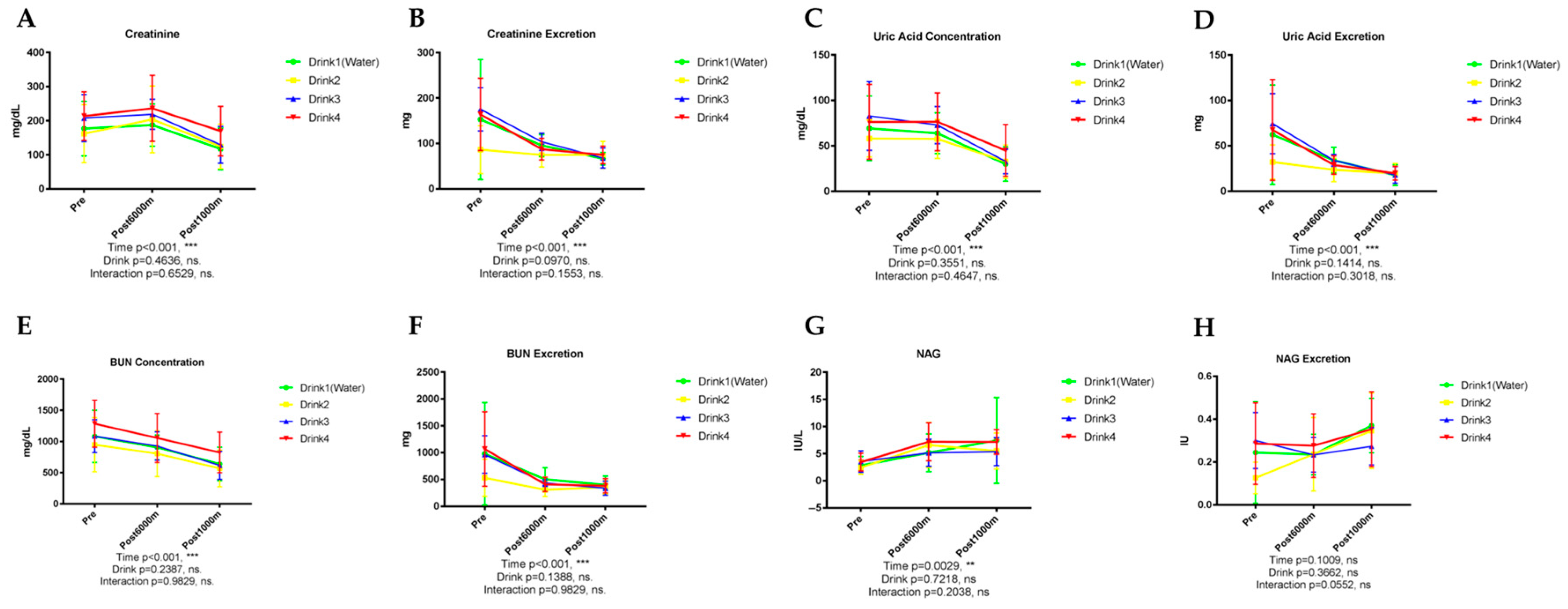
3.3.1. Creatinine
3.3.2. Uric Acid
3.3.3. Urea Nitrogen
3.3.4. N-Acetyl-β-D-Glucosaminidase (NAG)
3.4. Electrolyte Excretion
3.4.1. Sodium (Na+)
3.4.2. Potassium (K+)
3.4.3. Calcium (Ca2+)
3.4.4. Chloride (Cl−)
3.4.5. Inorganic Phosphorus
3.5. Muscle and Visceral Injury Markers
3.5.1. Titin
3.5.2. Intestinal Fatty Acid-Binding Protein (I-FABP)
3.6. Inflammatory Markers, Cytokines and Chemokines Analysis
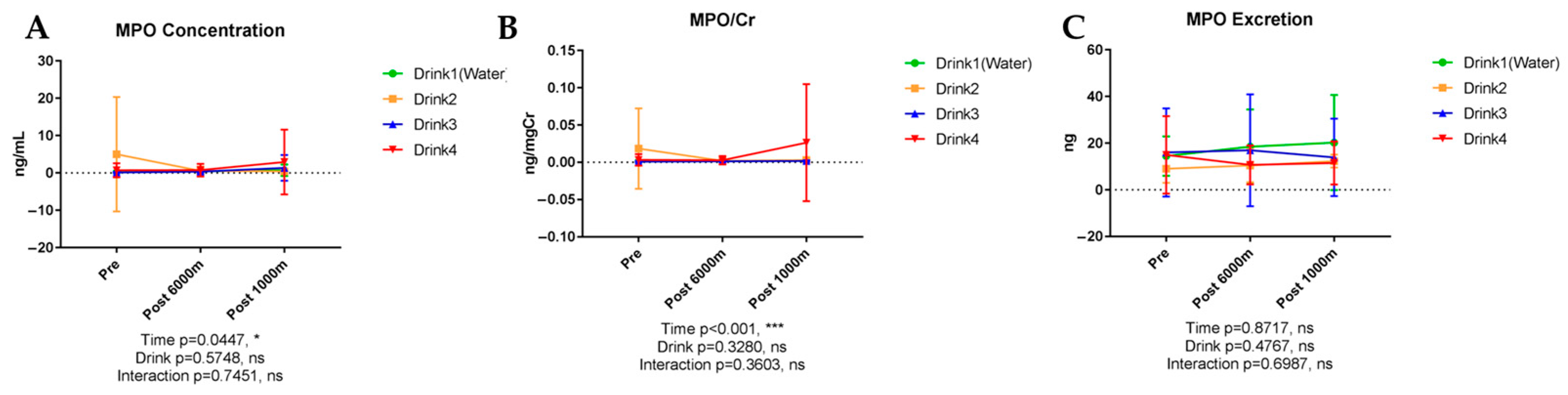
3.6.1. Myeloperoxidase (MPO)
3.6.2. Calprotectin
3.6.3. Complement Component 5a (C5a)
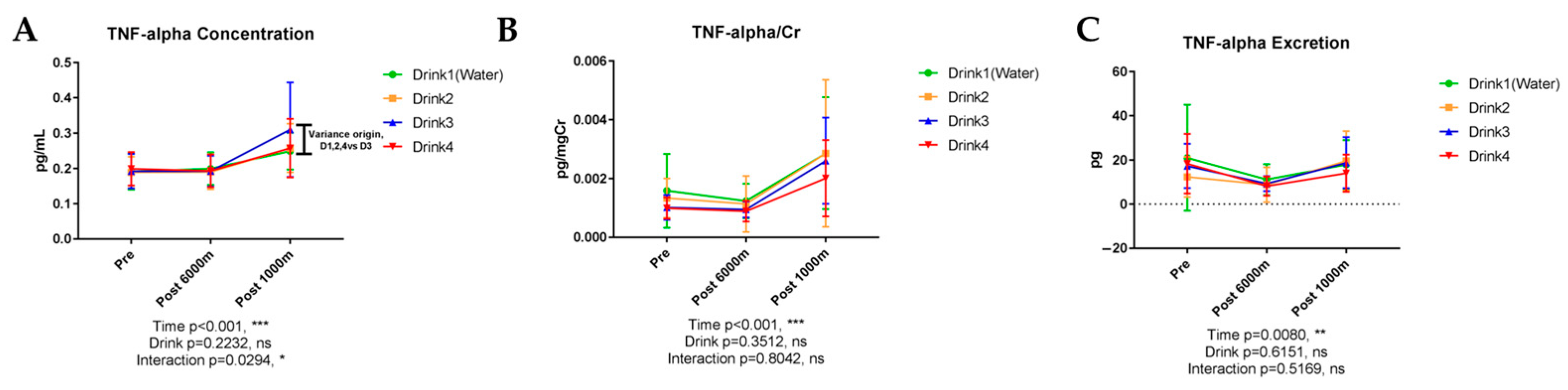
3.6.4. Tumor Necrosis Factor-α (TNF-α)
3.6.5. Interleukin-1β (IL-1β)
3.6.6. Interleukin-6 (IL-6)
3.6.7. Interleukin-8 (IL-8)
3.6.8. Interleukin-1 Receptor Antagonist (IL-1ra)
3.6.9. Interleukin-4 (IL-4)
3.6.10. Interleukin-10 (IL-10)
3.6.11. Interleukin-2 (IL-2)
3.6.12. Interferon-γ (IFN-γ)
3.6.13. Interleukin-12p40 (IL-12p40)
3.6.14. Monocyte Chemoattractant Protein-1 (MCP-1)
3.6.15. Macrophage Colony-Stimulating Factor (M-CSF)
4. Discussion
4.1. Performance Parameters and Methodological Considerations
4.2. Beverage-Specific Effects on Waste Product Excretion
ORS and Reduced Waste Product Elimination
4.3. Intestinal Protection and Damage Biomarkers
I-FABP and Beverage-Dependent Intestinal Protection
4.4. Inflammatory Cytokine Modulation: Novel Beverage-Specific Effects
4.4.1. IL-4 Enhancement: Promoting Resolution of Inflammation
4.4.2. Comparison with Previous Cytokine Research
4.5. Osmolarity and Electrolyte Composition: Mechanistic Insights
4.5.1. Beyond Osmolarity: Specific Compositional Effects
4.5.2. Sodium Content and Fluid Retention Mechanisms
4.6. Clinical and Practical Applications
4.6.1. Tailored Beverage Selection for Specific Scenarios
4.6.2. Implications for Beverage Formulation
4.7. Limitations and Methodological Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gann, J.J.; Andre, T.L.; Gallucci, A.R.; Willoughby, D.S. Effects of Hypohydration on Muscular Strength, Endurance, and Power in Women. J. Strength Cond. Res. 2021, 35, S102–S106. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, M.; Motti, M.L.; Meccariello, R.; Mazzeo, F. Resveratrol and Physical Activity: A Successful Combination for the Maintenance of Health and Wellbeing? Nutrients 2025, 17, 837. [Google Scholar] [CrossRef] [PubMed]
- Ganu, G.; Nagore, D.; Rede, S.M.; Lohani, M.R.; Aniket, M. Beyond the Hype: A Scientific Assessment of Sports Supplements. Int. J. Clin. Stud. Med. 2024, 45, 003. [Google Scholar]
- De Brier, N.; Borra, V.; Berry, D.C.; Zideman, D.; Singletary, E.; De Buck, E.; International Liaison Committee on Resuscitation First Aid Task Force. Oral Rehydration Beverages for Treating Exercise-Associated Dehydration: A Systematic Review, Part II. The Effectiveness of Alternatives to Carbohydrate-Electrolyte Drinks. J. Athl. Train. 2025, 60, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Borra, V.; De Brier, N.; Berry, D.C.; Zideman, D.; Singletary, E.; De Buck, E.; International Liaison Committee on Resuscitation First Aid Task Force. Oral rehydration beverages for treating exercise-associated dehydration: A systematic review, Part I. Carbohydrate-electrolyte solutions. J. Athl. Train. 2025, 60, 34–54. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Characterization of exercise-induced cytokine release, the impacts on the body, the mechanisms and modulations. Int. J. Sports Exerc. Med. 2019, 5, 10–23937. [Google Scholar] [CrossRef]
- Ikemura, T.; Suzuki, K.; Nakamura, N.; Yada, K.; Hayashi, N. Fluid intake restores retinal blood flow early after exhaustive exercise in healthy subjects. Eur. J. Appl. Physiol. 2018, 118, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Trangmar, S.J.; Chiesa, S.T.; Llodio, I.; Garcia, B.; Kalsi, K.K.; Secher, N.H.; González-Alonso, J. Dehydration accelerates reductions in cerebral blood flow during prolonged exercise in the heat without compromising brain metabolism. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H1598–H1607. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T.; Ma, S.; Sugama, K.; Kanda, K.; Omae, C.; Choi, W.; Hashimoto, S.; Aoyama, K.; Yoshikai, Y.; Suzuki, K. Changes in urinary biomarkers of organ damage, inflammation, oxidative stress, and bone turnover following a 3000-m time trial. Antioxidants 2021, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Nalin, D.R.; Hirschhorn, N.; Greenough, W., III; Fuchs, G.J.; Cash, R.A. Clinical concerns about reduced-osmolarity oral rehydration solution. Jama 2004, 291, 2632–2635. [Google Scholar] [CrossRef] [PubMed]
- Kung, S.; Vakula, M.N.; Kim, Y.; England, D.L.; Bergeson, J.; Bressel, E.; Lefevre, M.; Ward, R. No effect of a dairy-based, high flavonoid pre-workout beverage on exercise-induced intestinal injury, permeability, and inflammation in recreational cyclists: A randomized controlled crossover trial. PLoS ONE 2022, 17, e0277453. [Google Scholar] [CrossRef] [PubMed]
- Castell, L.M.; Nieman, D.C.; Bermon, S.; Peeling, P. Exercise-induced illness and inflammation: Can immunonutrition and iron help? Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Belval, L.N.; Hosokawa, Y.; Casa, D.J.; Adams, W.M.; Armstrong, L.E.; Baker, L.B.; Burke, L.; Cheuvront, S.; Chiampas, G.; González-Alonso, J.; et al. Practical hydration solutions for sports. Nutrients 2019, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.J.; Chan, A.W.; Wu, P.; Vail, A.; Guyatt, G.H.; Altman, D.G. Design, analysis, and presentation of crossover trials. Trials 2009, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Zhukovsky, D.S.; Bruera, E. Which treatment is better? Ascertaining patient preferences with crossover randomized controlled trials. J. Pain Symptom Manag. 2015, 49, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Louis, T.A.; Philip, W.L.; Polansky, M. Crossover and self-controlled designs in clinical research. In Medical Uses of Statistics; CRC Press: Boca Raton, FL, USA, 2019; pp. 83–103. [Google Scholar]
- Kanda, K.; Sakuma, J.; Akimoto, T.; Kawakami, Y.; Suzuki, K. Detection of titin fragments in urine in response to exercise-induced muscle damage. PLoS ONE 2017, 12, e0181623. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.S.; Kopetschny, B.H.; Badenhorst, C.E. The hydrating effects of hypertonic, isotonic and hypotonic sports drinks and waters on central hydration during continuous exercise: A systematic meta-analysis and perspective. Sports Med. 2022, 52, 349–375. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Idárraga, A.; Aragón-Vargas, L.F. Postexercise rehydration: Potassium-rich drinks versus water and a sports drink. Appl. Physiol. Nutr. Metab. 2014, 39, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Snipe, R.M.; Khoo, A.; Kitic, C.M.; Gibson, P.R.; Costa, R.J. Carbohydrate and protein intake during exertional heat stress ameliorates intestinal epithelial injury and small intestine permeability. Appl. Physiol. Nutr. Metab. 2017, 42, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.; Camões-Costa, V.; Snipe, R.M.; Dixon, D.; Russo, I.; Huschtscha, Z. Impact of exercise-induced hypohydration on gastrointestinal integrity, function, symptoms, and systemic endotoxin and inflammatory profile. J. Appl. Physiol. 2019, 126, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- van Wijck, K.; Lenaerts, K.; Grootjans, J.; Wijnands, K.A.; Poeze, M.; Van Loon, L.J.; Dejong, C.H.; Buurman, W.A. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: Strategies for evaluation and prevention. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 303, G155–G168. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Shiraishi, K.; Yoshitani, K.; Sugama, K.; Kometani, T. Effect of a sports drink based on highly-branched cyclic dextrin on cytokine responses to exhaustive endurance exercise. J. Sports Med. Phys. Fit. 2014, 54, 622–630. [Google Scholar]
- Peake, J.M.; Neubauer, O.; Walsh, N.P.; Simpson, R.J. Recovery of the immune system after exercise. J. Appl. Physiol. 2017, 122, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017, 31, 1273. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.H.; James, L.J.; Shirreffs, S.M.; Maughan, R.J. Optimizing the restoration and maintenance of fluid balance after exercise-induced dehydration. J. Appl. Physiol. 2017, 122, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Watson, P.; Cordery, P.A.; Walsh, N.P.; Oliver, S.J.; Dolci, A.; Rodriguez-Sanchez, N.; Galloway, S.D. Sucrose and sodium but not caffeine content influence the retention of beverages in humans under euhydrated conditions. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Heaton, L.E.; Davis, J.K.; Rawson, E.S.; Nuccio, R.P.; Witard, O.C.; Stein, K.W.; Baar, K.; Carter, J.M.; Baker, L.B. Selected in-season nutritional strategies to enhance recovery for team sport athletes: A practical overview. Sports Med. 2017, 47, 2201–2218. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; Jeukendrup, A.E. Optimal composition of fluid-replacement beverages. Compr. Physiol. 2014, 4, 575–620. [Google Scholar] [CrossRef] [PubMed]
- Roumelioti, M.E.; Glew, R.H.; Khitan, Z.J.; Rondon-Berrios, H.; Argyropoulos, C.P.; Malhotra, D.; Raj, D.S.; Agaba, E.I.; Rohrscheib, M.; Murata, G.H.; et al. Fluid balance concepts in medicine: Principles and practice. World J. Nephrol. 2018, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Prowle, J.R.; Kirwan, C.J.; Bellomo, R. Fluid management for the prevention and attenuation of acute kidney injury. Nat. Rev. Nephrol. 2014, 10, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Orrù, S.; Imperlini, E.; Nigro, E.; Alfieri, A.; Cevenini, A.; Polito, R.; Daniele, A.; Buono, P.; Mancini, A. Role of functional beverages on sport performance and recovery. Nutrients 2018, 10, 1470. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Abraham, J. A comprehensive study on sports and energy drinks. In Sports and Energy Drinks; Woodhead Publishing: Sawston, UK, 2019; pp. 515–537. [Google Scholar]
- Can, S.; Can, H. Functional Nutritional Strategies for Peak Athletic Performance. In Evaluating the Effectiveness of Functional Ingredients in Sports Nutrition; IGI Global Scientific Publishing: Hershey, PA, USA, 2025; pp. 1–34. [Google Scholar]
- Sutehall, S.; Muniz-Pardos, B.; Bosch, A.N.; Di Gianfrancesco, A.; Pitsiladis, Y.P. Sports drinks on the edge of a new era. Curr. Sports Med. Rep. 2018, 17, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, D.L.; Hopkins, W.G.; Jeukendrup, A. Effects of Hypotonic and Isotonic Sports Drinks on Endurance Performance and Physiology. Sportscience 2010, 14, 63. [Google Scholar]
- Keegan, A.D.; Leonard, W.J.; Zhu, J. Recent advances in understanding the role of IL-4 signaling. Fac. Rev. 2021, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Ishijima, T.; Hayashida, H.; Suzuki, K.; Higuchi, M. Menstrual cycle phase and carbohydrate ingestion alter immune response following endurance exercise and high intensity time trial performance test under hot conditions. J. Int. Soc. Sports Nutr. 2014, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Giustiniani, P.; Rodriguez-Sanchez, N.; Galloway, S.D. Fluid and electrolyte balance considerations for female athletes. Eur. J. Sport Sci. 2022, 22, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Vela, J.; Fontana, L.; Casuso, R.A.; Plaza-Díaz, J.; Huertas, J.R. Differential inflammatory response of men and women subjected to an acute resistance exercise. Biomed. J. 2021, 44, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Kenward, M.G.; Jones, B. Crossover trials. Methods Appl. Stat. Clin. Trials Concepts Princ. Trials Des. 2014, 1, 310–319. [Google Scholar]
- Nakagawa, S.; Cuthill, I.C. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. 2007, 82, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Périard, J.D.; Eijsvogels, T.M.; Daanen, H.A. Exercise under heat stress: Thermoregulation, hydration, performance implications, and mitigation strategies. Physiol. Rev. 2021, 101, 1873–1979. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.L.; Johnson, B.D.; Parker, M.D.; Hostler, D.; Pryor, R.R.; Schlader, Z. Kidney physiology and pathophysiology during heat stress and the modification by exercise, dehydration, heat acclimation and aging. Temperature 2021, 8, 108–159. [Google Scholar] [CrossRef] [PubMed]
- Bossi, A.H.; Naumann, U.; Passfield, L.; Hopker, J. Modelling inter-individual variability in acute and adaptive responses to interval training: Insights into exercise intensity normalisation. Eur. J. Appl. Physiol. 2024, 124, 1201–1216. [Google Scholar] [CrossRef] [PubMed]







| Component | Drink 1 | Drink 2 | Drink 3 | Drink 4 |
|---|---|---|---|---|
| Description | Water (Control) | Hypotonic Sports Drink | Oral Rehydration Solution | Modified Hypotonic |
| Glucose (mmol/L) | 0 | 39 | 100 | 81 |
| Na+ (mmol/L) | 0 | 17.4 | 50 | 41 |
| Ca2+ (mmol/L) | 0 | 0.6 | ― | ― |
| Cl− (mmol/L) | 0 | 13 | 50 | 31 |
| K+ (mmol/L) | 0 | 1.2 | 20 | 20 |
| Mg2+ (mmol/L) | 0 | 0.5 | 1.0 | ― |
| Osmolarity (mOsm/L) | 0 | 200 | 270 | 200 |
| Carbohydrate Content | 0 g/100 mL | 2.9 g/100 mL | 2.5 g/100 mL | 1.5 g/100 mL |
| Category | Biomarker | Time Effect Concentration | Time Effect Excretion | Beverage Effect Concentration | Beverage Effect Excretion | Time × Beverage Interaction | Key Significant Findings |
|---|---|---|---|---|---|---|---|
| Basic Urinary Parameters | Protein | <0.001 * | <0.001 * | 0.882 | NS | - | Baseline < post-6000 m < post-1000 m |
| Albumin | <0.001 * | 0.006 | 0.788 | NS | - | Exercise-induced elevation | |
| Glucose | 0.001 | 0.003 | NS | NS | - | Exercise-induced elevation | |
| pH | <0.001 * | - | 0.599 | - | - | Exercise-induced reduction | |
| Specific Gravity | <0.001 * | - | 0.164 | - | - | Exercise-induced reduction | |
| Osmolarity | <0.001 * | - | 0.098 | - | - | Exercise-induced reduction | |
| Renal Function | Creatinine | <0.001 * | <0.001 * | 0.464 | 0.097 | - | Post-1000 m < baseline, post-6000 m |
| Uric Acid | <0.001 * | <0.001 * | 0.355 | 0.141 | - | Exercise-induced reduction | |
| Urea Nitrogen | <0.001 * | <0.001 * | 0.239 | 0.139 | - | Exercise-induced reduction | |
| NAG | 0.003 | NS | 0.722 | 0.366 | - | Exercise-induced elevation | |
| Electrolytes | Na+ | 0.002 | 0.004 | 0.415 | 0.661 | - | Exercise-induced reduction |
| K+ | <0.001 * | 0.005 | 0.439 | 0.829 | - | Exercise-induced reduction | |
| Ca2+ | <0.001 * | 0.003 | 0.038 * | 0.092 | - | Beverage effect (Drink 2) | |
| Cl− | <0.001 * | <0.001 * | 0.442 | 0.442 | - | Exercise-induced reduction | |
| Inorganic P | 0.002 | NS | 0.451 | NS | 0.026 * | Drink 4 effect post-1000 m | |
| Muscle/Visceral Injury | Titin | 0.356 | 0.001 | 0.417 | NS | - | Creatinine-corrected: <0.001 * |
| I-FABP | <0.001 * | <0.001 * | 0.774 | NS | 0.006 cr | Exercise elevation; creatinine-corrected interaction | |
| Inflammatory Markers | MPO | 0.045 | NS | NS | NS | - | Creatinine-corrected: <0.001 * |
| Calprotectin | NS | NS | NS | NS | - | No significant effects | |
| C5a | 0.004 | 0.007 | NS | NS | - | Exercise-induced elevation | |
| Pro-inflammatory Cytokines | TNF-α | <0.001 * | 0.008 | 0.223 | NS | 0.029 * | Exercise elevation; Drink 3 interaction |
| IL-1β | 0.005 | <0.001 * | NS | NS | - | Exercise-induced elevation | |
| IL-6 | 0.042 | NS | NS | NS | - | Creatinine-corrected: <0.001 * | |
| IL-8 | NS | NS | NS | NS | - | Creatinine-corrected: 0.003 | |
| Anti-inflammatory Cytokines | IL-1ra | NS | NS | NS | NS | - | No significant effects |
| IL-4 | NS | 0.020 | 0.047 * | NS | 0.027 *,cr | BEVERAGE MAIN EFFECT: Drink 4 > others | |
| IL-10 | NS | 0.010 | NS | NS | - | Exercise-induced elevation | |
| Immunomodulatory Cytokines | IL-2 | NS | NS | NS | NS | - | Creatinine-corrected: 0.007 |
| IFN-γ | NS | 0.033 | NS | NS | - | Exercise-induced elevation | |
| IL-12p40 | NS | NS | NS | NS | - | No significant effects | |
| Chemokines/ Growth Factors | MCP-1 | <0.001 * | NS | NS | NS | - | Exercise-induced elevation |
| M-CSF | 0.024 | NS | NS | NS | - | Exercise-induced elevation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, K.; Kanda, K.; Ma, S. Beverage-Specific Modulation of Urinary Inflammatory Biomarkers After Endurance Running in Trained Males. Nutrients 2025, 17, 2379. https://doi.org/10.3390/nu17142379
Suzuki K, Kanda K, Ma S. Beverage-Specific Modulation of Urinary Inflammatory Biomarkers After Endurance Running in Trained Males. Nutrients. 2025; 17(14):2379. https://doi.org/10.3390/nu17142379
Chicago/Turabian StyleSuzuki, Katsuhiko, Kazue Kanda, and Sihui Ma. 2025. "Beverage-Specific Modulation of Urinary Inflammatory Biomarkers After Endurance Running in Trained Males" Nutrients 17, no. 14: 2379. https://doi.org/10.3390/nu17142379
APA StyleSuzuki, K., Kanda, K., & Ma, S. (2025). Beverage-Specific Modulation of Urinary Inflammatory Biomarkers After Endurance Running in Trained Males. Nutrients, 17(14), 2379. https://doi.org/10.3390/nu17142379








