Dietary Nitrogen and Its Role in the Gut Microbiome and Inflammatory Bowel Disease: A Narrative Review
Abstract
1. Introduction
2. Sources of Dietary Nitrogen
2.1. Amino Acids
2.2. Nitrates, Nitrites, and Nitric Oxide
2.3. Ammonia and Urea
3. Dietary Nitrogen and Their Roles in IBD Pathogenesis
3.1. Effects on Gut Permeability
3.2. Effect of Nitrogen on Impaired Mucus Layers → Exposure to Nitrogen
3.3. Gut Immune System
4. Effects of Nitrogen-Containing Compounds on Gut Microbiota and Their Roles in IBD
5. Other Notable Compounds and Their Effects on the Gut Microbiota and IBD
5.1. Lectins
5.2. Purines
6. Potential Therapeutic Implications
7. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Front. Cell. Infect. Microbiol. 2022, 12, 733992. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Sun, Q.; Zhang, J.; Ng, S.C. The role of gut microbiome in inflammatory bowel disease diagnosis and prognosis. United Eur. Gastroenterol. J. 2022, 10, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Gyriki, D.; Nikolaidis, C.; Stavropoulou, E.; Bezirtzoglou, I.; Tsigalou, C.; Vradelis, S.; Bezirtzoglou, E. Exploring the Gut Microbiome’s Role in Inflammatory Bowel Disease: Insights and Interventions. J. Pers. Med. 2024, 14, 507. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Kerby, R.L.; Zhang, Q.; Pradhan, M.; Mehrabian, M.; Lusis, A.J.; Bergstrom, G.; Backhed, F.; Rey, F.E. Gut bacterial metabolism contributes to host global purine homeostasis. Cell Host Microbe 2023, 31, 1038–1053.e1010. [Google Scholar] [CrossRef] [PubMed]
- Choden, T.; Cohen, N.A. The gut microbiome and the immune system. Explor. Med. 2022, 3, 219–233. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Igai, K.; Itakura, M.; Nishijima, S.; Tsurumaru, H.; Suda, W.; Tsutaya, T.; Tomitsuka, E.; Tadokoro, K.; Baba, J.; Odani, S.; et al. Nitrogen fixation and nifH diversity in human gut microbiota. Sci. Rep. 2016, 6, 31942. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Soltero, R.; Bailen, M.; de Lucas, B.; Ramirez-Goercke, M.I.; Pareja-Galeano, H.; Larrosa, M. Role of Oral and Gut Microbiota in Dietary Nitrate Metabolism and Its Impact on Sports Performance. Nutrients 2020, 12, 3611. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, P.; Stahl, B.; Knol, J.; Belzer, C. The infant gut microbiota: In pursuit of non-protein nitrogen. Gut Microbes 2023, 15, 2211917. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; Zhu, Y.; Ouyang, J.; Wang, Y.; Lei, Q.; Xia, J.; Guan, Y.; Zhang, J.; Guo, J.; He, Y.; et al. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome 2020, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, A.; Elemba, E.; Zhong, Q.; Sun, Z. Gastrointestinal Interaction between Dietary Amino Acids and Gut Microbiota: With Special Emphasis on Host Nutrition. Curr. Protein Pept. Sci. 2020, 21, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Diether, N.E.; Willing, B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet(-)Microbe(-)Host Interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Shen, T.-C.D.; Chen, E.Z.; Bittinger, K.; Bailey, A.; Roggiani, M.; Sirota-Madi, A.; Friedman, E.S.; Chau, L.; Lin, A.; et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci. Transl. Med. 2017, 9, eaah6888. [Google Scholar] [CrossRef] [PubMed]
- Andriamihaja, M.; Lan, A.; Beaumont, M.; Audebert, M.; Wong, X.; Yamada, K.; Yin, Y.; Tome, D.; Carrasco-Pozo, C.; Gotteland, M.; et al. The deleterious metabolic and genotoxic effects of the bacterial metabolite p-cresol on colonic epithelial cells. Free Radic. Biol. Med. 2015, 85, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Calvez, J.; Azzout-Marniche, D.; Tome, D. Protein quality, nutrition and health. Front. Nutr. 2024, 11, 1406618. [Google Scholar] [CrossRef] [PubMed]
- Silk, D.B.; Fairclough, P.D.; Clark, M.L.; Hegarty, J.E.; Marrs, T.C.; Addison, J.M.; Burston, D.; Clegg, K.M.; Matthews, D.M. Use of a peptide rather than free amino acid nitrogen source in chemically defined “elemental” diets. JPEN J. Parenter. Enter. Nutr. 1980, 4, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.; Kleiner, M. Dietary protein and the intestinal microbiota: An understudied relationship. iScience 2022, 25, 105313. [Google Scholar] [CrossRef] [PubMed]
- Carlstrom, M.; Moretti, C.H.; Weitzberg, E.; Lundberg, J.O. Microbiota, diet and the generation of reactive nitrogen compounds. Free Radic. Biol. Med. 2020, 161, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Bhat, Z.F.; Gounder, R.S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Ding, Y.; Bekhit, A.E.A. Effect of Dietary Protein and Processing on Gut Microbiota-A Systematic Review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Iyer, A.; Russell, W.R. Impact of protein on the composition and metabolism of the human gut microbiota and health. Proc. Nutr. Soc. 2021, 80, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary Protein and Gut Microbiota Composition and Function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
- Chalvon-Demersay, T.; Luise, D.; Le Floc’h, N.; Tesseraud, S.; Lambert, W.; Bosi, P.; Trevisi, P.; Beaumont, M.; Corrent, E. Functional Amino Acids in Pigs and Chickens: Implication for Gut Health. Front. Vet. Sci. 2021, 8, 663727. [Google Scholar] [CrossRef] [PubMed]
- Broer, S. Intestinal Amino Acid Transport and Metabolic Health. Annu. Rev. Nutr. 2023, 43, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tomar, M.; Punia, S.; Dhakane-Lad, J.; Dhumal, S.; Changan, S.; Senapathy, M.; Berwal, M.K.; Sampathrajan, V.; Sayed, A.A.S.; et al. Plant-based proteins and their multifaceted industrial applications. LWT 2022, 154, 112620. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef] [PubMed]
- Nimni, M.E.; Han, B.; Cordoba, F. Are we getting enough sulfur in our diet? Nutr. Metab. 2007, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. What are U.S. Standards and Regulations for Nitrates and Nitrites Exposure? Registry, Agency for Toxic Substances and Disease Registry. Centers for Disease Control and Prevention: 2013. Available online: https://archive.cdc.gov/www_atsdr_cdc_gov/csem/nitrate-nitrite/standards.html (accessed on 1 March 2025).
- Karwowska, M.; Kononiuk, A. Nitrates/Nitrites in Food-Risk for Nitrosative Stress and Benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.M.; Beaver, L.; Prater, M.C.; Hord, N.G. Dietary Nitrate and Nitrite Concentrations in Food Patterns and Dietary Supplements. Nutr. Today 2020, 55, 218–226. [Google Scholar] [CrossRef]
- Schlagenhauf, U. On the Role of Dietary Nitrate in the Maintenance of Systemic and Oral Health. Dent. J. 2022, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Kamalian, A.; Sohrabi Asl, M.; Dolatshahi, M.; Afshari, K.; Shamshiri, S.; Momeni Roudsari, N.; Momtaz, S.; Rahimi, R.; Abdollahi, M.; Abdolghaffari, A.H. Interventions of natural and synthetic agents in inflammatory bowel disease, modulation of nitric oxide pathways. World J. Gastroenterol. 2020, 26, 3365–3400. [Google Scholar] [CrossRef] [PubMed]
- Jakhar, D.; Sarin, S.K.; Kaur, S. Gut microbiota and dynamics of ammonia metabolism in liver disease. npj Gut Liver 2024, 1, 11. [Google Scholar] [CrossRef]
- Barmore, W.; Azad, F.; Stone, W.L. Physiology, Urea Cycle. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513323/ (accessed on 1 March 2025).
- Lapierre, H.; Lobley, G.E. Nitrogen Recycling in the Ruminant: A Review. J. Dairy Sci. 2001, 84, E223–E236. [Google Scholar] [CrossRef]
- Wu, G.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, W.; Liu, C.; Wang, B.; Wang, J.; Yin, Y. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids 2013, 44, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wang, K.; Yin, J.; Chen, S.; Liu, G.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Yin, Y. Glutamine-Induced Secretion of Intestinal Secretory Immunoglobulin A: A Mechanistic Perspective. Front. Immunol. 2016, 7, 503. [Google Scholar] [CrossRef] [PubMed]
- Perna, S.; Alalwan, T.A.; Alaali, Z.; Alnashaba, T.; Gasparri, C.; Infantino, V.; Hammad, L.; Riva, A.; Petrangolini, G.; Allegrini, P.; et al. The Role of Glutamine in the Complex Interaction between Gut Microbiota and Health: A Narrative Review. Int. J. Mol. Sci. 2019, 20, 5232. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Qiao, S.Y.; Li, D.F. Amino acids and gut function. Amino Acids 2009, 37, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Fotiadis, C.; Adamis, S.; Misiakos, E.P.; Genetzakis, M.; Antonakis, P.T.; Tsekouras, D.K.; Gorgoulis, V.G.; Zografos, G.C.; Papalois, A.; Fotinou, M.; et al. The prophylactic effect of L-arginine in acute ischaemic colitis in a rat model of ischaemia/reperfusion injury. Acta Chir. Belg. 2007, 107, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Coburn, L.A.; Gong, X.; Singh, K.; Asim, M.; Scull, B.P.; Allaman, M.M.; Williams, C.S.; Rosen, M.J.; Washington, M.K.; Barry, D.P.; et al. L-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS ONE 2012, 7, e33546. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Blachier, F. Amino Acids in Intestinal Physiology and Health. Adv. Exp. Med. Biol. 2020, 1265, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Stoll, R.; Lugering, N.; Domschke, W. Circulating antiinflammatory cytokine IL-10 in patients with inflammatory bowel disease (IBD). Clin. Exp. Immunol. 1995, 100, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Matsuda, S. Gut Protective Effect from D-Methionine or Butyric Acid against DSS and Carrageenan-Induced Ulcerative Colitis. Molecules 2023, 28, 4392. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Meng, S.; Motooka, D.; Sato, H.; Arao, Y.; Tsuji, Y.; Yabumoto, T.; Doki, Y.; Eguchi, H.; Uchida, S.; et al. Fat and proteolysis due to methionine, tryptophan, and niacin deficiency leads to alterations in gut microbiota and immune modulation in inflammatory bowel disease. Cancer Sci. 2024, 115, 2473–2485. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yu, L.; Fang, J.; Andy Hu, C.-A.; Yin, J.; Ni, H.; Ren, W.; Duraipandiyan, V.; Chen, S.; Abdullah Al-Dhabi, N.; et al. Methionine restriction on oxidative stress and immune response in dss-induced colitis mice. Oncotarget 2017, 8, 44511–44520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Hu, C.A. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients 2017, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Muro, P.; Zhang, L.; Li, S.; Zhao, Z.; Jin, T.; Mao, F.; Mao, Z. The emerging role of oxidative stress in inflammatory bowel disease. Front. Endocrinol. 2024, 15, 1390351. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.J.; Pettei, M.J.; Valderrama, E.; Gold, D.M.; Kessler, B.H.; Trachtman, H. Nitric oxide and inflammatory bowel disease: Evidence for local intestinal production in children with active colonic disease. J. Pediatr. Gastroenterol. Nutr. 1998, 26, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.K.; Wilson, K.T. Nitric oxide in inflammatory bowel disease. Inflamm. Bowel Dis. 2003, 9, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Cerrudo, V.; Audebert, M.; Dumont, F.; Mancano, G.; Khodorova, N.; Andriamihaja, M.; et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: A randomized, parallel, double-blind trial in overweight humans. Am. J. Clin. Nutr. 2017, 106, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Konozy, E.H.E.; Osman, M.E.M. From inflammation to immune regulation: The dual nature of dietary lectins in health and disease. Heliyon 2024, 10, e39471. [Google Scholar] [CrossRef] [PubMed]
- Banwell, J.G.; Howard, R.; Kabir, I.; Costerton, J.W. Bacterial overgrowth by indigenous microflora in the phytohemagglutinin-fed rat. Can. J. Microbiol. 1988, 34, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.; Pusztai, A. Toxicity of kidney bean (Phaseolus vulgaris) in rats: Changes in intestinal permeability. Digestion 1985, 32, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.J.; Han, S.M.; Lau, P.; Guisado, D.; Liang, Y.; Nakashige, T.G.; Ali, T.; Chiang, D.; Rahman, A.; Brady, S.F. Unraveling function and diversity of bacterial lectins in the human microbiome. Nat. Commun. 2022, 13, 3101. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Wang, R.X.; Goldberg, M.S.; Clifford, G.P.; Kao, D.J.; Colgan, S.P. Microbiota-Sourced Purines Support Wound Healing and Mucous Barrier Function. iScience 2020, 23, 101226. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Tao, Y.; Wang, Q.; Pei, Y.; Zhao, Z.; Yang, W.; Lu, Y. Utilizing an integrated bioinformatics and machine learning approach to uncover biomarkers linking ulcerative colitis to purine metabolism-related genes. Heliyon 2024, 10, e38403. [Google Scholar] [CrossRef] [PubMed]
- Worledge, C.S.; Kostelecky, R.E.; Zhou, L.; Bhagavatula, G.; Colgan, S.P.; Lee, J.S. Allopurinol Disrupts Purine Metabolism to Increase Damage in Experimental Colitis. Cells 2024, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Loening-Baucke, V.; Theissig, F.; Engelhardt, H.; Bengmark, S.; Koch, S.; Lochs, H.; Dorffel, Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 2007, 56, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Liao, W.; Huang, J.; Liu, Y.; Li, Z.; Tang, J. Gut microbiota remodeling: A promising therapeutic strategy to confront hyperuricemia and gout. Front. Cell. Infect. Microbiol. 2022, 12, 935723. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Alsabbagh Alchirazi, K.; Eltelbany, A.; Nanah, R.; Regueiro, M. Increased prevalence of gout in patients with inflammatory bowel disease: A population-based study. JGH Open 2023, 7, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lewis, P.; Samuelson, D.; Liboni, K.; Neu, J. Glutamine regulates Caco-2 cell tight junction proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G726–G733. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Neu, J. Glutamine deprivation alters intestinal tight junctions via a PI3-K/Akt mediated pathway in Caco-2 cells. J. Nutr. 2009, 139, 710–714. [Google Scholar] [CrossRef] [PubMed]
- De-Souza, D.A.; Greene, L.J. Intestinal permeability and systemic infections in critically ill patients: Effect of glutamine. Crit. Care Med. 2005, 33, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Guo, Y.C.; Zhou, H.F.; Yue, T.T.; Wang, F.X.; Sun, F.; Wang, W.Z. Arginine metabolism regulates the pathogenesis of inflammatory bowel disease. Nutr. Rev. 2023, 81, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Coburn, L.A.; Barry, D.P.; Asim, M.; Scull, B.P.; Allaman, M.M.; Lewis, N.D.; Washington, M.K.; Rosen, M.J.; Williams, C.S.; et al. Deletion of cationic amino acid transporter 2 exacerbates dextran sulfate sodium colitis and leads to an IL-17-predominant T cell response. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G225–G240. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.K.; Maltz, B.E.; Coburn, L.A.; Slaughter, J.C.; Chaturvedi, R.; Schwartz, D.A.; Wilson, K.T. Increased serum levels of L-arginine in ulcerative colitis and correlation with disease severity. Inflamm. Bowel Dis. 2010, 16, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Coburn, L.A.; Horst, S.N.; Allaman, M.M.; Brown, C.T.; Williams, C.S.; Hodges, M.E.; Druce, J.P.; Beaulieu, D.B.; Schwartz, D.A.; Wilson, K.T. L-Arginine Availability and Metabolism Is Altered in Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 22, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.E.R.; Barros, P.A.V.d.; Menta, P.L.d.R.; Costa, G.M.F.; Miranda, S.E.M.; Leocádio, P.C.L.; Almeida-Leite, C.M.d.; Generoso, S.d.V.; Leite, J.I.A.; Cardoso, V.N. Arginine supplementation reduces colonic injury, inflammation and oxidative stress of DSS-induced colitis in mice. J. Funct. Foods 2019, 52, 360–369. [Google Scholar] [CrossRef]
- Kruidenier, L.; Kuiper, I.; Lamers, C.B.; Verspaget, H.W. Intestinal oxidative damage in inflammatory bowel disease: Semi-quantification, localization, and association with mucosal antioxidants. J. Pathol. 2003, 201, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Dudzinska, E.; Gryzinska, M.; Ognik, K.; Gil-Kulik, P.; Kocki, J. Oxidative Stress and Effect of Treatment on the Oxidation Product Decomposition Processes in IBD. Oxid. Med. Cell Longev. 2018, 2018, 7918261. [Google Scholar] [CrossRef] [PubMed]
- Jahanian, R. Immunological responses as affected by dietary protein and arginine concentrations in starting broiler chicks. Poult. Sci. 2009, 88, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Tome, S.; Ortega Moreno, L.; Chaparro, M.; Gisbert, J.P. Gut Microbiota and Dietary Factors as Modulators of the Mucus Layer in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 10224. [Google Scholar] [CrossRef] [PubMed]
- Blander, J.M.; Longman, R.S.; Iliev, I.D.; Sonnenberg, G.F.; Artis, D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017, 18, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Tome, S.; Marin, A.C.; Ortega Moreno, L.; Baldan-Martin, M.; Mora-Gutierrez, I.; Lanas-Gimeno, A.; Moreno-Monteagudo, J.A.; Santander, C.; Sanchez, B.; Chaparro, M.; et al. Immunomodulatory Effect of Gut Microbiota-Derived Bioactive Peptides on Human Immune System from Healthy Controls and Patients with Inflammatory Bowel Disease. Nutrients 2019, 11, 2605. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tomé, S.; Hernández-Ledesma, B.; Chaparro, M.; Indiano-Romacho, P.; Bernardo, D.; Gisbert, J.P. Role of food proteins and bioactive peptides in inflammatory bowel disease. Trends Food Sci. Technol. 2019, 88, 194–206. [Google Scholar] [CrossRef]
- Colgan, S.P.; Curtis, V.F.; Campbell, E.L. The inflammatory tissue microenvironment in IBD. Inflamm. Bowel Dis. 2013, 19, 2238–2244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Q.; Xing, J.; Yang, Y.; Zhu, M.; Lin, L.; Yu, Y.; Cai, X.; Wang, X. Tannic acid and zinc ion coordination of nanase for the treatment of inflammatory bowel disease by promoting mucosal repair and removing reactive oxygen and nitrogen species. Acta Biomater. 2024, 177, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Boughton-Smith, N.K.; Evans, S.M.; Hawkey, C.J.; Cole, A.T.; Balsitis, M.; Whittle, B.J.; Moncada, S. Nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Lancet 1993, 342, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Seril, D.N.; Liao, J.; Yang, G.Y.; Yang, C.S. Oxidative stress and ulcerative colitis-associated carcinogenesis: Studies in humans and animal models. Carcinogenesis 2003, 24, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Stettner, N.; Rosen, C.; Bernshtein, B.; Gur-Cohen, S.; Frug, J.; Silberman, A.; Sarver, A.; Carmel-Neiderman, N.N.; Eilam, R.; Biton, I.; et al. Induction of Nitric-Oxide Metabolism in Enterocytes Alleviates Colitis and Inflammation-Associated Colon Cancer. Cell Rep. 2018, 23, 1962–1976. [Google Scholar] [CrossRef] [PubMed]
- Abot, A.; Fried, S.; Cani, P.D.; Knauf, C. Reactive Oxygen Species/Reactive Nitrogen Species as Messengers in the Gut: Impact on Physiology and Metabolic Disorders. Antioxid. Redox Signal. 2022, 37, 394–415. [Google Scholar] [CrossRef] [PubMed]
- Linares, R.; Frances, R.; Gutierrez, A.; Juanola, O. Bacterial Translocation as Inflammatory Driver in Crohn’s Disease. Front. Cell Dev. Biol. 2021, 9, 703310. [Google Scholar] [CrossRef] [PubMed]
- Stummer, N.; Feichtinger, R.G.; Weghuber, D.; Kofler, B.; Schneider, A.M. Role of Hydrogen Sulfide in Inflammatory Bowel Disease. Antioxidants 2023, 12, 1570. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Kye, B.H.; Ko, S.H.; Yoo, R.N. Sulfur Metabolism of the Gut Microbiome and Colorectal Cancer: The Threat to the Younger Generation. Nutrients 2023, 15, 1966. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Andriamihaja, M.; Lan, A.; Khodorova, N.; Audebert, M.; Blouin, J.M.; Grauso, M.; Lancha, L.; Benetti, P.H.; Benamouzig, R.; et al. Detrimental effects for colonocytes of an increased exposure to luminal hydrogen sulfide: The adaptive response. Free Radic. Biol. Med. 2016, 93, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F.; Andriamihaja, M.; Larraufie, P.; Ahn, E.; Lan, A.; Kim, E. Production of hydrogen sulfide by the intestinal microbiota and epithelial cells and consequences for the colonic and rectal mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G125–G135. [Google Scholar] [CrossRef] [PubMed]
- Braccia, D.J.; Jiang, X.; Pop, M.; Hall, A.B. The Capacity to Produce Hydrogen Sulfide (H(2)S) via Cysteine Degradation Is Ubiquitous in the Human Gut Microbiome. Front. Microbiol. 2021, 12, 705583. [Google Scholar] [CrossRef] [PubMed]
- Cremin, J.D., Jr.; Fitch, M.D.; Fleming, S.E. Glucose alleviates ammonia-induced inhibition of short-chain fatty acid metabolism in rat colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G105–G114. [Google Scholar] [CrossRef] [PubMed]
- Andriamihaja, M.; Davila, A.M.; Eklou-Lawson, M.; Petit, N.; Delpal, S.; Allek, F.; Blais, A.; Delteil, C.; Tome, D.; Blachier, F. Colon luminal content and epithelial cell morphology are markedly modified in rats fed with a high-protein diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1030–G1037. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F.; Andriamihaja, M. Effects of the L-tyrosine-derived bacterial metabolite p-cresol on colonic and peripheral cells. Amino Acids 2022, 54, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Toft, P.B.; Vanslette, A.M.; Trost, K.; Moritz, T.; Gillum, M.P.; Backhed, F.; Arora, T. Microbial metabolite p-cresol inhibits gut hormone expression and regulates small intestinal transit in mice. Front. Endocrinol. 2023, 14, 1200391. [Google Scholar] [CrossRef] [PubMed]
- Al Hinai, E.A.; Kullamethee, P.; Rowland, I.R.; Swann, J.; Walton, G.E.; Commane, D.M. Modelling the role of microbial p-cresol in colorectal genotoxicity. Gut Microbes 2019, 10, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Mimoun, S.; Andriamihaja, M.; Chaumontet, C.; Atanasiu, C.; Benamouzig, R.; Blouin, J.M.; Tome, D.; Bouillaud, F.; Blachier, F. Detoxification of H2S by differentiated colonic epithelial cells: Implication of the sulfide oxidizing unit and of the cell respiratory capacity. Antioxid. Redox Signal. 2012, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F.; Beaumont, M.; Andriamihaja, M.; Davila, A.M.; Lan, A.; Grauso, M.; Armand, L.; Benamouzig, R.; Tome, D. Changes in the Luminal Environment of the Colonic Epithelial Cells and Physiopathological Consequences. Am. J. Pathol. 2017, 187, 476–486. [Google Scholar] [CrossRef] [PubMed]
- De Preter, V.; Arijs, I.; Windey, K.; Vanhove, W.; Vermeire, S.; Schuit, F.; Rutgeerts, P.; Verbeke, K. Decreased mucosal sulfide detoxification is related to an impaired butyrate oxidation in ulcerative colitis. Inflamm. Bowel Dis. 2012, 18, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Zhang, S.; Chen, J.; Shen, Y.; Zhang, Z.; Huang, H.; Ma, Y.; Xiang, Y.; Liao, L.; Zhou, J.; et al. A mouse protozoan boosts antigen-specific mucosal IgA responses in a specific lipid metabolism- and signaling-dependent manner. Nat. Commun. 2024, 15, 7914. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rhoads, J.M. Communication between B-Cells and Microbiota for the Maintenance of Intestinal Homeostasis. Antibodies 2013, 2, 535–553. [Google Scholar] [CrossRef]
- Takeuchi, T.; Ohno, H. IgA in human health and diseases: Potential regulator of commensal microbiota. Front. Immunol. 2022, 13, 1024330. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Back, S.J.; Ruchelli, E.D.; Markowitz, J.; Mascarenhas, M.; Verma, R.; Piccoli, D.A.; Baldassano, R.N. Lamina propria and circulating interleukin-6 in newly diagnosed pediatric inflammatory bowel disease patients. Am. J. Gastroenterol. 2002, 97, 2603–2608. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Sufit, A.J.; Wischmeyer, P.E. Glutamine therapy improves outcome of in vitro and in vivo experimental colitis models. JPEN J. Parenter. Enter. Nutr. 2011, 35, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Katinios, G.; Casado-Bedmar, M.; Walter, S.A.; Vicario, M.; Gonzalez-Castro, A.M.; Bednarska, O.; Soderholm, J.D.; Hjortswang, H.; Keita, A.V. Increased Colonic Epithelial Permeability and Mucosal Eosinophilia in Ulcerative Colitis in Remission Compared With Irritable Bowel Syndrome and Health. Inflamm. Bowel Dis. 2020, 26, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; El-Mowafy, M.; Elgaml, A.; Ahmed, T.A.E.; Hassan, H.; Mottawea, W. Metabolic Influences of Gut Microbiota Dysbiosis on Inflammatory Bowel Disease. Front. Physiol. 2021, 12, 715506. [Google Scholar] [CrossRef] [PubMed]
- Michielan, A.; D’Inca, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediat. Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef] [PubMed]
- Reese, A.T.; Pereira, F.C.; Schintlmeister, A.; Berry, D.; Wagner, M.; Hale, L.P.; Wu, A.; Jiang, S.; Durand, H.K.; Zhou, X.; et al. Microbial nitrogen limitation in the mammalian large intestine. Nat. Microbiol. 2018, 3, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.J.; Chew, Y.V.; Colakoglu, F.; Cliff, J.B.; Klaassens, E.; Read, M.N.; Solon-Biet, S.M.; McMahon, A.C.; Cogger, V.C.; Ruohonen, K.; et al. Diet-Microbiome Interactions in Health Are Controlled by Intestinal Nitrogen Source Constraints. Cell Metab. 2017, 25, 140–151. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Ferrocino, I.; Calabrese, F.M.; De Filippis, F.; Cavallo, N.; Siragusa, S.; Rampelli, S.; Di Cagno, R.; Rantsiou, K.; Vannini, L.; et al. Diet influences the functions of the human intestinal microbiome. Sci. Rep. 2020, 10, 4247. [Google Scholar] [CrossRef] [PubMed]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M.; Bedu-Ferrari, C.; Etienne-Mesmin, L.; Mariadassou, M.; Lebreuilly, L.; Tran, S.L.; Brazeau, L.; Mayeur, C.; Delmas, J.; Rue, O.; et al. Nitric Oxide Impacts Human Gut Microbiota Diversity and Functionalities. mSystems 2021, 6, e0055821. [Google Scholar] [CrossRef] [PubMed]
- Davila, A.M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.H.; Sanz, Y.; Tome, D. Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host. Pharmacol. Res. 2013, 68, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Prindiville, T.P.; Sheikh, R.A.; Cohen, S.H.; Tang, Y.J.; Cantrell, M.C.; Silva, J., Jr. Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg. Infect. Dis. 2000, 6, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Steck, N.; Hoffmann, M.; Sava, I.G.; Kim, S.C.; Hahne, H.; Tonkonogy, S.L.; Mair, K.; Krueger, D.; Pruteanu, M.; Shanahan, F.; et al. Enterococcus faecalis Metalloprotease Compromises Epithelial Barrier and Contributes to Intestinal Inflammation. Gastroenterology 2011, 141, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, Z.; Wu, W.; Lin, Q.; Liang, Y. High animal protein diet and gut microbiota in human health. Crit. Rev. Food Sci. Nutr. 2022, 62, 6225–6237. [Google Scholar] [CrossRef] [PubMed]
- Tiso, M.; Schechter, A.N. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE 2015, 10, e0119712. [Google Scholar] [CrossRef]
- Wu, L.; Tang, Z.; Chen, H.; Ren, Z.; Ding, Q.; Liang, K.; Sun, Z. Mutual interaction between gut microbiota and protein/amino acid metabolism for host mucosal immunity and health. Anim. Nutr. 2021, 7, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Schanuel, C.; Dias, E.; Ferreira, A.; Bertges, K.; Bertges, L. Glutamine as A Therapeutic Strategy in Inflammatory Bowel Diseases: A Systematic Review. Gastroenterol. Hepatol. Dig. Disord. 2019, 2, 1–6. [Google Scholar] [CrossRef]
- Singh, K.; Gobert, A.P.; Coburn, L.A.; Barry, D.P.; Allaman, M.; Asim, M.; Luis, P.B.; Schneider, C.; Milne, G.L.; Boone, H.H.; et al. Dietary Arginine Regulates Severity of Experimental Colitis and Affects the Colonic Microbiome. Front. Cell. Infect. Microbiol. 2019, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Nciri, N.; Cho, N.; Bergaoui, N.; Mhamdi, F.E.; Ammar, A.B.; Trabelsi, N.; Zekri, S.; Guémira, F.; Mansour, A.B.; Sassi, F.H.; et al. Effect of White Kidney Beans (Phaseolus vulgaris L. var. Beldia) on Small Intestine Morphology and Function in Wistar Rats. J. Med. Food 2015, 18, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Kordás, K.; Szalmay, G.; Bardocz, S.; Pusztai, Á.; Varga, G. Phytohaemagglutinin inhibits gastric acid but not pepsin secretion in conscious rats. J. Physiol.-Paris 2001, 95, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Lucius, K. Dietary Lectins: Gastrointestinal and Immune Effects. Altern. Complement. Ther. 2020, 26, 168–174. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Wellens, J.; Vissers, E.; Matthys, C.; Vermeire, S.; Sabino, J. Personalized Dietary Regimens for Inflammatory Bowel Disease: Current Knowledge and Future Perspectives. Pharmgenom. Pers. Med. 2023, 16, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Jalanka, J.; Staudacher, H.M. Can Gut Microbiota Composition Predict Response to Dietary Treatments? Nutrients 2019, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Olendzki, B.; Bucci, V.; Cawley, C.; Maserati, R.; McManus, M.; Olednzki, E.; Madziar, C.; Chiang, D.; Ward, D.V.; Pellish, R.; et al. Dietary manipulation of the gut microbiome in inflammatory bowel disease patients: Pilot study. Gut Microbes 2022, 14, 2046244. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Romero-Mosquera, B.; Hernandez, V. Diet, Gut Microbiome and Epigenetics: Emerging Links with Inflammatory Bowel Diseases and Prospects for Management and Prevention. Nutrients 2017, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Haskey, N.; Estaki, M.; Ye, J.; Shim, R.K.; Singh, S.; Dieleman, L.A.; Jacobson, K.; Gibson, D.L. A Mediterranean Diet Pattern Improves Intestinal Inflammation Concomitant with Reshaping of the Bacteriome in Ulcerative Colitis: A Randomised Controlled Trial. J. Crohns Colitis 2023, 17, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.; Kim, Y.S.; Choi, C.H.; Sadowsky, M.J.; Unno, T. Current understanding of microbiota- and dietary-therapies for treating inflammatory bowel disease. J. Microbiol. 2018, 56, 189–198. [Google Scholar] [CrossRef] [PubMed]
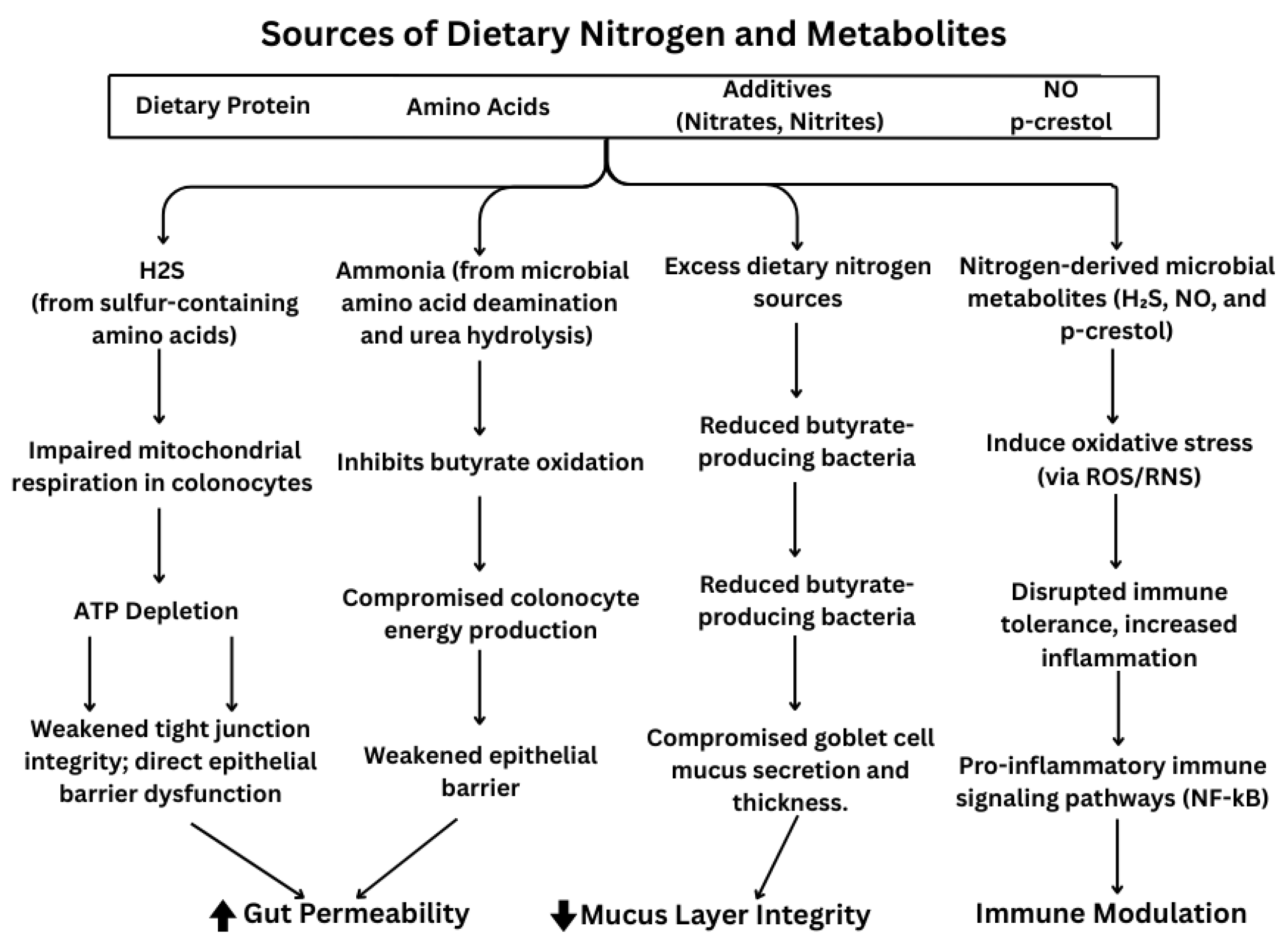
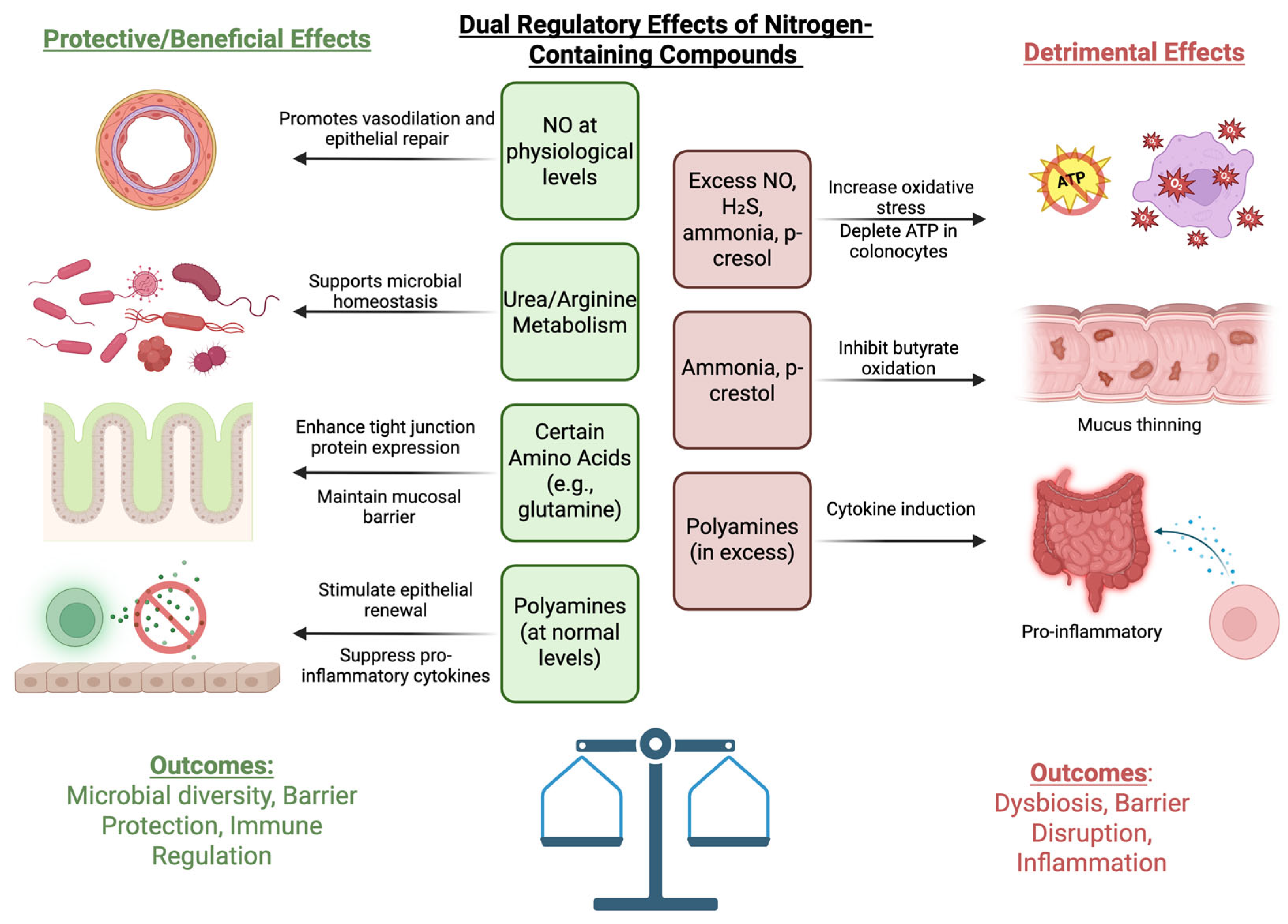
| Nitrogen Source | Primary Source | Microbial Metabolites | Effects on Gut Barrier | Effects on Inflammation | IBD-Specific Notes |
|---|---|---|---|---|---|
| Amino Acids (e.g., Glutamine, Arginine, Methionine) | Meat, fish, dairy, legumes, soy | SCFAs, ammonia, polyamines | ↑ Tight junction integrity (via mTOR, CAT1/2); ↑ mucus production [41,42,43,44,45,46] | ↓ Pro-inflammatory cytokines (e.g., IL-6); ↑ IL-10 [42,47] | Glutamine and arginine support mucosal repair; methionine has mixed effects [48,49,50,51,52] |
| Nitrates/Nitrites | Leafy greens, beets, cured meats | Nitric oxide (NO), N-nitroso compounds | Plant-based nitrates may preserve barrier; processed forms can ↑ permeability [35,36,53] | NO has dual roles: anti-inflammatory via PPAR-γ or damaging via iNOS [54,55,56] | NO production ↑ in IBD; excess favors dysbiosis and oxidative damage [54,57,58] |
| Urea/Ammonia | Endogenous metabolism, protein catabolism | Ammonia | ↑ Permeability when overproduced; toxic to epithelium [38,39] | ↑ Inflammatory cytokines, oxidative stress [18,59] | ↑ Urease-expressing bacteria in IBD; ammonia worsens barrier function [18,38,40] |
| Lectins | Beans, lentils, whole grains | — | Rodent studies: ↓ mucus thickness, ↑ epithelial permeability [60,61,62] | Mixed: Some prebiotic effects; others increase ROS & permeability [60,61] | Human studies limited; may alter barrier and immune signaling in IBD [60,63] |
| Purines | Seafood, organ meats, beer | Uric acid | ↓ Barrier function if depleted; required for cell proliferation [64,65] | Excess linked to inflammation and immune dysregulation [65,66,67] | High-purine diets → dysbiosis, ↑ IBD-gout comorbidity [68,69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, M.; Byerley, L.O. Dietary Nitrogen and Its Role in the Gut Microbiome and Inflammatory Bowel Disease: A Narrative Review. Nutrients 2025, 17, 2373. https://doi.org/10.3390/nu17142373
Herrera M, Byerley LO. Dietary Nitrogen and Its Role in the Gut Microbiome and Inflammatory Bowel Disease: A Narrative Review. Nutrients. 2025; 17(14):2373. https://doi.org/10.3390/nu17142373
Chicago/Turabian StyleHerrera, Matthew, and Lauri O. Byerley. 2025. "Dietary Nitrogen and Its Role in the Gut Microbiome and Inflammatory Bowel Disease: A Narrative Review" Nutrients 17, no. 14: 2373. https://doi.org/10.3390/nu17142373
APA StyleHerrera, M., & Byerley, L. O. (2025). Dietary Nitrogen and Its Role in the Gut Microbiome and Inflammatory Bowel Disease: A Narrative Review. Nutrients, 17(14), 2373. https://doi.org/10.3390/nu17142373






