The Impact of Tea Consumption on Prediabetes Regression and Progression: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
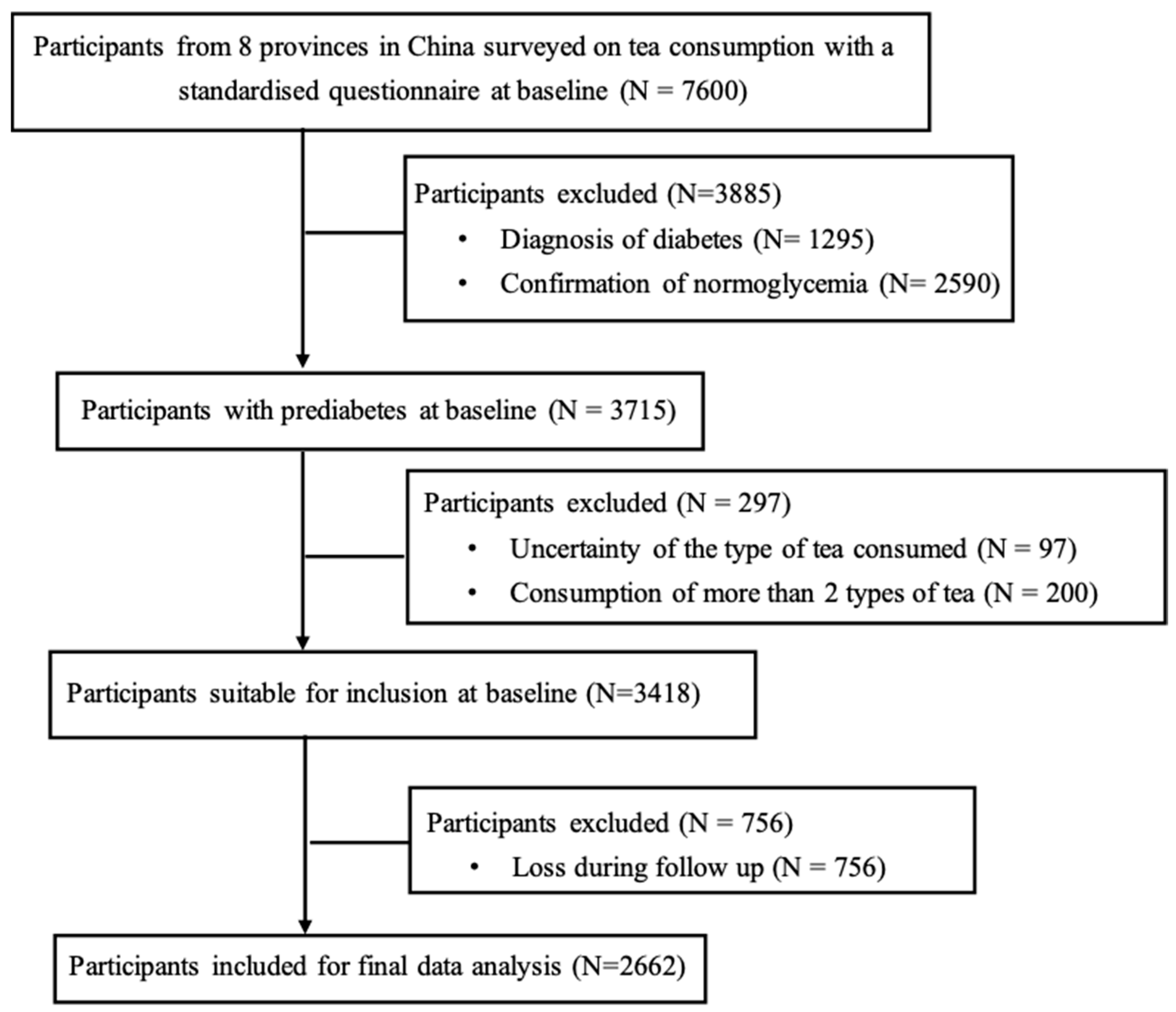
2.1. Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Tea Consumption and Prediabetes Progression and Regression
3.3. Frequency of Tea Consumption and Prediabetes Progression and Regression
3.4. Tea Consumption and Insulin Resistance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Teng, D.; Shi, X.; Qin, G.; Qin, Y.; Quan, H.; Shi, B.; Sun, H.; Ba, J.; Chen, B.; et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: National cross sectional study. BMJ 2020, 369, m997. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, P.; Zhang, M.; Huang, Z.; Zhang, D.; Deng, Q.; Li, Y.; Zhao, Z.; Qin, X.; Jin, D.; et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA 2017, 317, 2515–2523. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhang, Y.; Li, M.; Wu, J.H.; Mai, L.; Li, J.; Yang, Y.; Hu, Y.; Huang, Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: Updated meta-analysis. BMJ 2020, 370, m2297. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Qiu, Z.; Wang, F.; Deng, S.; Wang, Y.; Wang, Z.; Yin, P.; Huo, Y.; Zhou, M.; Liu, G.; et al. Associations of Diabetes and Prediabetes With Mortality and Life Expectancy in China: A National Study. Diabetes Care 2024, 47, 1969–1977. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, S.; Song, Q.; Wang, X. Reversion From Pre-Diabetes Mellitus to Normoglycemia and Risk of Cardiovascular Disease and All-Cause Mortality in a Chinese Population: A Prospective Cohort Study. J. Am. Heart Assoc. 2021, 10, e019045. [Google Scholar] [CrossRef] [PubMed]
- Glechner, A.; Keuchel, L.; Affengruber, L.; Titscher, V.; Sommer, I.; Matyas, N.; Wagner, G.; Kien, C.; Klerings, I.; Gartlehner, G. Effects of lifestyle changes on adults with prediabetes: A systematic review and meta-analysis. Prim. Care Diabetes 2018, 12, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Galaviz, K.I.; Weber, M.B.; Suvada, K.B.; Gujral, U.P.; Wei, J.; Merchant, R.; Dharanendra, S.; Haw, J.S.; Narayan, K.M.V.; Ali, M.K. Interventions for Reversing Prediabetes: A Systematic Review and Meta-Analysis. Am. J. Prev. Med. 2022, 62, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, W.; Qiu, S.; Vladmir, C.; Xu, X.; Wang, X.; Nian, X.; Chen, Q.; Wang, Q.; Tu, P.; et al. Tea consumption and risk of diabetes in the Chinese population: A multi-centre, cross-sectional study. Br. J. Nutr. 2020, 123, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sang, M.; Wang, J.; Sun, Z.; Wang, D.; Xie, C.; Huang, W.; Rayner, C.K.; Horowitz, M.; Qiu, S.; et al. Dark tea consumption is associated with a reduced risk of dysglycaemia and increased urinary glucose and sodium excretion in Chinese adults. Diabetes Obes. Metab. 2024, 26, 4705–4712. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, A.O.; Pereira, M.A.; Koh, W.P.; Arakawa, K.; Lee, H.P.; Yu, M.C. Coffee, tea, and incident type 2 diabetes: The Singapore Chinese Health Study. Am. J. Clin. Nutr. 2008, 88, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Hayashino, Y.; Fukuhara, S.; Okamura, T.; Tanaka, T.; Ueshima, H.; Group, H.-O.R. High oolong tea consumption predicts future risk of diabetes among Japanese male workers: A prospective cohort study. Diabet. Med. 2011, 28, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xia, G.; Luo, Z.; Liu, S. UHPLC analysis of major functional components in six types of Chinese teas: Constituent profile and origin consideration. LWT 2019, 102, 52–57. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Randisi, F.; Perletti, G.; Marras, E.; Gariboldi, M.B. Green Tea Components: In Vitro and In Vivo Evidence for Their Anticancer Potential in Colon Cancer. Cancers 2025, 17, 623. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Daglia, M.; Ciampaglia, R.; Novellino, E. Exploring the nutraceutical potential of polyphenols from black, green and white tea infusions—An overview. Curr. Pharm. Biotechnol. 2015, 16, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Date, C.; Wakai, K.; Fukui, M.; Tamakoshi, A.; Group, J.S. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann. Intern. Med. 2006, 144, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Yu, C.; Guo, Y.; Pei, P.; Chen, L.; Pang, Y.; Du, H.; Yang, L.; Chen, Y.; Yan, S.; et al. Tea consumption and long-term risk of type 2 diabetes and diabetic complications: A cohort study of 0.5 million Chinese adults. Am. J. Clin. Nutr. 2021, 114, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, A.; Jimba, M.; Pham, T.T.P.; Nguyen, C.Q.; Hoang, D.V.; Phan, T.V.; Yazawa, A.; Phan, D.C.; Hachiya, M.; Le, H.X.; et al. Association of green tea consumption with prediabetes, diabetes and markers of glucose metabolism in rural Vietnam: A cross-sectional study. Br. J. Nutr. 2024, 131, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, W.; Cai, H.; Gao, Y.T.; Li, H.; Ji, B.T.; Shu, X.; Wang, T.; Gerszten, R.E.; Zheng, W.; et al. Green tea consumption and risk of type 2 diabetes in Chinese adults: The Shanghai Women’s Health Study and the Shanghai Men’s Health Study. Int. J. Epidemiol. 2018, 47, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Malaga, G.; Golozar, A.; Khademi, H.; Kamangar, F.; Poutschi, H.; Islami, F.; Abnet, C.C.; Freedman, N.D.; Taylor, P.R.; Pharoah, P.; et al. Diabetes Mellitus and Its Correlates in an Iranian Adult Population. PLoS ONE 2011, 6, e26725. [Google Scholar] [CrossRef]
- Wang, Y.; Kan, Z.; Thompson, H.J.; Ling, T.; Ho, C.T.; Li, D.; Wan, X. Impact of Six Typical Processing Methods on the Chemical Composition of Tea Leaves Using a Single Camellia sinensis Cultivar, Longjing 43. J. Agric. Food Chem. 2019, 67, 5423–5436. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Le, T.; Wang, W.; Yu, L.; Yang, L.; Jiang, H. Effects of Key Components on the Antioxidant Activity of Black Tea. Foods 2023, 12, 3134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Z.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.M.; Xu, W.; Li, J.; Lin, H.Y.; Zhang, Z.; Xiao, J.B.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Le, M.; He, C.; Yang, C.S.; Ling, T. Dark tea: A popular beverage with possible medicinal application. Chin. Herb. Med. 2023, 15, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mao, Q.X.; Xu, H.X.; Ma, X.; Zeng, C.Y. Tea consumption and risk of type 2 diabetes mellitus: A systematic review and meta-analysis update. BMJ Open 2014, 4, e005632. [Google Scholar] [CrossRef] [PubMed]
- InterAct, C.; van Woudenbergh, G.J.; Kuijsten, A.; Drogan, D.; van der, A.D.; Romaguera, D.; Ardanaz, E.; Amiano, P.; Barricarte, A.; Beulens, J.W.; et al. Tea consumption and incidence of type 2 diabetes in Europe: The EPIC-InterAct case-cohort study. PLoS ONE 2012, 7, e36910. [Google Scholar] [CrossRef]
- Li, W.; Xie, B.; Qiu, S.; Huang, X.; Chen, J.; Wang, X.; Li, H.; Chen, Q.; Wang, Q.; Tu, P.; et al. Non-lab and semi-lab algorithms for screening undiagnosed diabetes: A cross-sectional study. EBioMedicine 2018, 35, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Xie, C.; Qiu, S.; Wang, X.; Horowitz, M.; Jones, K.L.; Rayner, C.K.; Sun, Z.; Wu, T. Cholecystectomy is associated with dysglycaemia: Cross-sectional and prospective analyses. Diabetes Obes. Metab. 2022, 24, 1656–1660. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sang, M.; Yuan, Y.; Du, Z.; Li, W.; Hu, H.; Wen, L.; Wang, F.; Guo, H.; Wang, B.; et al. Novel clusters of newly-diagnosed type 2 diabetes and their association with diabetic retinopathy: A 3-year follow-up study. Acta Diabetol. 2022, 59, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Vasques, A.C.; Novaes, F.S.; de Oliveira Mda, S.; Souza, J.R.; Yamanaka, A.; Pareja, J.C.; Tambascia, M.A.; Saad, M.J.; Geloneze, B. TyG index performs better than HOMA in a Brazilian population: A hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 2011, 93, e98–e100. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garcia, A.; Rodriguez-Gutierrez, R.; Mancillas-Adame, L.; Gonzalez-Nava, V.; Diaz Gonzalez-Colmenero, A.; Solis, R.C.; Alvarez-Villalobos, N.A.; Gonzalez-Gonzalez, J.G. Diagnostic Accuracy of the Triglyceride and Glucose Index for Insulin Resistance: A Systematic Review. Int. J. Endocrinol. 2020, 2020, 4678526. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Miles, J. Tolerance and Variance Inflation Factor. In Encyclopedia of Statistics in Behavioral Science; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Mackenzie, T.; Leary, L.; Brooks, W.B. The effect of an extract of green and black tea on glucose control in adults with type 2 diabetes mellitus: Double-blind randomized study. Metabolism 2007, 56, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Luo, L.; Zhao, J.; Wang, Y.; Luo, H. Biological potential and mechanisms of Tea’s bioactive compounds: An Updated review. J. Adv. Res. 2024, 65, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wei, Y.; Peng, L.; Wei, K.; Liu, Z.; Wei, X. State-of-the-art review of theabrownins: From preparation, structural characterization to health-promoting benefits. Crit. Rev. Food Sci. Nutr. 2024, 64, 11321–11340. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hu, W.; Zhou, S.; Tu, C.; Xia, X.; Zhang, J.; Dong, M. Increased Phenolic Content and Enhanced Antioxidant Activity in Fermented Glutinous Rice Supplemented with Fu Brick Tea. Molecules 2019, 24, 671. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xiao, C.; Hu, J.; Chen, B.; Wang, C.; Cui, B.; Deng, P.; Yang, J.; Deng, Z. Qing brick tea (QBT) aqueous extract protects monosodium glutamate-induced obese mice against metabolic syndrome and involves up-regulation Transcription Factor Nuclear Factor-Erythroid 2-Related Factor 2 (Nrf2) antioxidant pathway. Biomed. Pharmacother. 2018, 103, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.; Derbyshire, E. Tea Compounds and the Gut Microbiome: Findings from Trials and Mechanistic Studies. Nutrients 2019, 11, 2364. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, Q.; Yang, X. Fu Brick Tea Alleviates Chronic Kidney Disease of Rats with High Fat Diet Consumption through Attenuating Insulin Resistance in Skeletal Muscle. J. Agric. Food Chem. 2019, 67, 2839–2847. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hayashi, S.; Fang, C.; Hao, S.; Wang, X.; Nishiguchi, S.; Tsutsui, H.; Sheng, J. Pu’erh tea extract-mediated protection against hepatosteatosis and insulin resistance in mice with diet-induced obesity is associated with the induction of de novo lipogenesis in visceral adipose tissue. J. Gastroenterol. 2017, 52, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.M.; Nanri, A.; Kochi, T.; Kuwahara, K.; Tsuruoka, H.; Kurotani, K.; Akter, S.; Kabe, I.; Sato, M.; Hayabuchi, H.; et al. Coffee and green tea consumption is associated with insulin resistance in Japanese adults. Metabolism 2014, 63, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Bhupathiraju, S.N.; Chen, M.; van Dam, R.M.; Hu, F.B. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: A systematic review and a dose-response meta-analysis. Diabetes Care 2014, 37, 569–586. [Google Scholar] [CrossRef] [PubMed]
- van Dam, R.M.; Willett, W.C.; Manson, J.E.; Hu, F.B. Coffee, caffeine, and risk of type 2 diabetes: A prospective cohort study in younger and middle-aged U.S. women. Diabetes Care 2006, 29, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M. Coffee consumption and risk of type 2 diabetes mellitus in Asians: A meta-epidemiological study of population-based cohort studies. World J. Diabetes 2021, 12, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Kabthymer, R.H.; Wu, T.; Beigrezaei, S.; Franco, O.H.; Hodge, A.M.; de Courten, B. The association of sweetened beverage intake with risk of type 2 diabetes in an Australian population: A longitudinal study. Diabetes Metab. 2025, 101665, in press. [Google Scholar] [CrossRef] [PubMed]
- Rios-Leyvraz, M.; Montez, J.; World Health Organization. Health Effects of the Use of Non-Sugar Sweeteners: A Systematic Review and Meta-Analysis; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Fagherazzi, G.; Vilier, A.; Saes Sartorelli, D.; Lajous, M.; Balkau, B.; Clavel-Chapelon, F. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique aupres des femmes de la Mutuelle Generale de l’Education Nationale-European Prospective Investigation into Cancer and Nutrition cohort. Am. J. Clin. Nutr. 2013, 97, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Despres, J.P.; Hu, F.B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Ramne, S.; Drake, I.; Ericson, U.; Nilsson, J.; Orho-Melander, M.; Engstrom, G.; Sonestedt, E. Identification of Inflammatory and Disease-Associated Plasma Proteins that Associate with Intake of Added Sugar and Sugar-Sweetened Beverages and Their Role in Type 2 Diabetes Risk. Nutrients 2020, 12, 3129. [Google Scholar] [CrossRef] [PubMed]
| Tea Type | Processing Method | Key Steps | Major Composition Differences |
|---|---|---|---|
| Green tea | Unfermented/unoxidized | Steaming or pan-firing to inactivate enzymes Rolling Drying | High catechin content (especially EGCG) Low theaflavins and thearubigins Retains more polyphenols and vitamin C |
| Black tea | Fermented/oxidized | Withering Rolling Oxidation Drying | High in theaflavins and thearubigins (from catechin oxidation) Lower catechin levels Darker color and stronger flavor |
| Dark tea | Post-fermented | Microbial fermentation Aging Drying | High theabrownins and microbial metabolites Very low catechin levels Rich in polysaccharides and unique bioactive compounds |
| Regression to Normoglycemia (N = 716) | Remained as Prediabetes (N = 1647) | Progression to Diabetes (N = 299) | poverall | |
|---|---|---|---|---|
| Gender | <0.001 | |||
| Male (%) | 248 (34.6%) | 570 (34.6%) | 138 (46.2%) | |
| Female (%) | 468(65.4%) | 1077 (65.4%) | 161 (53.8%) | |
| Age (y) | 51.9 ± 9.1 *** | 54.3 ± 8.2 | 55.0 ± 8.4 | <0.001 |
| BMI (kg/m2) | 25.1 ± 4.0 ** | 25.8 ± 4.4 | 26.1 ± 4.5 | <0.001 |
| WC (cm) | 84.3 ± 9.4 * | 85.5 ± 10.3 | 81.1 ± 10.8 *** | <0.001 |
| SBP (mmHg) | 136.8 ± 19.8 *** | 137.1 ± 19.3 | 144.6 ± 20.4 *** | <0.001 |
| DBP (mmHg) | 82.6 ± 11.7 | 83.2 ± 11.4 | 86.6 ± 11.4 *** | <0.001 |
| FPG (mmol/L) | 5.7 ± 0.4 *** | 5.8 ± 0.5 | 6.1 ± 0.5 *** | <0.001 |
| 2hPG (mmol/L) | 6.8 ± 1.5 *** | 7.3 ± 1.6 | 8.3 ± 1.9 *** | <0.001 |
| HbA1c (%) | 5.3 ± 0.4 *** | 5.5 ± 0.4 | 5.7± 0.4 *** | <0.001 |
| TC (mmo/L) | 4.9 ± 1.0 *** | 5.1 ± 1.0 | 5.0 ± 1.0 | <0.001 |
| TG (mmo/L) | 1.6 ± 1.5 * | 1.8 ± 1.9 | 2.2 ± 2.8 ** | <0.001 |
| HDL-C (mmo/L) | 1.6 ± 0.4 * | 1.5 ± 0.4 | 1.5 ± 0.4 | <0.001 |
| LDL-C (mmo/L) | 2.7 ± 0.7 *** | 2.8 ± 0.8 | 2.8 ± 0.8 | <0.001 |
| eGFR (mL/min/1.73 m2) | 99.2 ± 15.1 | 97.9 ± 13.7 | 97.7 ± 15.2 | 0.11 |
| Ethnicity, n (%) | <0.001 | |||
| Han | 595 (83.1%) | 1311 (79.6%) | 271(90.6%) | |
| Other | 121 (16.9%) | 336 (20.4%) | 28 (9.4%) | |
| Vegetable consumption, n (%) | 0.85 | |||
| Minimal | 3 (0.4%) | 5 (0.3%) | 2 (0.7%) | |
| Low | 32 (4.5%) | 77 (4.7%) | 12 (4.0%) | |
| Moderate | 408 (57.0%) | 898 (54.5%) | 162 (54.2%) | |
| High | 273 (38.1%) | 667 (40.5%) | 123 (41.1%) | |
| Fruit consumption, n (%) | 0.26 | |||
| Minimal | 20 (2.8%) | 56 (3.4%) | 12 (4.0%) | |
| Low | 329 (45.9%) | 721 (43.8%) | 133 (44.5%) | |
| Moderate | 339 (47.3%) | 774 (47.0%) | 145 (48.5%) | |
| High | 28 (3.9%) | 95 (5.8%) | 9 (3.0%) | |
| Low-salt and low-fat diet, n (%) | 224 (31.3%) | 491 (29.8%) | 94 (31.4%) | 0.71 |
| Regular exercise, n (%) | 203 (28.4%) *** | 512 (31.1%) | 76 (25.4%) | 0.09 |
| Hypotensive medication, n (%) | 135 (18.9%) *** | 437 (26.5%) | 119 (39.8%) *** | <0.001 |
| Family history of diabetes, n (%) | 110 (15.4%) | 289 (17.5%) | 59 (19.7%) | 0.20 |
| Current smoker, n (%) | 116 (16.2%) | 273 (16.6%) | 77 (25.8%) *** | <0.001 |
| Habitual alcohol drinker, n (%) | 148 (20.7%) ** | 365 (22.2%) | 79 (26.4%) | 0.13 |
| N, Cases/Total | Model 1 | Model 2 | |||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Progression to Diabetes | |||||
| Type of Tea | |||||
| No tea | 126/1198 | 1.00 (Ref.) | 1.00 (Ref.) | ||
| Green tea | 141/1016 | 1.27 (0.97, 1.65) | 0.08 | 1.10 (0.83, 1.47) | 0.50 |
| Black tea | 9/138 | 0.59 (0.29, 1.20) | 0.15 | 0.61 (0.29, 1.27) | 0.19 |
| Dark tea | 5/140 | 0.30 (0.12, 0.75) | 0.01 | 0.28 (0.11, 0.72) | 0.01 |
| Other | 18/170 | 1.13 (0.66, 1.94) | 0.66 | 1.03 (0.59, 1.80) | 0.92 |
| Regression to Normoglycemia | |||||
| Type of Tea | |||||
| No tea | 344/1198 | 1.00 (Ref.) | 1.00 (Ref.) | ||
| Green tea | 232/1016 | 0.76 (0.63, 0.93) | 0.01 | 0.73 (0.59, 0.90) | 0.01 |
| Black tea | 41/138 | 0.99 (0.67, 1.46) | 0.94 | 0.98 (0.65, 1.48) | 0.92 |
| Dark tea | 39/140 | 0.86(0.58, 1.27) | 0.45 | 0.81 (0.53, 1.24) | 0.34 |
| Other | 60/170 | 1.38 (0.97, 1.96) | 0.07 | 1.19 (0.82, 1.71) | 0.36 |
| N, Cases/Total | Model 1 | Model 2 | |||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Progression to Diabetes | |||||
| Frequency of Green Tea Consumption | |||||
| Never | 158/1646 | 1.00 | 1.00 | ||
| Sometimes | 58/473 | 1.21 (0.87, 1.68) | 0.25 | 1.17 (0.83, 1.63) | 0.37 |
| Daily | 83/543 | 1.56 (1.16, 2.09) | 0.003 | 1.27 (0.92, 1.76) | 0.14 |
| Frequency of Dark Tea Consumption | |||||
| Never | 294/2522 | 1.00 | 1.00 | ||
| Sometimes | 2/31 | 0.42 (0.10, 1.79) | 0.24 | 0.49 (0.11, 2.15) | 0.35 |
| Daily | 3/109 | 0.22 (0.07, 0.71) | 0.01 | 0.22 (0.07, 0.71) | 0.01 |
| Regression to Normoglycemia | |||||
| Frequency of Green Tea Consumption | |||||
| Never | 484/1646 | 1.00 | 1.00 | ||
| Sometimes | 111/473 | 0.76 (0.59, 0.97) | 0.03 | 0.74 (0.58, 0.95) | 0.02 |
| Daily | 121/543 | 0.74 (0.59, 0.94) | 0.01 | 0.72 (0.56, 0.92) | 0.01 |
| Frequency of Dark Tea Consumption | |||||
| Never | 677/2522 | 1.00 | 1.00 | ||
| Sometimes | 4/31 | 0.37 (0.13, 1.06) | 0.06 | 0.31 (0.10, 0.91) | 0.03 |
| Daily | 35/109 | 1.13 (0.75, 1.71) | 0.57 | 1.14 (0.73, 1.78) | 0.58 |
| TyG | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| Mean (95% CI) | p | Mean (95% CI) | p | |
| Type of Tea | ||||
| No tea | 0.00 (Ref.) | 0.00 (Ref.) | ||
| Green tea | 0.08 (0.03, 0.13) | 0.001 | 0.05 (0.01, 1.00) | 0.05 |
| Black tea | −0.04 (−0.14, 0.06) | 0.43 | −0.06 (−0.17, 0.04) | 0.21 |
| Dark tea | −0.20 (−0.30, −0.10) | <0.001 | −0.23 (−0.34, −0.13) | <0.001 |
| Other | 0.08 (−0.14, 0.17) | 0.10 | 0.05 (−0.04, 0.15) | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Rayner, C.K.; Horowitz, M.; Jones, K.; Xie, C.; Huang, W.; Sun, Z.; Qiu, S.; Wu, T. The Impact of Tea Consumption on Prediabetes Regression and Progression: A Prospective Cohort Study. Nutrients 2025, 17, 2366. https://doi.org/10.3390/nu17142366
Li T, Rayner CK, Horowitz M, Jones K, Xie C, Huang W, Sun Z, Qiu S, Wu T. The Impact of Tea Consumption on Prediabetes Regression and Progression: A Prospective Cohort Study. Nutrients. 2025; 17(14):2366. https://doi.org/10.3390/nu17142366
Chicago/Turabian StyleLi, Tingting, Christopher K. Rayner, Michael Horowitz, Karen Jones, Cong Xie, Weikun Huang, Zilin Sun, Shanhu Qiu, and Tongzhi Wu. 2025. "The Impact of Tea Consumption on Prediabetes Regression and Progression: A Prospective Cohort Study" Nutrients 17, no. 14: 2366. https://doi.org/10.3390/nu17142366
APA StyleLi, T., Rayner, C. K., Horowitz, M., Jones, K., Xie, C., Huang, W., Sun, Z., Qiu, S., & Wu, T. (2025). The Impact of Tea Consumption on Prediabetes Regression and Progression: A Prospective Cohort Study. Nutrients, 17(14), 2366. https://doi.org/10.3390/nu17142366








