Effects of Ginger Supplementation on Markers of Inflammation and Functional Capacity in Individuals with Mild to Moderate Joint Pain †
Abstract
1. Introduction
2. Methods
2.1. Research Design
2.2. Study Participants
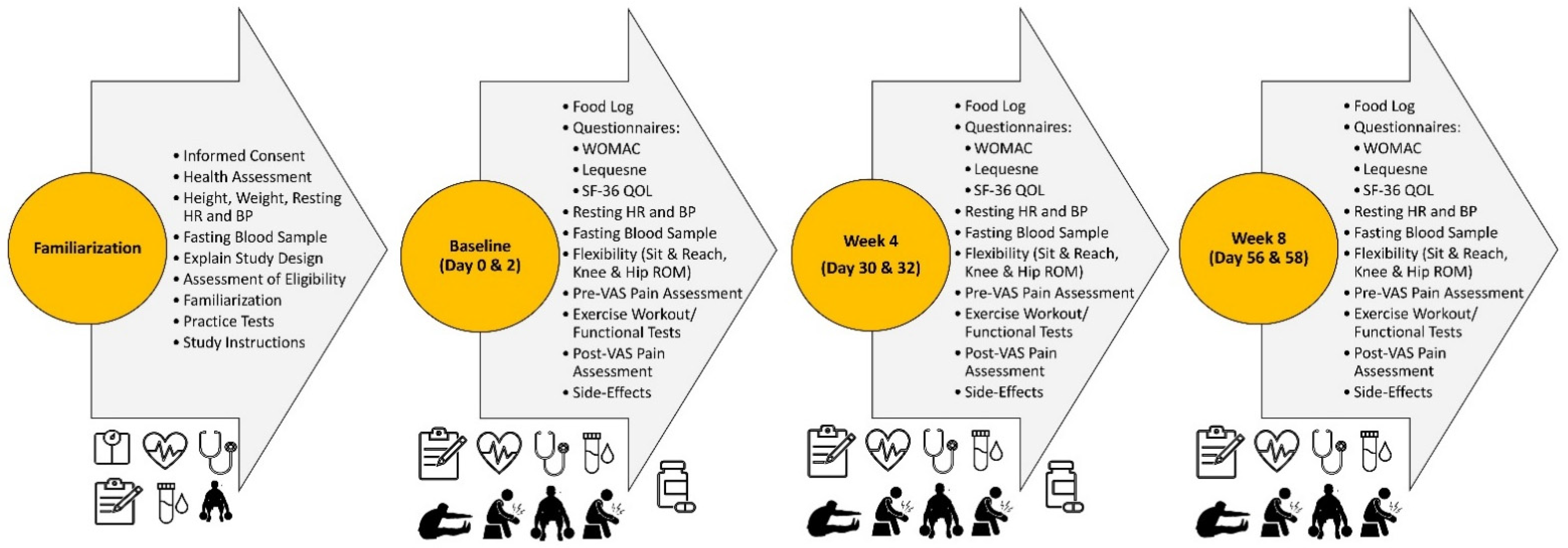
2.3. Familiarization
2.4. Experimental Session Testing Protocol
2.5. Functional Exercise Intervention
2.6. Supplementation Intervention
2.7. Diet Standardization
3. Procedures
3.1. Anthropometrics and Hemodynamics
3.2. Blood Sampling
3.3. Range of Motion and Flexibility Assessment
3.4. Pain Assessment
3.5. Quality of Life
3.6. Functional Capacity
3.7. Side Effects
3.8. Statistical Analysis
4. Results
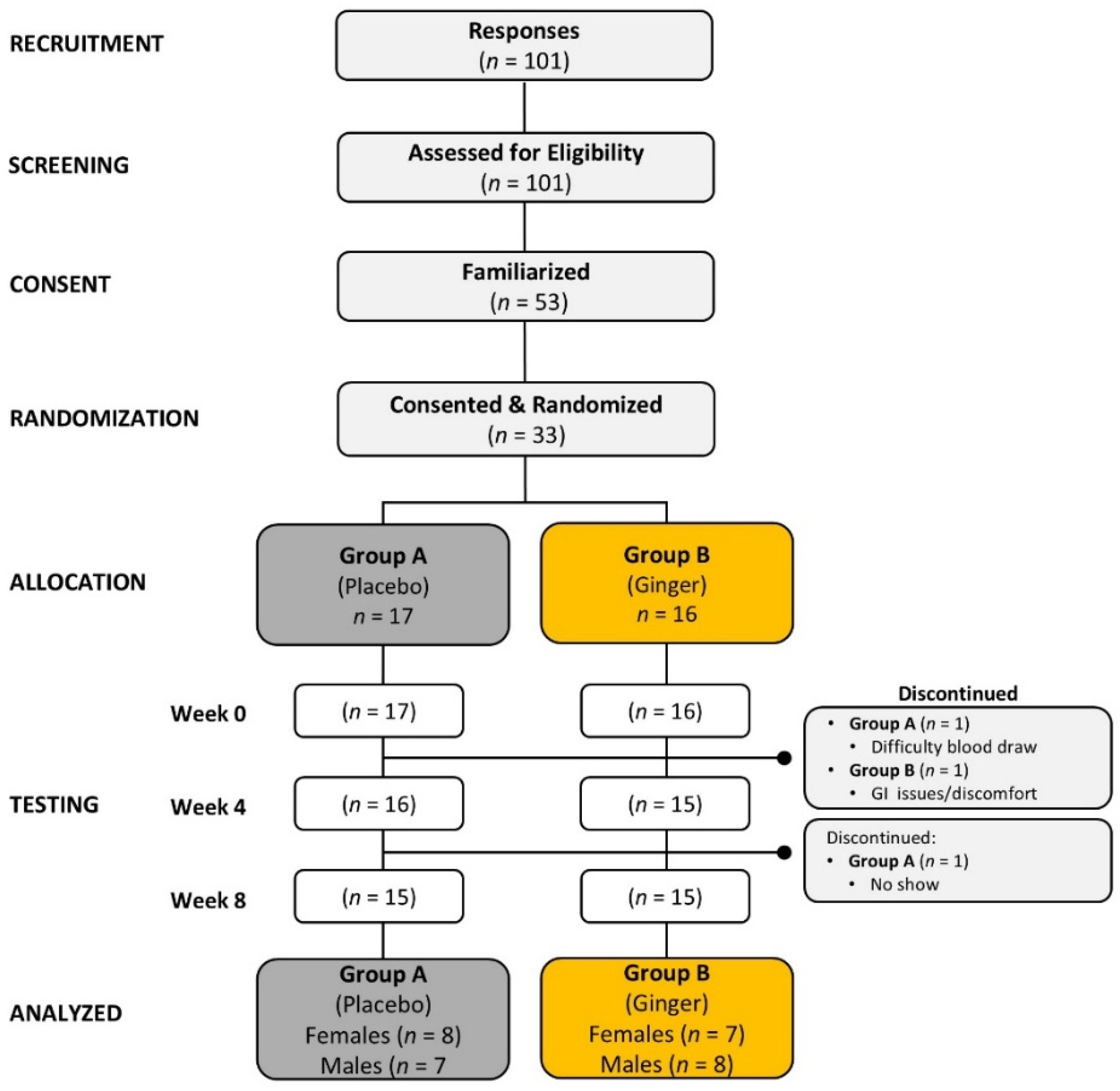
4.1. Participant Demographics
4.2. Exercise Stimulus
4.3. Primary Outcomes
4.3.1. Ratings of Muscle Pain
4.3.2. Osteoarthritis Severity Indices
4.3.3. Lequesne Index of Severity of Hip Osteoarthritis
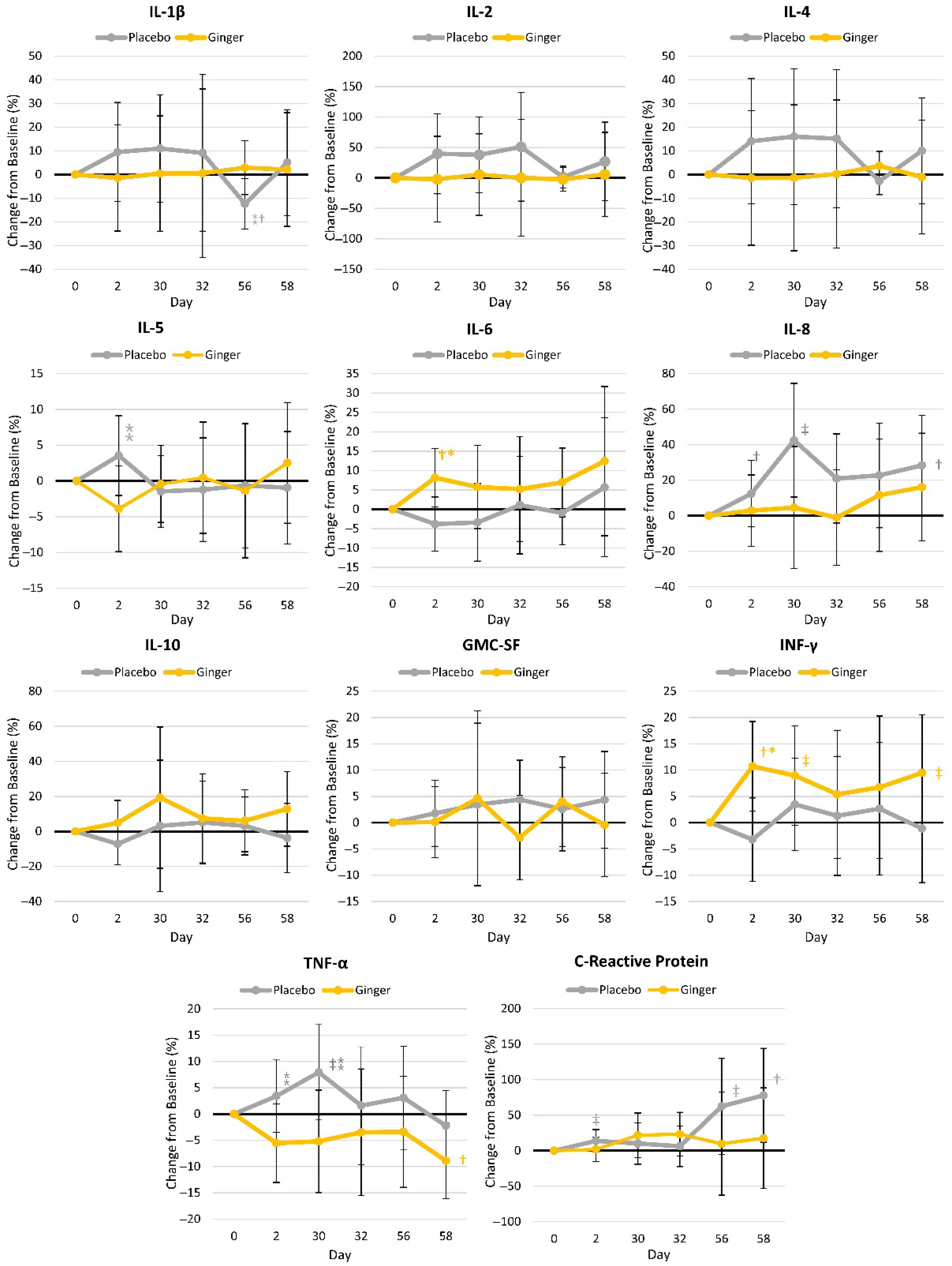
4.3.4. Inflammatory Markers
4.4. Secondary Outcomes
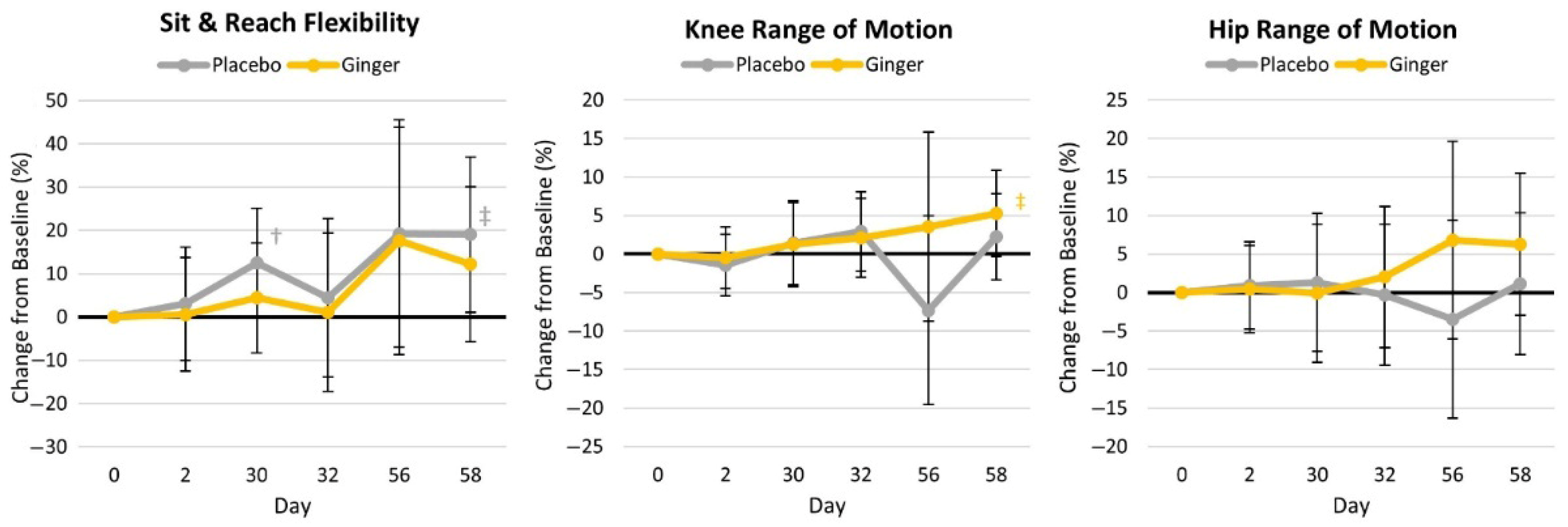
4.4.1. Flexibility and Range of Motion
4.4.2. Quality of Life
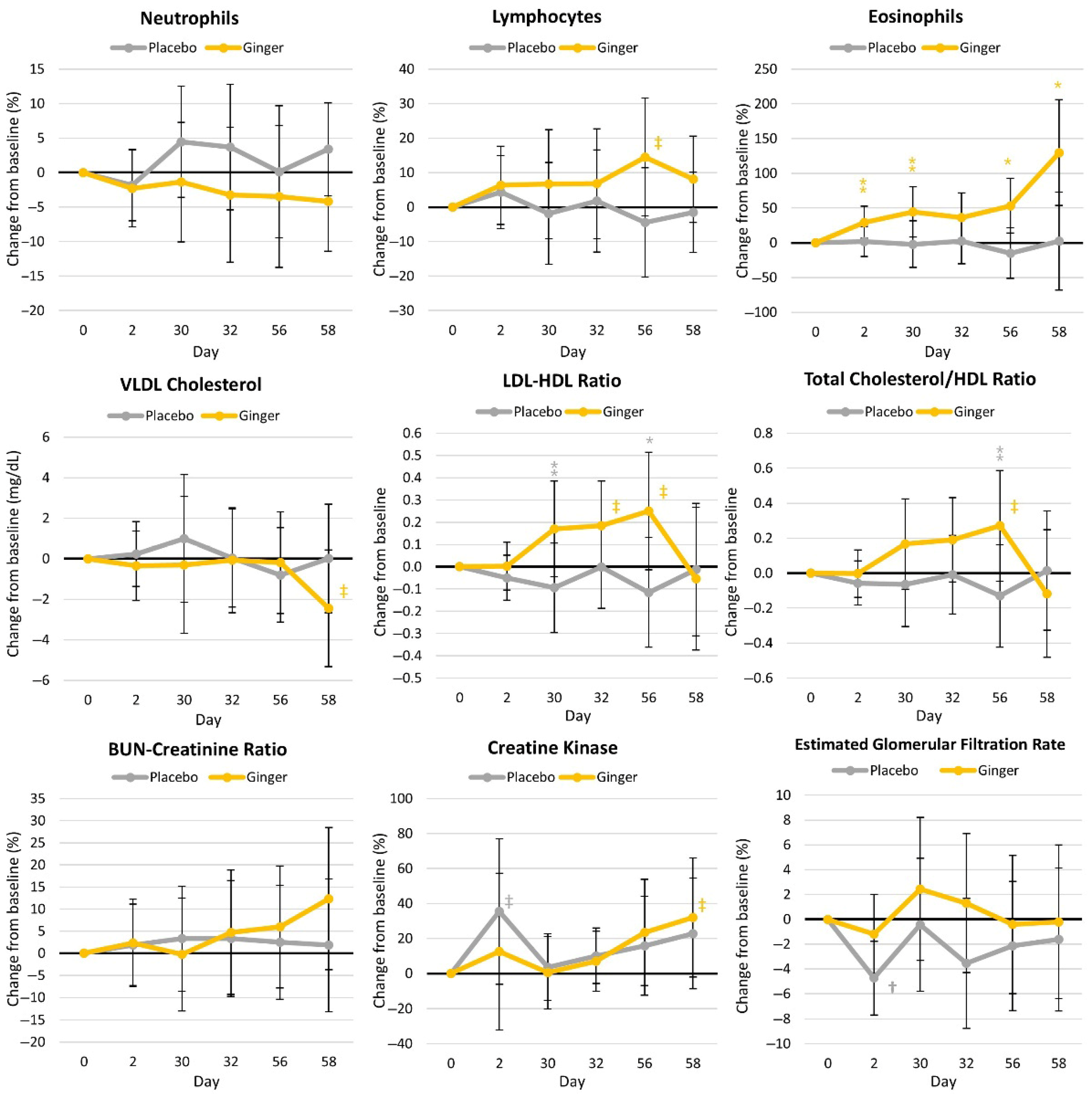
4.4.3. Blood Markers of Health
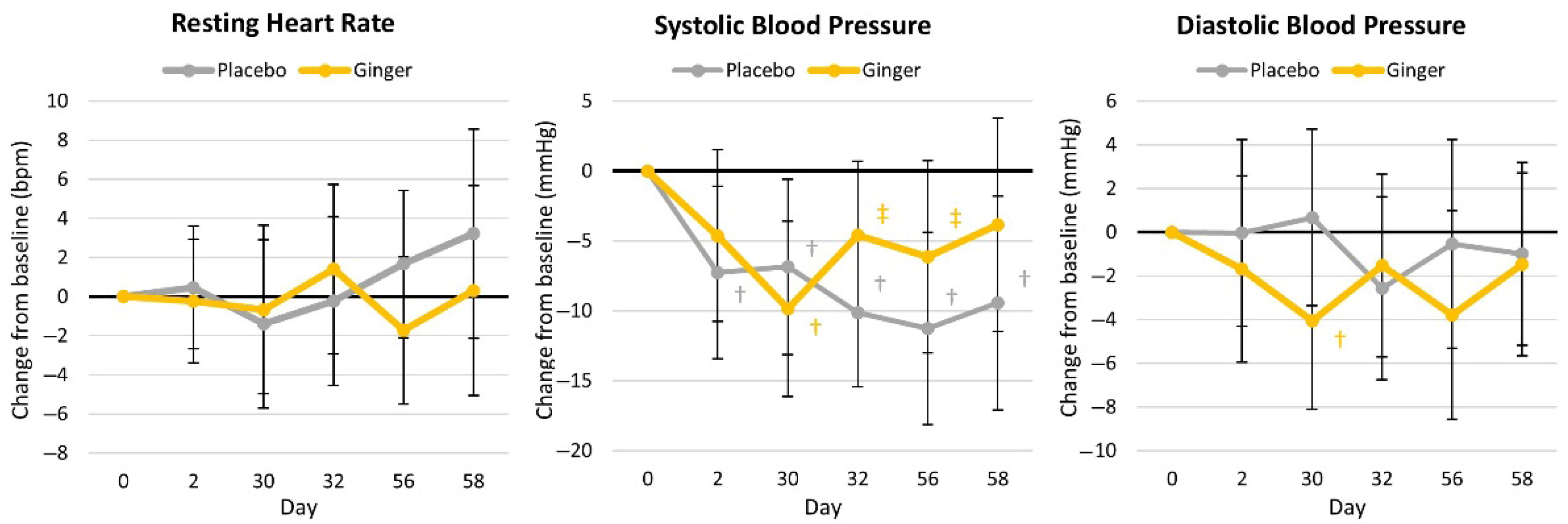
4.4.4. Resting Hemodynamics
4.4.5. Analgesic Use and Side Effects
5. Discussion
5.1. Primary Outcomes
5.1.1. Perceptions of Pain and Functional Capacity
5.1.2. Markers of Inflammation
5.2. Secondary Outcomes
5.3. Safety and Side Effects
5.4. Strengths, Limitations, and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein Kinase B |
| ANOVA | Analysis of variance |
| CI | Confidence interval |
| CONSORT | Consolidated Standards of Reporting Trials |
| COX-2 | Cyclooxygenase enzyme-2 isoenzyme |
| CK | Creatine kinase |
| DMARDS | Disease-modifying antirheumatic drugs |
| GLM | General linear model |
| GMC-SF | Granulocyte-macrophage colony-stimulating factor |
| HDL | High-density lipoprotein |
| HOMAIR | Homeostatic model assessment for insulin resistance |
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| LDL | Low-density lipoproteins |
| LSD | Least Significant Difference |
| NF-κβ | Nuclear Factor-kappa β |
| OTC | Over the counter |
| NSAIDS | Nonsteroidal anti-inflammatory drugs |
| QOL | Quality of life |
| ROM | Range of motion |
| SD | Standard deviation |
| SF-36 | Short Form Health Survey, 36-item |
| TNF-α | Tumor Necrosis Factor-α |
| TRPV1 | Vanilloid subtype 1 pain-sensitive receptor |
| VAS | Visual analog scale |
| VLDL | Very-low-density lipoprotein |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis Index |
References
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, J.; Grygiel-Górniak, B.; Cielecka-Piontek, J. Zingiber Officinale Roscoe: The Antiarthritic Potential of a Popular Spice—Preclinical and Clinical Evidence. Nutrients 2024, 16, 741. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Haniadka, R.; Pereira, M.M.; D’Souza, J.J.; Pallaty, P.L.; Bhat, H.P.; Popuri, S. Update on the chemopreventive effects of ginger and its phytochemicals. Crit. Rev. Food Sci. Nutr. 2011, 51, 499–523. [Google Scholar] [CrossRef] [PubMed]
- Mohd Yusof, Y.A. Gingerol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 177–207. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Wang, X.; Ji, R.; Liu, L.; Qiao, Y.; Lou, Z.; Ma, C.; Li, S.; Wang, H.; Ho, C.T. Occurrence, biological activity and metabolism of 6-shogaol. Food Funct. 2018, 9, 1310–1327. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Rehman, M.U.; Amin, I.; Arif, A.; Rasool, S.; Bhat, S.A.; Afzal, I.; Hussain, I.; Bilal, S.; Mir, M. A Review on Pharmacological Properties of Zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). Sci. World J. 2015, 2015, 816364. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; McGrath, K.C.; Tran, V.H.; Li, Y.M.; Duke, C.C.; Roufogalis, B.D.; Heather, A.K. Attenuation of Proinflammatory Responses by S-[6]-Gingerol via Inhibition of ROS/NF-Kappa B/COX2 Activation in HuH7 Cells. Evid. Based Complement. Altern. Med. 2013, 2013, 146142. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Maier, K.G.; Bruch, D.; Kittur, D.S. Effect of 6-gingerol on pro-inflammatory cytokine production and costimulatory molecule expression in murine peritoneal macrophages. J. Surg. Res. 2007, 138, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Black, C.D.; Herring, M.P.; Hurley, D.J.; O’Connor, P.J. Ginger (Zingiber officinale) reduces muscle pain caused by eccentric exercise. J. Pain 2010, 11, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, B.O.; Adedara, I.A.; Farombi, E.O. Protective mechanisms of 6-gingerol in dextran sulfate sodium-induced chronic ulcerative colitis in mice. Hum. Exp. Toxicol. 2018, 37, 1054–1068. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, P.; Zheng, B.; Zhang, M.; Zhang, Y.; Xue, Y.; Liu, C.; Chu, X.; Wang, X.; Sun, S.; et al. 6-Gingerol exerts a protective effect against hypoxic injury through the p38/Nrf2/HO-1 and p38/NF-kappaB pathway in H9c2 cells. J. Nutr. Biochem. 2022, 104, 108975. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, C.Y.; Lo, H.Y.; Huang, H.C.; Li, C.C.; Wu, S.L.; Ho, T.Y. Ginger extract and zingerone ameliorated trinitrobenzene sulphonic acid-induced colitis in mice via modulation of nuclear factor-kappaB activity and interleukin-1beta signalling pathway. Food Chem. 2013, 136, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Ballester, P.; Cerda, B.; Arcusa, R.; Marhuenda, J.; Yamedjeu, K.; Zafrilla, P. Effect of Ginger on Inflammatory Diseases. Molecules 2022, 27, 7223. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.M.; Ezzat, M.I.; Okba, M.M.; Menze, E.T.; Abdel-Naim, A.B. The hidden mechanism beyond ginger (Zingiber officinale Rosc.) potent in vivo and in vitro anti-inflammatory activity. J. Ethnopharmacol. 2018, 214, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Colletti, A.; Cicero, A.F.G. Nutraceutical Approach to Chronic Osteoarthritis: From Molecular Research to Clinical Evidence. Int. J. Mol. Sci. 2021, 22, 12920. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Dickenson, A.H. Neuropharmacological basis for multimodal analgesia in chronic pain. Postgrad. Med. 2022, 134, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Fossari, F.; Vecchio, V.; Gasparri, C.; Peroni, G.; Spadaccini, D.; Riva, A.; Petrangolini, G.; Iannello, G.; Nichetti, M.; et al. Clinical trials on pain lowering effect of ginger: A narrative review. Phytother. Res. 2020, 34, 2843–2856. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cheon, C.; Kim, B.; Kim, W. The Effect of Ginger and Its Sub-Components on Pain. Plants 2022, 11, 2296. [Google Scholar] [CrossRef] [PubMed]
- Fajrin, F.A.; Nugroho, A.E.; Nurrochmad, A.; Susilowati, R. Ginger extract and its compound, 6-shogaol, attenuates painful diabetic neuropathy in mice via reducing TRPV1 and NMDAR2B expressions in the spinal cord. J. Ethnopharmacol. 2020, 249, 112396. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Pittler, M.H.; Roufogalis, B.D. Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Coruzzi, G.; Venturi, N.; Spaggiari, S. Gastrointestinal safety of novel nonsteroidal antiinflammatory drugs: Selective COX-2 inhibitors and beyond. Acta Biomed. Ateneo Parm. 2007, 78, 96–110. [Google Scholar]
- Moodley, I. Review of the cardiovascular safety of COXIBs compared to NSAIDS. Cardiovasc. J. Afr. 2008, 19, 102–107. [Google Scholar] [PubMed]
- Hoffman, T. Ginger: An ancient remedy and modern miracle drug. Hawaii Med. J. 2007, 66, 326–327. [Google Scholar] [PubMed]
- Therkleson, T. Ginger compress therapy for adults with osteoarthritis. J. Adv. Nurs. 2010, 66, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.D.; Zavorsky, G.S.; Smoliga, J.M. The Effects of Pre-Exercise Ginger Supplementation on Muscle Damage and Delayed Onset Muscle Soreness. Phytother. Res. 2015, 29, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari-Khosravi, H.; Naderi, Z.; Dehghan, A.; Nadjarzadeh, A.; Fallah Huseini, H. Effect of Ginger Supplementation on Proinflammatory Cytokines in Older Patients with Osteoarthritis: Outcomes of a Randomized Controlled Clinical Trial. J. Nutr. Gerontol. Geriatr. 2016, 35, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Naderi, Z.; Mozaffari-Khosravi, H.; Dehghan, A.; Nadjarzadeh, A.; Huseini, H.F. Effect of ginger powder supplementation on nitric oxide and C-reactive protein in elderly knee osteoarthritis patients: A 12-week double-blind randomized placebo-controlled clinical trial. J. Tradit. Complement. Med. 2016, 6, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Riva, A.; Morazzoni, P.; Allegrini, P.; Faliva, M.A.; Naso, M.; Miccono, A.; Peroni, G.; Degli Agosti, I.; Perna, S. The effect and safety of highly standardized Ginger (Zingiber officinale) and Echinacea (Echinacea angustifolia) extract supplementation on inflammation and chronic pain in NSAIDs poor responders. A pilot study in subjects with knee arthrosis. Nat. Prod. Res. 2017, 31, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Aryaeian, N.; Mahmoudi, M.; Shahram, F.; Poursani, S.; Jamshidi, F.; Tavakoli, H. The effect of ginger supplementation on IL2, TNFalpha, and IL1beta cytokines gene expression levels in patients with active rheumatoid arthritis: A randomized controlled trial. Med. J. Islam. Repub. Iran 2019, 33, 154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilson, P.B. A Randomized Double-Blind Trial of Ginger Root for Reducing Muscle Soreness and Improving Physical Performance Recovery Among Experienced Recreational Distance Runners. J. Diet. Suppl. 2020, 17, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, Y.; Li, P.; Chen, X.; Liu, F.; Hou, Q. Ginger relieves intestinal hypersensitivity of diarrhea predominant irritable bowel syndrome by inhibiting proinflammatory reaction. BMC Complement. Med. Ther. 2020, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Unuofin, J.O.; Masuku, N.P.; Paimo, O.K.; Lebelo, S.L. Ginger from Farmyard to Town: Nutritional and Pharmacological Applications. Front. Pharmacol. 2021, 12, 779352. [Google Scholar] [CrossRef] [PubMed]
- Alshibani, N.; Al-Kattan, R.; Alssum, L.; Basudan, A.; Shaheen, M.; Alqutub, M.N.; Al Dahash, F. Postoperative Analgesic and Anti-inflammatory Effectiveness of Ginger (Zingiber officinale) and NSAIDs as Adjuncts to Nonsurgical Periodontal Therapy for the Management of Periodontitis. Oral Health Prev. Dent. 2022, 20, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Marcussen, K.C. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001, 44, 2531–2538. [Google Scholar] [CrossRef] [PubMed]
- Ozgoli, G.; Goli, M.; Moattar, F. Comparison of effects of ginger, mefenamic acid, and ibuprofen on pain in women with primary dysmenorrhea. J. Altern. Complement. Med. 2009, 15, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Anh, N.H.; Kim, S.J.; Long, N.P.; Min, J.E.; Yoon, Y.C.; Lee, E.G.; Kim, M.; Kim, T.J.; Yang, Y.Y.; Son, E.Y.; et al. Ginger on Human Health: A Comprehensive Systematic Review of 109 Randomized Controlled Trials. Nutrients 2020, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Cui, H.; Li, X.; Askar, G.; Yu, J.; Hayat, K.; Zhang, X.; Ho, C.T. Current progress in the ginger oleoresin: Bioactivities, extraction and encapsulation technologies. Crit. Rev. Food Sci. Nutr. 2025; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Oriani, V.B.; Alvim, I.D.; Paulino, B.N.; Procópio, F.R.; Pastore, G.M.; Hubinger, M.D. The influence of the storage temperature on the stability of lipid microparticles containing ginger oleoresin. Food Res. Int. 2018, 109, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.; Medicine, A.C.o.S. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Grubbs, F.E. Procedures for Detecting Outlying Observations in Samples. Technometrics 1969, 11, 1–21. [Google Scholar] [CrossRef]
- Denegar, C.R.; Perrin, D.H. Effect of transcutaneous electrical nerve stimulation, cold, and a combination treatment on pain, decreased range of motion, and strength loss associated with delayed onset muscle soreness. J. Athl. Train. 1992, 27, 200–206. [Google Scholar] [PubMed]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar] [PubMed]
- Faucher, M.; Poiraudeau, S.; Lefevre-Colau, M.M.; Rannou, F.; Fermanian, J.; Revel, M. Assessment of the test-retest reliability and construct validity of a modified Lequesne index in knee osteoarthritis. Jt. Bone Spine 2003, 70, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Linsell, L.; Doll, H.; Zondervan, K.; Rose, P.; Carr, A.; Randall, T.; Fitzpatrick, R. Assessment of the Lequesne index of severity for osteoarthritis of the hip in an elderly population. Osteoarthr. Cartil. 2005, 13, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Samuel, A.J.; Kanimozhi, D. Outcome measures used in patient with knee osteoarthritis: With special importance on functional outcome measures. Int. J. Health Sci. 2019, 13, 52–60. [Google Scholar]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Taft, C.; Karlsson, J.; Sullivan, M. Performance of the Swedish SF-36 version 2.0. Qual. Life Res. 2004, 13, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Çelik, D.; Çoban, Ö. Short Form Health Survey version-2.0 Turkish (SF-36v2) is an efficient outcome parameter in musculoskeletal research. Acta Orthop. Traumatol. Turc. 2016, 50, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Grubic, T.J.; Sowinski, R.J.; Nevares, B.E.; Jenkins, V.M.; Williamson, S.L.; Reyes, A.G.; Rasmussen, C.; Greenwood, M.; Murano, P.S.; Earnest, C.P.; et al. Comparison of ingesting a food bar containing whey protein and isomalto-oligosaccharides to carbohydrate on performance and recovery from an acute bout of resistance-exercise and sprint conditioning: An open label, randomized, counterbalanced, crossover pilot study. J. Int. Soc. Sports Nutr. 2019, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Sowinski, R.; Gonzalez, D.; Xing, D.; Yoo, C.; Jenkins, V.; Nottingham, K.; Dickerson, B.; Humphries, M.; Leonard, M.; Ko, J.; et al. Effects of Inositol-Enhanced Bonded Arginine Silicate Ingestion on Cognitive and Executive Function in Gamers. Nutrients 2021, 13, 3758. [Google Scholar] [CrossRef] [PubMed]
- Sowinski, R.J.; Grubic, T.J.; Dalton, R.L.; Schlaffer, J.; Reyes-Elrod, A.G.; Jenkins, V.M.; Williamson, S.; Rasmussen, C.; Murano, P.S.; Earnest, C.P.; et al. An Examination of a Novel Weight Loss Supplement on Anthropometry and Indices of Cardiovascular Disease Risk. J. Diet. Suppl. 2021, 18, 478–506. [Google Scholar] [CrossRef] [PubMed]
- Earnest, C.P.; Roberts, B.M.; Harnish, C.R.; Kutz, J.L.; Cholewa, J.M.; Johannsen, N.M. Reporting Characteristics in Sports Nutrition. Sports 2018, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, E. Applications of confidence limits and effect sizes in sport research. Open Sports Sci. J. 2008, 1, 3–4. [Google Scholar] [CrossRef]
- Grabowski, B. “p < 0.05” Might Not Mean What You Think: American Statistical Association Clarifies P Values. J. Natl. Cancer Inst. 2016, 108, djw194. [Google Scholar] [CrossRef] [PubMed]
- Page, P. Beyond statistical significance: Clinical interpretation of rehabilitation research literature. Int. J. Sports Phys. Ther. 2014, 9, 726. [Google Scholar] [PubMed]
- Sharma, H. Statistical significance or clinical significance? A researcher’s dilemma for appropriate interpretation of research results. Saudi J. Anaesth. 2021, 15, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.E.; Dickerson, B.L.; Johnson, S.E.; Woodruff, K.E.; Leonard, M.; Yoo, C.; Ko, J.; Xing, D.; Martinez, V.; Kendra, J.; et al. Impact of astaxanthin supplementation on markers of cardiometabolic health and tactical performance among firefighters. J. Int. Soc. Sports Nutr. 2024, 21, 2427751. [Google Scholar] [CrossRef] [PubMed]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum: Hillsdale, NJ, USA, 1988; pp. 75–108. [Google Scholar]
- Kang, H. The prevention and handling of the missing data. Korean J. Anesthesiol. 2013, 64, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Quintero, M.; LeBoulluec, A. Missing Data Imputation for Ordinal Data. Int. J. Comput. Appl. 2018, 181, 10–16. [Google Scholar] [CrossRef]
- Kulkarni, R.A.; Deshpande, A.R. Anti-inflammatory and antioxidant effect of ginger in tuberculosis. J. Complement. Integr. Med. 2016, 13, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Zehsaz, F.; Farhangi, N.; Mirheidari, L. The effect of Zingiber officinale R. rhizomes (ginger) on plasma pro-inflammatory cytokine levels in well-trained male endurance runners. Cent. Eur. J. Immunol. 2014, 39, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Wigler, I.; Grotto, I.; Caspi, D.; Yaron, M. The effects of Zintona EC (a ginger extract) on symptomatic gonarthritis. Osteoarthr. Cartil. 2003, 11, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, Z.; Izadi, S.; Bari, Z.; Soltani, F.; Narouie, B.; Ghasemi-rad, M. Evaluating the effects of ginger extract on knee pain, stiffness and difficulty in patients with knee osteoarthritis. J. Med. Plants Res. 2011, 5, 3375–3379. [Google Scholar]
- Black, C.D.; Oconnor, P.J. Acute effects of dietary ginger on quadriceps muscle pain during moderate-intensity cycling exercise. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi, N.S.; Ghiasvand, R.; Askari, G.; Feizi, A.; Hariri, M.; Darvishi, L.; Barani, A.; Taghiyar, M.; Shiranian, A.; Hajishafiee, M. Influence of ginger and cinnamon intake on inflammation and muscle soreness endued by exercise in Iranian female athletes. Int. J. Prev. Med. 2013, 4, S11–S15. [Google Scholar] [PubMed]
- Hoseinzadeh, K.; Daryanoosh, F.; Baghdasar, P.J.; Alizadeh, H. Acute effects of ginger extract on biochemical and functional symptoms of delayed onset muscle soreness. Med. J. Islam. Repub. Iran 2015, 29, 261. [Google Scholar] [PubMed]
- Dominguez-Balmaseda, D.; Diez-Vega, I.; Larrosa, M.; San Juan, A.F.; Issaly, N.; Moreno-Perez, D.; Burgos, S.; Sillero-Quintana, M.; Gonzalez, C.; Bas, A.; et al. Effect of a Blend of Zingiber officinale Roscoe and Bixa orellana L. Herbal Supplement on the Recovery of Delayed-Onset Muscle Soreness Induced by Unaccustomed Eccentric Resistance Training: A Randomized, Triple-Blind, Placebo-Controlled Trial. Front. Physiol. 2020, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Bartels, E.M.; Folmer, V.N.; Bliddal, H.; Altman, R.D.; Juhl, C.; Tarp, S.; Zhang, W.; Christensen, R. Efficacy and safety of ginger in osteoarthritis patients: A meta-analysis of randomized placebo-controlled trials. Osteoarthr. Cartil. 2015, 23, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, M.; Md, A.; Msc, S. Comparing the Effects of ginger (Zingiber officinale) extract and ibuprofen On patients with osteoarthritis. Arch. Iran. Med. 2005, 8, 267–271. [Google Scholar]
- Bliddal, H.; Rosetzsky, A.; Schlichting, P.; Weidner, M.S.; Andersen, L.A.; Ibfelt, H.H.; Christensen, K.; Jensen, O.N.; Barslev, J. A randomized, placebo-controlled, cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthr. Cartil. 2000, 8, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.H.; Makpol, S.; Abdul Hamid, N.A.; Das, S.; Ngah, W.Z.; Yusof, Y.A. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics 2008, 63, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Mahluji, S.; Ostadrahimi, A.; Mobasseri, M.; Ebrahimzade Attari, V.; Payahoo, L. Anti-inflammatory effects of Zingiber officinale in type 2 diabetic patients. Adv. Pharm. Bull. 2013, 3, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Atashak, S.; Peeri, M.; Azarbayjani, M.A.; Stannard, S.R.; Haghighi, M.M. Obesity-related cardiovascular risk factors after long- term resistance training and ginger supplementation. J. Sports Sci. Med. 2011, 10, 685–691. [Google Scholar] [PubMed]
- Arablou, T.; Naheed, A.; Majid, V.; Faranak, S.; AghaFatemeh, H.; Djalali, M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int. J. Food Sci. Nutr. 2014, 65, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Azimi, P.; Ghiasvand, R.; Feizi, A.; Hariri, M.; Abbasi, B. Effects of Cinnamon, Cardamom, Saffron, and Ginger Consumption on Markers of Glycemic Control, Lipid Profile, Oxidative Stress, and Inflammation in Type 2 Diabetes Patients. Rev. Diabet. Stud. 2014, 11, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Niempoog, S.; Pawa, K.K.; Amatyakul, C. The efficacy of powdered ginger in osteoarthritis of the knee. J. Med. Assoc. Thai 2012, 95 (Suppl. 1), S59–S64. [Google Scholar]


| Group | Week 0 | χ2 p-Level | Week 4 | χ2 p-Level | Week 8 | χ2 p-Level | Total Used Analgesics | Percent Used Analgesics |
|---|---|---|---|---|---|---|---|---|
| Placebo | 5 | 0.195 | 6 | 0.232 | 7 | 0.713 | 11 | 73.3% |
| Ginger | 2 | 3 | 6 | 7 | 46.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broeckel, J.; Estes, L.; Leonard, M.; Dickerson, B.L.; Gonzalez, D.E.; Purpura, M.; Jäger, R.; Sowinski, R.J.; Rasmussen, C.J.; Kreider, R.B. Effects of Ginger Supplementation on Markers of Inflammation and Functional Capacity in Individuals with Mild to Moderate Joint Pain. Nutrients 2025, 17, 2365. https://doi.org/10.3390/nu17142365
Broeckel J, Estes L, Leonard M, Dickerson BL, Gonzalez DE, Purpura M, Jäger R, Sowinski RJ, Rasmussen CJ, Kreider RB. Effects of Ginger Supplementation on Markers of Inflammation and Functional Capacity in Individuals with Mild to Moderate Joint Pain. Nutrients. 2025; 17(14):2365. https://doi.org/10.3390/nu17142365
Chicago/Turabian StyleBroeckel, Jacob, Landry Estes, Megan Leonard, Broderick L. Dickerson, Drew E. Gonzalez, Martin Purpura, Ralf Jäger, Ryan J. Sowinski, Christopher J. Rasmussen, and Richard B. Kreider. 2025. "Effects of Ginger Supplementation on Markers of Inflammation and Functional Capacity in Individuals with Mild to Moderate Joint Pain" Nutrients 17, no. 14: 2365. https://doi.org/10.3390/nu17142365
APA StyleBroeckel, J., Estes, L., Leonard, M., Dickerson, B. L., Gonzalez, D. E., Purpura, M., Jäger, R., Sowinski, R. J., Rasmussen, C. J., & Kreider, R. B. (2025). Effects of Ginger Supplementation on Markers of Inflammation and Functional Capacity in Individuals with Mild to Moderate Joint Pain. Nutrients, 17(14), 2365. https://doi.org/10.3390/nu17142365







