Effect of Dealcoholized Muscadine Wine on the Development of Spontaneous Colitis and Gut Microbiome in IL-10−/− Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPLC Analysis of Dealcoholized Muscadine Wine
2.3. Proximate Analysis of DMW and Diet Formulation
2.4. Animal Care and Experimental Design
2.5. Quantitative Reverse Transcription PCR (RT-qPCR)
2.6. Analysis of Serum IL-6, IL-1β and TNF-α
2.7. Gut Microbiome Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. DMW Contained Anthocyanins, Ellagic Acid, and Flavonols
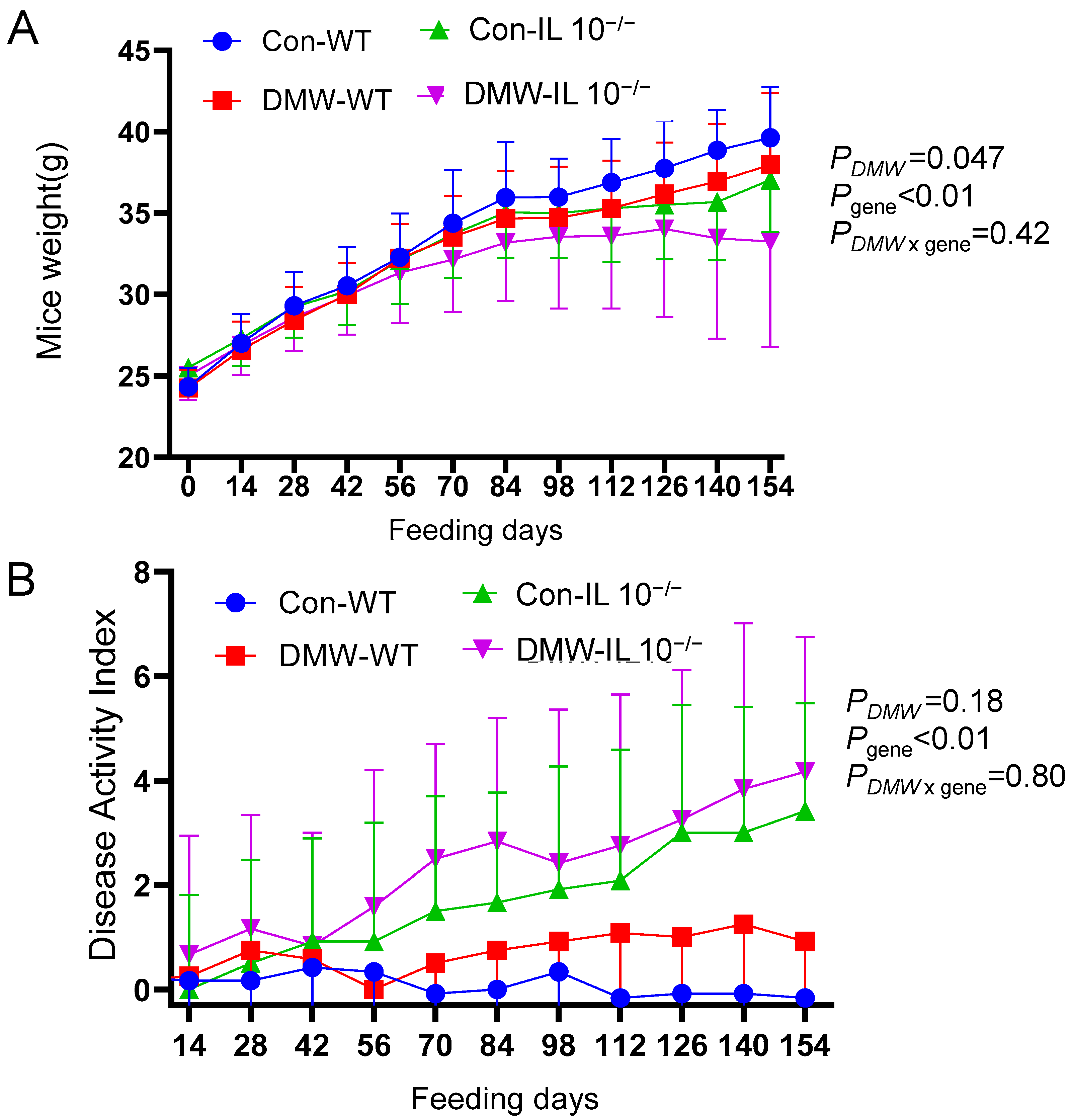
3.2. DMW Significantly Affected Body Weight but Not DAI
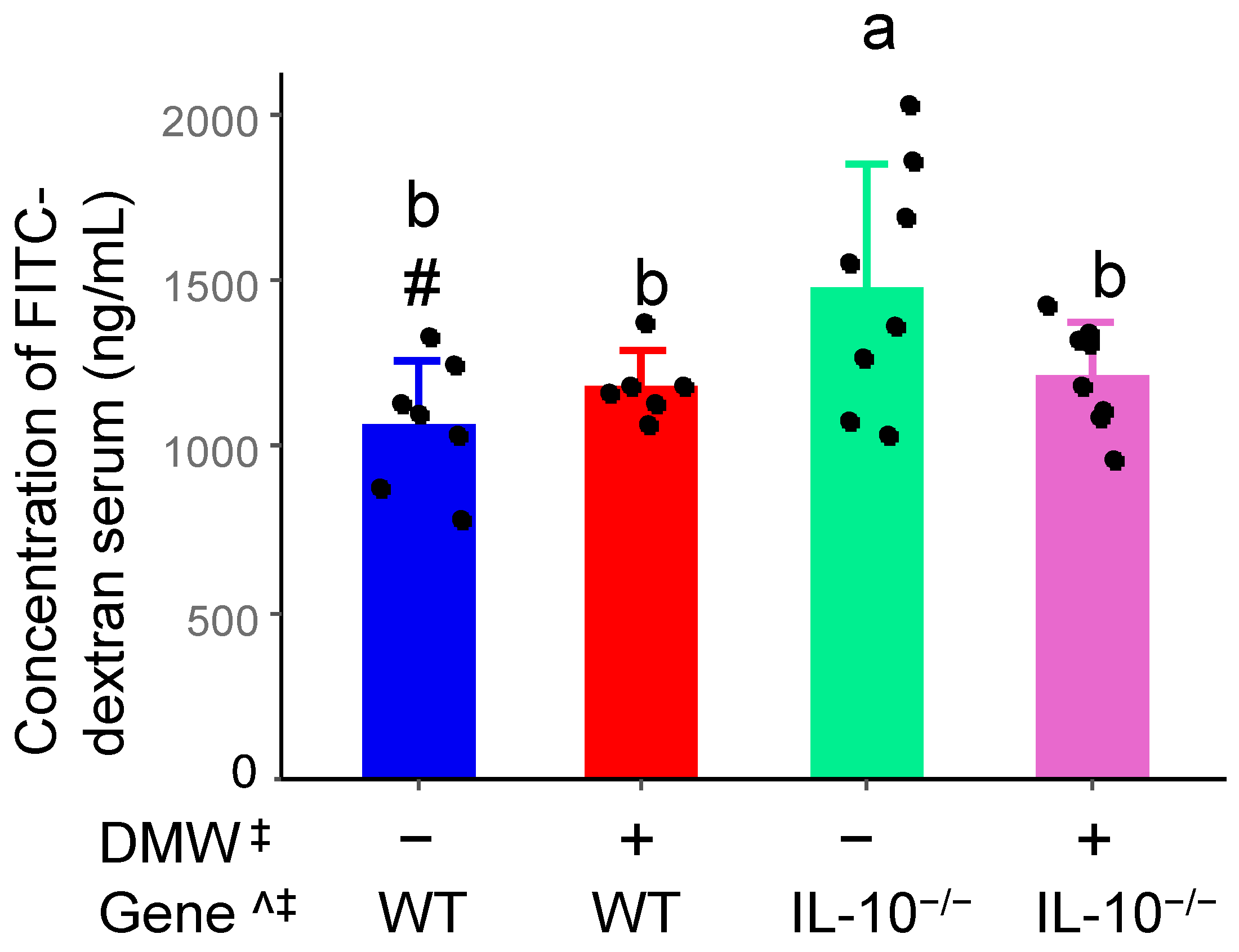
3.3. DMW Interacted with Gene to Reduce Intestinal Permeability in IL-10−/− Mice
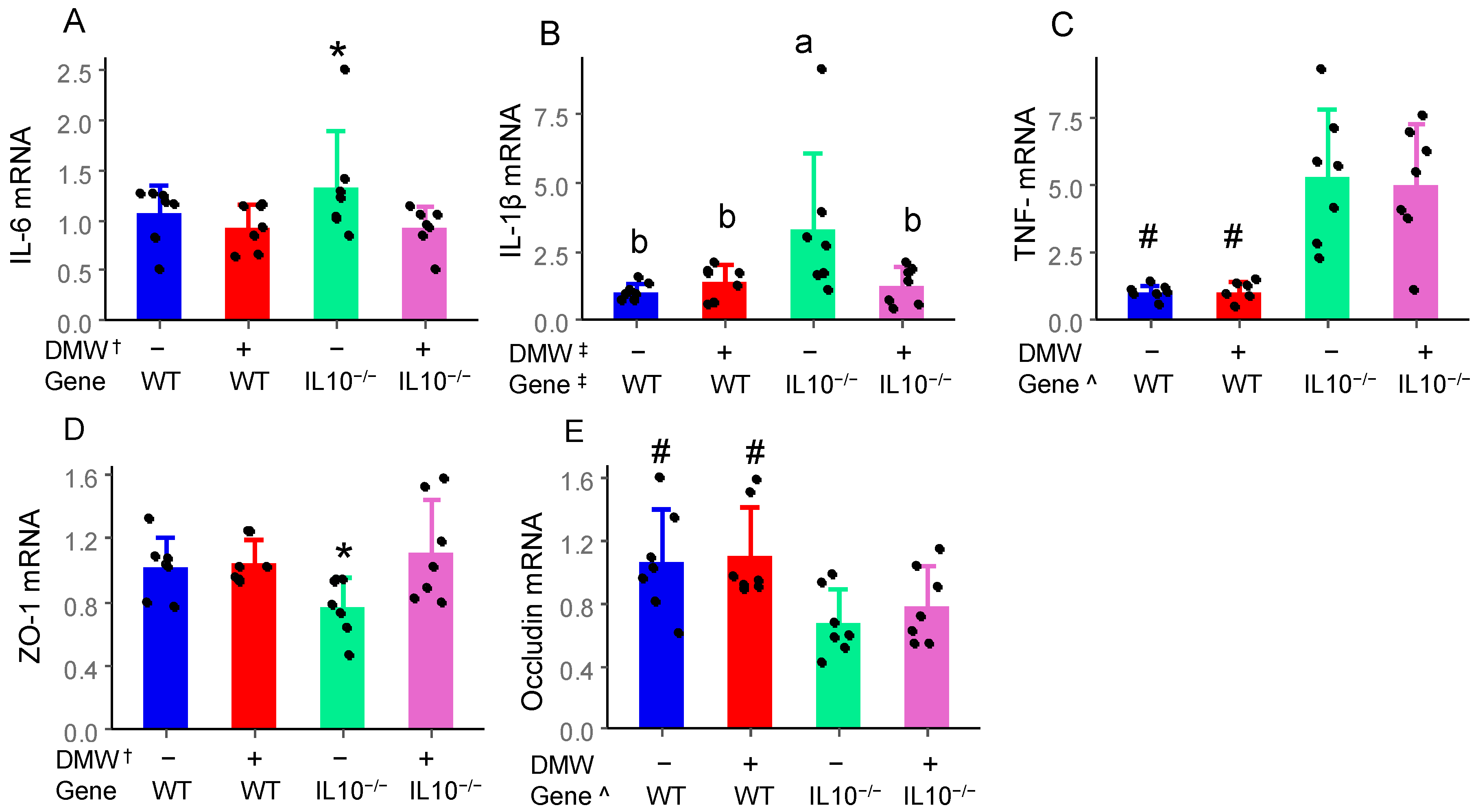
3.4. DMW, Gene, and Their Interactions Differentially Affect Gene Expression of Cytokines and Tight Junction Proteins
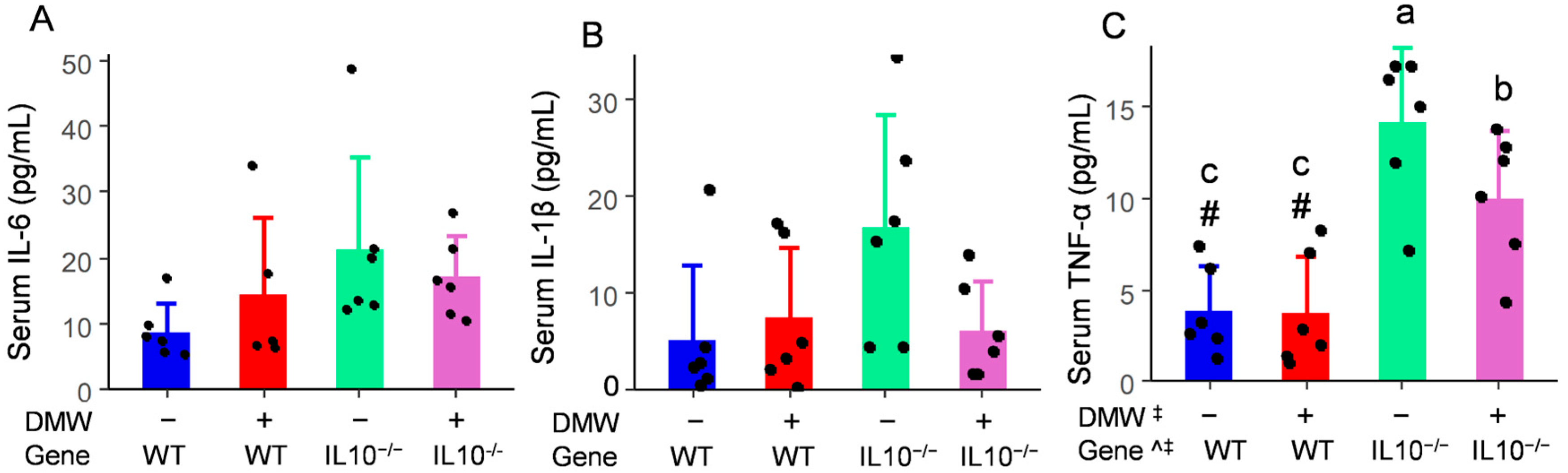
3.5. DMW × Gene Interaction Reduced the Serum TNF-α in IL-10−/− Mice
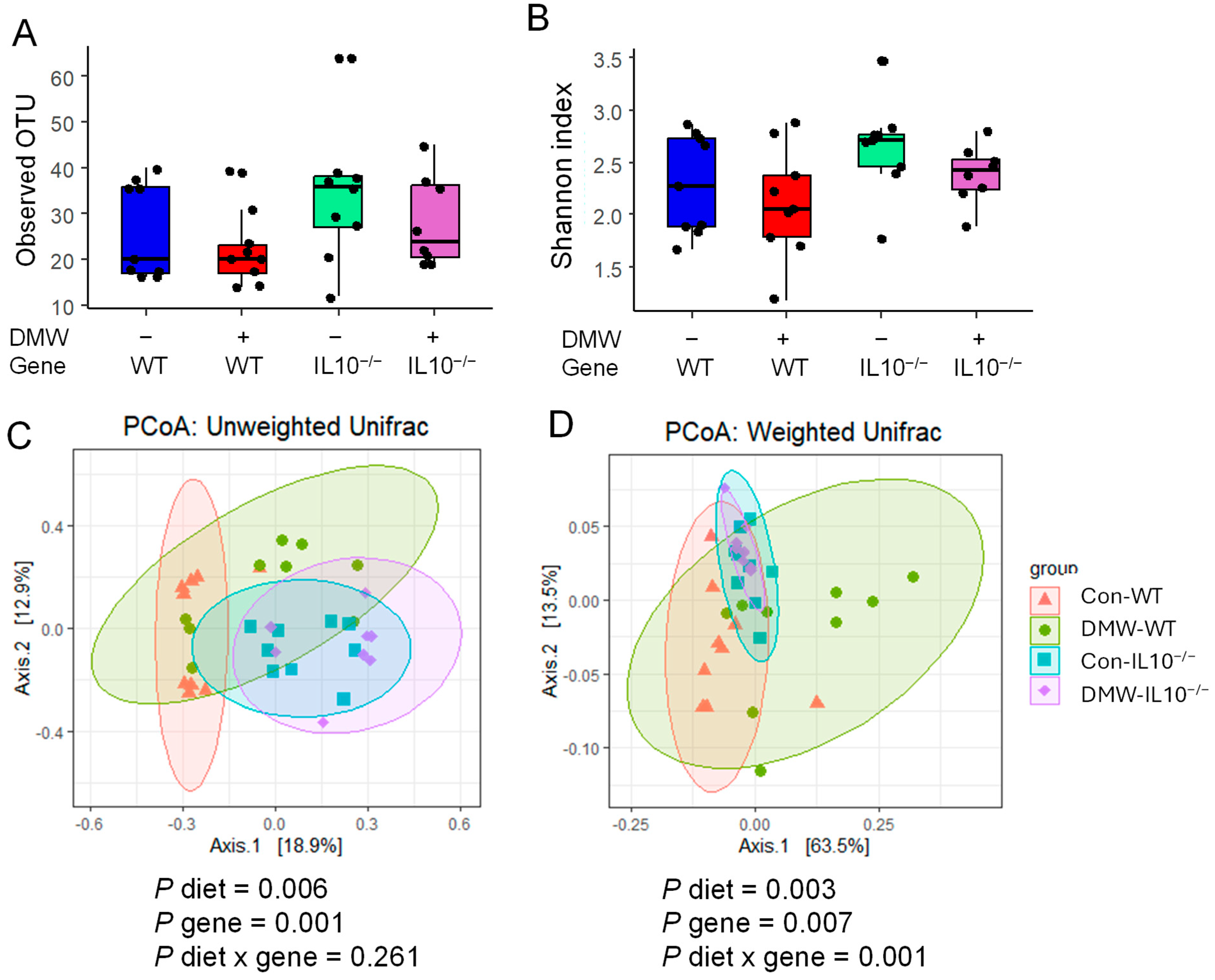
3.6. DMW Did Not Affect α-Diversity but Altered the Beta-Diversity of the Gut Microbiome
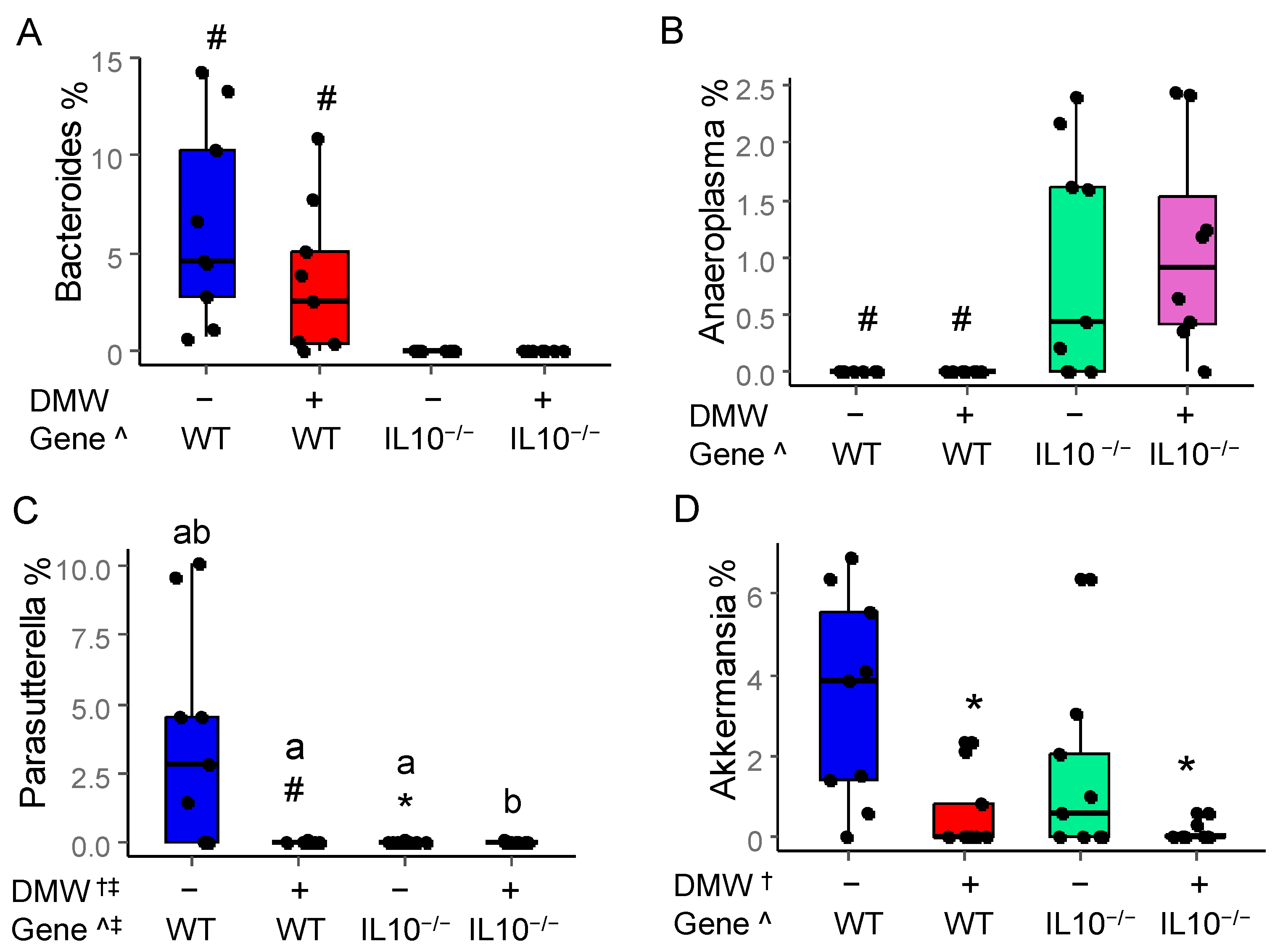
3.7. DMW and DMW × Gene Interaction Altered the Relative Abundance of Some Gut Microbes
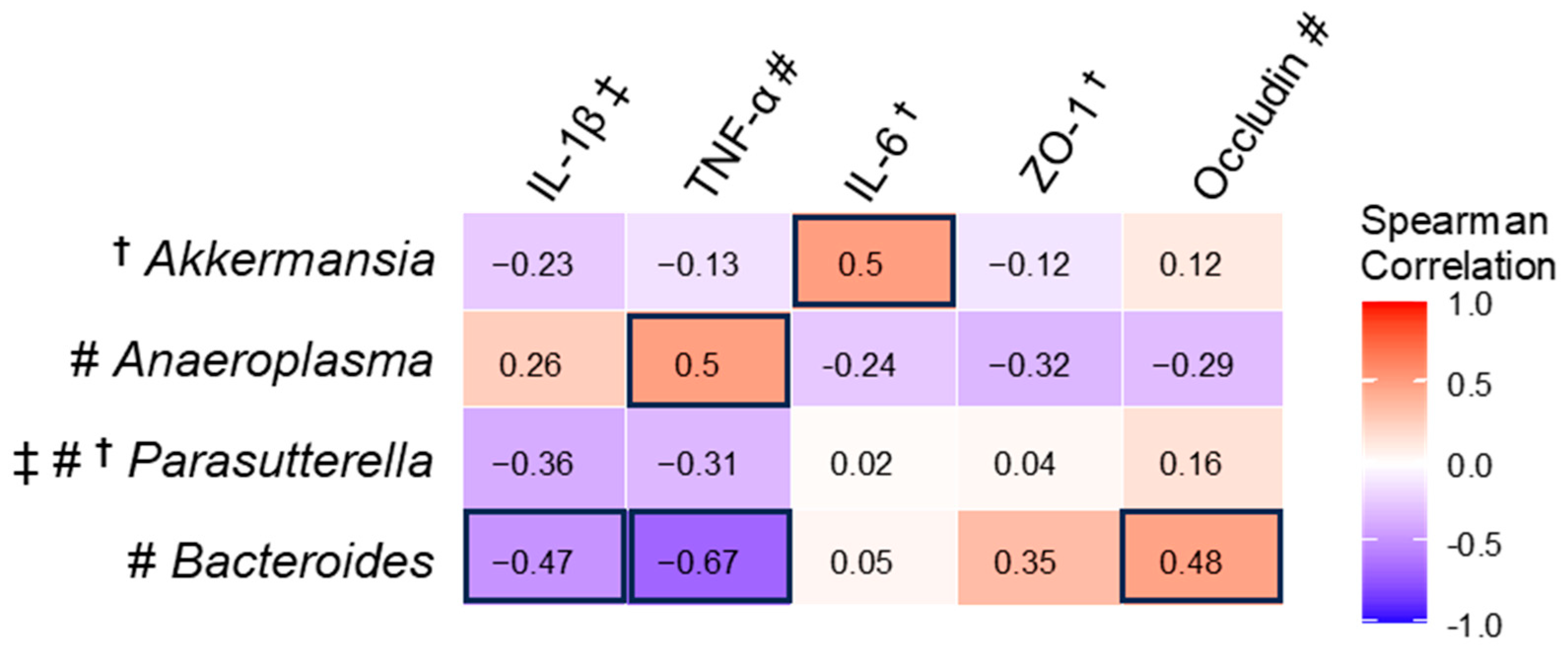
3.8. Spearman Correlation Between mRNA and Significantly Changed Gut Microbiota
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DAI | Disease activity index |
| DMW | Dealcoholized muscadine wine |
| DSS | Dextran sulfate sodium |
| FITC | Fluorescein isothiocyanate |
| IBD | Inflammatory bowel diseases |
| WT | Wild type |
References
- Hracs, L.; Windsor, J.W.; Gorospe, J.; Cummings, M.; Coward, S.; Buie, M.J.; Quan, J.; Goddard, Q.; Caplan, L.; Markovinović, A.; et al. Global IBD Visualization of Epidemiology Studies in the 21st Century (GIVES-21) Research Group Global evolution of inflammatory bowel disease across epidemiologic stages. Nature 2025, 642, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Fanizza, J.; Bencardino, S.; Allocca, M.; Furfaro, F.; Zilli, A.; Parigi, T.L.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S.; D’Amico, F. Inflammatory bowel disease and colorectal cancer. Cancers 2024, 16, 2943. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, M.; Wang, J.; Zhang, H.; Wang, Z.; Lei, Z.; Wang, C.; Chen, W. Hydroxytyrosol Ameliorates Colon Inflammation: Mechanistic Insights into Anti-Inflammatory Effects, Inhibition of the TLR4/NF-κB Signaling Pathway, Gut Microbiota Modulation, and Liver Protection. Foods 2025, 14, 1270. [Google Scholar] [CrossRef] [PubMed]
- Yaqin, Z.; Kehan, W.; Yi, Z.; Naijian, W.; Wei, Q.; Fei, M. Resveratrol alleviates inflammatory bowel disease by inhibiting JAK2/STAT3 pathway activity via the reduction of O-GlcNAcylation of STAT3 in intestinal epithelial cells. Toxicol. Appl. Pharmacol. 2024, 484, 116882. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Ren, Y.; Xue, P.; Sheng, Y.; Yang, Q.; Dai, Y.; Zhang, X.; Lin, Z.; Liu, T.; Geng, Y.; et al. Protective Effect of the Polyphenol Ligustroside on Colitis Induced with Dextran Sulfate Sodium in Mice. Nutrients 2024, 16, 522. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Kim, M.-H.; Sandhu, A.K.; Gao, C.; Gu, L. Muscadine grape (Vitis rotundifolia) or wine phytochemicals reduce intestinal inflammation in mice with dextran sulfate sodium-induced colitis. J. Agric. Food Chem. 2017, 65, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, P.; Bauer, J.D.; Pollock, S.H.; Gangemi, J.D.; Mayer, E.P.; Ghaffar, A.; Hargrove, J.L.; Hartle, D.K. Antiinflammatory properties of the muscadine grape (Vitis rotundifolia). J. Agric. Food Chem. 2005, 53, 8481–8484. [Google Scholar] [CrossRef] [PubMed]
- Bralley, E.E.; Hargrove, J.L.; Greenspan, P.; Hartle, D.K. Topical anti-inflammatory activities of Vitis rotundifolia (muscadine grape) extracts in the tetradecanoylphorbol acetate model of ear inflammation. J. Med. Food 2007, 10, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Keubler, L.M.; Buettner, M.; Häger, C.; Bleich, A. A Multihit Model: Colitis Lessons from the Interleukin-10-deficient Mouse. Inflamm. Bowel Dis. 2015, 21, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xue, Y.; Zhang, H.; Huang, Y.; Yang, G.; Du, M.; Zhu, M.-J. Dietary grape seed extract ameliorates symptoms of inflammatory bowel disease in IL10-deficient mice. Mol. Nutr. Food Res. 2013, 57, 2253–2257. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, G.P.; Whitlock, J.A.; Zhao, S.; Yagiz, Y.; Gu, L. Muscadine grapes (Vitis rotundifolia) and dealcoholized muscadine wine alleviated symptoms of colitis and protected against dysbiosis in mice exposed to dextran sulfate sodium. J. Funct. Foods 2020, 65, 103746. [Google Scholar] [CrossRef]
- Mishra, U.N.; Jena, D.; Sahu, C.; Devi, R.; Kumar, R.; Jena, R.; Irondi, E.A.; Rout, S.; Tiwari, R.K.; Lal, M.K.; et al. Nutrigenomics: An inimitable interaction amid genomics, nutrition and health. Innov. Food Sci. Emerg. Technol. 2022, 82, 103196. [Google Scholar] [CrossRef]
- Shaw, C.L.; Dolan, R.; Corsi, A.M.; Goodman, S.; Pearson, W. Exploring the barriers and triggers towards the adoption of low- and no-alcohol (NOLO) wines. Food Qual. Prefer. 2023, 110, 104932. [Google Scholar] [CrossRef]
- Shin, J.-M.; Hwang, Y.-O.; Tu, O.-J.; Jo, H.-B.; Kim, J.-H.; Chae, Y.-Z.; Rhu, K.-H.; Park, S.-K. Comparison of different methods to quantify fat classes in bakery products. Food Chem. 2013, 136, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Hurtubise, R.; Audiger, C.; Dominguez-Punaro, M.C.; Chabot-Roy, G.; Chognard, G.; Raymond-Marchand, L.; Coderre, L.; Chemtob, S.; Michnick, S.W.; Rioux, J.D.; et al. Induced and spontaneous colitis mouse models reveal complex interactions between IL-10 and IL-12/IL-23 pathways. Cytokine 2019, 121, 154738. [Google Scholar] [CrossRef] [PubMed]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yang, G.; Zhang, S.; Ross, C.F.; Zhu, M.-J. Goji Berry Modulates Gut Microbiota and Alleviates Colitis in IL-10-Deficient Mice. Mol. Nutr. Food Res. 2018, 62, e1800535. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.; Eibach, D.; Kops, F.; Brenneke, B.; Woltemate, S.; Schulze, J.; Bleich, A.; Gruber, A.D.; Muthupalani, S.; Fox, J.G.; et al. Intestinal microbiota composition of interleukin-10 deficient C57BL/6J mice and susceptibility to Helicobacter hepaticus-induced colitis. PLoS ONE 2013, 8, e70783. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, Y.; Fu, W.; Cox, A.D.; Lee, D.; Reddivari, L. Intermittent antibiotic treatment accelerated the development of colitis in IL-10 knockout mice. Biomed. Pharmacother. 2022, 146, 112486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Y.; Liu, G.; Hao, S.; Wang, C.; Wang, Y. Black rice anthocyanin-rich extract and rosmarinic acid, alone and in combination, protect against DSS-induced colitis in mice. Food Funct. 2018, 9, 2796–2808. [Google Scholar] [CrossRef] [PubMed]
- Larmonier, C.B.; Uno, J.K.; Lee, K.-M.; Karrasch, T.; Laubitz, D.; Thurston, R.; Midura-Kiela, M.T.; Ghishan, F.K.; Sartor, R.B.; Jobin, C.; et al. Limited effects of dietary curcumin on Th-1 driven colitis in IL-10 deficient mice suggest an IL-10-dependent mechanism of protection. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1079–G1091. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, X.; Zhang, R.; Ke, R.; Zhang, S.; Chen, Y. Intestinal barrier in inflammatory bowel disease: Mechanisms and treatment. J. Transl. Gastroenterol. 2025. [Google Scholar] [CrossRef]
- Yang, G.; Xue, Y.; Zhang, H.; Du, M.; Zhu, M.-J. Favourable effects of grape seed extract on intestinal epithelial differentiation and barrier function in IL10-deficient mice. Br. J. Nutr. 2015, 114, 15–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, N.; Shang, Y.-X. Epigallocatechin gallate ameliorates airway inflammation by regulating Treg/Th17 imbalance in an asthmatic mouse model. Int. Immunopharmacol. 2019, 72, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wang, L.; Yu, H.; Chen, D.; Zhu, W.; Sun, C. Pharmacological Effects of Polyphenol Phytochemicals on the JAK-STAT Signaling Pathway. Front. Pharmacol. 2021, 12, 716672. [Google Scholar] [CrossRef] [PubMed]
- Elhennawy, M.G.; Abdelaleem, E.A.; Zaki, A.A.; Mohamed, W.R. Cinnamaldehyde and hesperetin attenuate TNBS-induced ulcerative colitis in rats through modulation of the JAk2/STAT3/SOCS3 pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22730. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wu, Y.; Lu, D.; Pang, J.; Hu, J.; Zhang, X.; Wang, Z.; Zhang, G.; Wang, J. Polyphenol-rich diet mediates interplay between macrophage-neutrophil and gut microbiota to alleviate intestinal inflammation. Cell Death Dis. 2023, 14, 656. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, 6234. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, T.; Wu, B.; Fu, W.; Xu, B.; Pamuru, R.R.; Kennett, M.; Vanamala, J.K.P.; Reddivari, L. Anthocyanin-containing purple potatoes ameliorate DSS-induced colitis in mice. J. Nutr. Biochem. 2021, 93, 108616. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Mughal, R.S.; Warburton, P.; O’Regan, D.J.; Ball, S.G.; Porter, K.E. Mechanism of TNFalpha-induced IL-1alpha, IL-1beta and IL-6 expression in human cardiac fibroblasts: Effects of statins and thiazolidinediones. Cardiovasc. Res. 2007, 76, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Ni, J.; Zhang, M.; Xu, Y.; Li, Y.; Karim, N.; Chen, W. Mulberry Anthocyanins Ameliorate DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota. Antioxidants 2022, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Freitas, V.; Almeida, L.; Laranjinha, J. Red wine extract preserves tight junctions in intestinal epithelial cells under inflammatory conditions: Implications for intestinal inflammation. Food Funct. 2019, 10, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Christman, L.M.; Li, R.; Gu, L. Synergic interactions between polyphenols and gut microbiota in mitigating inflammatory bowel diseases. Food Funct. 2020, 11, 4878–4891. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, F.; He, X.; Fu, S.; Fan, B. Anthocyanin extract from black rice attenuates chronic inflammation in DSS-induced colitis mouse model by modulating the gut microbiota. Open Chem. 2023, 21, 20220288. [Google Scholar] [CrossRef]
- Moon, H.-J.; Cha, Y.-S.; Kim, K.-A. Blackcurrant Alleviates Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Foods 2023, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Germano, P.M.; Oh, S.; Wang, S.; Wang, J.; Lee, R.; Paige, H.; Yang, S.; Henning, S.M.; Zhong, J.; et al. Pomegranate Extract Improves Colitis in IL-10 Knockout Mice Fed a High Fat High Sucrose Diet. Mol. Nutr. Food Res. 2022, 66, e2100730. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Saier, M.H. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; He, Z.; Zhuo, Y.; Li, S.; Yang, W.; Hu, L.; Zhong, H. Rubidium chloride modulated the fecal microbiota community in mice. BMC Microbiol. 2021, 21, 46. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Peng, Y.; Chen, G.; Xie, M.; Dai, Z.; Huang, K.; Dong, W.; Zeng, X.; Sun, Y. Modulation of gut microbiota by Ilex kudingcha improves dextran sulfate sodium-induced colitis. Food Res. Int. 2019, 126, 108595. [Google Scholar] [CrossRef] [PubMed]
- Seregin, S.S.; Golovchenko, N.; Schaf, B.; Chen, J.; Pudlo, N.A.; Mitchell, J.; Baxter, N.T.; Zhao, L.; Schloss, P.D.; Martens, E.C.; et al. NLRP6 Protects Il10-/- Mice from Colitis by Limiting Colonization of Akkermansia muciniphila. Cell Rep. 2017, 19, 733–745. [Google Scholar] [CrossRef] [PubMed]
- López-Cauce, B.; Puerto, M.; García, J.J.; Ponce-Alonso, M.; Becerra-Aparicio, F.; Del Campo, R.; Peligros, I.; Fernández-Aceñero, M.J.; Gómez-Navarro, Y.; Lara, J.M.; et al. Akkermansia deficiency and mucin depletion are implicated in intestinal barrier dysfunction as earlier event in the development of inflammation in interleukin-10-deficient mice. Front. Microbiol. 2022, 13, 1083884. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Price, C.E.; Valls, R.A.; Ramsey, A.R.; Loeven, N.A.; Jones, J.T.; Barrack, K.E.; Schwartzman, J.D.; Royce, D.B.; Cramer, R.A.; Madan, J.C.; et al. Intestinal Bacteroides modulates inflammation, systemic cytokines, and microbial ecology via propionate in a mouse model of cystic fibrosis. MBio 2024, 15, e0314423. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Niu, M.; Bi, J.; Du, N.; Liu, S.; Yang, K.; Li, H.; Yao, J.; Du, Y.; Duan, Y. Protective effects of a new generation of probiotic Bacteroides fragilis against colitis in vivo and in vitro. Sci. Rep. 2023, 13, 15842. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, W.; Wang, Q.; Yang, L.; Bian, X.; Jiang, X.; Lv, L.; Yan, R.; Xia, J.; Han, S.; et al. The negative effect of Akkermansia muciniphila-mediated post-antibiotic reconstitution of the gut microbiota on the development of colitis-associated colorectal cancer in mice. Front. Microbiol. 2022, 13, 932047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, Y.; Jiang, Y.; Chen, M.; Ma, X.; Yu, X.; Ren, D.; Jiang, B. Akkermansia muciniphila ONE effectively ameliorates dextran sulfate sodium (DSS)-induced ulcerative colitis in mice. Npj Sci. Food 2024, 8, 97. [Google Scholar] [CrossRef] [PubMed]
| Polyphenols | Concentration (µg/mL) |
|---|---|
| Delphinidin-3,5-diglucoside | 1008.3 ± 62.7 |
| Cyanidin 3,5-diglucoside | 1000.5 ± 62.2 |
| Petunidin 3,5-diglucoside | 562.3 ± 36.7 |
| Pelargonidin 3,5-diglucoside | 49.9 ± 1.8 |
| Peonidin 3,5-diglucoside | 590.3 ± 39.6 |
| Malvidin 3,5-diglucoside | 243.5 ± 15.5 |
| Total anthocyanins | 3454.8 ± 217.9 |
| Myricetin | 106.1 ± 18.3 |
| Quercetin | 17.7 ± 2.5 |
| Kaempeferol | 3.7 ± 0.7 |
| Ellagic acid | 348.9 ± 16.9 |
| Total other polyphenols | 476.4 ± 36.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Gu, L. Effect of Dealcoholized Muscadine Wine on the Development of Spontaneous Colitis and Gut Microbiome in IL-10−/− Mice. Nutrients 2025, 17, 2327. https://doi.org/10.3390/nu17142327
Li H, Gu L. Effect of Dealcoholized Muscadine Wine on the Development of Spontaneous Colitis and Gut Microbiome in IL-10−/− Mice. Nutrients. 2025; 17(14):2327. https://doi.org/10.3390/nu17142327
Chicago/Turabian StyleLi, Hao, and Liwei Gu. 2025. "Effect of Dealcoholized Muscadine Wine on the Development of Spontaneous Colitis and Gut Microbiome in IL-10−/− Mice" Nutrients 17, no. 14: 2327. https://doi.org/10.3390/nu17142327
APA StyleLi, H., & Gu, L. (2025). Effect of Dealcoholized Muscadine Wine on the Development of Spontaneous Colitis and Gut Microbiome in IL-10−/− Mice. Nutrients, 17(14), 2327. https://doi.org/10.3390/nu17142327




