Cardiovascular Risk Factors, Alzheimer’s Disease, and the MIND Diet: A Narrative Review from Molecular Mechanisms to Clinical Outcomes
Abstract
1. Background
2. CVD Risk Factors and AD
2.1. Hypertension
2.2. Dyslipidemia
2.3. Diabetes Mellitus
2.4. Obesity
2.5. Smoking
2.6. Physical Inactivity
3. MIND Dietary Pattern and CVD Risk Factors
3.1. MIND Dietary Pattern and Hypertension
3.2. MIND Dietary Pattern and Dyslipidemia
3.3. MIND Dietary Pattern and Diabetes Mellitus
3.4. MIND Dietary Pattern and Obesity
4. MIND Dietary Pattern and AD
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-beta |
| AD | Alzheimer’s Disease |
| APOE | Apolipoprotein E |
| APOJ | Apolipoprotein J |
| APP | Amyloid Precursor Protein |
| BBB | Blood–Brain Barrier |
| BMI | Body Mass Index |
| CVDs | Cardiovascular Diseases |
| DASH | Dietary Approaches to Stop Hypertension |
| DM | Diabetes Mellitus |
| GSK-3β | glycogen synthase kinase-3β |
| HDL-C | High-Density Lipoprotein Cholesterol |
| HLP | Hyperlipidemia |
| HTN | Hypertension |
| LPSs | Lipopolysaccharides |
| LV | Left Ventricle |
| METs | Metabolic Equivalents |
| MIND | Mediterranean–DASH Intervention for Neurodegenerative Delay |
| MUFAs | Monounsaturated Fatty Acids |
| NF-κB | Nuclear Factor kappa B |
| NFTs | Neurofibrillary Tangles |
| NO | Nitric Oxide |
| PUFAs | Polyunsaturated Fatty Acids |
| RCTs | Randomized Controlled Trials |
| RAGE | Receptors for Advanced Glycation End |
| ROS | Reactive Oxygen Species |
| SCFAs | Short-Chain Fatty Acids |
| SORL1 | Sortilin-Related Receptor |
| T2DM | Type 2 Diabetes Mellitus |
| TCIM | Transcriptional and Immune-response Modulator |
| TG | Triglyceride |
| VLDL | Very Low-Density Lipoprotein |
| Wnt/β-catenin | wingless-related integration site/beta-catenin |
References
- Woodruff, R.C.; Tong, X.; Khan, S.S.; Shah, N.S.; Jackson, S.L.; Loustalot, F.; Vaughan, A.S. Trends in Cardiovascular Disease Mortality Rates and Excess Deaths, 2010–2022. Am. J. Prev. Med. 2024, 66, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Heart Disease Remains Leading Cause of Death as Key Health Risk Factors Continue to Rise: American Heart Association. 2025. Available online: https://newsroom.heart.org/news/heart-disease-remains-leading-cause-of-death-as-key-health-risk-factors-continue-to-rise (accessed on 27 January 2025).
- Kazi, D.S.; Elkind, M.S.V.; Deutsch, A.; Dowd, W.N.; Heidenreich, P.; Khavjou, O.; Mark, D.; Mussolino, M.E.; Ovbiagele, B.; Patel, S.S.; et al. Forecasting the Economic Burden of Cardiovascular Disease and Stroke in the United States Through 2050: A Presidential Advisory From the American Heart Association. Circulation 2024, 150, e89–e101. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Gerhardt, T.E.; Kwon, E. Risk Factors for Coronary Artery Disease. In StatPearls; Copyright © 2024, StatPearls Publishing LLC.; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Artola Arita, V.; Beigrezaei, S.; Franco, O.H. Risk factors for cardiovascular disease: The known unknown. Eur. J. Prev. Cardiol. 2023, 31, e106–e107. [Google Scholar] [CrossRef] [PubMed]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Rodríguez Guzmán, R.; Centofanti, F.; Doldo, E.; Céspedes Miranda, E.M.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Merdler, I.; Case, B.C.; Waksman, O.; Porto, I. Targeting inflammation in atherosclerosis: Overview, strategy and directions. EuroIntervention 2024, 20, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Sitia, S.; Tomasoni, L.; Atzeni, F.; Ambrosio, G.; Cordiano, C.; Catapano, A.; Tramontana, S.; Perticone, F.; Naccarato, P.; Camici, P.; et al. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev. 2010, 9, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Attems, J.; Jellinger, K.A. The overlap between vascular disease and Alzheimer’s disease--lessons from pathology. BMC Med. 2014, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Casserly, I.; Topol, E. Convergence of atherosclerosis and Alzheimer’s disease: Inflammation, cholesterol, and misfolded proteins. Lancet 2004, 363, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 2024, 20, 3708–3821. [CrossRef]
- Park, S.H.; Kim, J.H.; Choi, K.H.; Jang, Y.J.; Bae, S.S.; Choi, B.T.; Shin, H.K. Hypercholesterolemia accelerates amyloid β-induced cognitive deficits. Int. J. Mol. Med. 2013, 31, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, D.; Mascio, G.; D’Andrea, I.; Fardella, V.; Bell, R.D.; Branchi, I.; Pallante, F.; Zlokovic, B.; Yan, S.S.; Lembo, G. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 2012, 60, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; O’Bryant, S.E.; Johnson, L.A.; Rissman, R.A.; Yaffe, K.; The Health and Aging Brain Study (HABS-HD) Study Team. Association of cardiovascular risk factors and blood biomarkers with cognition: The HABS-HD study. Alzheimers Dement. 2023, 15, e12394. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Jia, Y.; Sowers, J.R. Contribution of Maladaptive Adipose Tissue Expansion to Development of Cardiovascular Disease. Compr. Physiol. 2016, 7, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, T.; Li, P.; Wei, N.; Zhao, Z.; Liang, H.; Ji, X.; Chen, W.; Xue, M.; Wei, J. The Ambiguous Relationship of Oxidative Stress, Tau Hyperphosphorylation, and Autophagy Dysfunction in Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2015, 2015, 352723. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, S.; Hermkens, D.M.A.; van der Weerd, L.; de Vries, H.E.; Daemen, M.J.A.P. Vascular Hypothesis of Alzheimer Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1265–1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, M.; Li, W.; Liu, X.; Zhu, M.; Qin, H. Biomarkers associated with the pathogenesis of Alzheimer’s disease. Front. Cell. Neurosci. 2023, 17, 1279046. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, S.; Hu, X.; Chen, F.; Li, D. A Review of Healthy Dietary Choices for Cardiovascular Disease: From Individual Nutrients and Foods to Dietary Patterns. Nutrients 2023, 15, 4898. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Przytuła, A.; Szymańska, M.; Popiołek-Kalisz, J. Dietary strategies for cardiovascular disease risk factors prevention. Curr. Probl. Cardiol. 2024, 49, 102746. [Google Scholar] [CrossRef] [PubMed]
- Zampelas, A.; Magriplis, E. Dietary patterns and risk of cardiovascular diseases: A review of the evidence. Proc. Nutr. Soc. 2020, 79, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Appel Lawrence, J.; Moore Thomas, J.; Obarzanek, E.; Vollmer William, M.; Svetkey Laura, P.; Sacks Frank, M.; Bray George, A.; Vogt Thomas, M.; Cutler Jeffrey, A.; Windhauser Marlene, M.; et al. A Clinical Trial of the Effects of Dietary Patterns on Blood Pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimers Dement. J. Alzheimers Assoc. 2015, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Barnes, L.L.; Bennett, D.; Aggarwal, N. O2-02-04: Mind Diet Score More Predictive than Dash or Mediterranean Diet Scores. Alzheimers Dement. 2014, 10, P166. [Google Scholar] [CrossRef]
- Kheirouri, S.; Alizadeh, M. MIND diet and cognitive performance in older adults: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8059–8077. [Google Scholar] [CrossRef] [PubMed]
- van Soest, A.P.M.; Beers, S.; van de Rest, O.; de Groot, L.C. The Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) Diet for the Aging Brain: A Systematic Review. Adv. Nutr. 2024, 15, 100184. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, A.; Ziaei, R.; Talebi, S.; Barghchi, H.; Nattagh-Eshtivani, E.; Moradi, S.; Rahbarinejad, P.; Mohammadi, H.; Ghasemi-Tehrani, H.; Marx, W.; et al. Soluble Fiber Supplementation and Serum Lipid Profile: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Lecumberri, E.; Goya, L.; Mateos, R.; Alía, M.; Ramos, S.; Izquierdo-Pulido, M.; Bravo, L. A diet rich in dietary fiber from cocoa improves lipid profile and reduces malondialdehyde in hypercholesterolemic rats. Nutrition 2007, 23, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Luo, F. Dietary Fiber: An Opportunity for a Global Control of Hyperlipidemia. Oxidative Med. Cell. Longev. 2021, 2021, 5542342. [Google Scholar] [CrossRef] [PubMed]
- Dayib, M.; Larson, J.; Slavin, J. Dietary fibers reduce obesity-related disorders: Mechanisms of action. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Deehan, E.C.; Mocanu, V.; Madsen, K.L. Effects of dietary fibre on metabolic health and obesity. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Houston, M. The role of magnesium in hypertension and cardiovascular disease. J. Clin. Hypertens 2011, 13, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Capuano, A.W.; Agarwal, P.; Arvanitakis, Z.; Wang, Y.; De Jager, P.L.; Schneider, J.A.; Tasaki, S.; de Paiva Lopes, K.; Hu, F.B.; et al. The MIND diet, brain transcriptomic alterations, and dementia. Alzheimers Dement. 2024, 20, 5996–6007. [Google Scholar] [CrossRef] [PubMed]
- Akbar, Z.; Fituri, S.; Ouagueni, A.; Alalwani, J.; Sukik, A.; Al-Jayyousi, G.F.; Bassil, M.; Tayyem, R. Associations of the MIND Diet with Cardiometabolic Diseases and Their Risk Factors: A Systematic Review. Diabetes Metab. Syndr. Obes. 2023, 16, 3353–3371. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kirabo, A. Hypertensive heart disease: Risk factors, complications and mechanisms. Front. Cardiovasc. Med. 2023, 10, 1205475. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, R.A.; Sidney, S.; Selby, J.; Johnston, S.C.; Yaffe, K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005, 64, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Skoog, I.; Gustafson, D. Update on hypertension and Alzheimer’s disease. Neurol. Res. 2006, 28, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Lennon, M.J.; Koncz, R.; Sachdev, P.S. Hypertension and Alzheimer’s disease: Is the picture any clearer? Curr. Opin. Psychiatry 2021, 34, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Hannesdottir, K.; Nitkunan, A.; Charlton, R.A.; Barrick, T.R.; MacGregor, G.A.; Markus, H.S. Cognitive impairment and white matter damage in hypertension: A pilot study. Acta Neurol. Scand. 2009, 119, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.; Tarantini, S.; Springo, Z.; Tucsek, Z.; Gautam, T.; Giles, C.B.; Wren, J.D.; Koller, A.; Sonntag, W.E.; Csiszar, A.; et al. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: Role of resveratrol treatment in vasoprotection. Aging Cell 2015, 14, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, A.; Conant, K. Matrix metalloproteinases, synaptic injury, and multiple sclerosis. Front. Psychiatry 2010, 1, 130. [Google Scholar] [CrossRef] [PubMed]
- Papuć, E.; Rejdak, K. The role of myelin damage in Alzheimer’s disease pathology. Arch. Med. Sci. 2020, 16, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.S.; Li, T.D.; Zeng, Z.H. Mechanisms underlying direct actions of hyperlipidemia on myocardium: An updated review. Lipids Health Dis. 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C. Dyslipidemia and the risk of Alzheimer’s disease. Curr. Atheroscler. Rep. 2013, 15, 307. [Google Scholar] [CrossRef] [PubMed]
- Notkola, I.L.; Sulkava, R.; Pekkanen, J.; Erkinjuntti, T.; Ehnholm, C.; Kivinen, P.; Tuomilehto, J.; Nissinen, A. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology 1998, 17, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kalmijn, S.; Foley, D.; White, L.; Burchfiel, C.M.; Curb, J.D.; Petrovitch, H.; Ross, G.W.; Havlik, R.J.; Launer, L.J. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2255–2260. [Google Scholar] [CrossRef] [PubMed]
- Hayden, K.M.; Zandi, P.P.; Lyketsos, C.G.; Khachaturian, A.S.; Bastian, L.A.; Charoonruk, G.; Tschanz, J.T.; Norton, M.C.; Pieper, C.F.; Munger, R.G.; et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: The Cache County study. Alzheimer Dis. Assoc. Disord. 2006, 20, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Pitas, R.E.; Boyles, J.K.; Lee, S.H.; Hui, D.; Weisgraber, K.H. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B,E(LDL) receptors in the brain. J. Biol. Chem. 1987, 262, 14352–14360. [Google Scholar] [CrossRef] [PubMed]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Cheng, R.; Rogaeva, E.; Lee, J.H.; Tokuhiro, S.; Zou, F.; Bettens, K.; Sleegers, K.; Tan, E.K.; Kimura, R.; et al. Meta-analysis of the association between variants in SORL1 and Alzheimer disease. Arch. Neurol. 2011, 68, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Gelissen, I.C.; Hochgrebe, T.; Wilson, M.R.; Easterbrook-Smith, S.B.; Jessup, W.; Dean, R.T.; Brown, A.J. Apolipoprotein J (clusterin) induces cholesterol export from macrophage-foam cells: A potential anti-atherogenic function? Biochem. J. 1998, 331 Pt 1, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Kim, W.S.; Kwok, J.B.; Hill, A.F.; Cappai, R.; Rye, K.A.; Garner, B. ATP-binding cassette transporter A7 regulates processing of amyloid precursor protein in vitro. J. Neurochem. 2008, 106, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Grziwa, B.; Grimm, M.O.; Masters, C.L.; Beyreuther, K.; Hartmann, T.; Lichtenthaler, S.F. The transmembrane domain of the amyloid precursor protein in microsomal membranes is on both sides shorter than predicted. J. Biol. Chem. 2003, 278, 6803–6808. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.W. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol. Life Sci. 2003, 60, 1158–1171. [Google Scholar] [CrossRef] [PubMed]
- Green, R.C.; McNagny, S.E.; Jayakumar, P.; Cupples, L.A.; Benke, K.; Farrer, L.A. Statin use and the risk of Alzheimer’s disease: The MIRAGE study. Alzheimers Dement. 2006, 2, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Larson, E.B.; Sonnen, J.A.; Shofer, J.B.; Petrie, E.C.; Schantz, A.; Peskind, E.R.; Raskind, M.A.; Breitner, J.C.; Montine, T.J. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology 2007, 69, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Masse, I.; Bordet, R.; Deplanque, D.; Al Khedr, A.; Richard, F.; Libersa, C.; Pasquier, F. Lipid lowering agents are associated with a slower cognitive decline in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Kirkland, S.; Hogan, D.B.; MacKnight, C.; Merry, H.; Verreault, R.; Wolfson, C.; McDowell, I. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch. Neurol. 2002, 59, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.W.; Kivipelto, M.; Feldman, H.; Sparks, L.; Doody, R.; Waters, D.D.; Hey-Hadavi, J.; Breazna, A.; Schindler, R.J.; Ramos, H. The Atorvastatin/Donepezil in Alzheimer’s Disease Study (LEADe): Design and baseline characteristics. Alzheimers Dement. 2008, 4, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.H.; Doody, R.S.; Kivipelto, M.; Sparks, D.L.; Waters, D.D.; Jones, R.W.; Schwam, E.; Schindler, R.; Hey-Hadavi, J.; DeMicco, D.A.; et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology 2010, 74, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Trompet, S.; van Vliet, P.; de Craen, A.J.; Jolles, J.; Buckley, B.M.; Murphy, M.B.; Ford, I.; Macfarlane, P.W.; Sattar, N.; Packard, C.J.; et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J. Neurol. 2010, 257, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L.; Connor, D.J.; Sabbagh, M.N.; Petersen, R.B.; Lopez, J.; Browne, P. Circulating cholesterol levels, apolipoprotein E genotype and dementia severity influence the benefit of atorvastatin treatment in Alzheimer’s disease: Results of the Alzheimer’s Disease Cholesterol-Lowering Treatment (ADCLT) trial. Acta Neurol. Scand. 2006, 114, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, S.J.; Looker, H.C.; Hothersall, E.J.; Wild, S.H.; Lindsay, R.S.; Chalmers, J.; Cleland, S.; Leese, G.P.; McKnight, J.; Morris, A.D.; et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012, 9, e1001321. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, M.; Anderson, K.M.; Wilson, P.W.; Levy, D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am. J. Cardiol. 1991, 68, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Basu, A.K.; Roychowdhury, P.; Banerjee, R.; Singhania, P.; Singh, S.; Datta, U.K. Comparison of left ventricular mass in normotensive type 2 diabetes mellitus patients with that in the nondiabetic population. J. Cardiovasc. Dis. Res. 2011, 2, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Barouch, L.A.; Berkowitz, D.E.; Harrison, R.W.; O’Donnell, C.P.; Hare, J.M. Disruption of Leptin Signaling Contributes to Cardiac Hypertrophy Independently of Body Weight in Mice. Circulation 2003, 108, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Oh, J.K.; Sakata, S.; Liang, I.; Park, W.; Hajjar, R.J.; Lebeche, D. Role of resistin in cardiac contractility and hypertrophy. J. Mol. Cell Cardiol. 2008, 45, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.; Gheorghiade, M. Management of the patient with diabetes mellitus and myocardial infarction: Clinical trials update. Am. J. Med. 2004, 116 (Suppl. S5A), 47s–63s. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J. Type 2 diabetes mellitus and Alzheimer’s disease. World J. Diabetes 2014, 5, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Yang, F.; Li, J.; Guo, W.; Zhang, C.; Gao, F.; Sun, X.; Zhou, Y.; Zhang, W. The relationship between diabetes and the dementia risk: A meta-analysis. Diabetol. Metab. Syndr. 2024, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, W. Molecular links between Alzheimer’s disease and diabetes mellitus. Neuroscience 2013, 250, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Vekrellis, K.; Ye, Z.; Qiu, W.Q.; Walsh, D.; Hartley, D.; Chesneau, V.; Rosner, M.R.; Selkoe, D.J. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J. Neurosci. 2000, 20, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Hamzé, R.; Delangre, E.; Tolu, S.; Moreau, M.; Janel, N.; Bailbé, D.; Movassat, J. Type 2 Diabetes Mellitus and Alzheimer’s Disease: Shared Molecular Mechanisms and Potential Common Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 15287. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M. Contributions of brain insulin resistance and deficiency in amyloid-related neurodegeneration in Alzheimer’s disease. Drugs 2012, 72, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Rorbach-Dolata, A.; Piwowar, A. Neurometabolic Evidence Supporting the Hypothesis of Increased Incidence of Type 3 Diabetes Mellitus in the 21st Century. BioMed Res. Int. 2019, 2019, 1435276. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cuevas, J.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Monroy-Ramírez, H.C.; Galicia-Moreno, M.; García-Bañuelos, J.; Santos, A.; Armendariz-Borunda, J. Molecular Mechanisms of Obesity-Linked Cardiac Dysfunction: An Up-Date on Current Knowledge. Cells 2021, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Paz Ocaranza, M.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Razay, G.; Vreugdenhil, A.; Wilcock, G. Obesity, Abdominal Obesity and Alzheimer Disease. Dement. Geriatr. Cogn. Disord. 2006, 22, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-Analysis of Alzheimer’s Disease Risk with Obesity, Diabetes, and Related Disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Picone, P.; Di Carlo, M.; Nuzzo, D. Obesity and Alzheimer’s disease: Molecular bases. Eur. J. Neurosci. 2020, 52, 3944–3950. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Huang, S.M.; Zhang, B. Mechanisms Underlying Obesity-induced Aβ Accumulation in Alzheimer’s Disease: A Qualitative Review. J. Integr. Neurosci. 2024, 23, 163. [Google Scholar] [CrossRef] [PubMed]
- Puig, K.L.; Floden, A.M.; Adhikari, R.; Golovko, M.Y.; Combs, C.K. Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS ONE 2012, 7, e30378. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Martin, J.M.; Maple, R.L.; Tharp, W.G.; Pratley, R.E. Plasma amyloid-beta peptide levels correlate with adipocyte amyloid precursor protein gene expression in obese individuals. Neuroendocrinology 2009, 90, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Khan Minhas, A.M.; Sedhom, R.; Jean, E.D.; Shapiro, M.D.; Panza, J.A.; Alam, M.; Virani, S.S.; Ballantyne, C.M.; Abramov, D. Global burden of cardiovascular disease attributable to smoking, 1990–2019: An analysis of the 2019 Global Burden of Disease Study. Eur. J. Prev. Cardiol. 2024, 31, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Shimu, S.J.; Patil, S.M.; Dadzie, E.; Tesfaye, T.; Alag, P.; Więckiewicz, G. Exploring Health Informatics in the Battle against Drug Addiction: Digital Solutions for the Rising Concern. J. Pers. Med. 2024, 14, 556. [Google Scholar] [CrossRef] [PubMed]
- Messner, B.; Bernhard, D. Smoking and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Talukder, M.A.; Johnson, W.M.; Varadharaj, S.; Lian, J.; Kearns, P.N.; El-Mahdy, M.A.; Liu, X.; Zweier, J.L. Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H388–H396. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, D.; Wang, X.L. Smoking, oxidative stress and cardiovascular diseases--do anti-oxidative therapies fail? Curr. Med. Chem. 2007, 14, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Ott, A.; Slooter, A.J.C.; Hofman, A.; van Harskamp, F.; Witteman, J.C.M.; Van Broeckhoven, C.; van Duijn, C.M.; Breteler, M.M.B. Smoking and risk of dementia and Alzheimer’s disease in a population-based cohort study: The Rotterdam Study. Lancet 1998, 351, 1840–1843. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.A.; Trares, K.; Kohl, M.; Jansen, E.; Brenner, H.; Schöttker, B. Long-term effects of smoking on serum concentrations of oxidative stress biomarkers: Results of a large, population-based cohort study. Environ. Res. 2022, 204, 111923. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, T.C.; Mattsson, N.; Weiner, M.W.; Alzheimer’s Disease Neuroimaging, I. Smoking and increased Alzheimer’s disease risk: A review of potential mechanisms. Alzheimers Dement. 2014, 10, S122–S145. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, G.T.; Chami, B.; Youssef, P.; Witting, P.K. Oxidative stress in Alzheimer’s disease: Primary villain or physiological by-product? Redox Rep. 2013, 18, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D.; Clark, C.M.; Liun, F.; Rokach, J.; Lee, V.Y.; Trojanowski, J.Q. Increase of brain oxidative stress in mild cognitive impairment: A possible predictor of Alzheimer disease. Arch. Neurol. 2002, 59, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Giunta, B.; Deng, J.; Jin, J.; Sadic, E.; Rum, S.; Zhou, H.; Sanberg, P.; Tan, J. EVALUATION OF HOW CIGARETTE SMOKE IS A DIRECT RISK FACTOR FOR ALZHEIMER’S DISEASE. Technol. Innov. 2012, 14, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Friedenreich, C.; Shiroma, E.J.; Lee, I.M. Physical inactivity and non-communicable disease burden in low-income, middle-income and high-income countries. Br. J. Sports Med. 2022, 56, 101. [Google Scholar] [CrossRef] [PubMed]
- Swindell, N.; Mackintosh, K.; McNarry, M.; Stephens, J.W.; Sluik, D.; Fogelholm, M.; Drummen, M.; MacDonald, I.; Martinez, J.A.; Handjieva-Darlenska, T.; et al. Objectively Measured Physical Activity and Sedentary Time Are Associated with Cardiometabolic Risk Factors in Adults with Prediabetes: The PREVIEW Study. Diabetes Care 2018, 41, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Di Raimondo, D.; Musiari, G.; Rizzo, G.; Tuttolomondo, A.; Pinto, A. Effects of physical inactivity in cardiovascular biomarkers. J. Lab. Precis. Med. 2020, 5, 21. [Google Scholar] [CrossRef]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Fu, W.; Wang, C.; Mao, J.; Liu, B.; Zou, L.; Lv, C. Association between sedentary behavior and the risk of dementia: A systematic review and meta-analysis. Transl. Psychiatry 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Thivel, D.; Tremblay, A.; Genin, P.M.; Panahi, S.; Rivière, D.; Duclos, M. Physical Activity, Inactivity, and Sedentary Behaviors: Definitions and Implications in Occupational Health. Front. Public Health 2018, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M.; On Behalf Of SBRN Terminology Consensus Project Participants. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Diniz, D.G.; Bento-Torres, J.; da Costa, V.O.; Carvalho, J.P.; Tomás, A.M.; Galdino de Oliveira, T.C.; Soares, F.C.; de Macedo, L.D.; Jardim, N.Y.; Bento-Torres, N.V.; et al. The Hidden Dangers of Sedentary Living: Insights into Molecular, Cellular, and Systemic Mechanisms. Int. J. Mol. Sci. 2024, 25, 10757. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, M.; Foss, J.F.; Giordano, T. Neuroinflammation, Its Role in Alzheimer’s Disease and Therapeutic Strategies. J. Prev. Alzheimers Dis. 2023, 10, 686–698. [Google Scholar] [CrossRef] [PubMed]
- López-Ortiz, S.; Pinto-Fraga, J.; Valenzuela, P.L.; Martín-Hernández, J.; Seisdedos, M.M.; García-López, O.; Toschi, N.; Di Giuliano, F.; Garaci, F.; Mercuri, N.B.; et al. Physical Exercise and Alzheimer’s Disease: Effects on Pathophysiological Molecular Pathways of the Disease. Int. J. Mol. Sci. 2021, 22, 2897. [Google Scholar] [CrossRef] [PubMed]
- Spielman, L.J.; Little, J.P.; Klegeris, A. Physical activity and exercise attenuate neuroinflammation in neurological diseases. Brain Res. Bull. 2016, 125, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Mee-Inta, O.; Zhao, Z.W.; Kuo, Y.M. Physical Exercise Inhibits Inflammation and Microglial Activation. Cells 2019, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Odum, J.; Shunnarah, J.G.; Austin, N.; Kaddoumi, A. Blood-Brain Barrier Breakdown in Alzheimer’s Disease: Mechanisms and Targeted Strategies. Int. J. Mol. Sci. 2023, 24, 16288. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chang, Z.g.; Cui, K.; Song, C.; Cai, Z.; Shi, B.; Dong, Q.; Dou, K. The value of the MIND diet in the primary and secondary prevention of hypertension: A cross-sectional and longitudinal cohort study from NHANES analysis. Front. Nutr. 2023, 10, 1129667. [Google Scholar] [CrossRef] [PubMed]
- Zare, S.; Eftekhari, M.H.; Arjmand, G.; Zare, M. Adherence to Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) Dietary Pattern in Elderly with Type 2 Diabetes and the Correlation with Cognitive Functions and Metabolic Profile. Int. J. Nutr. Sci. 2023, 8, 102–108. [Google Scholar] [CrossRef]
- Holthaus, T.A.; Sethi, S.; Cannavale, C.N.; Aguiñaga, S.; Burd, N.A.; Holscher, H.D.; Khan, N.A. MIND dietary pattern adherence is inversely associated with visceral adiposity and features of metabolic syndrome. Nutr. Res. 2023, 116, 69–79. [Google Scholar] [CrossRef]
- Yau, K.Y.; Law, P.S.; Wong, C.N. Cardiac and Mental Benefits of Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet plus Forest Bathing (FB) versus MIND Diet among Older Chinese Adults: A Randomized Controlled Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 14665. [Google Scholar] [CrossRef] [PubMed]
- Gholami, Z.; Maracy, M.R.; Paknahad, Z. The effects of MIND diet and propolis supplementation on metabolic syndrome: A randomized controlled clinical trial. Heliyon 2024, 10, e34493. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, G.; Abbas-Zadeh, M.; Fardaei, M.; Eftekhari, M.H. The Effect of Short-term Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet on Hunger Hormones, Anthropometric Parameters, and Brain Structures in Middle-aged Overweight and Obese Women: A Randomized Controlled Trial. Iran. J. Med. Sci. 2022, 47, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.M.; Rabiee, A.; El Refaye, G.E.; Elsisi, H.F. Aerobic Exercise with Mediterranean-DASH Intervention for Neurodegenerative Delay Diet Promotes Brain Cells’ Longevity despite Sex Hormone Deficiency in Postmenopausal Women: A Randomized Controlled Trial. Oxidative Med. Cell. Longev. 2022, 2022, 4146742. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.L.; Dhana, K.; Liu, X.; Carey, V.J.; Ventrelle, J.; Johnson, K.; Hollings, C.S.; Bishop, L.; Laranjo, N.; Stubbs, B.J.; et al. Trial of the MIND Diet for Prevention of Cognitive Decline in Older Persons. N. Engl. J. Med. 2023, 389, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C.; Harper, K.J. Potassium, magnesium, and calcium: Their role in both the cause and treatment of hypertension. J. Clin. Hypertens. 2008, 10 (Suppl. S2), 3–11. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, A.; Miguel, M. Dietary fiber and blood pressure control. Food Funct. 2016, 7, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Elijovich, F.; Weinberger, M.H.; Anderson, C.A.; Appel, L.J.; Bursztyn, M.; Cook, N.R.; Dart, R.A.; Newton-Cheh, C.H.; Sacks, F.M.; Laffer, C.L. Salt Sensitivity of Blood Pressure: A Scientific Statement From the American Heart Association. Hypertension 2016, 68, e7–e46. [Google Scholar] [CrossRef] [PubMed]
- Susic, D.; Frohlich, E.D.; Kobori, H.; Shao, W.; Seth, D.; Navar, L.G. Salt-induced renal injury in SHRs is mediated by AT1 receptor activation. J. Hypertens. 2011, 29, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, M.S.; Yatabe, J.; Yoneda, M.; Watanabe, T.; Otsuki, M.; Felder, R.A.; Jose, P.A.; Sanada, H. Salt sensitivity is associated with insulin resistance, sympathetic overactivity, and decreased suppression of circulating renin activity in lean patients with essential hypertension. Am. J. Clin. Nutr. 2010, 92, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Naska, A.; Kasdagli, M.I.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Malavolti, M.; Orsini, N.; Whelton, P.K.; et al. Potassium Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2020, 9, e015719. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; Green, T.; Harrison, T.N.; Reynolds, K. Dietary approaches to prevent hypertension. Curr. Hypertens. Rep. 2013, 15, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Bueno, M.; Arroyo, J.P.; Gamba, G. Independent regulation of Na+ and K+ balance by the kidney. Med. Princ. Pract. 2012, 21, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Barbagallo, M. Magnesium and Hypertension in Old Age. Nutrients 2021, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Sontia, B.; Touyz, R.M. Role of magnesium in hypertension. Arch. Biochem. Biophys. 2007, 458, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.E.; O’Donnell, A.A.; Himali, J.J.; Rajendran, I.; Melo van Lent, D.; Ataklte, F.; Jacques, P.F.; Beiser, A.S.; Seshadri, S.; Vasan, R.S.; et al. Associations of the Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay diet with cardiac remodelling in the community: The Framingham Heart Study. Br. J. Nutr. 2021, 126, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, S.; Ghorbaninejad, P.; Janbozorgi, N.; Shab-Bidar, S. Associations between adherence to MIND diet and metabolic syndrome and general and abdominal obesity: A cross-sectional study. Diabetol. Metab. Syndr. 2020, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Fats and fatty acids in human nutrition. Report of an expert consultation. FAO Food Nutr. Pap. 2010, 91, 1–166.
- Yu-Poth, S.; Etherton, T.D.; Reddy, C.C.; Pearson, T.A.; Reed, R.; Zhao, G.; Jonnalagadda, S.; Wan, Y.; Kris-Etherton, P.M. Lowering dietary saturated fat and total fat reduces the oxidative susceptibility of LDL in healthy men and women. J. Nutr. 2000, 130, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.d.O.B.; Dos Santos, C.A.; Leite, J.I.; Caldas, A.P.; Bressan, J. Impact of nutrients and food components on dyslipidemias: What is the evidence? Adv. Nutr. 2015, 6, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Tison, S.E.; Shikany, J.M.; Long, D.L.; Carson, A.P.; Cofield, S.S.; Pearson, K.E.; Howard, G.; Judd, S.E. Differences in the Association of Select Dietary Measures With Risk of Incident Type 2 Diabetes. Diabetes Care 2022, 45, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, A.M.; Vahdat, S.; Hojati, A.; Moradi, H.; Tousi, A.Z.; Ebrahimzadeh, F.; Farhangi, M.A. Evaluating the association between the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet, mental health, and cardio-metabolic risk factors among individuals with obesity. BMC Endocr. Disord. 2023, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Kanwugu, O.N.; Glukhareva, T.V.; Danilova, I.G.; Kovaleva, E.G. Natural antioxidants in diabetes treatment and management: Prospects of astaxanthin. Crit. Rev. Food Sci. Nutr. 2022, 62, 5005–5028. [Google Scholar] [CrossRef] [PubMed]
- Shafras, M.; Sabaragamuwa, R.; Suwair, M. Role of dietary antioxidants in diabetes: An overview. Food Chem. Adv. 2024, 4, 100666. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, W.; Guo, S. Regulation of Macronutrients in Insulin Resistance and Glucose Homeostasis during Type 2 Diabetes Mellitus. Nutrients 2023, 15, 4671. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Čolak, E.; Pap, D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J. Med. Biochem. 2021, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chami, L.; Checler, F. BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and β-amyloid production in Alzheimer’s disease. Mol. Neurodegener. 2012, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Atlante, A.; Amadoro, G.; Bobba, A.; Latina, V. Functional Foods: An Approach to Modulate Molecular Mechanisms of Alzheimer’s Disease. Cells 2020, 9, 2347. [Google Scholar] [CrossRef] [PubMed]
- Daak, A.A.; Elderdery, A.Y.; Elbashir, L.M.; Mariniello, K.; Mills, J.; Scarlett, G.; Elbashir, M.I.; Ghebremeskel, K. Omega 3 (n-3) fatty acids down-regulate nuclear factor-kappa B (NF-κB) gene and blood cell adhesion molecule expression in patients with homozygous sickle cell disease. Blood Cells Mol. Dis. 2015, 55, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Novak, T.E.; Babcock, T.A.; Jho, D.H.; Helton, W.S.; Espat, N.J. NF-kappa B inhibition by omega-3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L84–L89. [Google Scholar] [CrossRef] [PubMed]
- Chami, L.; Buggia-Prévot, V.; Duplan, E.; Del Prete, D.; Chami, M.; Peyron, J.F.; Checler, F. Nuclear factor-κB regulates βAPP and β- and γ-secretases differently at physiological and supraphysiological Aβ concentrations. J. Biol. Chem. 2012, 287, 24573–24584. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.P.; Vidya, C.S.; Pereira, P.; Jayaram, S.; Yadav, A.K.; Sujatha, P. Elevated Homocysteine Level and Brain Atrophy Changes as Markers to Screen the Alzheimer Disease: Case Series. Ann. Geriatr. Med. Res. 2024, 28, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bao, G.; Liu, D.; Yang, Y.; Li, X.; Cai, G.; Liu, Y.; Wu, Y. The Association Between Folate and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Neurosci. 2021, 15, 661198. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, Y.; Xu, T.; Li, Q.; Wang, D.; Zhang, L.; Fan, B.; Wang, F.; Liu, X. Genistein Ameliorates Scopolamine-Induced Amnesia in Mice Through the Regulation of the Cholinergic Neurotransmission, Antioxidant System and the ERK/CREB/BDNF Signaling. Front. Pharmacol. 2018, 9, 1153. [Google Scholar] [CrossRef] [PubMed]
- Shentu, Y.P.; Hu, W.T.; Liang, J.W.; Liuyang, Z.Y.; Wei, H.; Qun, W.; Wang, X.C.; Wang, J.Z.; Westermarck, J.; Liu, R. Genistein Decreases APP/tau Phosphorylation and Ameliorates Aβ Overproduction Through Inhibiting CIP2A. Curr. Alzheimer Res. 2019, 16, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Pierzynowska, K.; Gaffke, L.; Cyske, Z.; Węgrzyn, G. Genistein induces degradation of mutant huntingtin in fibroblasts from Huntington’s disease patients. Metab. Brain Dis. 2019, 34, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, V.; Kumar, A.; Singh, T.G. Genistein: A promising ally in combating neurodegenerative disorders. Eur. J. Pharmacol. 2025, 991, 177273. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Escudero, J.; Baquero, M.; Cebrián, M.; Carbonell-Asíns, J.A.; Muñoz, J.E.; Satorres, E.; Meléndez, J.C.; Ferrer-Rebolleda, J.; Cózar-Santiago, M.d.P.; et al. Genistein effect on cognition in prodromal Alzheimer’s disease patients. The GENIAL clinical trial. Alzheimers Res. Ther. 2022, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Jiang, K.; Zhang, Y.F.; Zhuang, J.; Ku, C.; Yang, J.; Zhang, Y. Improvement of Cardiovascular Risk Factors by Genistein Supplementation: A Systematic Review and Meta-Analysis in Diverse Population-Based RCTs. J. Nutr. Metab. 2025, 2025, 1827252. [Google Scholar] [CrossRef] [PubMed]
- Palomer, E.; Buechler, J.; Salinas, P.C. Wnt Signaling Deregulation in the Aging and Alzheimer’s Brain. Front. Cell Neurosci. 2019, 13, 227. [Google Scholar] [CrossRef] [PubMed]
- Hadi, F.; Akrami, H.; Shahpasand, K.; Fattahi, M.R. Wnt signalling pathway and tau phosphorylation: A comprehensive study on known connections. Cell Biochem. Funct. 2020, 38, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Den, H.; Dong, X.; Chen, M.; Zou, Z. Efficacy of probiotics on cognition, and biomarkers of inflammation and oxidative stress in adults with Alzheimer’s disease or mild cognitive impairment—A meta-analysis of randomized controlled trials. Aging 2020, 12, 4010–4039. [Google Scholar] [CrossRef] [PubMed]
- Sowmiya, S.; Dhivya, L.S.; Harikrishnan, N.; Ankul Singh, S. Exploring the potential of probiotics in Alzheimer’s disease and gut dysbiosis. IBRO Neurosci. Rep. 2024, 17, 441–455. [Google Scholar] [CrossRef]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, L.B.; Dohgu, S.; Sultana, R.; Lynch, J.L.; Owen, J.B.; Erickson, M.A.; Shah, G.N.; Price, T.O.; Fleegal-Demotta, M.A.; Butterfield, D.A.; et al. Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: A mechanism for inflammation in the progression of Alzheimer’s disease. Brain Behav. Immun. 2009, 23, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Healy, E. Impact of the MIND Diet on Cognition in Individuals with Dementia. J Alzheimers Dis. 2023, 96, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Ellouze, I.; Sheffler, J.; Nagpal, R.; Arjmandi, B. Dietary Patterns and Alzheimer’s Disease: An Updated Review Linking Nutrition to Neuroscience. Nutrients 2023, 15, 3204. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, G.; Abbas-Zadeh, M.; Eftekhari, M.H. Effect of MIND diet intervention on cognitive performance and brain structure in healthy obese women: A randomized controlled trial. Sci. Rep. 2022, 12, 2871. [Google Scholar] [CrossRef] [PubMed]
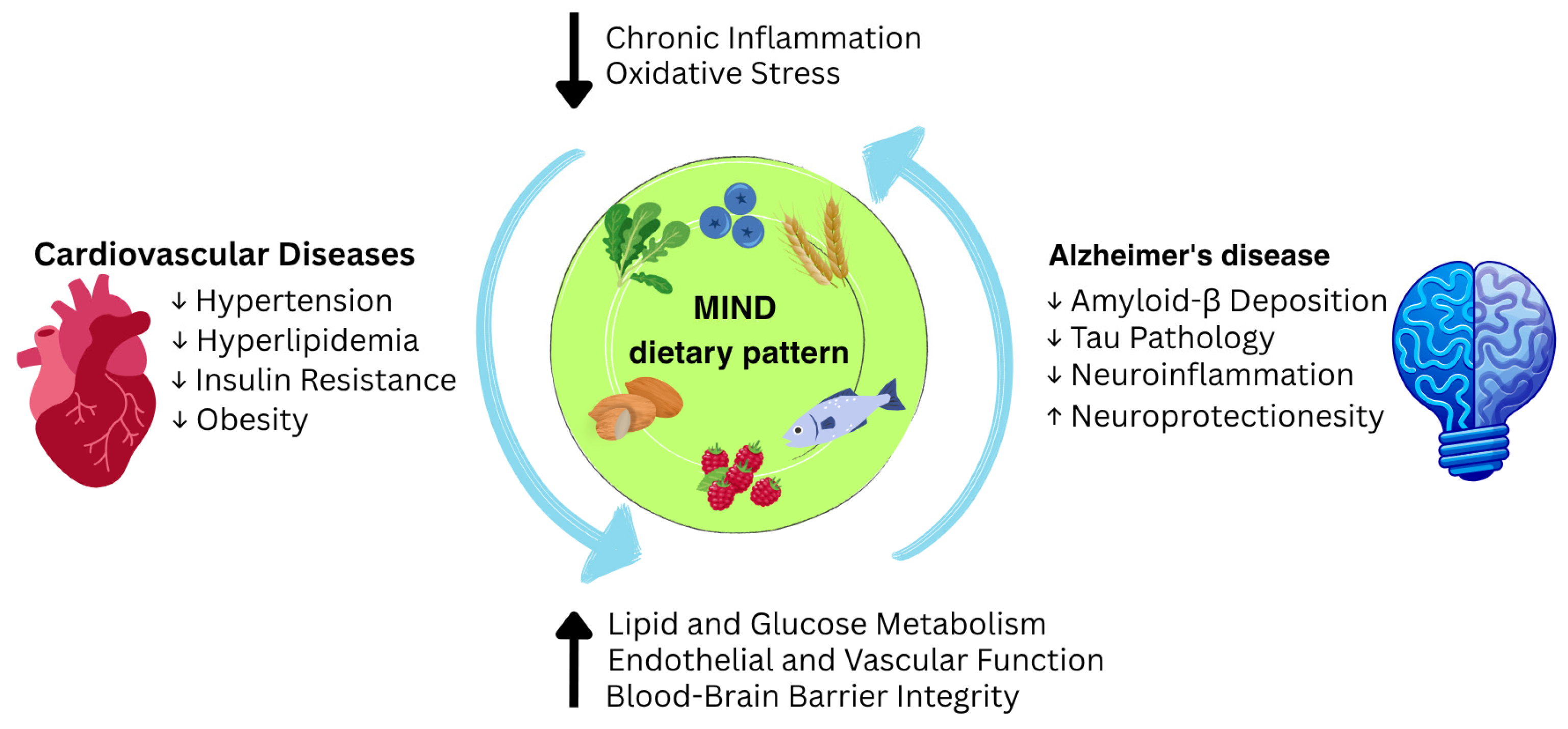
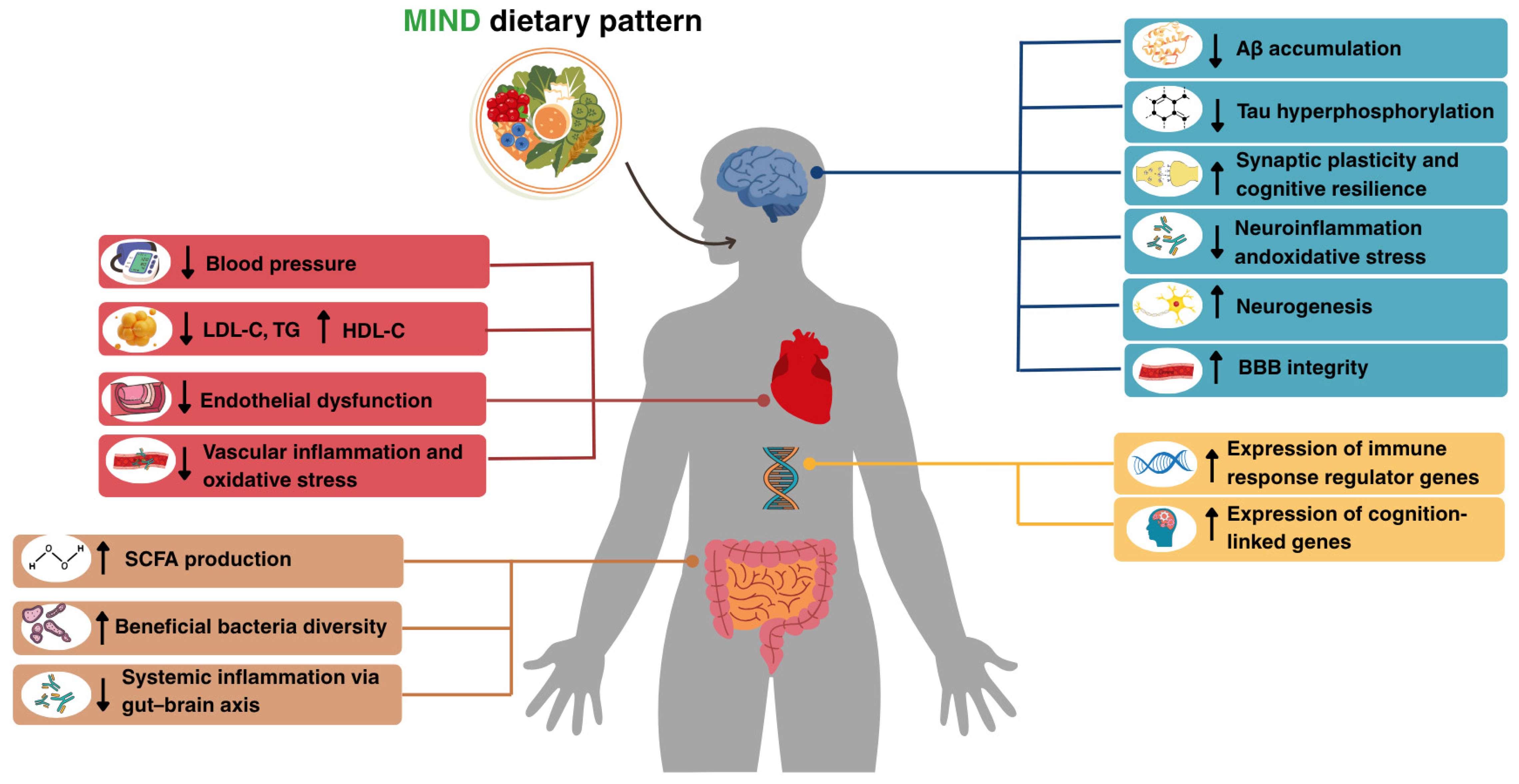
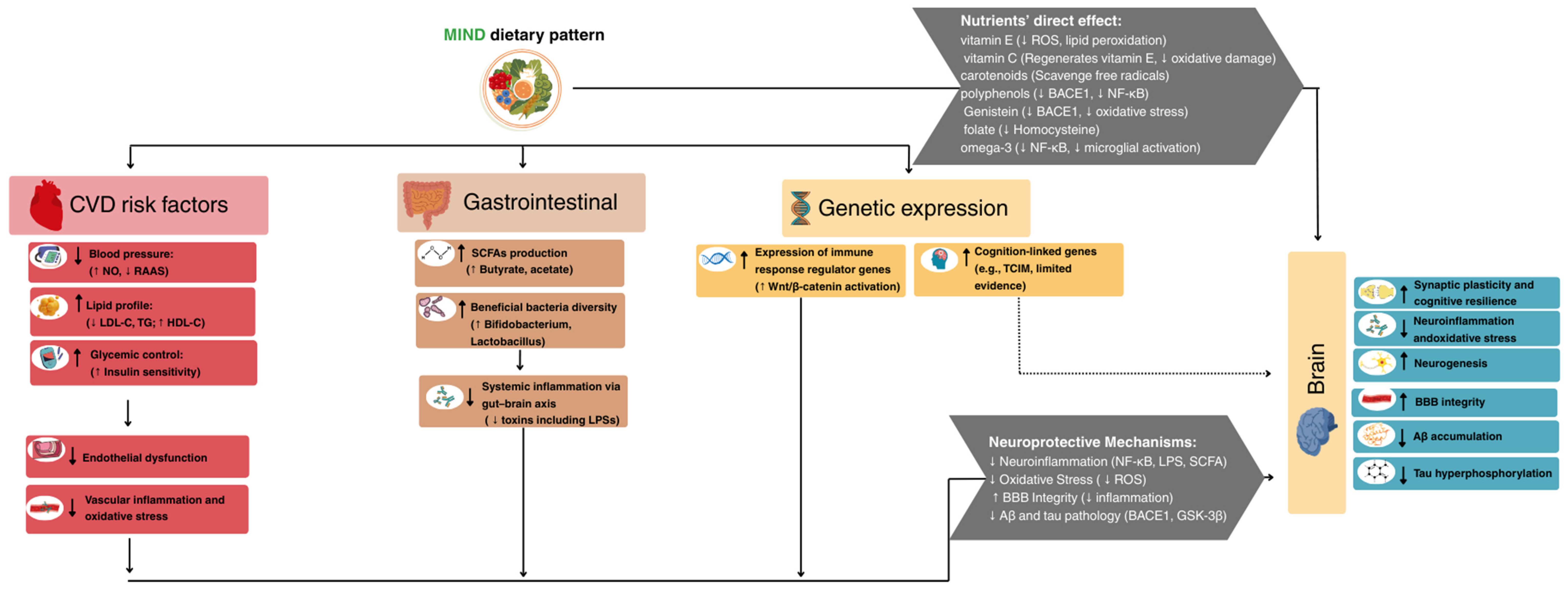
| CVD Risk Factor | CVD-Related Mechanisms | AD-Related Mechanisms | Evidence Type | Strength of Evidence | Reversibility |
|---|---|---|---|---|---|
| HTN | Endothelial dysfunction, cerebral hypoperfusion, BBB disruption | Elevated Aβ and tau pathology; damage to myelin and synapses | Human, Animal | Strong | Partially reversible with blood pressure control |
| Dyslipidemia | Lipid accumulation, oxidative stress, mitochondrial dysfunction | Alters Aβ production; associated with cholesterol metabolism genes (e.g., APOE, SORL1) | Human, Animal, Genetic | Moderate to Strong | Partially reversible with statins/diet |
| DM | Insulin resistance, cardiac remodeling, increased inflammation | Enhances Aβ accumulation (via reduced insulin-degrading enzyme activity); promotes tau hyperphosphorylation | Human, Animal | Strong | Partially reversible with glycemic control |
| Obesity | Adipokine dysregulation, oxidative stress, RAAS activation | Increased APP and Aβ in adipose tissue; elevated plasma Aβ; BBB disruption; mitochondrial dysfunction | Human, Animal | Moderate | Partially reversible with weight loss |
| Smoking | Endothelial damage, inflammation, oxidative stress | Increases Aβ aggregation and tau pathology via oxidative stress | Human, Animal | Strong | Largely irreversible, but further damage preventable |
| Physical Inactivity | Impaired glucose/lipid metabolism, endothelial dysfunction | Increase neuroinflammation, accelerating the accumulation of Aβ and tau protein; reduces BBB integrity | Human, Animal | Moderate | Reversible with regular physical activity |
| Study | Country | Sample Size | Population | Duration | Outcomes Measured | Key Findings |
|---|---|---|---|---|---|---|
| Yau et al. (2022) [122] | China | 78 | Older Chinese adults | 4 weeks | BP, glucose, HDL-C, mental health | ↓ BP, ↓ glucose, ↑ HDL-C, improved mental well-being |
| Gholami et al. (2024) [123] | Iran | 84 | Adults with metabolic syndrome | 12 weeks | Weight, BMI, WC, SBP, DBP, FBS, HDL-C, TG | ↓ BMI, WC, BP, FBS, TG; ↑ HDL-C. |
| Arjmand et al. (2022) [124] | Iran | 40 | Middle-aged overweight/obese women | 12 weeks | Cognitive performance, brain MRI (IFG surface area), BMI, WHR, body weight | ↑ working memory, attention, verbal memory; ↑ IFG surface area; ↓ BMI, WHR, weight |
| Elsayed et al. (2022) [125] | Egypt | 68 | Postmenopausal women with hormone deficiency | 12 weeks | Cognitive & functional level, sex hormone markers | ↑ cognition and functionality with MIND + aerobic exercise vs MIND alone |
| Barnes et al. (2023) [126] | United States | 604 | Older overweight adults | 3 years | Global cognition, MRI brain markers (WMH, hippocampal volume) | No significant difference in cognition or MRI outcomes vs control; both groups improved slightly |
| Dietary Component | Key Nutrients | Proposed Effects on CVD | Proposed Effects on AD |
|---|---|---|---|
| Green leafy vegetables | Folate, potassium, magnesium, fiber | Lower BP via vasodilation and endothelial support | Reduces oxidative stress, lowers homocysteine levels, supports cognitive resilience |
| Berries | Polyphenols, flavonoids | Anti-inflammatory, improves lipid profile | Protects against Aβ accumulation and oxidative damage |
| Nuts | MUFAs, vitamin E, polyphenols | Improves HDL-C, lowers LDL-C, reduces inflammation | Enhances synaptic function, reduces tau pathology |
| Whole grains | Fiber, B vitamins, antioxidants | Lowers cholesterol, improves glycemic control | Produces SCFAs, reduces inflammation, improves gut-brain axis |
| Fish | Omega-3 PUFAs | Reduces TGs and inflammation | Downregulates NF-κB, lowers BACE1 activity, reduces Aβ and tau production |
| Olive oil | MUFAs, polyphenols, vitamin E | Improves lipid profile, lowers BP, reduces oxidative stress | Has antioxidant and anti-amyloidogenic effects |
| Beans and legumes | Folate, fiber, magnesium | Supports lipid and glucose metabolism | Reduces oxidative stress and inflammation |
| Restricted items (e.g., red/processed meats, sweets, butter) | Saturated fats, sodium, refined sugars | Reduces risk of obesity, dyslipidemia, HTN | Promotes Aβ accumulation and cognitive decline |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ataei Kachouei, A.; Singar, S.; Wood, A.; Flatt, J.D.; Rosenkranz, S.K.; Rosenkranz, R.R.; Akhavan, N.S. Cardiovascular Risk Factors, Alzheimer’s Disease, and the MIND Diet: A Narrative Review from Molecular Mechanisms to Clinical Outcomes. Nutrients 2025, 17, 2328. https://doi.org/10.3390/nu17142328
Ataei Kachouei A, Singar S, Wood A, Flatt JD, Rosenkranz SK, Rosenkranz RR, Akhavan NS. Cardiovascular Risk Factors, Alzheimer’s Disease, and the MIND Diet: A Narrative Review from Molecular Mechanisms to Clinical Outcomes. Nutrients. 2025; 17(14):2328. https://doi.org/10.3390/nu17142328
Chicago/Turabian StyleAtaei Kachouei, Amirhossein, Saiful Singar, Amber Wood, Jason D. Flatt, Sara K. Rosenkranz, Richard R. Rosenkranz, and Neda S. Akhavan. 2025. "Cardiovascular Risk Factors, Alzheimer’s Disease, and the MIND Diet: A Narrative Review from Molecular Mechanisms to Clinical Outcomes" Nutrients 17, no. 14: 2328. https://doi.org/10.3390/nu17142328
APA StyleAtaei Kachouei, A., Singar, S., Wood, A., Flatt, J. D., Rosenkranz, S. K., Rosenkranz, R. R., & Akhavan, N. S. (2025). Cardiovascular Risk Factors, Alzheimer’s Disease, and the MIND Diet: A Narrative Review from Molecular Mechanisms to Clinical Outcomes. Nutrients, 17(14), 2328. https://doi.org/10.3390/nu17142328









