High Serum Ferritin Levels Are Associated with Sarcopenia in Patients Undergoing Chronic Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Covariates
2.3. Assessment of Skeletal Muscle Mass
2.4. Assessment of Skeletal Muscle Strength
2.5. Assessment of the Physical Performance
2.6. Definition of Sarcopenia
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
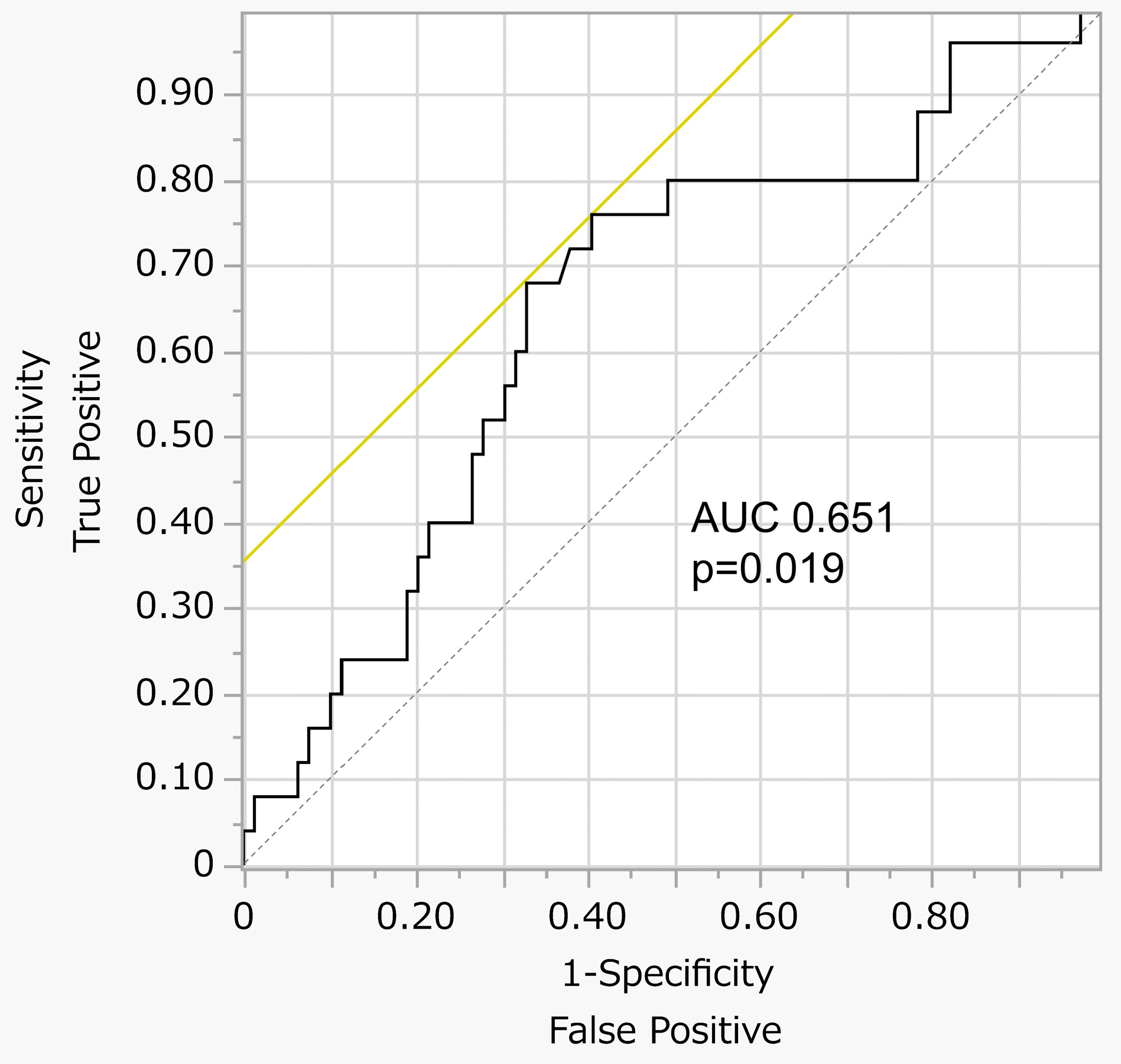
3.2. Serum Ferritin Levels and Sarcopenia
3.3. Association Between High Serum Ferritin Levels and Sarcopenia
3.4. Association Between Patient Characteristics and Components of Sarcopenia Criteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Alb | Albumin |
| AWGS | Asian Working Group for Sarcopenia |
| CI | Confidence interval |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| ESAs | Erythropoiesis-stimulating agents |
| ESRD | End-stage renal disease |
| Hb | Hemoglobin |
| HD | Hemodialysis |
| IDA | Iron-deficiency anemia |
| OR | Odds ratio |
| PKD | Polycystic kidney disease |
| ROC | Receiver operating characteristic |
| SMI | Skeletal muscle mass index |
| TIBC | Total iron-binding capacity |
| TSAT | Transferrin saturation |
References
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing acts: Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Daru, J.; Colman, K.; Stanworth, S.J.; De La Salle, B.; Wood, E.M.; Pasricha, S.R. Serum ferritin as an indicator of iron status: What do we need to know? Am. J. Clin. Nutr. 2017, 106, 1634S–1639S. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, E.K.; Kapitsinou, P.; Pergola, P.E.; Kovesdy, C.P.; Jalal, D.I. Iron deficiency in chronic kidney disease: Updates on pathophysiology, diagnosis, and treatment. J. Am. Soc. Nephrol. 2020, 31, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Bailie, G.R.; Larkina, M.; Goodkin, D.A.; Li, Y.; Pisoni, R.L.; Bieber, B.; Mason, N.; Tong, L.; Locatelli, F.; Marshall, M.R.; et al. Variation in intravenous iron use internationally and over time: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transplant. 2013, 28, 2570–2579. [Google Scholar] [CrossRef]
- Alves, F.M.; Ayton, S.; Bush, A.I.; Lynch, G.S.; Koopman, R. Age-related changes in skeletal muscle iron homeostasis. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 16–24. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International clinical practice guidelines for sarcopenia (ICFSR): Screening, diagnosis and management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Ren, H.; Gong, D.; Jia, F.; Xu, B.; Liu, Z. Sarcopenia in patients undergoing maintenance hemodialysis: Incidence rate, risk factors and its effect on survival risk. Ren. Fail. 2016, 38, 364–371. [Google Scholar] [CrossRef]
- Isoyama, N.; Qureshi, A.R.; Avesani, C.M.; Lindholm, B.; Bàràny, P.; Heimbürger, O.; Cederholm, T.; Stenvinkel, P.; Carrero, J.J. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1720–1728. [Google Scholar] [CrossRef]
- Mori, K.; Nishide, K.; Okuno, S.; Shoji, T.; Emoto, M.; Tsuda, A.; Nakatani, S.; Imanishi, Y.; Ishimura, E.; Yamakawa, T.; et al. Impact of diabetes on sarcopenia and mortality in patients undergoing hemodialysis. BMC Nephrol. 2019, 20, 105. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, S.G.; Oh, J.E.; Lee, Y.-K.; Noh, J.-W.; Kim, H.J.; Song, Y.R. Impact of sarcopenia on long-term mortality and cardiovascular events in patients undergoing hemodialysis. Korean J. Intern. Med. 2019, 34, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Z.; Gilbertson, D.T.; Foley, R.N.; Collins, A.J. An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int. 2010, 77, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Chen, L.-Y.; Hsu, P.-S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Hughes, G. Youden’s index and the weight of evidence revisited. Methods Inf. Med. 2015, 54, 198–199. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Collamati, A.; D’Angelo, E.; Pahor, M.; Bernabei, R.; et al. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017, 29, 11–17. [Google Scholar] [CrossRef]
- Stugiewicz, M.; Tkaczyszyn, M.; Kasztura, M.; Banasiak, W.; Ponikowski, P.; Jankowska, E.A. The influence of iron deficiency on the functioning of skeletal muscles: Experimental evidence and clinical implications. Eur. J. Heart Fail. 2016, 18, 762–773. [Google Scholar] [CrossRef]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Trinder, D. Athletic induced iron deficiency: New insights into the role of inflammation, cytokines and hormones. Eur. J. Appl. Physiol. 2008, 103, 381–391. [Google Scholar] [CrossRef]
- Shu, X.; Lin, T.; Wang, H.; Zhao, Y.; Jiang, T.; Peng, X.; Yue, J. Diagnosis, prevalence, and mortality of sarcopenia in dialysis patients: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 145–158. [Google Scholar] [CrossRef]
- Nakagawa, C.; Inaba, M.; Ishimura, E.; Yamakawa, T.; Shoji, S.; Okuno, S. Association of increased serum ferritin with impaired muscle strength/quality in hemodialysis patients. J. Ren. Nutr. 2016, 26, 253–257. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, E.L.; Iwasaki, K.; Tsuji, Y. Intracellular iron transport and storage: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 997–1030. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.W.; Kwak, D.; Liu, H.M.; Thompson, L.V. Age-induced oxidative stress: How does it influence skeletal muscle quantity and quality? J. Appl. Physiol. 2016, 121, 1047–1052. [Google Scholar] [CrossRef]
- Picca, A.; Mankowski, R.T.; Kamenov, G.; Anton, S.D.; Manini, T.M.; Buford, T.W.; Saini, S.K.; Calvani, R.; Landi, F.; Bernabei, R.; et al. Advanced age is associated with iron dyshomeostasis and mitochondrial DNA damage in human skeletal muscle. Cells 2019, 8, 1525. [Google Scholar] [CrossRef]
- Reardon, T.F.; Allen, D.G. Iron injections in mice increase skeletal muscle iron content, induce oxidative stress and reduce exercise performance. Exp. Physiol. 2009, 94, 720–730. [Google Scholar] [CrossRef]
- Zitt, E.; Sturm, G.; Kronenberg, F.; Neyer, U.; Knoll, F.; Lhotta, K.; Weiss, G.; Pantopoulos, K. Iron supplementation and mortality in incident dialysis patients: An observational study. PLoS ONE 2014, 9, e114144. [Google Scholar] [CrossRef]
- Kohgo, Y.; Ikuta, K.; Ohtake, T.; Torimoto, Y.; Kato, J. Body iron metabolism and pathophysiology of iron overload. Int. J. Hematol. 2008, 88, 7–15. [Google Scholar] [CrossRef]
- Kuragano, T.; Matsumura, O.; Matsuda, A.; Hara, T.; Kiyomoto, H.; Murata, T.; Kitamura, K.; Fujimoto, S.; Hase, H.; Joki, N.; et al. Association between hemoglobin variability, serum ferritin levels, and adverse events/mortality in maintenance hemodialysis patients. Kidney Int. 2014, 86, 845–854. [Google Scholar] [CrossRef]
- Van Buren, P.; Velez, R.L.; Vaziri, N.D.; Zhou, X.J. Iron overdose: A contributor to adverse outcomes in randomized trials of anemia correction in CKD. Int. Urol. Nephrol. 2012, 44, 499–507. [Google Scholar] [CrossRef]
- KDIGO Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2012, 2, 279–335. [Google Scholar]
- National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am. J. Kidney Dis. 2006, 47, S11–S145. [Google Scholar]
- National Clinical Guideline Centre (UK). Anaemia management in chronic kidney disease: Partial update 2015. In National Institute for Health and Care Excellence (NICE): Clinical Guideline; Royal College of Physicians: London, UK, 2015. [Google Scholar]
- Mikhail, A.; Brown, C.; Williams, J.A.; Mathrani, V.; Shrivastava, R.; Evans, J.; Isaac, H.; Bhandari, S. Renal association clinical practice guideline on anaemia of chronic kidney disease. BMC Nephrol. 2017, 18, 345. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Nishi, S.; Tomo, T.; Masakane, I.; Saito, K.; Nangaku, M.; Hattori, M.; Suzuki, T.; Morita, S.; Ashida, A.; et al. 2015 Japanese society for dialysis therapy: Guidelines for renal anemia in chronic kidney disease. Ren. Replace. Ther. 2017, 3, 1–46. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Regidor, D.L.; McAllister, C.J.; Michael, B.; Warnock, D.G. Time-dependent associations between iron and mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2005, 16, 3070–3080. [Google Scholar] [CrossRef]
- Shoji, T.; Niihata, K.; Fukuma, S.; Fukuhara, S.; Akizawa, T.; Inaba, M. Both low and high serum ferritin levels predict mortality risk in hemodialysis patients without inflammation. Clin. Exp. Nephrol. 2017, 21, 685–693. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Rodriguez, R.A.; Humphreys, M.H. Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 141–149. [Google Scholar] [CrossRef]
- Bazeley, J.; Bieber, B.; Li, Y.; Morgenstern, H.; de Sequera, P.; Combe, C.; Yamamoto, H.; Gallagher, M.; Port, F.K.; Robinson, B.M. C-reactive protein and prediction of 1-year mortality in prevalent hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2452–2461. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Tong, L.; Robinson, B.M.; Sen, A.; Fukuhara, S.; Kurokawa, K.; Canaud, B.; Lameire, N.; Port, F.K.; Pisoni, R.L. C-reactive protein and mortality in hemodialysis patients: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephron Clin. Pract. 2011, 117, c167–c178. [Google Scholar] [CrossRef]
- Rostoker, G.; Griuncelli, M.; Loridon, C.; Magna, T.; Machado, G.; Drahi, G.; Dahan, H.; Janklewicz, P.; Cohen, Y.; Barretti, P. Reassessment of iron biomarkers for prediction of dialysis iron overload: An MRI study. PLoS ONE 2015, 10, e0132006. [Google Scholar] [CrossRef]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- Kim, T.; Streja, E.; Soohoo, M.; Rhee, C.M.; Eriguchi, R.; Kim, T.W.; Chang, T.I.; Obi, Y.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum ferritin variations and mortality in incident hemodialysis patients. Am. J. Nephrol. 2017, 46, 120–130. [Google Scholar] [CrossRef] [PubMed]
| All Patients n = 104 | Sarcopenia n = 25 | Non-Sarcopenia n = 79 | p-Value | |
|---|---|---|---|---|
| Age (years) | 67.9 ± 11.8 | 75.2 ± 10.1 | 64.8 ± 11.3 | <0.001 |
| Sex (%), male | 78.8 | 80.0 | 78.4 | 0.87 |
| BMI (kg/m2) | 22.0 ± 3.4 | 20.4 ± 2.5 | 22.6 ± 3.5 | 0.005 |
| Dialysis vintage (years) | 7.6 (4.7–14.3) | 6.2 (4.1–15.0) | 8.2 (5.2–14.3) | 0.29 |
| Serum ferritin (ng/mL) | 131.3 (59.4–192.3) | 170.6 (116.4–222.5) | 92 (58.5–182.8) | 0.023 |
| Serum iron (µg/dL) | 62.7 ± 20.7 | 61.9 ± 20.9 | 63.0 ± 20.8 | 0.79 |
| TIBC (µg/dL) | 234 (201.5–260) | 237 (211–253.5) | 232 (198–260) | 0.63 |
| TSAT (%) | 27.6 ± 10.6 | 26.8 ± 10.8 | 27.8 ± 10.6 | 0.81 |
| CRP (mg/dL) | 0.096 (0.047–0.28) | 0.14 (0.063–0.37) | 0.083 (0.046–0.28) | 0.30 |
| Serum Alb (g/dL) | 3.5 ± 0.2 | 3.5 ± 0.2 | 3.5 ± 0.2 | 0.59 |
| Hemoglobin (g/dL) | 11.7 ± 1.2 | 11.4 ± 0.9 | 11.8 ± 1.2 | 0.16 |
| Intact PTH (pg/mL) | 157 (93.2–225.5) | 171 (99–268.5) | 155 (81–205) | 0.37 |
| ESRD cause | 0.28 | |||
| Diabetes | 40.0 | 52.0 | 34.1 | |
| Glomerulonephritis | 22.0 | 8.0 | 25.3 | |
| Hypertensive nephropathy | 17.0 | 16.0 | 16.4 | |
| PKD | 6.0 | 4.0 | 6.3 | |
| Other or unknown | 19.0 | 20.0 | 17.7 | |
| Comorbidity index | 4 (2–7) | 5 (4–8) | 4 (2–7) | 0.032 |
| Diabetes (%) | 45.1 | 56.0 | 41.7 | 0.21 |
| CVD (%) | 29.8 | 36.0 | 27.8 | 0.44 |
| Malignancy (%) | 10.6 | 20.0 | 7.6 | 0.098 |
| Liver disease (%) | 2.8 | 8.0 | 1.3 | 0.11 |
| Use of iron supplementation (%) | 51.9 | 52.0 | 51.9 | 0.99 |
| oral | 26.0 | 24.0 | 26.6 | 0.79 |
| intravenous | 27.9 | 28.0 | 27.9 | 0.98 |
| Use of ESA (%) | 73.1 | 92.0 | 67.1 | 0.007 |
| Variables | Univariate | Model 1 * | Model 2 ** | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Serum ferritin (per 10 ng/mL) | 1.06 (1.00–1.11) | 0.021 | 1.05 (0.99–1.12) | 0.071 | 1.06 (1.00–1.12) | 0.046 |
| Serum iron (per 10 µg/dL) | 0.97 (0.78–1.21) | 0.81 | 1.02 (0.79–1.31) | 0.85 | 1.08 (0.82–1.43) | 0.57 |
| TIBC (per 10 µg/dL) | 1.03 (0.93–1.13) | 0.50 | 1.11 (0.97–1.26) | 0.11 | 1.05 (0.93–1.19) | 0.36 |
| TSAT (%) | 0.99 (0.94–1.03) | 0.68 | 0.99 (0.94–1.05) | 0.92 | 1.00 (0.95–1.06) | 0.73 |
| Serum ferritin ≥ 132 ng/mL | 4.65 (1.67–12.92) | 0.003 | 5.02 (1.47–17.06) | 0.009 | 5.45 (1.54–19.23) | 0.008 |
| Serum Ferritin ≥ 132 ng/mL n = 51 | Serum Ferritin < 132 ng/mL n = 53 | p-Value | |
|---|---|---|---|
| Age (years) | 70.3 ± 11.3 | 64.5 ± 11.8 | 0.007 |
| Sex (%), male | 72.5 | 84.9 | 0.12 |
| Sarcopenia (%) | 37.3 | 11.3 | 0.001 |
| BMI (kg/m2) | 21.1 ± 2.7 | 22.9 ± 3.8 | 0.011 |
| Dialysis vintage (years) | 7.5 (4.7–12.9) | 7.7 (4.5–14.8) | 0.67 |
| CRP (mg/dL) | 0.13 (0.050–0.34) | 0.079 (0.046–0.26) | 0.30 |
| Serum Alb (g/dL) | 3.6 ± 0.2 | 3.5 ± 0.2 | 0.31 |
| Hemoglobin (g/dL) | 11.5 ± 0.8 | 11.9 ± 1.4 | 0.099 |
| Intact PTH (pg/mL) | 161 (103–230) | 154 (66.5–228.5) | 0.41 |
| ESRD cause | 0.63 | ||
| Diabetes | 35.3 | 41.5 | |
| Glomerulonephritis | 27.4 | 15.1 | |
| Hypertensive nephropathy | 15.7 | 17.0 | |
| PKD | 5.9 | 5.7 | |
| Other or unknown | 15.7 | 20.7 | |
| Comorbidity index | 4 (2–6) | 4 (3–7) | 0.14 |
| Diabetes (%) | 39.2 | 50.9 | 0.22 |
| CVD (%) | 27.5 | 32.0 | 0.60 |
| Malignancy (%) | 13.7 | 7.6 | 0.30 |
| Liver disease (%) | 3.9 | 1.9 | 0.53 |
| Use of iron supplementation (%) | 62.8 | 41.5 | 0.029 |
| oral | 33.3 | 18.9 | 0.091 |
| intravenous | 33.3 | 22.6 | 0.22 |
| Use of ESA (%) | 88.2 | 58.5 | <0.001 |
| Variables | SMI | Hand Grip | SPBB | |||
|---|---|---|---|---|---|---|
| ρ | p-Value | ρ | p-Value | ρ | p-Value | |
| Age | −0.432 | <0.001 | −0.398 | <0.001 | −0.236 | 0.015 |
| Sex, male | 0.584 | <0.001 | 0.534 | <0.001 | 0.054 | 0.58 |
| BMI | 0.622 | <0.001 | 0.354 | <0.001 | 0.0089 | 0.92 |
| Comorbidity index | 0.128 | 0.19 | 0.078 | 0.42 | −0.204 | 0.037 |
| Serum ferritin | −0.343 | <0.001 | −0.253 | 0.009 | −0.148 | 0.13 |
| Serum iron | −0.028 | 0.77 | 0.050 | 0.61 | 0.071 | 0.47 |
| TIBC | 0.160 | 0.10 | 0.086 | 0.38 | 0.151 | 0.12 |
| TSAT | −0.110 | 0.26 | −0.0018 | 0.98 | 0.012 | 0.89 |
| CRP | 0.063 | 0.51 | −0.070 | 0.47 | −0.156 | 0.11 |
| Serum Alb | 0.164 | 0.094 | −0.022 | 0.82 | 0.021 | 0.82 |
| Hemoglobin | 0.070 | 0.48 | 0.177 | 0.071 | 0.0085 | 0.93 |
| Intact PTH | −0.084 | 0.39 | −0.156 | 0.11 | 0.082 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hori, M.; Takahashi, H.; Kondo, C.; Takeda, A.; Morozumi, K.; Maruyama, S. High Serum Ferritin Levels Are Associated with Sarcopenia in Patients Undergoing Chronic Hemodialysis. Nutrients 2025, 17, 2323. https://doi.org/10.3390/nu17142323
Hori M, Takahashi H, Kondo C, Takeda A, Morozumi K, Maruyama S. High Serum Ferritin Levels Are Associated with Sarcopenia in Patients Undergoing Chronic Hemodialysis. Nutrients. 2025; 17(14):2323. https://doi.org/10.3390/nu17142323
Chicago/Turabian StyleHori, Mayuko, Hiroshi Takahashi, Chika Kondo, Asami Takeda, Kunio Morozumi, and Shoichi Maruyama. 2025. "High Serum Ferritin Levels Are Associated with Sarcopenia in Patients Undergoing Chronic Hemodialysis" Nutrients 17, no. 14: 2323. https://doi.org/10.3390/nu17142323
APA StyleHori, M., Takahashi, H., Kondo, C., Takeda, A., Morozumi, K., & Maruyama, S. (2025). High Serum Ferritin Levels Are Associated with Sarcopenia in Patients Undergoing Chronic Hemodialysis. Nutrients, 17(14), 2323. https://doi.org/10.3390/nu17142323






