Sea Cucumber Egg Oligopeptides Ameliorate Cognitive Impairments and Pathology of Alzheimer’s Disease Through Regulating HDAC3 and BDNF/NT3 via the Microbiota–Gut–Brain Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Approach
2.2. Materials
2.3. Preparation of Sea Cucumber Egg Peptide
2.4. Observation and Sampling of Mice
2.5. Morris Water Maze Test
2.6. Y-Maze Test
2.7. Individual Nesting Test
2.8. 16S Ribosomal RNA Gene Sequencing Analysis
2.9. Hematoxylin–Eosin Staining
2.10. Western Blotting
2.11. Nissl Staining
2.12. Immunohistochemistry and Immunofluorescence
2.13. Enzyme-Linked Immunosorbent Assay (ELISA)
2.14. Fecal Microbiota Transplantation Experiment
2.15. Quantification of Short-Chain Fatty Acids
2.16. Statistical Analysis
3. Results
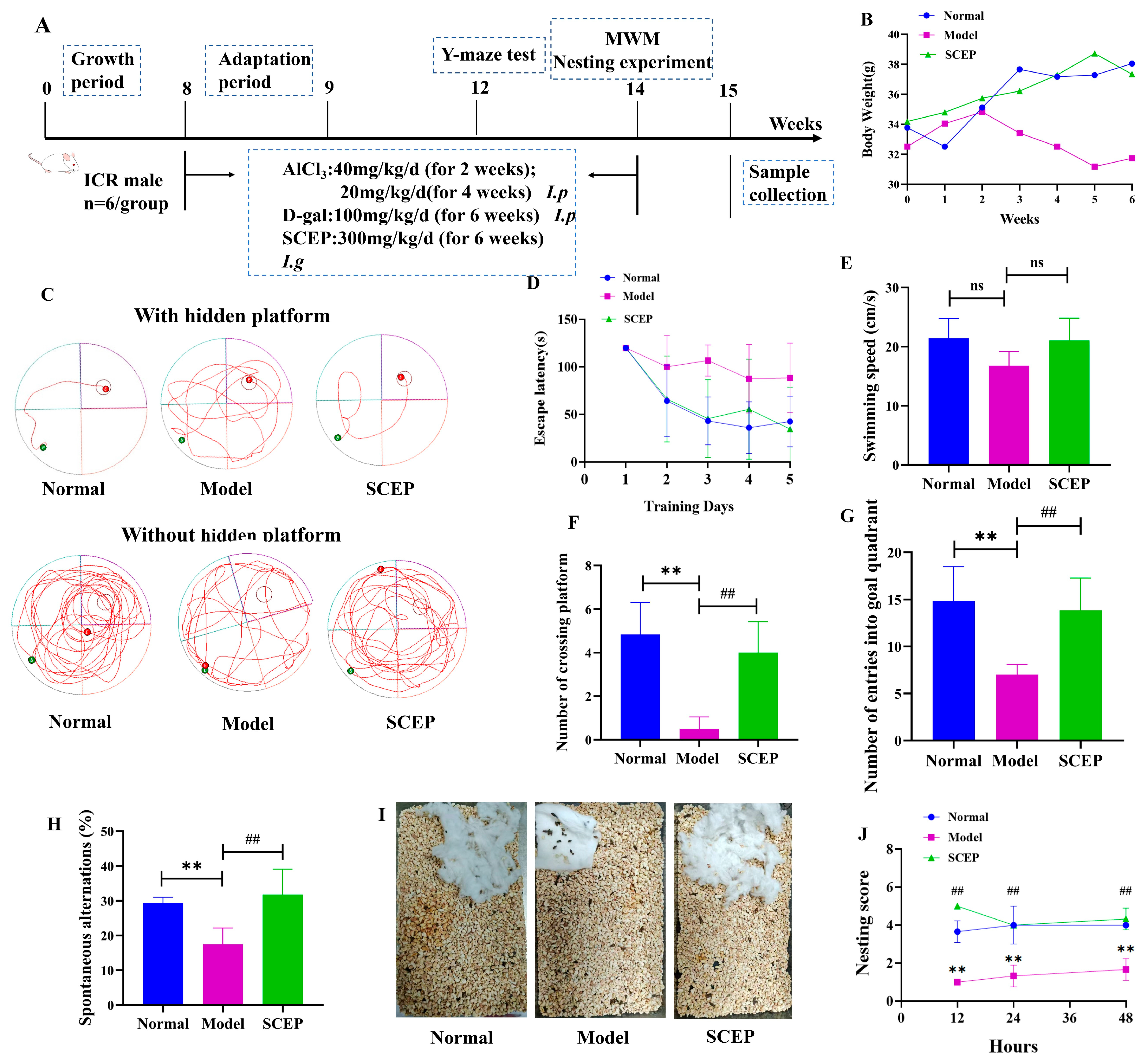
3.1. Effects of Gavage Administration of SCEPs on Cognitive and Emotional Deficits in the AD Mouse Model
3.2. Effects of Gavage Administration of SCEP on the Pathology of the AD Mouse Model
3.3. Effects of Gavage Administration of SCEP on Gut Dysbiosis in AD Model Mice
3.4. FMT from the SCEP Treatment Mice Also Attenuated Spatial Memory, Cognitive Deficits, and Pathology in the AD Mouse Model
3.5. Comparison of the Similarity of Mice Gut Microbiota Profiling After SCEP and FMT_SCEP Treatment
3.6. Gavage Administration of SCEP Improved the Levels and Distribution of Gut Microbial Metabolite SCFAs and Regulated the Transporter of MCT-1
3.7. Gavage Administration of SCEPs Improved the Intestinal and Blood–Brain Barrier Function of AD Mice
3.8. Gavage Administration of SCEP Suppressed HDAC3 Expression, in Turn Upregulating BDNF and NT3 Levels in the AD Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCEP | Sea cucumber eggs oligopeptides |
| AD | Alzheimer’s disease |
| FMT | Fecal microbiota transplantation |
| AlCl3 | Aluminum chloride |
| D-gal | D-galactose |
| Aβ | β-Amyloid |
| P-Tau | Phosphorylated tau protein |
| SCFAs | Short-chain fatty acids |
| BCA | Bicinchoninic Acid Assay |
| TJ | Tight junctions |
| CNS | Central nervous system |
| MWM | Morris water maze |
| HE | Hematoxylin–eosin staining |
| ZO-1 | Right junction protein 1 |
| claudin 5 | Compact linking protein 5 |
| HDAC3 | Histone deacetylase 3 |
| GFAP | Glial fibrillary acidic protein |
| NF-L | Neurofilament light chain |
| MCT-1 | Monocarboxylate transporter-1 |
| MUC4 | Mucin-4 |
| LC-MS | Liquid chromatography–mass spectrometry |
| PVDF | Polyvinylidene difluoride |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| DG | Dentate gyrus |
| BBB | Blood–brain barrier |
| NT3 | Neurotrophin-3 |
| BDNF | Brain-derived neurotrophic factor |
| DAPI | 4′,6-Diamidino-2′-phenylindole |
| PD | Parkinson’s disease |
References
- Marco-Contelles, J. Facts, Results, and Perspectives of the Current Alzheimer’s Disease Research. ACS Chem. Neurosci. 2019, 10, 1127–1128. [Google Scholar] [CrossRef] [PubMed]
- van der Kant, R.; Goldstein, L.S.B.; Ossenkoppele, R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 2020, 21, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.Q.; Su, Z.R.; Yang, W.; Zhong, M.; Xian, Y.F.; Lin, Z.X. Patchouli alcohol attenuates the cognitive deficits in a transgenic mouse model of Alzheimer’s disease via modulating neuropathology and gut microbiota through suppressing C/EBPβ/AEP pathway. J. Neuroinflamm. 2023, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, X.; Ma, J.; Wang, M.; Liu, W.; Wang, G.; Ding, Y.; Lin, Z.; Li, Y. Chemical substances and their activities in sea cucumber Apostichopus japonicus: A review. Arch. Der Pharm. 2024, 357, e2300427. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, N.; Dong, L.; Gao, Y.; Lin, S. Production of Bioactive Peptides from Sea Cucumber and Its Potential Health Benefits: A Comprehensive Review. J. Agric. Food Chem. 2022, 70, 7607–7625. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Shen, F.; Du, J.; Feng, F. Sea cucumber (Acaudina leucoprocta) peptides exhibit anti-aging effect in Drosophila melanogaster via regulation of microbiota dysbiosis and metabolic disorder. Food Biosci. 2024, 60, 104476. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, J.; Xu, X.; Liu, R.; Lin, S. Regulation mechanisms of sea cucumber peptides against scopolamine-induced memory disorder and novel memory-improving peptides identification. Eur. J. Pharmacol. 2024, 968, 176430. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides From Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Gan, M.Q.; Poh, J.M.; Lim, S.J.; Chang, L.S. The Potential of Protein Hydrolysates from Marine By-products: Mechanisms, Health Benefits, Applications, Future Prospects, and Challenges. Process Biochem. 2024, 147, 489–504. [Google Scholar] [CrossRef]
- Chen, J.; Gao, K.; Liu, S.; Wang, S.; Elango, J.; Bao, B.; Dong, J.; Liu, N.; Wu, W. Fish Collagen Surgical Compress Repairing Characteristics on Wound Healing Process In Vivo. Mar. Drugs 2019, 17, 33. [Google Scholar] [CrossRef]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, Characterization and Evaluation of Collagen from Jellyfish Rhopilema esculentum Kishinouye for Use in Hemostatic Applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef] [PubMed]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.; Chokshi, H.; Prajapati, B. Impact of marine collagen on nanocosmetics: A comprehensive review. Nano-Struct. Nano-Objects 2024, 40, 101394. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Zhang, Y.; Lu, Y.; Wang, M.; Wang, G.; Liu, X. Purification and antioxidant ability of peptide from egg in sea cucumber Apostichopus japonicus. Int. J. Food Prop. 2016, 20, 306–317. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Kasper, L.H. The Second Brain: Is the Gut Microbiota a Link Between Obesity and Central Nervous System Disorders? Curr. Obes. Rep. 2016, 5, 51–64. [Google Scholar] [CrossRef]
- Zhou, B.; Yuan, Y.; Zhang, S.; Guo, C.; Li, X.; Li, G.; Xiong, W.; Zeng, Z. Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract. Front. Immunol. 2020, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Walrath, T.; Dyamenahalli, K.U.; Hulsebus, H.J.; McCullough, R.L.; Idrovo, J.P.; Boe, D.M.; McMahan, R.H.; Kovacs, E.J. Age-related changes in intestinal immunity and the microbiome. J. Leukoc. Biol. 2021, 109, 1045–1061. [Google Scholar] [CrossRef]
- He, Y.; Wang, K.; Su, N.; Yuan, C.; Zhang, N.; Hu, X.; Fu, Y.; Zhao, F. Microbiota-gut-brain axis in health and neurological disease: Interactions between gut microbiota and the nervous system. J. Cell. Mol. Med. 2024, 28, e70099. [Google Scholar] [CrossRef]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef]
- Singh, J.; Singh, A.; Biswal, S.; Zomuansangi, R.; Lalbiaktluangi, C.; Singh, B.P.; Singh, P.K.; Vellingiri, B.; Iyer, M.; Ram, H.; et al. Microbiota-brain axis: Exploring the role of gut microbiota in psychiatric disorders—A comprehensive review. Asian J. Psychiatry 2024, 97, 104068. [Google Scholar] [CrossRef]
- Ma, L.; Jiang, X.; Huang, Q.; Chen, W.; Zhang, H.; Pei, H.; Cao, Y.; Wang, H.; Li, H. Traditional Chinese medicine for the treatment of Alzheimer’s disease: A focus on the microbiota-gut-brain axis. Biomed. Pharmacother. 2023, 165, 115244. [Google Scholar] [CrossRef] [PubMed]
- Arifin, W.N.; Zahiruddin, W.M. Sample Size Calculation in Animal Studies Using Resource Equation Approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Laterza, L.; Rizzatti, G.; Gaetani, E.; Chiusolo, P.; Gasbarrini, A. The Gut Microbiota and Immune System Relationship in Human Graft-versus-Host Disease. Mediterr. J. Hematol. Infect Dis. 2016, 8, e2016025. [Google Scholar] [CrossRef]
- Du, L.; Chen, J.; Yan, J.; Xie, H.; Wang, L.; Wang, R.; Han, X.; Wang, Y. Lingguizhugan decoction ameliorates cognitive impairment in AD-like mice by influencing the microbiome-gut-brain axis mediated by SCFAs. Phytomed. Int. J. Phytother. Phytopharmac. 2024, 133, 155942. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zheng, S.J.; Cai, W.J.; Yu, L.; Yuan, B.F.; Feng, Y.Q. Stable isotope labeling combined with liquid chromatography-tandem mass spectrometry for comprehensive analysis of short-chain fatty acids. Anal. Chim. Acta 2019, 1070, 51–59. [Google Scholar] [CrossRef]
- Rani, S.; Dhar, S.B.; Khajuria, A.; Gupta, D.; Jaiswal, P.K.; Singla, N.; Kaur, M.; Singh, G.; Barnwal, R.P. Advanced Overview of Biomarkers and Techniques for Early Diagnosis of Alzheimer’s Disease. Cell. Mol. Neurobiol. 2023, 43, 2491–2523. [Google Scholar] [CrossRef]
- Lu, C.; Tang, S.; Han, J.; Fan, S.; Huang, Y.; Zhang, Z.; Zhou, J.; Ming, T.; Li, Y.; Su, X. Apostichopus japonicus Oligopeptide Induced Heterogeneity in the Gastrointestinal Tract Microbiota and Alleviated Hyperuricemia in a Microbiota-Dependent Manner. Mol. Nutr. Food Res. 2021, 65, e2100147. [Google Scholar] [CrossRef]
- Leite, G.G.S.; Weitsman, S.; Parodi, G.; Celly, S.; Sedighi, R.; Sanchez, M.; Morales, W.; Villanueva-Millan, M.J.; Barlow, G.M.; Mathur, R.; et al. Mapping the Segmental Microbiomes in the Human Small Bowel in Comparison with Stool: A REIMAGINE Study. Dig. Dis. Sci. 2020, 65, 2595–2604. [Google Scholar] [CrossRef]
- Gao, J.; Song, G.; Shen, H.; Wu, Y.; Zhao, C.; Zhang, Z.; Jiang, Q.; Li, X.; Ma, X.; Tan, B.; et al. Allicin Improves Intestinal Epithelial Barrier Function and Prevents LPS-Induced Barrier Damages of Intestinal Epithelial Cell Monolayers. Front. Immunol. 2022, 13, 847861. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, Y.; Fan, Y.; He, Y.; Liu, K.; Deng, S.; Liu, Y. Sodium Humate-Derived Gut Microbiota Ameliorates Intestinal Dysfunction Induced by Salmonella Typhimurium in Mice. Microbiol. Spectr. 2023, 11, e0534822. [Google Scholar] [CrossRef]
- Liang, M.; Sun, X.; Guo, M.; Wu, H.; Zhao, L.; Zhang, J.; He, J.; Ma, X.; Yu, Z.; Yong, Y.; et al. Baicalin methyl ester prevents the LPS—Induced mice intestinal barrier damage in vivo and in vitro via P65/TNF-α/MLCK/ZO-1 signal pathway. Biomed. Pharmacother. 2024, 180, 117417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zeng, D.; Liu, S.; Huang, Y.; Lv, F.; Zhou, W. Histone deacetylase 3 inhibition alleviates 2,4-dinitrochlorobenzene-induced atopic dermatitis via epigenetically upregulating Nrf2/HO-1 signaling pathway. Int. Immunopharmacol. 2024, 126, 111107. [Google Scholar] [CrossRef]
- Bagheri, A.; Habibzadeh, P.; Razavipour, S.F.; Volmar, C.H.; Chee, N.T.; Brothers, S.P.; Wahlestedt, C.; Mowla, S.J.; Faghihi, M.A. HDAC Inhibitors Induce BDNF Expression and Promote Neurite Outgrowth in Human Neural Progenitor Cells-Derived Neurons. Int. J. Mol. Sci. 2019, 20, 1109. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.; Taylor, B.; Abelleira-Hervas, L.; Karimian-Marnani, N.; Aleksynas, R.; Syed, N.; Di Giovanni, S.; Palmisano, I.; Sastre, M. Histone deacetylase-3 regulates the expression of the amyloid precursor protein and its inhibition promotes neuroregenerative pathways in Alzheimer’s disease models. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Bio. 2024, 38, e23659. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Choi, H.; Jung, E.S.; Lee, W.; Oh, S.; Jeon, N.L.; Mook-Jung, I. HDAC6 inhibitor blocks amyloid beta-induced impairment of mitochondrial transport in hippocampal neurons. PLoS ONE 2012, 7, e42983. [Google Scholar] [CrossRef]
- Gao, D.; Li, P.; Gao, F.; Feng, Y.; Li, X.; Li, D.; Li, Y.; Xiao, Y. Preparation and Multitarget Anti-AD Activity Study of Chondroitin Sulfate Lithium in AD Mice Induced by Combination of D-Gal/AlCl3. Oxid. Med. Cell. Longev. 2022, 2022, 9466166. [Google Scholar] [CrossRef]
- Du, H.M.; Wang, Y.J.; Liu, X.; Wang, S.L.; Wu, S.M.; Yuan, Z.; Zhu, X.K. Defective Central Immune Tolerance Induced by High-Dose D-Galactose Resembles Aging. Biochemistry. Biokhimiia 2019, 84, 617–626. [Google Scholar] [CrossRef]
- Birla, H.; Minocha, T.; Kumar, G.; Misra, A.; Singh, S.K. Role of Oxidative Stress and Metal Toxicity in the Progression of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 552–562. [Google Scholar] [CrossRef]
- Mahady, L.; Nadeem, M.; Malek-Ahmadi, M.; Chen, K.; Perez, S.E.; Mufson, E.J. Frontal Cortex Epigenetic Dysregulation During the Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 115–131. [Google Scholar] [CrossRef]
- Wongpun, J.; Chanmanee, T.; Tocharus, C.; Chokchaisiri, R.; Chantorn, S.; Pabuprapap, W.; Suksamrarn, A.; Tocharus, J. The effects of festidinol treatment on the D-galactose and aluminum chloride-induced Alzheimer-like pathology in mouse brain. Phytomed. Int. J. Phytother. Phytopharmacol. 2022, 98, 153925. [Google Scholar] [CrossRef]
- Lin, X.; Yao, M.; Lu, J.H.; Wang, Y.; Yin, X.; Liang, M.; Yuan, E.; Ren, J. Identification of novel oligopeptides from the simulated digestion of sea cucumber (Stichopus japonicus) to alleviate Aβ aggregation progression. J. Funct. Foods 2019, 60, 103412. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, X.; Li, D.; Sun, N.; Lin, S. Sea Cucumber Peptides Attenuated the Scopolamine-Induced Memory Impairment in Mice and Rats and the Underlying Mechanism. J. Agric. Food Chem. 2022, 70, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lu, Z.; Xu, X.; Sun, N.; Lin, S. Sea Cucumber-Derived Peptide Attenuates Scopolamine-Induced Cognitive Impairment by Preventing Hippocampal Cholinergic Dysfunction and Neuronal Cell Death. J. Agric. Food Chem. 2022, 70, 567–576. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber IV, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbio. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 2018, 120, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Paley, E.L.; Merkulova-Rainon, T.; Faynboym, A.; Shestopalov, V.I.; Aksenoff, I. Geographical Distribution and Diversity of Gut Microbial NADH:Ubiquinone Oxidoreductase Sequence Associated with Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 61, 1531–1540. [Google Scholar] [CrossRef]
- Ling, Z.; Zhu, M.; Yan, X.; Cheng, Y.; Shao, L.; Liu, X.; Jiang, R.; Wu, S. Structural and Functional Dysbiosis of Fecal Microbiota in Chinese Patients with Alzheimer’s Disease. Front. Cell Dev. Biol. 2021, 8, 634069. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Xiayu, X.; Shi, C.; Chen, W.; Song, N.; Fu, X.; Zhou, R.; Xu, Y.F.; Huang, L.; et al. Altered Gut Microbiota in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 60, 1241–1257. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Ling, Y.; Wang, F.; Gong, T.; Yang, C.; Ye, S.; Ye, K.; Wei, D.; Song, Z.; et al. Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Trans. Psychiatr. 2019, 9, 189. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 2023, 63, 12073–12088. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef]
- Opeyemi, O.M.; Rogers, M.B.; Firek, B.A.; Janesko-Feldman, K.; Vagni, V.; Mullett, S.J.; Wendell, S.G.; Nelson, B.P.; New, L.A.; Mariño, E.; et al. Sustained Dysbiosis and Decreased Fecal Short-Chain Fatty Acids after Traumatic Brain Injury and Impact on Neurologic Outcome. J. Neurotrauma 2021, 38, 2610–2621. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Duan, L. Butyrate-producing Bacteria increase gut microbiota diversity to delay intestinal inflammaging. Innov. Aging 2023, 7 (Suppl. S1), 931. [Google Scholar] [CrossRef]
- Fock, E.; Parnova, R. Mechanisms of Blood-Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef]
- Nehra, G.; Bauer, B.; Hartz, A.M.S. Blood-brain barrier leakage in Alzheimer’s disease: From discovery to clinical relevance. Pharmacol. Ther. 2022, 234, 108119. [Google Scholar] [CrossRef]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef]
- Kuo, W.T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Berndt, P.; Winkler, L.; Cording, J.; Breitkreuz-Korff, O.; Rex, A.; Dithmer, S.; Rausch, V.; Blasig, R.; Richter, M.; Sporbert, A.; et al. Tight junction proteins at the blood-brain barrier: Far more than claudin-5. Cell. Mol. Life Sci. 2019, 76, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, G.; Daws, S.E.; Ozkan, E.D.; Rojas, C.S.; Hubbs, C.R.; Aceti, M.; Kilgore, M.; Kudugunti, S.; Puthanveettil, S.V.; Sweatt, J.D.; et al. Pharmacological Selectivity Within Class I Histone Deacetylases Predicts Effects on Synaptic Function and Memory Rescue. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2015, 40, 2307–2316. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Sartor, G.C.; Malvezzi, A.M.; Kumar, A.; Andrade, N.S.; Wiedner, H.J.; Vilca, S.J.; Janczura, K.J.; Bagheri, A.; Al-Ali, H.; Powell, S.K.; et al. Enhancement of BDNF Expression and Memory by HDAC Inhibition Requires BET Bromodomain Reader Proteins. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.H.; Li, S.H.; Gao, Z.; Zou, S.F.; Li, H.Y.; Tao, Z.Y.; Song, J.; Yang, J.X. Neurotrophin-3 promotes proliferation and cholinergic neuronal differentiation of bone marrow- derived neural stem cells via notch signaling pathway. Life Sci. 2016, 166, 131–138. [Google Scholar] [CrossRef]
- Gupta, R.; Ambasta, R.K.; Kumar, P. Pharmacological intervention of histone deacetylase enzymes in the neurodegenerative disorders. Life Sci. 2020, 243, 117278. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Shi, X.; Wang, H.; Si, C.; Liu, Q.; Du, Y. Neurotrophin-3 Promotes the Neuronal Differentiation of BMSCs and Improves Cognitive Function in a Rat Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 629356. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, F.; Yu, Z.; Guo, S.; Liu, N.; Jiang, Y.; Lo, E.H.; Xu, Y.; Wang, X. HDAC3 inhibition prevents blood-brain barrier permeability through Nrf2 activation in type 2 diabetes male mice. J. Neuroinflamm. 2019, 16, 103. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, Z.; Zhang, F.; Huang, L.; Xing, C.; Liu, N.; Xu, Y.; Wang, X. HDAC3 inhibition prevents oxygen glucose deprivation/reoxygenation-induced transendothelial permeability by elevating PPARγ activity in vitro. J. Neurochem. 2019, 149, 298–310. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Q. Short-Chain Fatty Acids Attenuate Renal Fibrosis and Enhance Autophagy of Renal Tubular Cells in Diabetic Mice Through the HDAC2/ULK1 Axis. Endocrinol. Metab. 2022, 37, 432–443. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Dou, Y.; Xie, H.; Pu, D.; Wang, L.; Wang, R.; Han, X. Sea Cucumber Egg Oligopeptides Ameliorate Cognitive Impairments and Pathology of Alzheimer’s Disease Through Regulating HDAC3 and BDNF/NT3 via the Microbiota–Gut–Brain Axis. Nutrients 2025, 17, 2312. https://doi.org/10.3390/nu17142312
Zhang G, Dou Y, Xie H, Pu D, Wang L, Wang R, Han X. Sea Cucumber Egg Oligopeptides Ameliorate Cognitive Impairments and Pathology of Alzheimer’s Disease Through Regulating HDAC3 and BDNF/NT3 via the Microbiota–Gut–Brain Axis. Nutrients. 2025; 17(14):2312. https://doi.org/10.3390/nu17142312
Chicago/Turabian StyleZhang, Guifeng, Yanjie Dou, Huiwen Xie, Dan Pu, Longxing Wang, Renjun Wang, and Xiaofei Han. 2025. "Sea Cucumber Egg Oligopeptides Ameliorate Cognitive Impairments and Pathology of Alzheimer’s Disease Through Regulating HDAC3 and BDNF/NT3 via the Microbiota–Gut–Brain Axis" Nutrients 17, no. 14: 2312. https://doi.org/10.3390/nu17142312
APA StyleZhang, G., Dou, Y., Xie, H., Pu, D., Wang, L., Wang, R., & Han, X. (2025). Sea Cucumber Egg Oligopeptides Ameliorate Cognitive Impairments and Pathology of Alzheimer’s Disease Through Regulating HDAC3 and BDNF/NT3 via the Microbiota–Gut–Brain Axis. Nutrients, 17(14), 2312. https://doi.org/10.3390/nu17142312




