Associations Between Sleep, Appetite, and Food Reward over 6 Months in Black Emerging Adults—Findings from the Sleep, Health Outcomes and Body Weight (SHOW) Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measures
2.3. Statistical Analyses
3. Results
Partial Correlations Between Changes in Sleep with Changes in Appetite and Food Reward
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hopkins, M.; Blundell, J.E. Energy balance, body composition, sedentariness and appetite regulation: Pathways to obesity. Clin. Sci. 2016, 130, 1615–1628. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Hu, F.B. Short Sleep Duration and Weight Gain: A Systematic Review. Obesity 2008, 16, 643. [Google Scholar] [CrossRef]
- Zhu, B.; Shi, C.; Park, C.G.; Zhao, X.; Reutrakul, S. Effects of sleep restriction on metabolism-related parameters in healthy adults: A comprehensive review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2019, 45, 18–30. [Google Scholar] [CrossRef]
- Rutters, F.; Gonnissen, H.K.; Hursel, R.; Lemmens, S.G.; Martens, E.A.; Westerterp-Plantenga, M.S. Distinct associations between energy balance and the sleep characteristics slow wave sleep and rapid eye movement sleep. Int. J. Obes. 2012, 36, 1346–1352. [Google Scholar] [CrossRef]
- McNeil, J.; Forest, G.; Hintze, L.J.; Brunet, J.-F.; Finlayson, G.; Blundell, J.E.; Doucet, É. The effects of partial sleep restriction and altered sleep timing on appetite and food reward. Appetite 2017, 109, 48–56. [Google Scholar] [CrossRef]
- Shechter, A.; O’Keeffe, M.; Roberts, A.L.; Zammit, G.K.; RoyChoudhury, A.; St-Onge, M.-P. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R883–R889. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, E.; Yoshimura, E.; Hatamoto, Y.; Shiratsuchi, H.; Tanaka, S.; Shimoda, S. Acute Sleep Curtailment Increases Sweet Taste Preference, Appetite and Food Intake in Healthy Young Adults: A Randomized Crossover Trial. Behav. Sci. 2020, 10, 47. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; McReynolds, A.; Trivedi, Z.B.; Roberts, A.L.; Sy, M.; Hirsch, J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli1234. Am. J. Clin. Nutr. 2012, 95, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Brooks, S.J.; O’Daly, O.G.; Almèn, M.S.; Morell, A.; Åberg, K.; Gingnell, M.; Schultes, B.; Hallschmid, M.; Broman, J.-E.; et al. Acute sleep deprivation enhances the brain’s response to hedonic food stimuli: An fMRI study. J. Clin. Endocrinol. Metab. 2012, 97, E443–E447. [Google Scholar] [CrossRef]
- Tasali, E.; Chapotot, F.; Wroblewski, K.; Schoeller, D. The Effects of Extended Bedtimes on Sleep Duration and Food Desire in Overweight Young Adults: A Home-Based Intervention. Appetite 2014, 80, 220–224. [Google Scholar] [CrossRef]
- Lewis, C.E.; Jacobs, D.R., Jr.; McCreath, H.; Kiefe, C.I.; Schreiner, P.J.; Smith, D.E.; Williams, O.D. Weight Gain Continues in the 1990s: 10-year Trends in Weight and Overweight from the CARDIA Study. Am. J. Epidemiol. 2000, 151, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Schell, R.C.; Just, D.R.; Levitsky, D.A. Predicted Lifetime Third-Party Costs of Obesity for Black and White Adolescents with Race-Specific Age-Related Weight Gain. Obesity 2020, 28, 397–403. [Google Scholar] [CrossRef]
- Maslowsky, J.; Ozer, E. Developmental trends in sleep duration in adolescence and young adulthood: Evidence from a national US sample. J. Adolesc. Health 2014, 54, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Sa, J.; Choe, S.; Cho, B.; Chaput, J.-P.; Kim, G.; Park, C.-H.; Chung, J.; Choi, Y.; Nelson, B.; Kim, Y. Relationship between sleep and obesity among U.S. and South Korean college students. BMC Public Health 2020, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Walsemann, K.M.; Ailshire, J.A.; Gee, G.C. Student loans and racial disparities in self-reported sleep duration: Evidence from a nationally representative sample of US young adults. J. Epidemiol. Community Health 2016, 70, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Rowell, T.E.; Curtis, D.S.; El-Sheikh, M.; Duke, A.M.; Ryff, C.D.; Zgierska, A.E. Racial discrimination mediates race differences in sleep problems: A longitudinal analysis. Cult. Divers. Ethn. Minor. Psychol. 2017, 23, 165–173. [Google Scholar] [CrossRef]
- Williams, D.L.; Mohammed, S. Discrimination and racial disparities in health: Evidence and needed research. J. Behav. Med. 2009, 32, 20. [Google Scholar] [CrossRef]
- Dorling, J.L.; Church, T.S.; Myers, C.A.; Höchsmann, C.; White, U.A.; Hsia, D.S.; Martin, C.K.; Apolzan, J.W. Racial Variations in Appetite-Related Hormones, Appetite, and Laboratory-Based Energy Intake from the E-MECHANIC Randomized Clinical Trial. Nutrients 2019, 11, 2018. [Google Scholar] [CrossRef]
- McNeil, J.; Clark, K.G.; Adams, W.M.; Pickett, S.; Edwards, K.; Exford, T.; Hemphill, M.A.; Wideman, L. The Sleep, Health Outcomes and Body Weight (SHOW) Study: A Measurement Burst Design Study on Sleep and Risk Factors for Obesity in Black Emerging Adults. BMJ Open 2024, 14, e087950. [Google Scholar] [CrossRef]
- Koch, H.R.; Sims, J.N.L.; Pickett, S.; Wideman, L.; McNeil, J. Associations between Sleep, Energy Balance and Obesity Markers over 6 Months in Black Emerging Adults—Pilot Study Findings from the Sleep, Health Outcomes and Body Weight (SHOW) Study. Appl. Physiol. Nutr. Metab. 2025, 50, 1–13. [Google Scholar] [CrossRef]
- Choi, L.; Liu, Z.; Matthews, C.E.; Buchowski, M.S. Validation of Accelerometer Wear and Nonwear Time Classification Algorithm. Med. Sci. Sports Exerc. 2011, 43, 357–364. [Google Scholar] [CrossRef]
- Sadeh, A.; Sharkey, K.M.; Carskadon, M.A. Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep 1994, 17, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.W.; Hong, I.H.; Baik, I.; Shin, H.W. Evaluation of the feasibility and preference of Nox-A1 type 2 ambulatory device for unattended home sleep test: A randomized crossover study. Sleep Biol. Rhythm. 2019, 17, 297–304. [Google Scholar] [CrossRef]
- Troester, M.M.; Quan, S.F.; Berry, R.B. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Darien, IL, USA, 2023. [Google Scholar]
- Marsh-Richard, D.M.; Hatzis, E.S.; Mathias, C.W.; Venditti, N.; Dougherty, D.M. Adaptive Visual Analog Scales (AVAS): A modifiable software program for the creation, administration, and scoring of visual analog scales. Behav. Res. Methods 2009, 41, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Magson, L.D.; Blundell, J.E. Hunger and palatability: Tracking ratings of subjective experience before, during and after the consumption of preferred and less preferred food. Appetite 1984, 5, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Doucet, E.; St-Pierre, S.; Alméras, N.; Tremblay, A. Relation between appetite ratings before and after a standard meal and estimates of daily energy intake in obese and reduced obese individuals. Appetite 2003, 40, 137–143. [Google Scholar] [CrossRef]
- Finlayson, G.; King, N.; Blundell, J.E. Is it possible to dissociate “liking” and “wanting” for foods in humans? A novel experimental procedure. Physiol. Behav. 2007, 90, 36–42. [Google Scholar] [CrossRef]
- Berridge, K.C. Food reward: Brain substrates of wanting and liking. Neurosci. Biobehav. Rev. 1996, 20, 1–25. [Google Scholar] [CrossRef]
- French, S.A.; Mitchell, N.R.; Wolfson, J.; Finlayson, G.; Blundell, J.E.; Jeffery, R.W. Questionnaire and laboratory measures of eating behavior. Associations with energy intake and BMI in a community sample of working adults. Appetite 2014, 72, 50–58. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kim, S. ppcor: Partial Correlation. R Package Version 1.1. [Internet]. 2015. Available online: https://CRAN.R-project.org/package=ppcor (accessed on 10 June 2024).
- Brondel, L.; Romer, M.A.; Nougues, P.M.; Touyarou, P.; Davenne, D. Acute partial sleep deprivation increases food intake in healthy men123. Am. J. Clin. Nutr. 2010, 91, 1550–1559. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief Communication: Sleep Curtailment in Healthy Young Men Is Associated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Ann. Intern. Med. 2004, 141, 846. [Google Scholar] [CrossRef] [PubMed]
- Hibi, M.; Kubota, C.; Mizuno, T.; Aritake, S.; Mitsui, Y.; Katashima, M.; Uchida, S. Effect of shortened sleep on energy expenditure, core body temperature, and appetite: A human randomised crossover trial. Sci. Rep. 2017, 7, 39640. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, E.; Yoshimura, E.; Tobina, T.; Yamashita, T.; Kume, K.; Hatamoto, Y.; Shimoda, S. Effects of sleep restriction on food intake and appetite under free-living conditions: A randomized crossover trial. Appetite 2023, 189, 106998. [Google Scholar] [CrossRef]
- Ehlers, C.; Kupfer, D. Slow-wave sleep: Do young adult men and women age differently? J. Sleep Res. 1997, 6, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Monk, T.H.; Buysse, D.J.; Rose, L.R.; Hall, J.A.; Kupfer, D.J. The sleep of healthy people—A diary study. Chronobiol. Int. 2000, 17, 49–60. [Google Scholar] [CrossRef]
- Kracht, C.L.; Chaput, J.-P.; Martin, C.K.; Champagne, C.M.; Katzmarzyk, P.T.; Staiano, A.E. Associations of Sleep with Food Cravings, Diet, and Obesity in Adolescence. Nutrients 2019, 11, 2899. [Google Scholar] [CrossRef]
- McNeil, J.; Cadieux, S.; Finlayson, G.; Blundell, J.E.; Doucet, É. Associations between sleep parameters and food reward. J. Sleep Res. 2015, 24, 346–350. [Google Scholar] [CrossRef]
- Yang, C.-L.; Schnepp, J.; Tucker, R.M. Increased Hunger, Food Cravings, Food Reward, and Portion Size Selection after Sleep Curtailment in Women Without Obesity. Nutrients 2019, 11, 663. [Google Scholar] [CrossRef]
| Baseline | 6 Months | Difference Between BLN and 6MO | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Z, p Value, r | |
| Anthropometrics | |||
| Body weight (kg) † | 72.4 ± 12.6 | 75.0 ± 12.0 | −3.0, <0.01, 0.76 |
| Fat mass (kg) † | 19.6 ± 11.0 | 21.5 ± 10.6 | −1.70, 0.09, 0.44 |
| Waist circumference (cm) † | 77.7 ± 8.0 | 80.1 ± 7.9 | −2.93, 0.03, 0.76 |
| Actigraph Sleep Variables | |||
| Sleep duration (min) † | 395.4 ± 70.2 | 357.8 ± 48.5 | 1.99, 0.05, 0.51 |
| Sleep efficiency (%) † | 72.6 ± 7.5 | 75.8 ± 7.8 | −1.76, 0.08, 0.45 |
| PSG Sleep Variables | |||
| Sleep duration (min) † | 469.9 ± 68.2 | 463.5 ± 52.8 | 0.51, 0.63, 0.13 |
| Sleep efficiency (%) † | 84.5 ± 14.6 | 88.7 ± 8.7 | −0.85, 0.41, 0.22 |
| REM duration (min) † | 75.6 ± 35.4 | 83.8 ± 42.1 | −1.08, 0.49, 0.28 |
| N2 duration (min) † | 284.0 ± 62.0 | 260.0 ± 60.0 | 1.65, 0.11, 0.43 |
| N3 duration (min) † | 104.7 ± 30.6 | 104.7 ± 27.0 | 0.06, 0.98, 0.02 |
| Appetite Scores | |||
| Fasting desire to eat | 59.0 ± 35.0 | 67.3 ± 38.6 | −1.87, 0.06, 0.48 |
| Desire to eat post-meal AUC | 8260.3 ± 3784.7 | 8596.3 ± 3262.3 | −0.28, 0.80, 0.07 |
| Fasting hunger | 53.4 ± 28.3 | 58.3 ± 31.0 | −1.42, 0.16, 0.37 |
| Hunger post-meal AUC | 7045.0 ± 3253.9 | 7865 ± 2856.8 | −0.97, 0.35, 0.25 |
| Fasting fullness | 28.5 ± 28.9 | 19.6 ± 27.2 | 2.02, <0.01, 0.52 |
| Fullness post-meal AUC | 8198.0 ± 2529.1 | 8341.5 ± 3013.2 | 0.57, 0.59, 0.15 |
| Fasting prospective food consumption | 58.4 ± 24.3 | 61.3 ± 32.0 | −0.85, 0.41, 0.22 |
| Prospective food consumption post-meal AUC | 7958.1 ± 2650.6 | 8542.5 ± 3378.9 | −0.68, 0.51, 0.18 |
| Food Reward | |||
| Fat—explicit liking | −9.1 ± 18.6 | −11.0 ± 14.6 | 0.74, 0.48, 0.19 |
| Fat—explicit wanting | −11.6 ± 18.0 | −10.4 ± 14.4 | −0.03, 1.00, 0.01 |
| Fat—implicit wanting | −18.3 ± 35.0 | −17.3 ± 31.5 | −0.34, 0.75, 0.09 |
| Fat preference | −5.8 ± 12.1 | −5.7 ± 11.3 | −0.20, 0.86, 0.05 |
| Sweet—explicit liking | −7.3 ± 23.1 | 4.0 ± 14.8 | −2.56, 0.01, 0.66 |
| Sweet—explicit wanting | −8.5 ± 23.0 | 2.8 ± 17.6 | −2.56, 0.01, 0.66 |
| Sweet—implicit wanting | −17.8 ± 41.3 | 0.6 ± 30.6 | −2.33, 0.02, 0.60 |
| Sweet preference | −5.8 ± 12.7 | 0.5 ± 11.1 | −2.78, 0.02, 0.72 |
| Sleep Variable | Coefficient (r) | p-Value |
|---|---|---|
| Actigraph—Total sleep duration (min) | ||
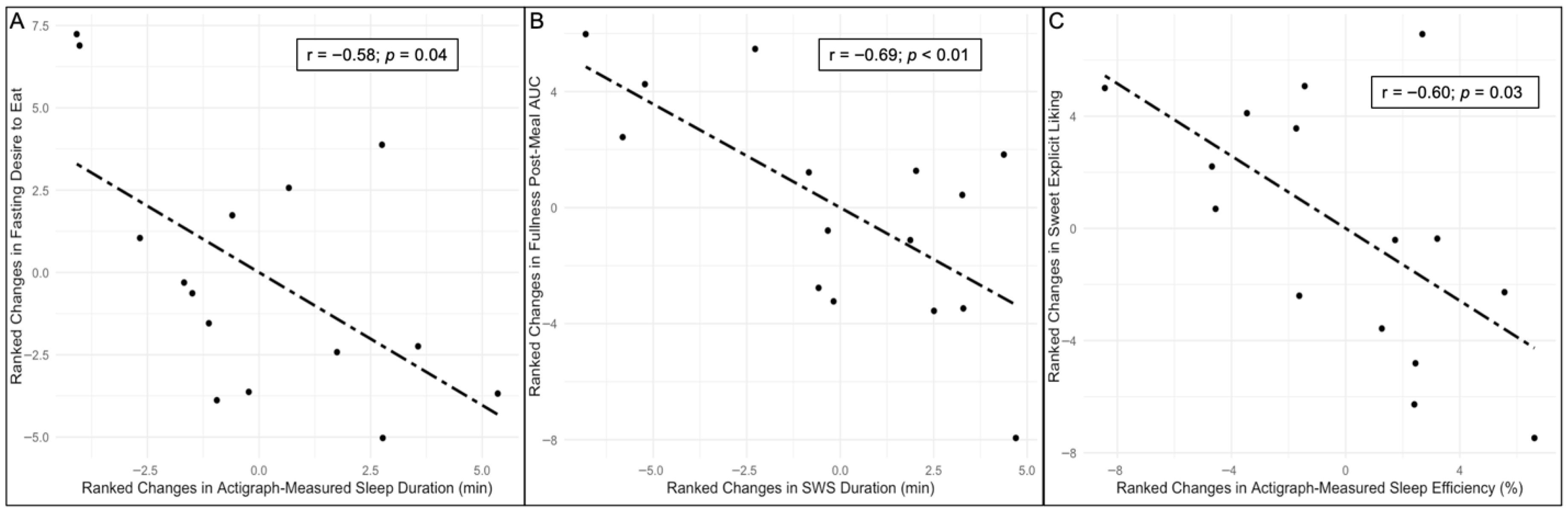
| Fasting desire to eat | −0.58 | 0.04 |
| Desire to eat post-meal AUC | −0.05 | 0.87 |
| Fasting hunger | −0.47 | 0.11 |
| Hunger post-meal AUC | 0.03 | 0.93 |
| Fasting fullness | 0.34 | 0.26 |
| Fullness post-meal AUC | −0.19 | 0.54 |
| Fasting prospective food consumption | −0.44 | 0.13 |
| Prospective food consumption post-meal AUC | 0.15 | 0.63 |
| Actigraph—Sleep efficiency (%) | ||
| Fasting desire to eat | 0.34 | 0.25 |
| Desire to eat post-meal AUC | 0.07 | 0.83 |
| Fasting hunger | −0.10 | 0.75 |
| Hunger post-meal AUC | −0.01 | 0.97 |
| Fasting fullness | 0.08 | 0.78 |
| Fullness post-meal AUC | −0.27 | 0.38 |
| Fasting prospective food consumption | 0.42 | 0.15 |
| Prospective food consumption post-meal AUC | 0.37 | 0.22 |
| Polysomnography—Total sleep duration (min) | ||
| Fasting desire to eat | −0.26 | 0.40 |
| Desire to eat post-meal AUC | −0.26 | 0.40 |
| Fasting hunger | −0.08 | 0.79 |
| Hunger post-meal AUC | −0.09 | 0.77 |
| Fasting fullness | 0.18 | 0.56 |
| Fullness post-meal AUC | 0.36 | 0.23 |
| Fasting prospective food consumption | −0.37 | 0.21 |
| Prospective food consumption post-meal AUC | −0.33 | 0.28 |
| Polysomnography—Sleep efficiency (%) | ||
| Fasting desire to eat | 0.05 | 0.87 |
| Desire to eat post-meal AUC | −0.14 | 0.64 |
| Fasting hunger | −0.18 | 0.56 |
| Hunger post-meal AUC | 0.05 | 0.88 |
| Fasting fullness | −0.03 | 0.93 |
| Fullness post-meal AUC | −0.05 | 0.87 |
| Fasting prospective food consumption | 0.01 | 0.98 |
| Prospective food consumption post-meal AUC | 0.04 | 0.91 |
| Stage 2 sleep duration (min) | ||
| Fasting desire to eat | −0.10 | 0.75 |
| Desire to eat post-meal AUC | −0.08 | 0.80 |
| Fasting hunger | −0.21 | 0.48 |
| Hunger post-meal AUC | 0.09 | 0.78 |
| Fasting fullness | 0.07 | 0.82 |
| Fullness post-meal AUC | 0.01 | 0.98 |
| Fasting prospective food consumption | 0.27 | 0.36 |
| Prospective food consumption post-meal AUC | 0.02 | 0.96 |
| Slow-wave sleep duration (min) | ||
| Fasting desire to eat | −0.03 | 0.91 |
| Desire to eat post-meal AUC | 0.05 | 0.88 |
| Fasting hunger | −0.26 | 0.39 |
| Hunger post-meal AUC | 0.40 | 0.17 |
| Fasting fullness | −0.45 | 0.13 |
| Fullness post-meal AUC | −0.69 | <0.01 |
| Fasting prospective food consumption | −0.43 | 0.15 |
| Prospective food consumption post-meal AUC | 0.19 | 0.53 |
| Rapid eye movement sleep duration (min) | ||
| Fasting desire to eat | −0.33 | 0.28 |
| Desire to eat post-meal AUC | −0.06 | 0.85 |
| Fasting hunger | 0.23 | 0.45 |
| Hunger post-meal AUC | −0.10 | 0.75 |
| Fasting fullness | 0.16 | 0.61 |
| Fullness post-meal AUC | 0.49 | 0.09 |
| Fasting prospective food consumption | −0.36 | 0.23 |
| Prospective food consumption post-meal AUC | −0.26 | 0.39 |
| Sleep Variable | Coefficient (r) | p-Value |
|---|---|---|
| Actigraphy—Total sleep duration (min) | ||
| Fat explicit liking | 0.42 | 0.15 |
| Fat explicit wanting | 0.55 | 0.05 |
| Fat implicit wanting | 0.42 | 0.15 |
| Fat preference | 0.48 | 0.09 |
| Sweet explicit liking | 0.43 | 0.15 |
| Sweet explicit wanting | 0.36 | 0.22 |
| Sweet implicit wanting | 0.44 | 0.13 |
| Sweet preference | 0.43 | 0.14 |
| Actigraphy—Sleep efficiency (%) | ||
| Fat explicit liking | −0.36 | 0.23 |
| Fat explicit wanting | −0.28 | 0.35 |
| Fat implicit wanting | −0.39 | 0.19 |
| Fat preference | −0.30 | 0.32 |
| Sweet explicit liking | −0.60 | 0.03 |
| Sweet explicit wanting | −0.14 | 0.65 |
| Sweet implicit wanting | −0.18 | 0.55 |
| Sweet preference | −0.41 | 0.16 |
| Polysomnography—Total sleep duration (min) | ||
| Fat explicit liking | 0.23 | 0.45 |
| Fat explicit wanting | 0.44 | 0.13 |
| Fat implicit wanting | 0.15 | 0.63 |
| Fat preference | 0.03 | 0.91 |
| Sweet explicit liking | 0.43 | 0.14 |
| Sweet explicit wanting | 0.07 | 0.83 |
| Sweet implicit wanting | −0.13 | 0.67 |
| Sweet preference | 0.20 | 0.52 |
| Polysomnography—Sleep efficiency (%) | ||
| Fat explicit liking | 0.09 | 0.77 |
| Fat explicit wanting | 0.24 | 0.42 |
| Fat implicit wanting | 0.03 | 0.93 |
| Fat preference | 0.16 | 0.59 |
| Sweet explicit liking | 0.22 | 0.47 |
| Sweet explicit wanting | 0.51 | 0.08 |
| Sweet implicit wanting | 0.08 | 0.80 |
| Sweet preference | −0.05 | 0.87 |
| Stage 2 sleep duration (min) | ||
| Fat explicit liking | −0.07 | 0.81 |
| Fat explicit wanting | 0.05 | 0.86 |
| Fat implicit wanting | −0.07 | 0.82 |
| Fat preference | −0.20 | 0.52 |
| Sweet explicit liking | −0.03 | 0.91 |
| Sweet explicit wanting | 0.00 | 1.00 |
| Sweet implicit wanting | −0.21 | 0.50 |
| Sweet preference | 0.01 | 0.97 |
| Slow-wave sleep duration (min) | ||
| Fat explicit liking | 0.32 | 0.28 |
| Fat explicit wanting | 0.39 | 0.19 |
| Fat implicit wanting | 0.14 | 0.65 |
| Fat preference | 0.15 | 0.62 |
| Sweet explicit liking | 0.31 | 0.31 |
| Sweet explicit wanting | 0.30 | 0.32 |
| Sweet implicit wanting | 0.51 | 0.08 |
| Sweet preference | 0.52 | 0.07 |
| Rapid eye movement sleep duration (min) | ||
| Fat explicit liking | 0.08 | 0.79 |
| Fat explicit wanting | 0.16 | 0.61 |
| Fat implicit wanting | 0.18 | 0.55 |
| Fat preference | 0.17 | 0.59 |
| Sweet explicit liking | 0.36 | 0.23 |
| Sweet explicit wanting | −0.14 | 0.64 |
| Sweet implicit wanting | −0.18 | 0.57 |
| Sweet preference | 0.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, H.R.; Sims, J.N.L.; Pickett, S.; Finlayson, G.; Wideman, L.; McNeil, J. Associations Between Sleep, Appetite, and Food Reward over 6 Months in Black Emerging Adults—Findings from the Sleep, Health Outcomes and Body Weight (SHOW) Pilot Study. Nutrients 2025, 17, 2305. https://doi.org/10.3390/nu17142305
Koch HR, Sims JNL, Pickett S, Finlayson G, Wideman L, McNeil J. Associations Between Sleep, Appetite, and Food Reward over 6 Months in Black Emerging Adults—Findings from the Sleep, Health Outcomes and Body Weight (SHOW) Pilot Study. Nutrients. 2025; 17(14):2305. https://doi.org/10.3390/nu17142305
Chicago/Turabian StyleKoch, Hannah R., Jesse N. L. Sims, Stephanie Pickett, Graham Finlayson, Laurie Wideman, and Jessica McNeil. 2025. "Associations Between Sleep, Appetite, and Food Reward over 6 Months in Black Emerging Adults—Findings from the Sleep, Health Outcomes and Body Weight (SHOW) Pilot Study" Nutrients 17, no. 14: 2305. https://doi.org/10.3390/nu17142305
APA StyleKoch, H. R., Sims, J. N. L., Pickett, S., Finlayson, G., Wideman, L., & McNeil, J. (2025). Associations Between Sleep, Appetite, and Food Reward over 6 Months in Black Emerging Adults—Findings from the Sleep, Health Outcomes and Body Weight (SHOW) Pilot Study. Nutrients, 17(14), 2305. https://doi.org/10.3390/nu17142305









