Perioperative Immunonutrition in Gastrointestinal Oncology: A Comprehensive Umbrella Review and Meta-Analysis on Behalf of TROGSS—The Robotic Global Surgical Society
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
2.5. Assessment of Methodological Quality
2.6. Statistical Analysis
3. Results
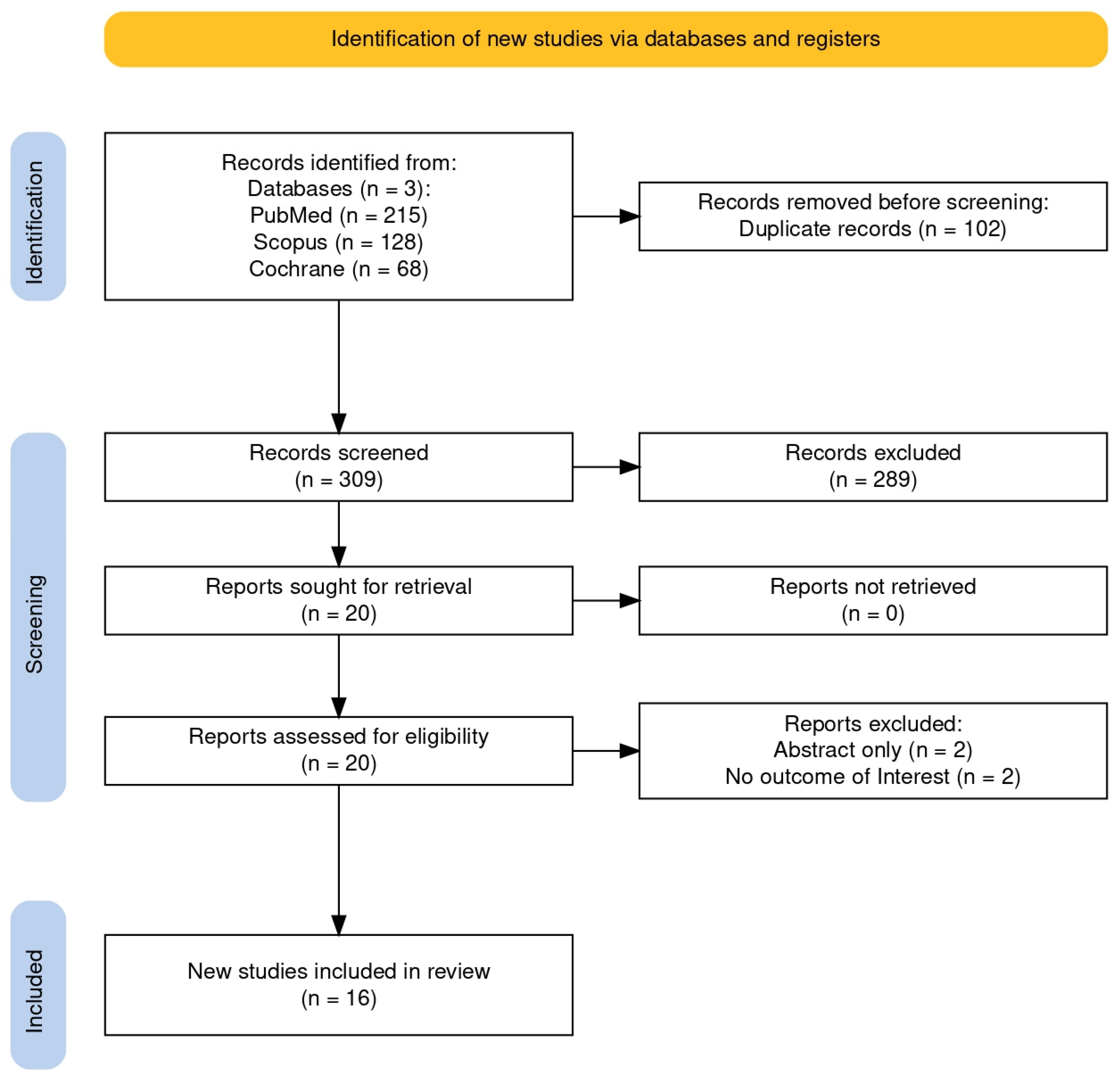
3.1. Selected Studies
3.2. Study Characteristics
3.3. Postoperative Infectious Complications
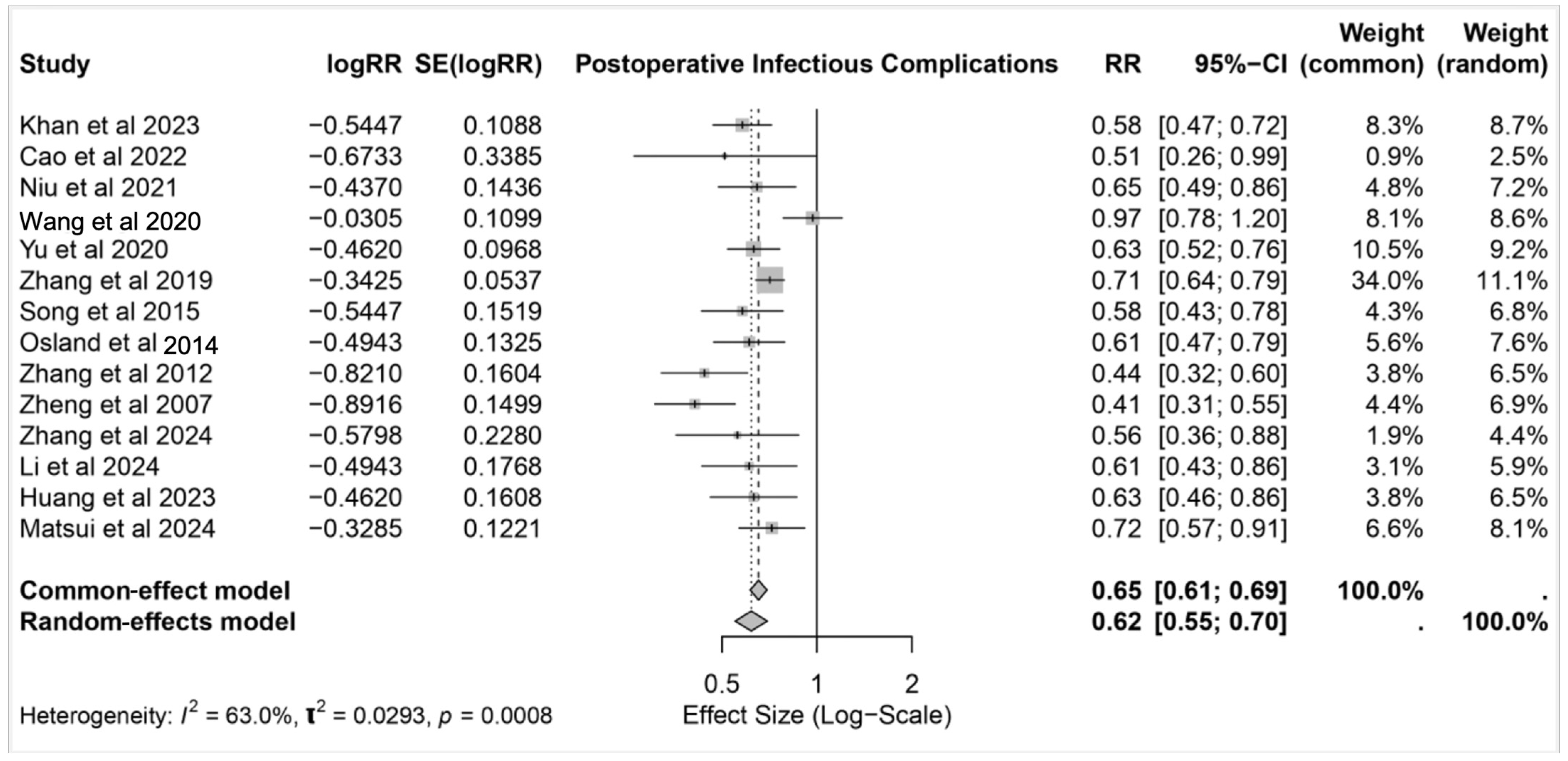
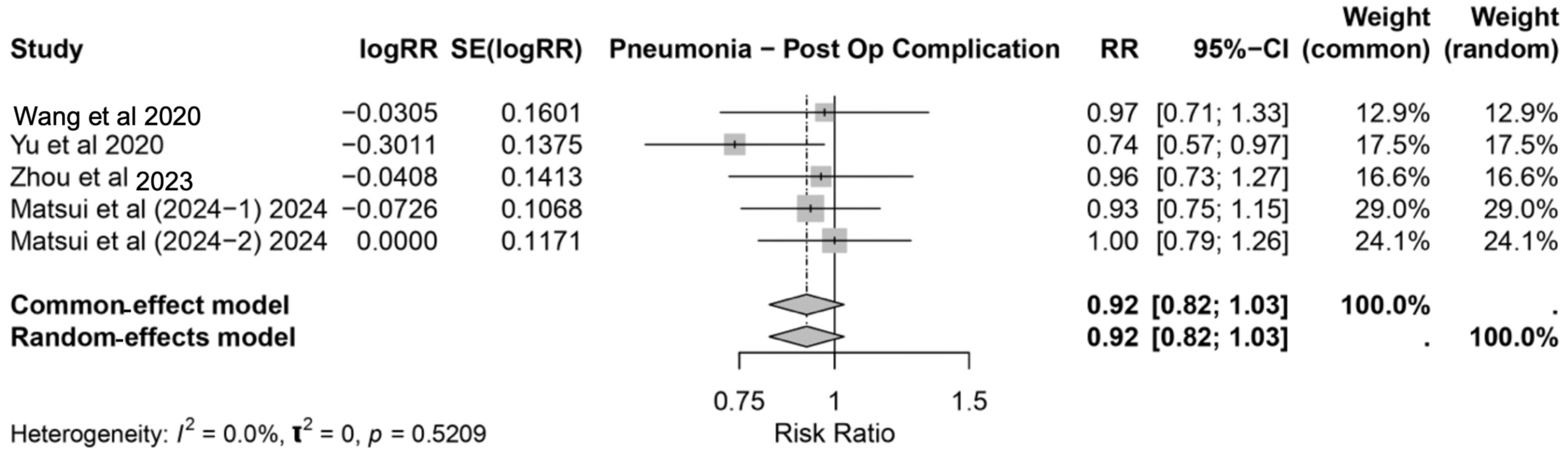
3.3.1. Pneumonia
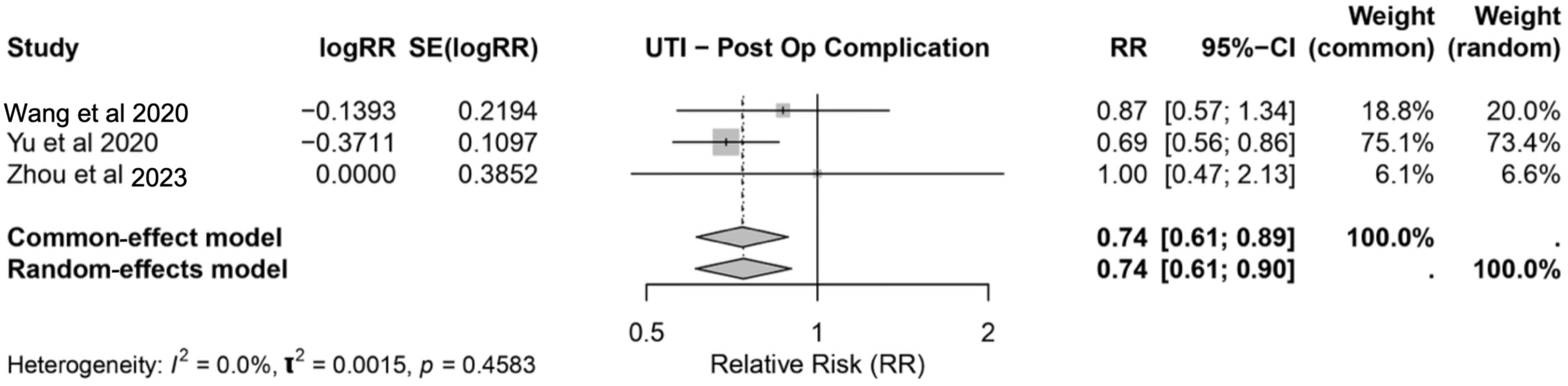
3.3.2. Urinary Tract Infection
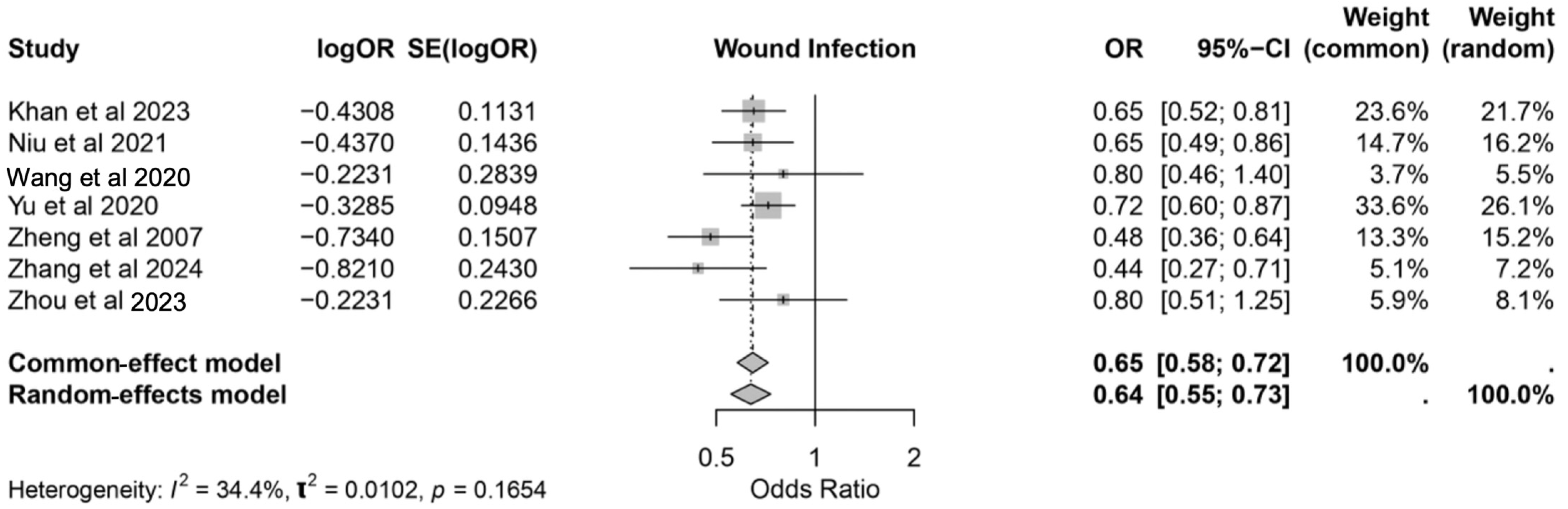
3.3.3. Wound Infection
3.4. Postoperative Noninfectious Complications
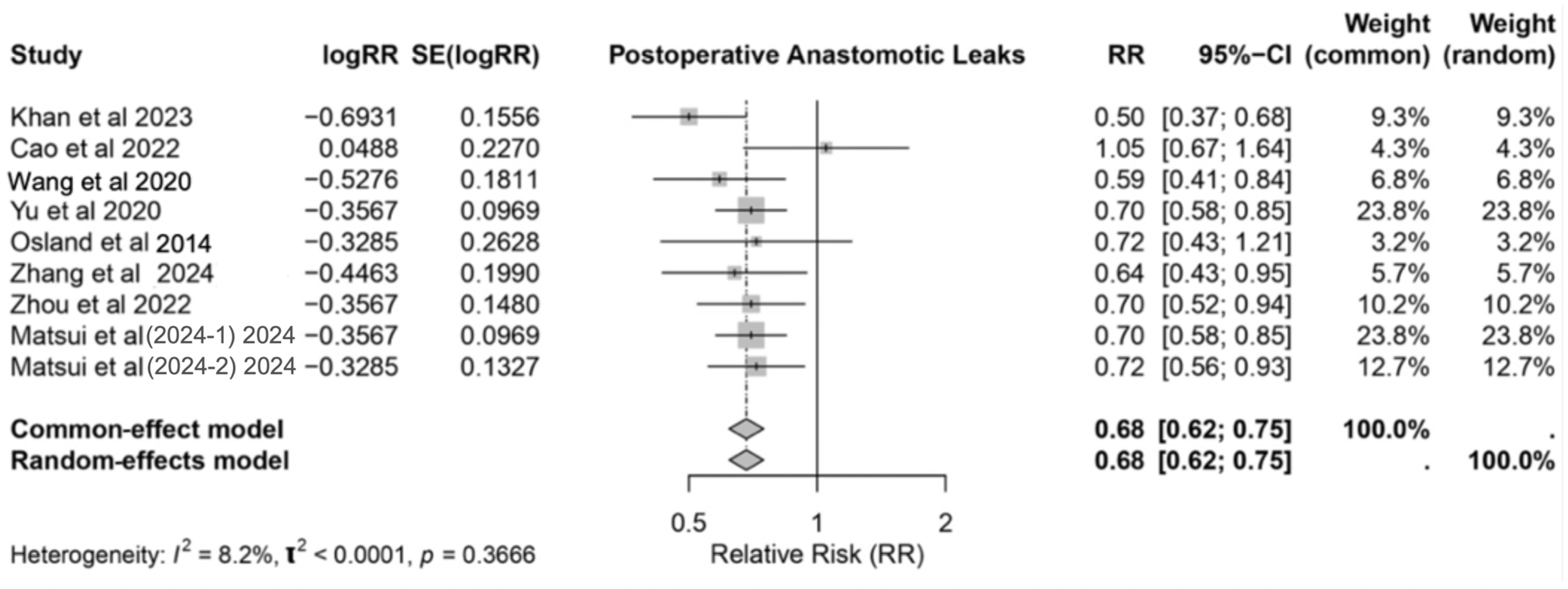
3.5. Anastomotic Leaks
3.6. Length of Hospital Stay
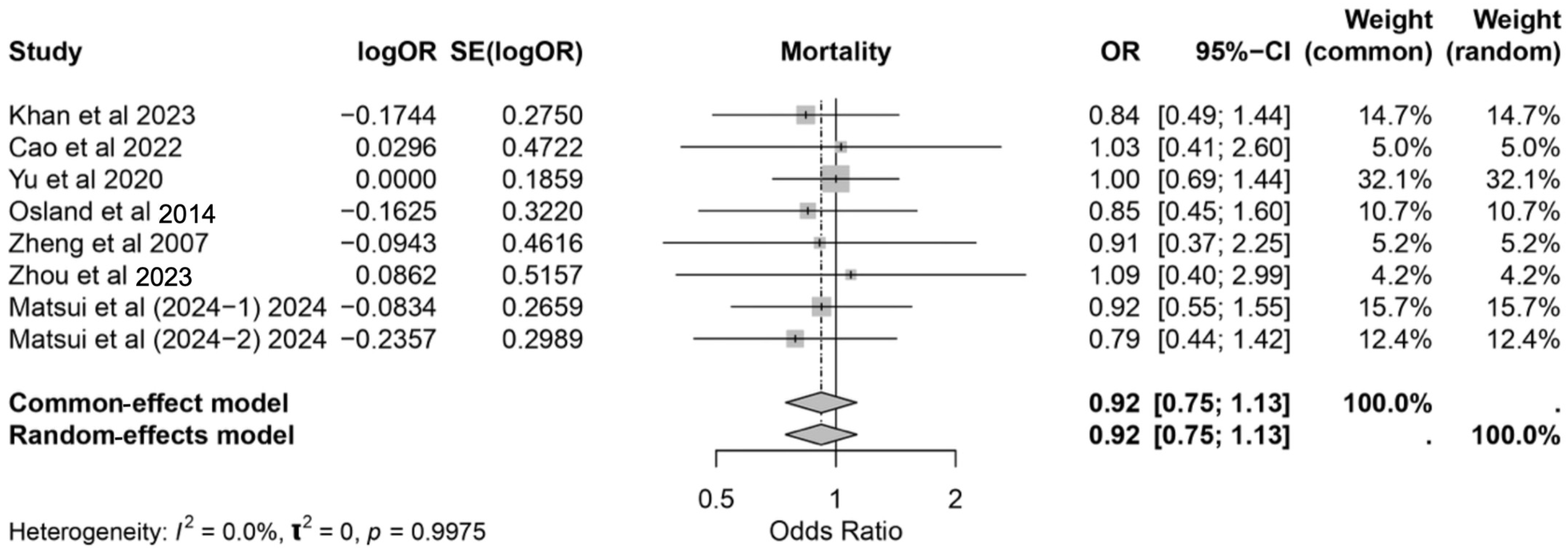
3.7. Mortality Rate
3.8. Nutritional Status Improvement
3.9. Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Felice, F.; Cattaneo, C.G.; Poto, G.E.; Antropoli, C.; Brillantino, A.; Carbone, L.; Brunetti, O.; De Luca, R.; Desideri, I.; Incorvaia, L.; et al. Mapping the Landscape of Immunonutrition and Cancer Research: A Comprehensive Bibliometric Analysis on Behalf of NutriOnc Research Group. Int. J. Surg. 2024, 110, 395–405. [Google Scholar] [CrossRef]
- Xu, J.; Yunshi, Z.; Li, R. Immunonutrition in Surgical Patients. Curr. Drug Targets 2009, 10, 771–777. [Google Scholar] [CrossRef]
- Matsui, R.; Sagawa, M.; Sano, A.; Sakai, M.; Hiraoka, S.; Tabei, I.; Imai, T.; Matsumoto, H.; Onogawa, S.; Sonoi, N.; et al. Impact of Perioperative Immunonutrition on Postoperative Outcomes for Patients Undergoing Head and Neck or Gastrointestinal Cancer Surgeries: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Surg. 2024, 279, 419–428. [Google Scholar] [CrossRef]
- Riso, S.; Aluffi, P.; Brugnani, M.; Farinetti, F.; Pia, F.; D’Andrea, F. Postoperative Enteral Immunonutrition in Head and Neck Cancer Patients. Clin. Nutr. 2000, 19, 407–412. [Google Scholar] [CrossRef]
- Yu, J.; Yuan, A.; Liu, Q.; Wang, W.; Sun, Y.; Li, Z.; Meng, C.; Zhou, Y.; Cao, S. Effect of Preoperative Immunonutrition on Postoperative Short-Term Clinical Outcomes in Patients with Gastric Cancer Cachexia: A Prospective Randomized Controlled Trial. World J. Surg. Oncol. 2024, 22, 101. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef]
- Chen, C.-L.; Hsu, S.-C.; Ann, D.K.; Yen, Y.; Kung, H.-J. Arginine Signaling and Cancer Metabolism. Cancers 2021, 13, 3541. [Google Scholar] [CrossRef]
- Howes, N.; Atkinson, C.; Thomas, S.; Lewis, S.J. Immunonutrition for Patients Undergoing Surgery for Head and Neck Cancer. Cochrane Database Syst. Rev. 2018, 8, CD010954. [Google Scholar] [CrossRef]
- García-Malpartida, K.; Aragón-Valera, C.; Botella-Romero, F.; Ocón-Bretón, M.J.; López-Gómez, J.J. Effects of Immunonutrition on Cancer Patients Undergoing Surgery: A Scoping Review. Nutrients 2023, 15, 1776. [Google Scholar] [CrossRef]
- Klek, S.; Szybinski, P.; Szczepanek, K. Perioperative Immunonutrition in Surgical Cancer Patients: A Summary of a Decade of Research. World J. Surg. 2014, 38, 803–812. [Google Scholar] [CrossRef]
- Ma, M.; Zheng, Z.; Zeng, Z.; Li, J.; Ye, X.; Kang, W. Perioperative Enteral Immunonutrition Support for the Immune Function and Intestinal Mucosal Barrier in Gastric Cancer Patients Undergoing Gastrectomy: A Prospective Randomized Controlled Study. Nutrients 2023, 15, 4566, Erratum in Nutrients 2024, 16, 3800. [Google Scholar] [CrossRef]
- Smith, V.; Devane, D.; Begley, C.M.; Clarke, M. Methodology in Conducting a Systematic Review of Systematic Reviews of Healthcare Interventions. BMC Med. Res. Methodol. 2011, 11, 15. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- PRISMA-P Group; Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Khan, A.; Wong, J.; Riedel, B.; Laing, E.; Beaumont, A.; Kong, J.; Warrier, S.; Heriot, A. The Impact of Peri-Operative Enteral Immunonutrition on Post-Operative Complications in Gastrointestinal Cancer Surgery: A Meta-Analysis. Ann. Surg. Oncol. 2023, 30, 3619–3631. [Google Scholar] [CrossRef]
- Cao, Y.; Han, D.; Zhou, X.; Han, Y.; Zhang, Y.; Li, H. Effects of Preoperative Nutrition on Postoperative Outcomes in Esophageal Cancer: A Systematic Review and Meta-Analysis. Dis. Esophagus 2022, 35, doab028. [Google Scholar] [CrossRef]
- Niu, J.-W.; Zhou, L.; Liu, Z.-Z.; Pei, D.-P.; Fan, W.-Q.; Ning, W. A Systematic Review and Meta-Analysis of the Effects of Perioperative Immunonutrition in Gastrointestinal Cancer Patients. Nutr. Cancer 2021, 73, 252–261. [Google Scholar] [CrossRef]
- Wang, M.L.; Ke, Z.Y.; Fan, F.F.; Wang, H.Z.; Li, Y.X. Perioperative Immunonutrition in Esophageal Cancer Patients Undergoing Esophagectomy: The First Meta-Analysis of Randomized Clinical Trials. Dis. Esophagus 2020, 33, doz111. [Google Scholar] [CrossRef]
- Yu, K.; Zheng, X.; Wang, G.; Liu, M.; Li, Y.; Yu, P.; Yang, M.; Guo, N.; Ma, X.; Bu, Y.; et al. Immunonutrition vs Standard Nutrition for Cancer Patients: A Systematic Review and Meta-Analysis (Part 1). J. Parenter. Enter. Nutr. 2020, 44, 742–767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Najarali, Z.; Ruo, L.; Alhusaini, A.; Solis, N.; Valencia, M.; Sanchez, M.I.P.; Serrano, P.E. Effect of Perioperative Nutritional Supplementation on Postoperative Complications—Systematic Review and Meta-Analysis. J. Gastrointest. Surg. 2019, 23, 1682–1693. [Google Scholar] [CrossRef]
- Song, G.-M.; Tian, X.; Zhang, L.; Ou, Y.-X.; Yi, L.-J.; Shuai, T.; Zhou, J.-G.; Zeng, Z.; Yang, H.-L. Immunonutrition Support for Patients Undergoing Surgery for Gastrointestinal Malignancy: Preoperative, Postoperative, or Perioperative? A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Medicine 2015, 94, e1225. [Google Scholar] [CrossRef]
- Osland, E.; Hossain, M.B.; Khan, S.; Memon, M.A. Effect of Timing of Pharmaconutrition (Immunonutrition) Administration on Outcomes of Elective Surgery for Gastrointestinal Malignancies: A Systematic Review and Meta-Analysis. J. Parenter. Enter. Nutr. 2014, 38, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, Y.; Guo, T.; Li, Y.; Cai, H. Perioperative Immunonutrition for Gastrointestinal Cancer: A Systematic Review of Randomized Controlled Trials. Surg. Oncol. 2012, 21, e87–e95. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, F.; Qi, B.; Luo, B.; Sun, H.; Liu, S.; Wu, X. Application of Perioperative Immunonutrition for Gastrointestinal Surgery: A Meta-Analysis of Randomized Controlled Trials. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. S1), 253–257. [Google Scholar]
- Zhang, M.; Chen, G.; Jin, X.; Wang, J.; Yu, S. Pre-Operative Immunonutrition Enhances Postoperative Outcomes and Elevates Tumor-Infiltrating Lymphocyte Counts in Colorectal Cancer Patients: A Meta-Analysis of Randomized Controlled Trials. Nutr. Cancer 2024, 76, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiang, Q.-L.; Zhu, J.-X.; Zhang, Y.-X.; Li, S.-Q. Comparison of Enteral Immunonutrition and Enteral Nutrition in Patients Undergoing Gastric Cancer Surgery: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. J. Int. Med. Res. 2024, 52, 03000605231220870. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, T.-T.; Yang, Z.; Tan, Z.-M.; Yang, C.-F.; Wang, Z. The Effect of Perioperative Immunonutrition on Patients Undergoing Esophagectomy: A Systematic Review and Updated Meta-Analysis. Nutr. Hosp. 2023, 40, 839–847. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, Q.; Li, W. A Meta-Analysis of Randomized Controlled Trials Comparing Enteral Immunonutrition (EIN) and Standard Enteral Nutrition Regarding Biochemical, Immunological, and Clinical Outcomes in Gastrectomy Patients with Gastric Cancer and Investigating Evidence Networks for EIN Formulae. Videosurgery Miniinvasive Tech. 2023, 18, 588–602. [Google Scholar] [CrossRef]
- Matsui, R.; Sagawa, M.; Inaki, N.; Fukunaga, T.; Nunobe, S. Impact of Perioperative Immunonutrition on Postoperative Outcomes in Patients with Upper Gastrointestinal Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2024, 16, 577. [Google Scholar] [CrossRef] [PubMed]
- Zabaglo, M.; Leslie, S.W.; Sharman, T. Postoperative Wound Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ma, C.; Tsai, H.; Su, W.; Sun, L.; Shih, Y.; Wang, J. Combination of Arginine, Glutamine, and Omega-3 Fatty Acid Supplements for Perioperative Enteral Nutrition in Surgical Patients with Gastric Adenocarcinoma or Gastrointestinal Stromal Tumor (GIST): A Prospective, Randomized, Double-Blind Study. J. Postgrad. Med. 2018, 64, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-S.; Xi, H.-Q.; Chen, L.; Wei, B. A Meta-Analysis of Robotic versus Laparoscopic Gastrectomy for Gastric Cancer. Surg. Endosc. 2014, 28, 2795–2802. [Google Scholar] [CrossRef]


| Authors | Year of Publication | Study Design | Country | Number of Studies Included | Time Period | Total Number of Patients Included | Type of Surgery | Type of Cancer | Interventions |
|---|---|---|---|---|---|---|---|---|---|
| Khan et al. [17] | 2023 | SR & MA | Australia | 37 RCT | 2000–2022 | 3793 | Elective GI Cancer | Upper GI, colorectal, and mixed GI cancers | Preoperative, perioperative, and postoperative enteral IMN: Arg, glutamine, ω-3-FA, and nucleotides. |
| Cao et al. [18] | 2022 | SR & MA | China | 5 RCTs and 10 observational | 1991–2018 | 1864 | Esophagectomy | Esophageal cancer | Preoperative IEN: Arg, ω-3-FA, and nucleotides |
| Niu et al. [19] | 2021 | SR & MA | China | 25 RCT | 1988–2011 | 2238 | Elective GI Cancer | Gastric, colorectal, and pancreatic cancers | Perioperative IMN: Arg, ω-3-FA, and nucleotides |
| Wang et al. [20] | 2020 | SR | China | 11 RCT | 2007–2019 | 606 | Esophagectomy | Esophageal Cancer | Perioperative EIN: Arg, RNA, and ω-3-FA (formulas like IMPACT, EPA, Oxepa) |
| Yu et al. [21] | 2020 | SR & MA | China | 61 RCT | 1994–2017 | 5983 | Elective surgical resection for gastrointestinal cancers. | Gastric, Colorectal, Esophageal, and Pancreatic | Perioperative IMN, including Arg, ω-3-FA, and RNA, compared to standard nutrition. |
| Zhang et al. [22] | 2019 | SR & MA | Canada | 56 RCT | 1995–2018 | 6370 | Elective GI Cancer | Gastric, colorectal, esophageal, and pancreatic cancer | Perioperative IMN: Arg, ω-3-FA, RNA, protein supplementation, and carbohydrate |
| Song et al. [23] | 2015 | SR & MA | China | 27 RCT | 1992–2013 | 2538 | Elective GI Cancer | Gastric, colorectal, esophageal, and pancreatic cancers | Preoperative, perioperative, and postoperative IMN: Arg, ω-3-FA, glutamine, and RNA |
| Osland et al. [24] | 2013 | SR & MA | Australia | 20 RCT | 1988–2011 | 2005 | Elective GI Cancer | Gastric, pancreatic, esophageal cancers | Arg-dominant pharmaconutrition vs standard enteral nutrition, delivered preoperatively, perioperatively, or postoperatively. |
| Zhang et al. [25] | 2012 | SR & MA | China | 19 RCT | 1995–2011 | 2331 | Elective GI Cancer | Gastric, colorectal, pancreatic, and esophageal cancers | Perioperative IMN: Arg, ω-3-FA, glutamine, RNA |
| Zheng et al. [26] | 2007 | MA | China | 13 RCT | 1992–2005 | 1269 | Elective GI Cancer | Gastric, colorectal, and pancreatic cancers | Perioperative IMN: Arg, glutamine, ω-3-FA, and RNA |
| Zhang et al. [27] | 2024 | SR & MA | China | 16 RCTs | 2002–2023 | 1416 patients | Colorectal cancer surgery | Colorectal cancer | Preoperative IMN: Arg, ω-3-FA, glutamine, RNA |
| Li et al. [28] | 2024 | SR & MA | China | 12 RCT | 2005–2022 | 1124 | Gastrectomy | Gastric Cancer | IMN: ω-3-FA, Arg, glutamine, nucleotides |
| Zhou et al. [29] | 2022 | SR & MA | China | 10 RCT | 2007–2020 | 1052 | Esophagectomy | Esophageal Cancer | EIN: Arg, glutamine, ω-3-FA and nucleotides |
| Matsui et al. [3] | 2024 | SR & MA | Japan | 48 RCT | 1981–2022 | 4825 | Elective Heck and Neck Cancer Surgery and Elective GI Cancer | Head and Neck Gastrointestinal Cancer | Preoperative, perioperative, and postoperative enteral IMN: Arg, ω-3-FA, or glutamine |
| Huang et al. [30] | 2023 | MA | China | 10 RCT | 2005–2022 | 1409 | Gastrectomy | Gastric Cancer | EIN: ω-3 fatty acids, glutamine, Arg, and nucleotides. |
| Matsui et al. [31] | 2024 | SR & MA | Japan | 23 RCT | 2000–2022 | 2249 | Elective GI Cancer | Upper GI cancer | Preoperative, perioperative, and postoperative enteral IMN: Arg, ω-3-FA, or glutamine |
| Study | Parameter | Effect Size | 95% CI | p-Value |
|---|---|---|---|---|
| Niu et al., 2021 [19] | CRP | Hedges’ g = −1.529 | [−2.250–0.809] | <0.001 |
| Zheng et al., 2007 [26] | Lymphocytes | WMD = 0.40 | [0.21–0.59] | <0.00001 |
| CD4 | WMD = 11.39 | [6.20–16.58] | <0.00001 | |
| Huang et al., 2023 [30] | Lymphocytes | SMD = 1.34 | [0.39–3.07] | 0.0001 |
| Li et al., 2024 [28] | CD4 | MD = 2.92 | [−3.84 to 9.69] | Not significant |
| CD8 | MD = 1.16 | [0.06–2.26] | 0.04 | |
| CD4/CD8 ratio | MD = 0.48 | [0.08–0.88] | 0.02 | |
| IgG | MD = 2.46 | [1.47–3.46] | <0.001 | |
| Zhang et al., 2024 [27] | IgA | MD = 0.58 | [0.31–0.85] | <0.01 |
| IgG | MD = 1.67 | [1.15–2.19] | <0.01 | |
| IgM | MD = 0.40 | [0.20–0.61] | <0.01 | |
| CD16 | MD = 0.04 | [0.02–0.06] | <0.001 | |
| CD56 | MD = 0.05 | [0.03–0.06] | <0.001 |
| AMSTAR 2 Domain | Khan et al. [17] | Cao et al. [18] | Niu et al. [19] | Wang et al. [20] | Yu et al. [21] | Zhang et al. [22] | Song et al. [23] | Osland et al. [24] | Zhang et al. [25] | Zheng et al. [26] | Zhang et al. [27] | Li et al. [28] | Zhou et al. [29] | Matsui et al. [3] | Huang et al. [30] | Matsui et al. [31] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 2 | Y | Y | N | N | Y | Y | N | N | N | N | Y | Y | N | Y | PY | PY |
| 3 | Y | Y | N | Y | N | Y | Y | Y | N | N | Y | Y | N | Y | N | Y |
| 4 | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY |
| 5 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 6 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 7 | N | N | N | N | N | N | N | N | N | N | PY | N | N | N | N | N |
| 8 | Y | Y | Y | Y | Y | PY | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| 9 | Y | Y | N | Y | Y | Y | Y | N | Y | N | Y | PY | Y | PY | PY | Y |
| 10 | Y | N | N | N | N | N | N | Y | N | N | N | N | N | N | N | N |
| 11 | Y | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| 12 | Y | Y | N | N | Y | Y | Y | N | N | N | Y | N | Y | Y | N | N |
| 13 | Y | Y | N | Y | Y | Y | Y | N | N | N | N | N | N | N | Y | N |
| 14 | Y | Y | N | Y | Y | Y | Y | Y | N | N | Y | N | N | N | N | N |
| 15 | N | Y | Y | Y | Y | Y | N | Y | N | N | Y | Y | N | Y | Y | Y |
| 16 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Score | Critical | Low | Critical | Critical | Low | Low | Critical | Critical | Critical | Critical | Low | Critical | Critical | Critical | Low | Critical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyal, A.; Macias, C.A.; Corzo, M.P.; Vargas, V.P.S.; Mendoza, M.; Guarecuco Castillo, J.E.; Garcia, A.; Morfin-Meza, K.D.; Fuentes-Orozco, C.; González-Ojeda, A.; et al. Perioperative Immunonutrition in Gastrointestinal Oncology: A Comprehensive Umbrella Review and Meta-Analysis on Behalf of TROGSS—The Robotic Global Surgical Society. Nutrients 2025, 17, 2304. https://doi.org/10.3390/nu17142304
Goyal A, Macias CA, Corzo MP, Vargas VPS, Mendoza M, Guarecuco Castillo JE, Garcia A, Morfin-Meza KD, Fuentes-Orozco C, González-Ojeda A, et al. Perioperative Immunonutrition in Gastrointestinal Oncology: A Comprehensive Umbrella Review and Meta-Analysis on Behalf of TROGSS—The Robotic Global Surgical Society. Nutrients. 2025; 17(14):2304. https://doi.org/10.3390/nu17142304
Chicago/Turabian StyleGoyal, Aman, Christian Adrian Macias, Maria Paula Corzo, Vanessa Pamela Salolin Vargas, Mathew Mendoza, Jesús Enrique Guarecuco Castillo, Andrea Garcia, Kathia Dayana Morfin-Meza, Clotilde Fuentes-Orozco, Alejandro González-Ojeda, and et al. 2025. "Perioperative Immunonutrition in Gastrointestinal Oncology: A Comprehensive Umbrella Review and Meta-Analysis on Behalf of TROGSS—The Robotic Global Surgical Society" Nutrients 17, no. 14: 2304. https://doi.org/10.3390/nu17142304
APA StyleGoyal, A., Macias, C. A., Corzo, M. P., Vargas, V. P. S., Mendoza, M., Guarecuco Castillo, J. E., Garcia, A., Morfin-Meza, K. D., Fuentes-Orozco, C., González-Ojeda, A., Suárez-Carreón, L. O., Ruiz-Úcar, E., Vashist, Y., Pérez Bonet, A., Abou-Mrad, A., Oviedo, R. J., & Marano, L. (2025). Perioperative Immunonutrition in Gastrointestinal Oncology: A Comprehensive Umbrella Review and Meta-Analysis on Behalf of TROGSS—The Robotic Global Surgical Society. Nutrients, 17(14), 2304. https://doi.org/10.3390/nu17142304








