Protective Effects of Deer Antler Peptides on D-Galactose-Induced Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. MWM Test
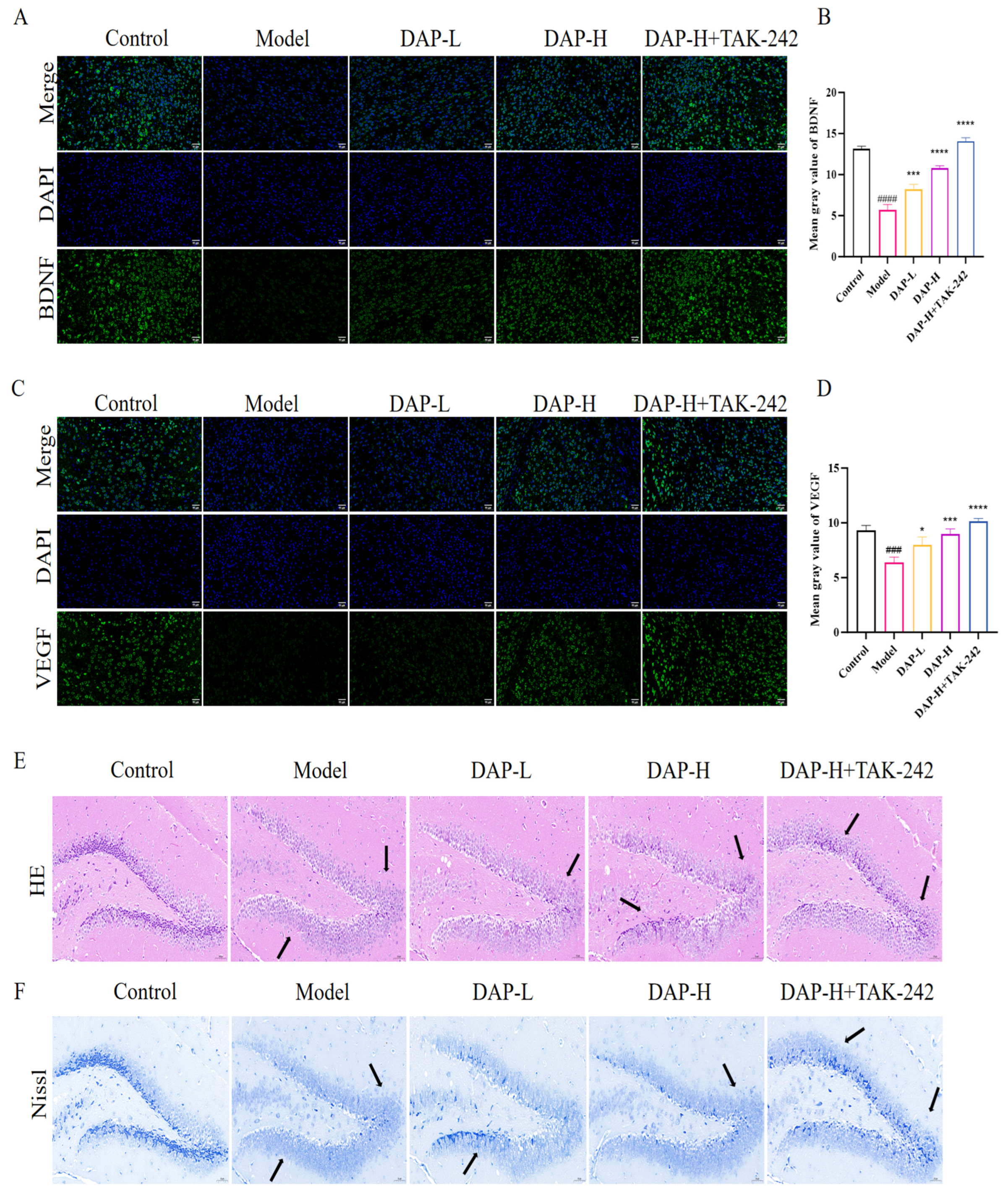
2.4. Immunofluorescence
2.5. HE and Nissl Staining
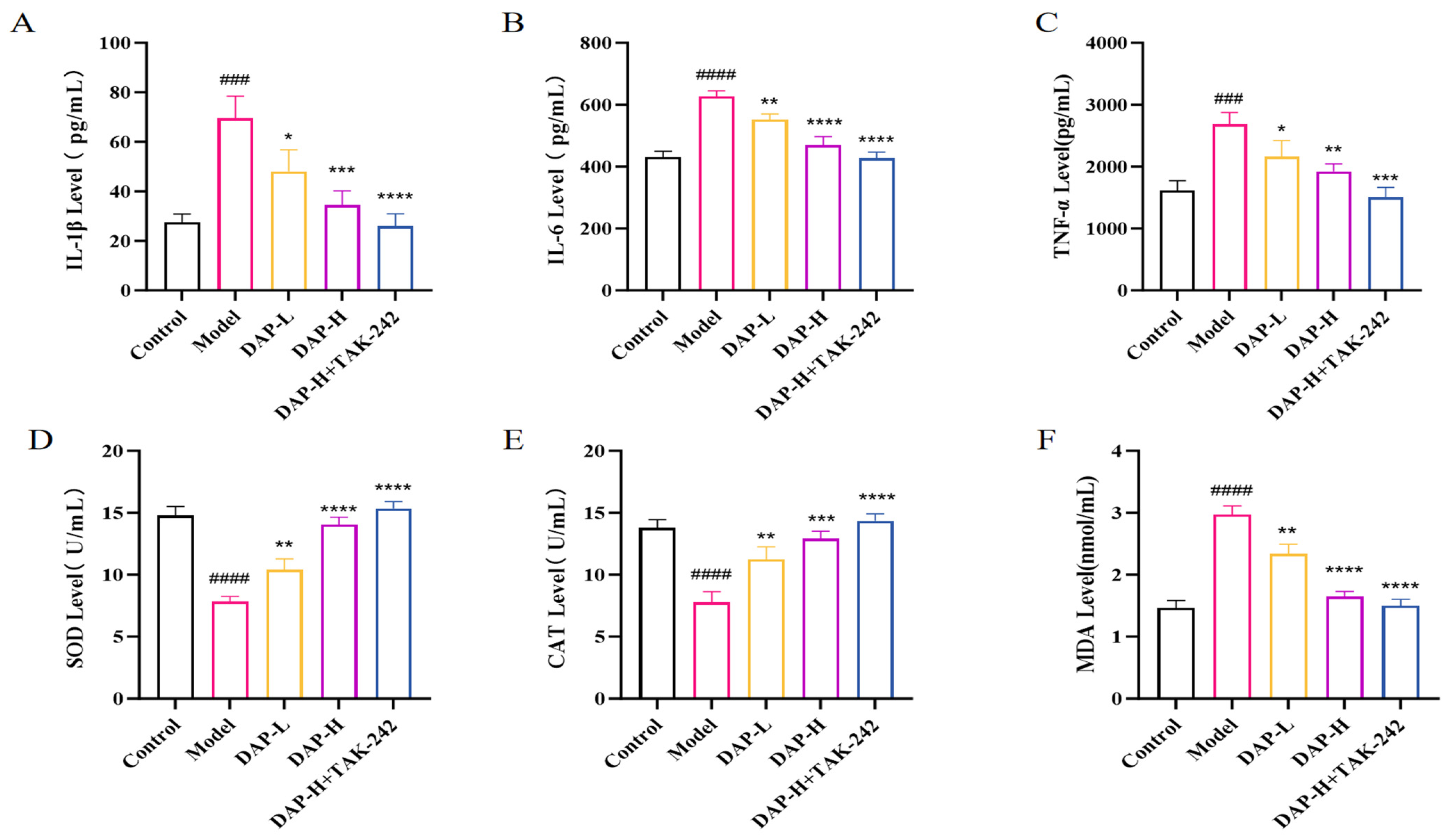
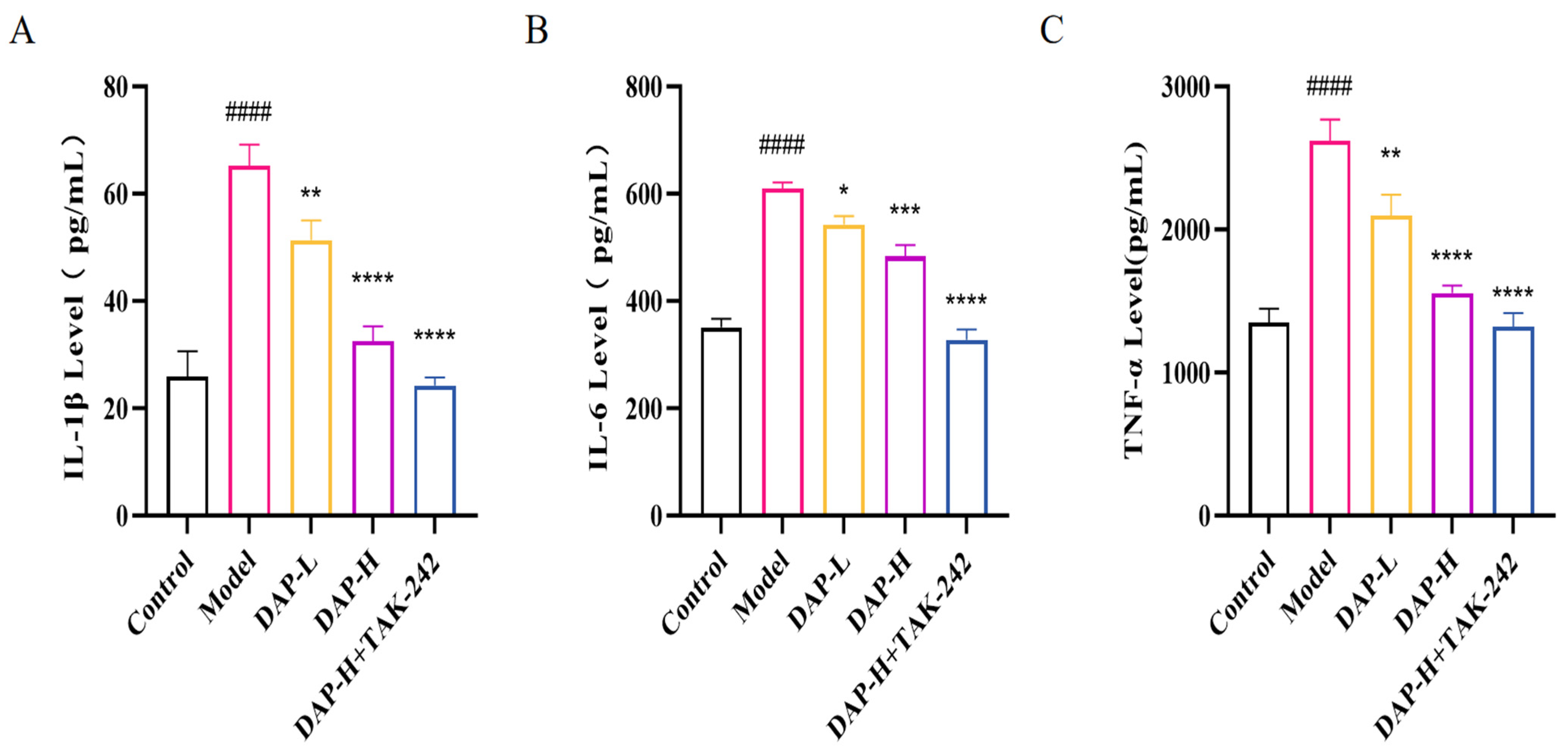
2.6. ELISA
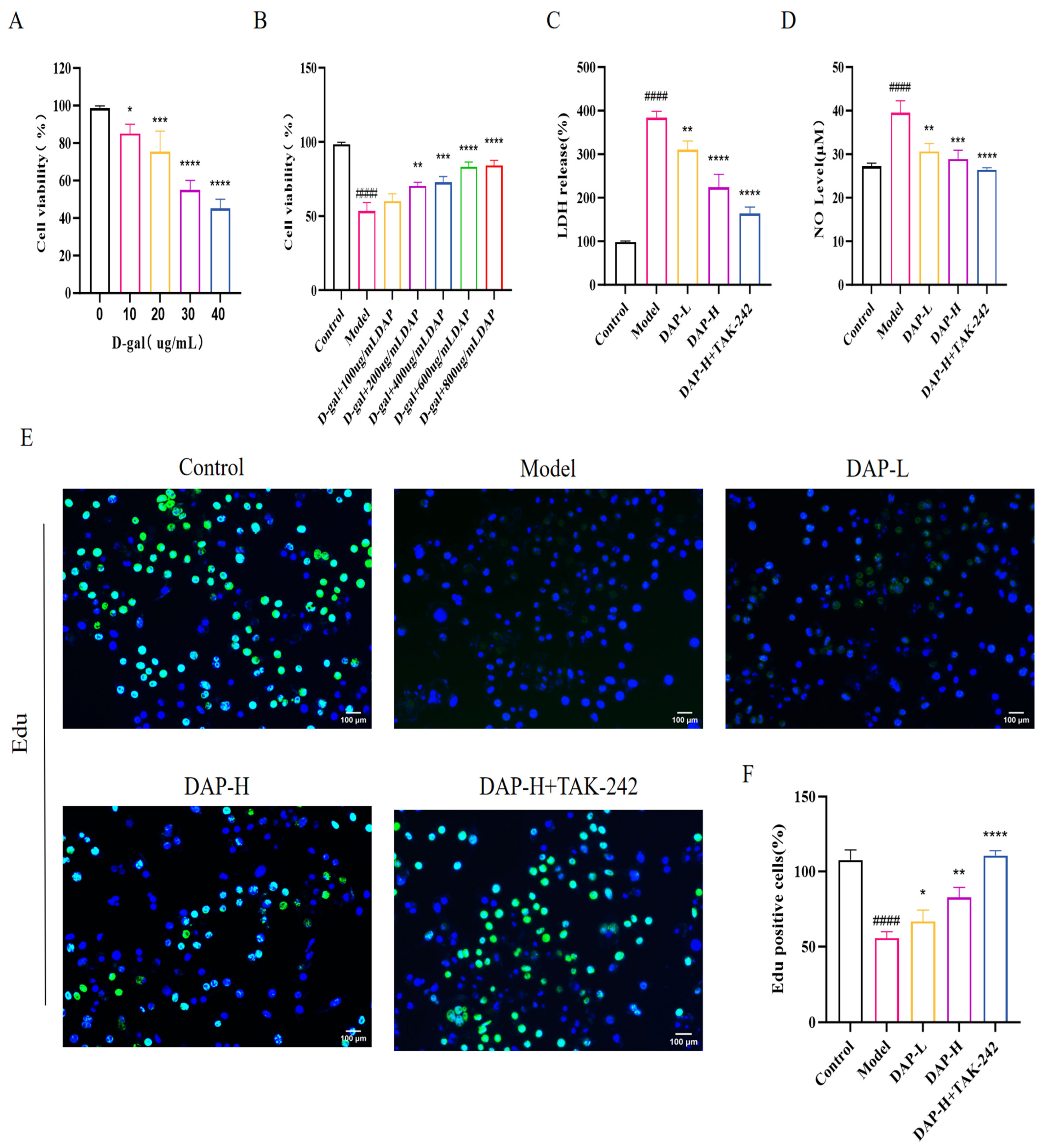
2.7. Cell Viability Detection
2.8. Determination of NO and LDH
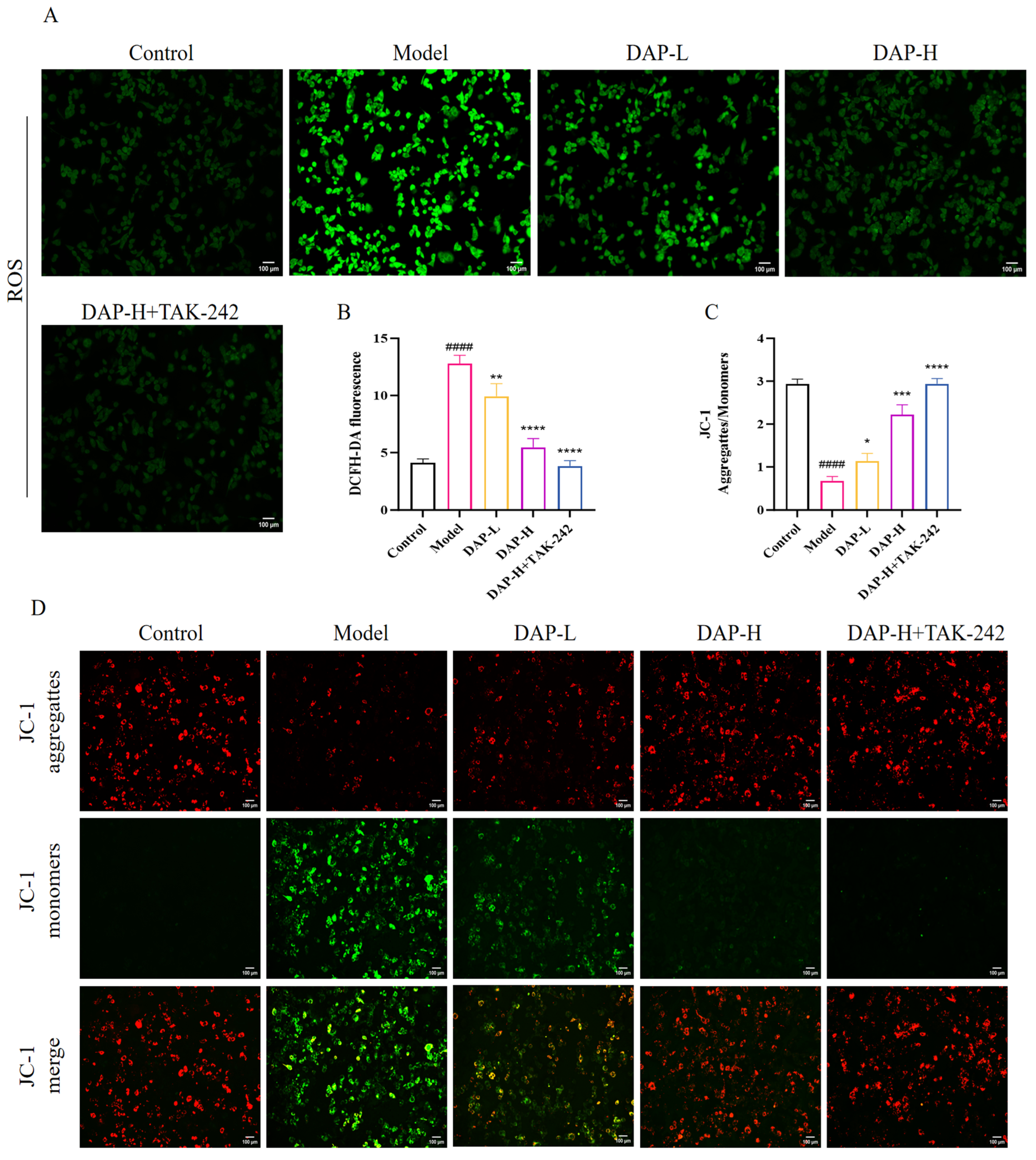
2.9. ROS, Cell Proliferation, Mitochondrial Membrane Potential Detection
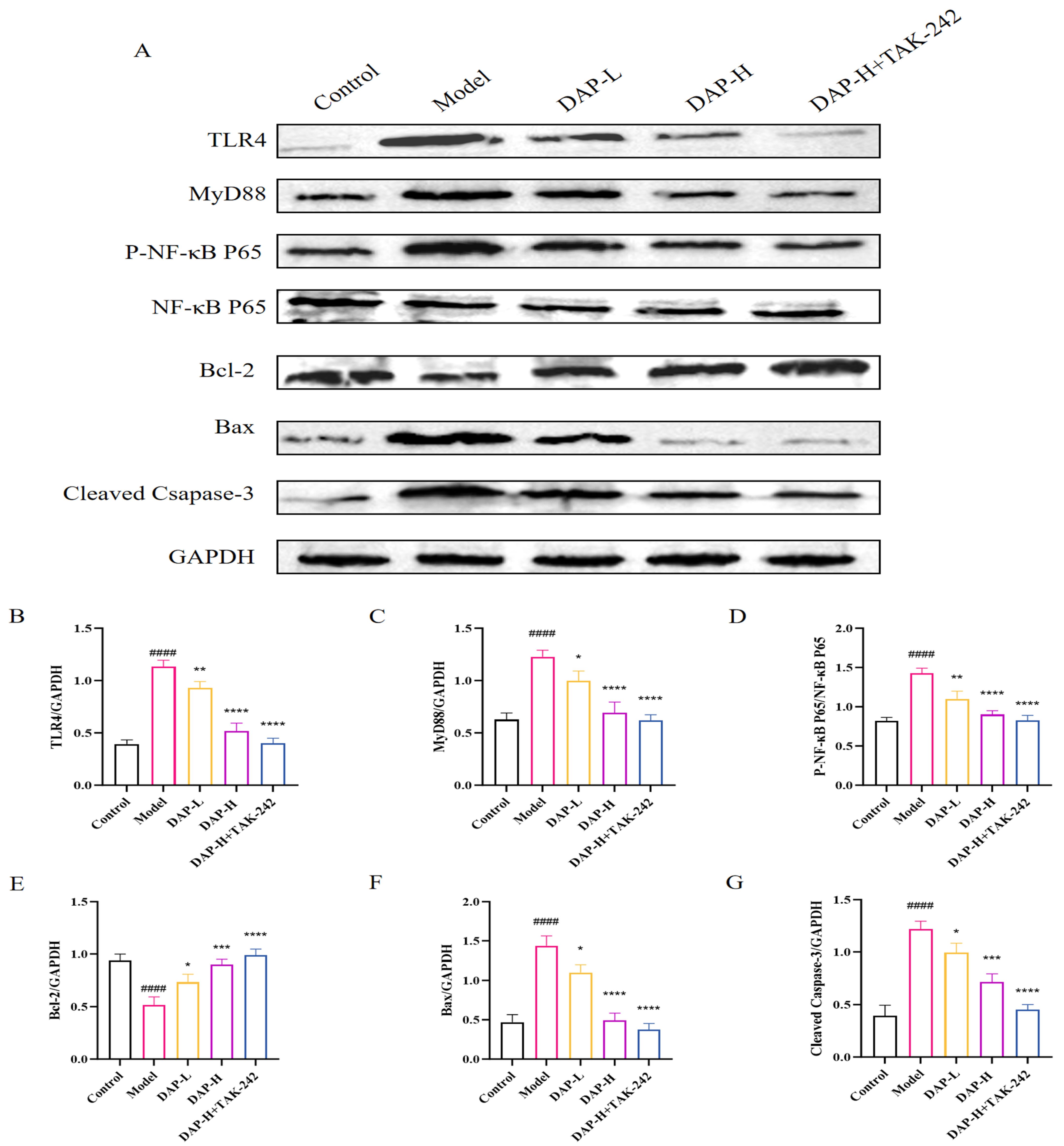
2.10. Western Blot
2.11. Statistical Analysis
3. Results
3.1. The Effect of DAP on Learning and Memory Ability in D-Gal-Induced Aging Mice
3.2. The Effect of DAP on Brain Tissue in D-Gal-Induced Aging Mice
3.3. The Effect of DAP on Serum Inflammatory Factors and Antioxidant Indices in D-Gal-Induced Aging Mice
3.4. The Effect of DAP on TLR4/MyD88/NF-κB Pathway in D-Gal-Induced Aging Mice Brain
3.5. The Effect of DAP on the Viability and Proliferation of D-Gal-Induced BV2 Microglia
3.6. The Effect of DAP on Pro-Inflammatory Factors of BV2 Microglia Induced by D-Gal
3.7. The Effect of DAP on Oxidative Stress in BV2 Microglia Induced by D-Gal
3.8. The Effect of DAP on TLR4/MyD88/NF-κB Signaling in D-Gal-Induced BV2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, Y.; Yu, Y.-H.; Wang, S.-T.; Ren, J.; Camer, D.; Hua, Y.-Z.; Zhang, Q.; Huang, J.; Xue, D.-L.; Zhang, X.-F.; et al. Chlorogenic Acid Protects d -Galactose-Induced Liver and Kidney Injury via Antioxidation and Anti-Inflammation Effects in Mice. Pharm. Biol. 2016, 54, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Zhou, Y.; Yang, J.; Leng, J.; Li, J.; Hu, J.; Liu, W.; Jiang, S.; Wang, Y.; Chen, C.; et al. Maltol (3-Hydroxy-2-Methyl-4-Pyrone) Slows d -Galactose-Induced Brain Aging Process by Damping the Nrf2/HO-1-Mediated Oxidative Stress in Mice. J. Agric. Food Chem. 2019, 67, 10342–10351. [Google Scholar] [CrossRef] [PubMed]
- Juan, S.M.A.; Adlard, P.A. Ageing and Cognition. In Biochemistry and Cell Biology of Ageing: Part II Clinical Science; Harris, J.R., Korolchuk, V.I., Eds.; Subcellular Biochemistry; Springer: Singapore, 2019; Volume 91. [Google Scholar]
- Blagosklonny, M.V. Disease or Not, Aging Is Easily Treatable. Aging 2018, 10, 3067–3078. [Google Scholar] [CrossRef] [PubMed]
- Walters, H.E.; Cox, L.S. mTORC Inhibitors as Broad-Spectrum Therapeutics for Age-Related Diseases. Int. J. Mol. Sci. 2018, 19, 2325. [Google Scholar] [CrossRef]
- Jia, L.; Du, Y.; Chu, L.; Zhang, Z.; Li, F.; Lyu, D.; Li, Y.; Li, Y.; Zhu, M.; Jiao, H.; et al. Prevalence, Risk Factors, and Management of Dementia and Mild Cognitive Impairment in Adults Aged 60 Years or Older in China: A Cross-Sectional Study. Lancet Public Health 2020, 5, e661–e671. [Google Scholar] [CrossRef]
- Sessa, F.; Maglietta, F.; Bertozzi, G.; Salerno, M.; Di Mizio, G.; Messina, G.; Montana, A.; Ricci, P.; Pomara, C. Human Brain Injury and miRNAs: An Experimental Study. Int. J. Mol. Sci. 2019, 20, 1546. [Google Scholar] [CrossRef]
- Moscatelli, F.; Messina, G.; Valenzano, A.; Petito, A.; Triggiani, A.I.; Ciliberti, M.A.P.; Monda, V.; Messina, A.; Tafuri, D.; Capranica, L.; et al. Relationship between RPE and Blood Lactate after Fatiguing Handgrip Exercise in Taekwondo and Sedentary Subjects. Biol. Med. 2015, 1, S3008. [Google Scholar]
- Huo, Y.; Huo, H.; Zhang, J. The Contribution of Deer Velvet Antler Research to the Modern Biological Medicine. Chin. J. Integr. Med. 2014, 20, 723–728. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, Z.; Ma, L.; Yang, L.; Wu, T.; Fu, Z. Pilose Antler Polypeptides Ameliorate Inflammation and Oxidative Stress and Improves Gut Microbiota in Hypoxic-Ischemic Injured Rats. Nutr. Res. 2019, 64, 93–108. [Google Scholar] [CrossRef]
- Xin, J.-L.; Zhang, Y.; Li, Y.; Zhang, L.-Z.; Lin, Y.; Zheng, L.-W. Protective Effects of Cervus Nippon Temminck Velvet Antler Polypeptides against MPP+-Induced Cytotoxicity in SH-SY5Y Neuroblastoma Cells. Mol. Med. Rep. 2017, 16, 5143–5515. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Li, H.; Lan, X.; Kan, M.; Lin, J.; Wang, J.; Zhang, Z.; Ming, S.; Li, Z.; et al. The Anti-Aging Effect of Velvet Antler Polypeptide Is Dependent on Modulation of the Gut Microbiota and Regulation of the PPARα/APOE4 Pathway. J. Integr. Neurosci. 2021, 20, 573–583. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Yang, M.; Guo, J.; Sun, Z.; Wang, S.; Li, R.; Pang, X.; Kim, Y.; Wang, X.; et al. Sika Deer Velvet Antler Peptide Exerts Neuroprotective Effect in a Parkinson’s Disease Model via Regulating Oxidative Damage and Gut Microbiota. Pharmaceuticals 2024, 17, 972. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; He, Z.; Du, R.; Yang, Y.; Wu, S.; Li, W.; Sheng, J.; Han, C. Polypeptide-PNP2 in Corn Cervi Pantotrichum Ameliorates Cognitive Impairment in Alzheimer’s Disease Mice by Inhibiting Microglial Cell Activation. Mol. Neurobiol. 2025, 62, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, Y.; Ye, R.; Wang, H.; Ge, Y. Velvet Antler Polypeptide (VAP) Protects against Cerebral Ischemic Injury through NF-κB Signaling Pathway in Vitro. J. Stroke Cerebrovasc. Dis. 2024, 33, 107666. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Du, R.; He, Z.; Yang, Y.; Wu, S.; Li, W.; Sheng, J.; Lv, Y.; Han, C. Protection of a Novel Velvet Antler Polypeptide PNP1 against Cerebral Ischemia-Reperfusion Injury. Int. J. Biol. Macromol. 2023, 247, 125815. [Google Scholar] [CrossRef]
- Wu, T.; Yang, L.; Chen, Y.; Ni, Y.; Jiang, J.; Zhang, W.; Zhou, Q.; Zheng, X.; Wang, Q.; Fu, Z.; et al. Pilose Antler Polypeptides Ameliorates Hypoxic-Ischemic Encephalopathy by Activated Neurotrophic Factors and SDF1/CXCR4 Axis in Rats. Acta Biochim. Biophys. Sin. 2018, 50, 254–262. [Google Scholar] [CrossRef]
- Li, X.-Y.; Zeng, W.-H.; Feng, H.; Cai, W.-F.; Chen, Q.-C.; Ni, Q.; Lin, S.-X.; Wu, M.-X.; Yi, Y.-K.; Liu, L.; et al. Flavonoids from Liriodendron Chinense Leaves Alleviate Oxidative Activity in Vitro and D-Galactose-Induced Brain Injury via AMPK/SIRT1 Pathway in Vivo. Ind. Crops Prod. 2024, 213, 118404. [Google Scholar] [CrossRef]
- Wang, X.; Kang, J.; Li, X.; Wu, P.; Huang, Y.; Duan, Y.; Feng, J.; Wang, J. Codonopsis Pilosula Water Extract Delays D-Galactose-Induced Aging of the Brain in Mice by Activating Autophagy and Regulating Metabolism. J. Ethnopharmacol. 2024, 327, 118016. [Google Scholar] [CrossRef]
- Qi, J.; Fu, L.-Y.; Liu, K.-L.; Li, R.-J.; Qiao, J.-A.; Yu, X.-J.; Yu, J.-Y.; Li, Y.; Feng, Z.-P.; Yi, Q.-Y.; et al. Resveratrol in the Hypothalamic Paraventricular Nucleus Attenuates Hypertension by Regulation of ROS and Neurotransmitters. Nutrients 2022, 14, 4177. [Google Scholar] [CrossRef]
- Hao, X.; Long, X.; Fan, L.; Gou, J.; Liu, Y.; Fu, Y.; Zhao, H.; Xie, X.; Wang, D.; Liang, G.; et al. Prenatal LPS Leads to Increases in RAS Expression within the PVN and Overactivation of Sympathetic Outflow in Offspring Rats. Hypertens. Res. 2024, 47, 2363–2376. [Google Scholar] [CrossRef]
- Radford-Smith, D.E.; Oke, K.; Costa, C.F.F.A.; Anthony, D.C. Systematic Review and Meta-Analysis of Microbiota-Gut-Astrocyte Axis Perturbation in Neurodegeneration, Brain Injury, and Mood Disorders. Brain Behav. Immun. Health 2025, 46, 101013. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, J.; Zhang, C.; Wang, H.; Wang, B.; Zhang, X. Poliumoside Alleviates Microglia-Mediated Inflammation and Blood-Brain Barrier Disruption via Modulating the Polarization of Microglia after Ischemic Stroke in Mice. Phytomedicine 2025, 143, 156881. [Google Scholar] [CrossRef]
- Otani, K.; Koyama, R.; Tsuyama, J.; Sakai, S.; Hase, K.; Shichita, T. Zoledronic Acid Attenuates Ischemic Brain Injury by Promoting ETS2 and MSR1 Expression. Int. Immunol. 2025, 37, 393–402. [Google Scholar] [CrossRef]
- Ibrahim, W.W.; Skalicka-Woźniak, K.; Budzyńska, B.; El Sayed, N.S. NLRP3 Inflammasome Inhibition and M1-to-M2 Microglial Polarization Shifting via Scoparone-Inhibited TLR4 Axis in Ovariectomy/D-Galactose Alzheimer’s Disease Rat Model. Int. Immunopharmacol. 2023, 119, 110239. [Google Scholar] [CrossRef]
- Cui, M.; Shan, X.; Yan, Y.; Zhao, T.; Sun, Y.; Hao, W.; Wang, Z.; Chang, Y.; Xie, Y.; Wei, B. Ganmaidazao Decoction Alleviated Cognitive Impairment on Alzheimer’s Disease Rats by Regulating Gut Microbiota and Their Corresponding Metabolites. Arab. J. Chem. 2023, 16, 104688. [Google Scholar] [CrossRef]
- Fan, X.; Wang, H.; Lv, X.; Wang, Q.; Yu, B.; Li, X.; Li, L.; Zhang, Y.; Ma, N.; Lu, Q.; et al. The pCREB/BDNF Pathway in the Hippocampus Is Involved in the Therapeutic Effect of Selective 5-HT Reuptake Inhibitors in Adult Male Rats Exposed to Blast Traumatic Brain Injury. Brain Sci. 2025, 15, 236. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, D.; Klang, A.; Thams, S.; Rostami, E. The Role of BDNF in Experimental and Clinical Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 3582. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, X.; Fang, J.; Li, Q. Intervention of Exogenous VEGF Protect Brain Microvascular Endothelial Cells from Hypoxia-Induced Injury by Regulating PLCγ/RAS/ERK and PI3K/AKT Pathways. Exp. Gerontol. 2024, 192, 112452. [Google Scholar] [CrossRef]
- Zhou, G.; Cao, Y.; Yan, Y.; Xu, H.; Zhang, X.; Yan, T.; Wan, H. Injectable Hydrogels Based on Hyaluronic Acid and Gelatin Combined with Salvianolic Acid B and Vascular Endothelial Growth Factor for Treatment of Traumatic Brain Injury in Mice. Molecules 2024, 29, 1705. [Google Scholar] [CrossRef]
- Long, Y.; Liu, S.; Wan, J.; Zhang, Y.; Li, D.; Yu, S.; Shi, A.; Li, N.; He, F. Brain Targeted Borneol-Baicalin Liposome Improves Blood-Brain Barrier Integrity after Cerebral Ischemia-Reperfusion Injury via Inhibiting HIF-1α/VEGF/eNOS/NO Signal Pathway. Biomed. Pharmacother. 2023, 160, 114240. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, T.; Gu, Y.; Wu, J.; Hu, Z.; Gong, P. Amelioration of Inflammation in D-Galactose-Induced Aging C57BL/6J Mice via Sialoglycan-Enriched Soybean Protein Isolate. Food Biosci. 2025, 69, 106964. [Google Scholar] [CrossRef]
- Xu, X.; Pang, Y.; Fan, X. Mitochondria in Oxidative Stress, Inflammation and Aging: From Mechanisms to Therapeutic Advances. Sig. Transduct. Target. Ther. 2025, 10, 190. [Google Scholar] [CrossRef]
- Zhai, L.; Shen, H.; Wu, S.; Guo, L.; Yang, Y.; Sheng, J.; Han, C. Deer Antler Polypeptides Inhibit Microglial Activation via TREM2 to Improve Behavior and Neuroinflammation in CUMS Mice. Int. Immunopharmacol. 2025, 150, 114284. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, H.; Zhang, W.; Xu, N.; Xu, J.; Li, Y.; Liu, W.; Lv, S. Protective Effects and Plausible Mechanisms of Antler-Velvet Polypeptide against Hydrogen Peroxide Induced Injury in Human Umbilical Vein Endothelial Cells. Can. J. Physiol. Pharmacol. 2017, 95, 610–619. [Google Scholar] [CrossRef]
- He, P.; Yan, S.; Zheng, J.; Gao, Y.; Zhang, S.; Liu, Z.; Liu, X.; Xiao, C. Eriodictyol Attenuates LPS-Induced Neuroinflammation, Amyloidogenesis, and Cognitive Impairments via the Inhibition of NF-κB in Male C57BL/6J Mice and BV2 Microglial Cells. J. Agric. Food Chem. 2018, 66, 10205–10214. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Xue, F.; Zhang, J.; Li, J.; Li, H.; Qiao, B. Cornel Iridoid Glycosides Exerted Neuroprotective Effects against Cerebral Ischemia/Reperfusion Injury in Rats via Inhibiting TLR4/MyD88/NF-κB Pathway. Eur. J. Pharmacol. 2025, 1001, 177742. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, J.; Pan, E.; Pang, L.; Zhou, X.; Che, Y. Paeonol Alleviates Subarachnoid Hemorrhage Injury in Rats Through Upregulation of SIRT1 and Inhibition of HMGB1/TLR4/MyD88/NF-κB Pathway. J. Biochem. Mol. Toxicol. 2024, 38, e70035. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, Y.; Liu, F.; Huang, Y.; Li, L.; Cheng, D.; Bai, S.; Sun, W. Β-elemene Attenuates IRI-AKI by Inhibiting Inflammation and Apoptosis via Suppression of the TLR4/MyD88/NF-κB/MAPK Signal Axis Activation. Mol. Med. Rep. 2025, 32, 221. [Google Scholar] [CrossRef]
- Wang, T.; Li, R.; Niu, P.; Wei, Z.; Xie, D.; Huang, H.; Pan, J.; Rong, C. Astaxanthin Relieves HT22 Cells from LPS-Induced Inflammation and Apoptosis by Inhibiting Oxygen Species and Regulating the TLR4/MyD88/NFκB Signaling Pathway. J. Funct. Foods 2025, 125, 106676. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zong, Y.; Li, J.; He, Z.; Du, R. Protective Effects of Deer Antler Peptides on D-Galactose-Induced Brain Injury. Nutrients 2025, 17, 2306. https://doi.org/10.3390/nu17142306
Chen S, Zong Y, Li J, He Z, Du R. Protective Effects of Deer Antler Peptides on D-Galactose-Induced Brain Injury. Nutrients. 2025; 17(14):2306. https://doi.org/10.3390/nu17142306
Chicago/Turabian StyleChen, Sihan, Ying Zong, Jianming Li, Zhongmei He, and Rui Du. 2025. "Protective Effects of Deer Antler Peptides on D-Galactose-Induced Brain Injury" Nutrients 17, no. 14: 2306. https://doi.org/10.3390/nu17142306
APA StyleChen, S., Zong, Y., Li, J., He, Z., & Du, R. (2025). Protective Effects of Deer Antler Peptides on D-Galactose-Induced Brain Injury. Nutrients, 17(14), 2306. https://doi.org/10.3390/nu17142306





