The Hypoglycaemic Effects of the New Zealand Pine Bark Extract on Sucrose Uptake and Glycaemic Responses in Healthy Adults—A Single-Blind, Randomised, Placebo-Controlled, Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Intervention Treatments
2.4. Sample Size
2.5. Statistical Analysis
3. Results
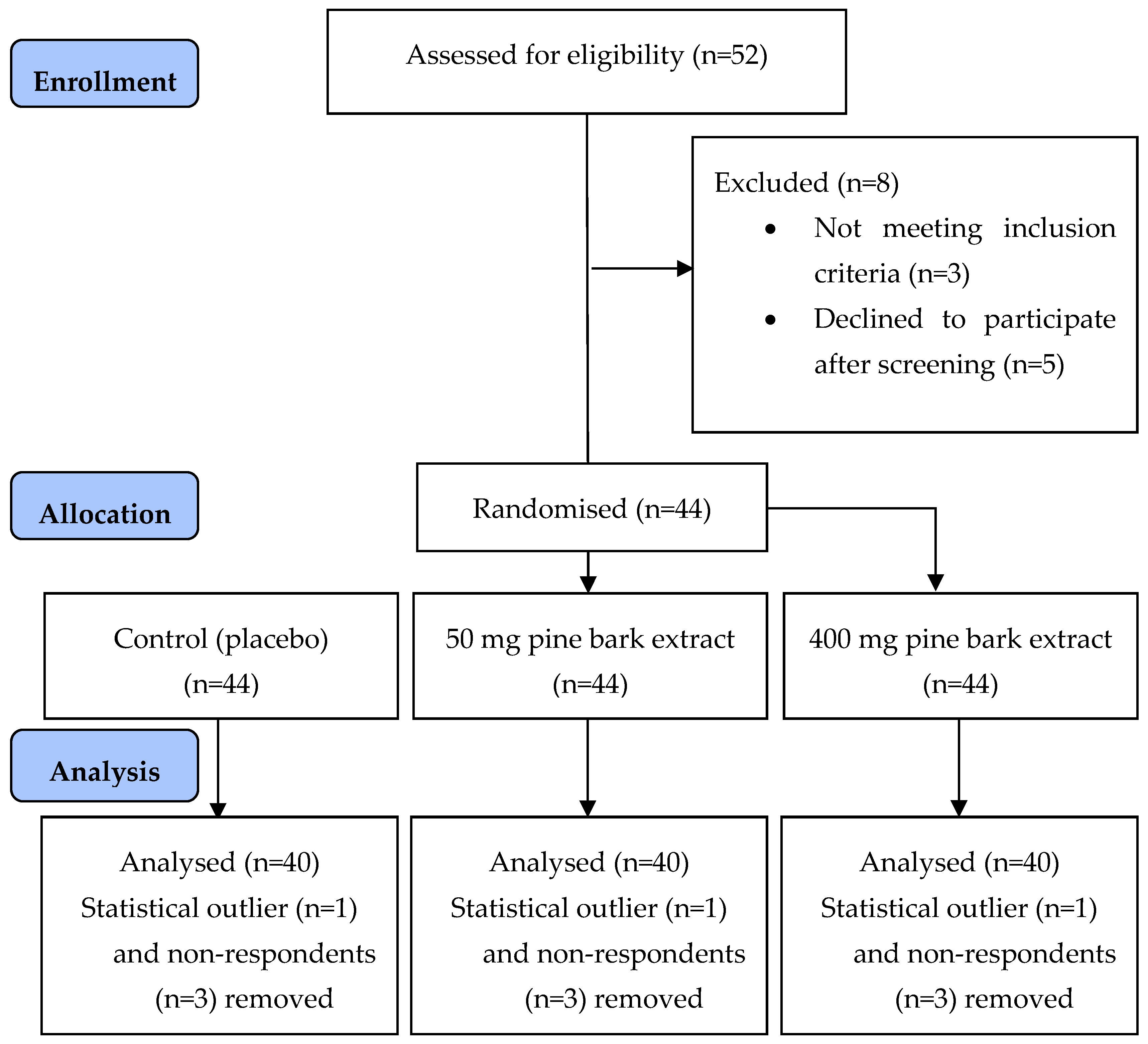
3.1. Participant Demographics
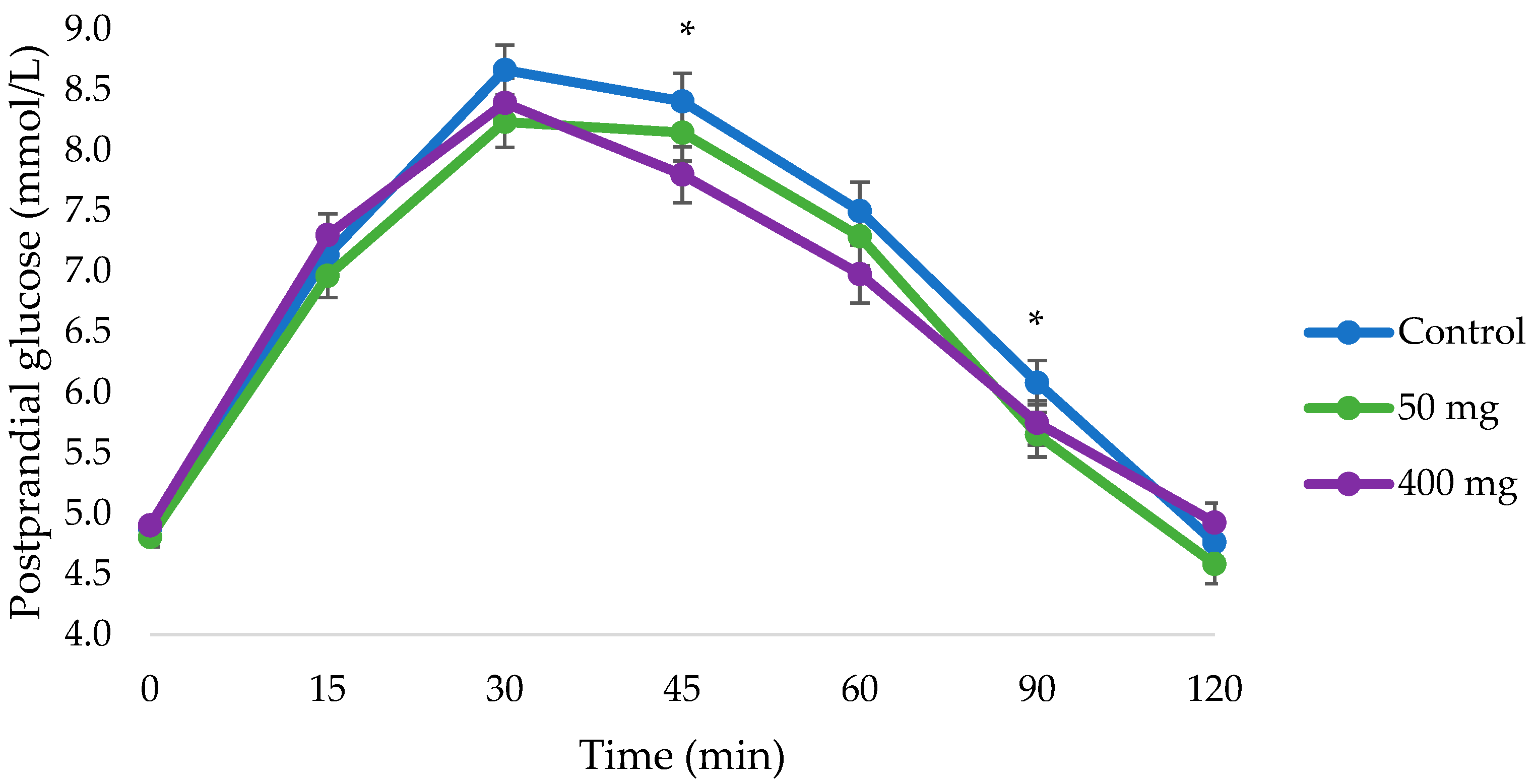
3.2. Changes in Postprandial Glucose
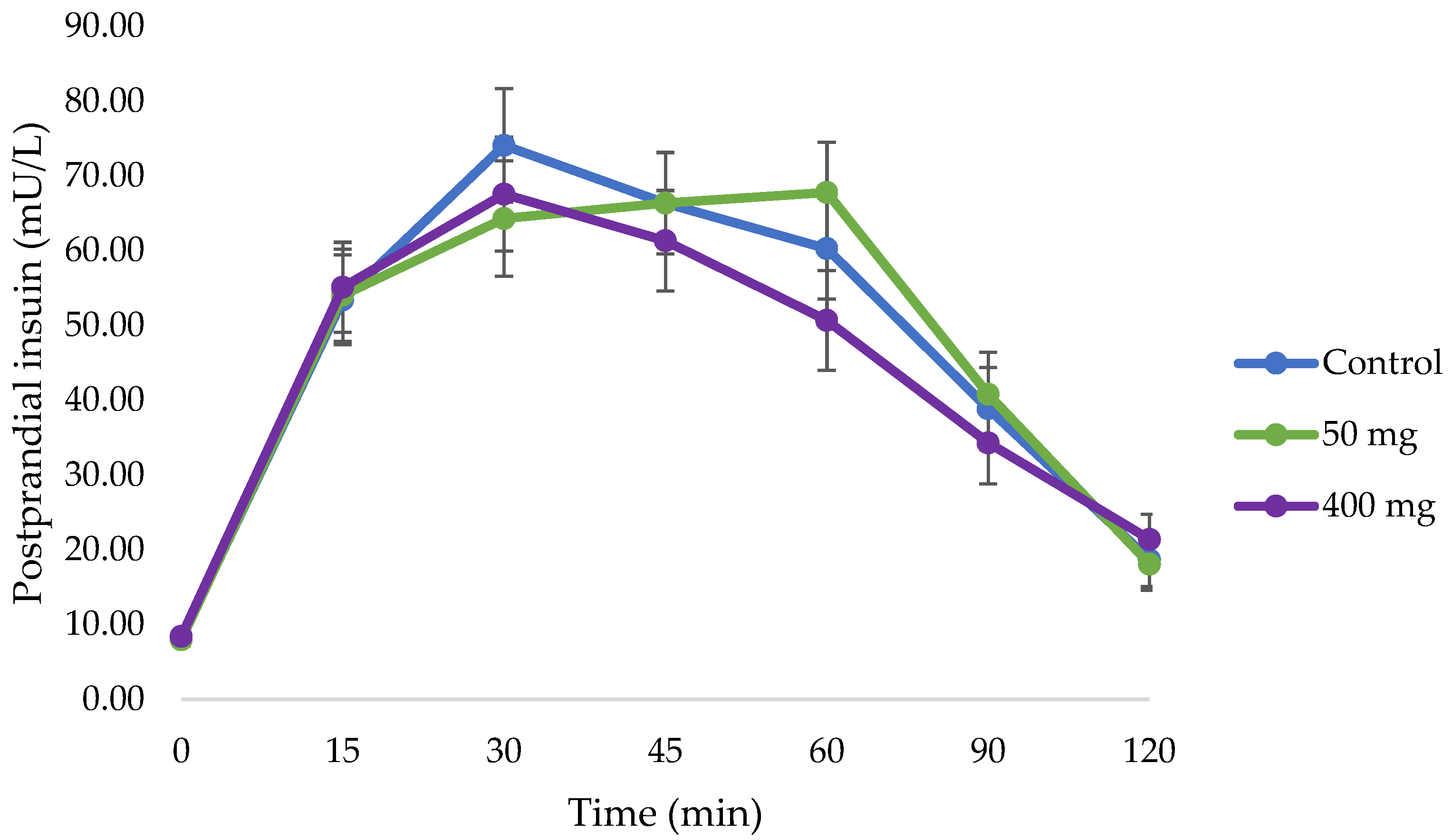
3.3. Changes in Postprandial Insulin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| DBP | Diastolic blood pressure |
| EAT-26 | The Eating Attitudes Testing (questionnaire) |
| FBG | Fasting blood glucose |
| HbA1c | Glycated haemoglobin A1c |
| HDL | High-density lipoprotein cholesterol |
| LDL | Low-density lipoprotein cholesterol |
| iAUC | Incremental area under the curve |
| iAUCglucose | Incremental area under the curve of postprandial glucose |
| IPAQ | International Physical Activity Questionnaire |
| OGTT | Oral glucose tolerance test |
| SBP | Systolic blood pressure |
| TC | Total cholesterol |
| TG | Triglyceride |
| 1hPG | 1 h postprandial glucose |
| 2hPG | 2 h postprandial glucose |
References
- International Diabetes Federation. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2025. [Google Scholar]
- Bray, G.A.; Popkin, B.M. Dietary sugar and body weight: Have we reached a crisis in the epidemic of obesity and diabetes? Health be damned! Pour on the sugar. Diabetes Care 2014, 37, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Hu, F.B.; Tappy, L.; Brand-Miller, J. Dietary carbohydrates: Role of quality and quantity in chronic disease. BMJ 2018, 361, k2340. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanan, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, C.F.; Wai, S.T.C.; Zhang, Y.B.; Portillo, M.P.; Paoli, P.; Wu, Y.J.; San Cheang, W.; Liu, B.; Carpene, C.; et al. Regulation of glucose metabolism by bioactive phytochemicals for the management of type 2 diabetes mellitus. Crit. Rev. Food Sci. Nutr. 2019, 59, 830–847. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.X.J.; Chepulis, L.; von Hurst, P.; Gammon, C.S.; Page, R.A. An Acute, Placebo-Controlled, Single-Blind, Crossover, Dose-Response, Exploratory Study to Assess the Effects of New Zealand Pine Bark Extract (Enzogenol®) on Glycaemic Responses in Healthy Participants. Nutrients 2020, 12, 497. [Google Scholar] [CrossRef]
- Lim, W.X.J.; Gammon, C.S.; von Hurst, P.R.; Chepulis, L.; Mugridge, O.; Page, R.A. Hypoglycemic effects of antioxidant-rich plant extracts on postprandial glycemic responses in participants with prediabetes (GLARE study). Funct. Foods Health Dis. 2021, 11, 604–626. [Google Scholar] [CrossRef]
- Frevel, M.A.; Pipingas, A.; Grigsby, W.J.; Frampton, C.M.; Gilchrist, N.L. Production, composition and toxicology studies of Enzogenol® Pinus radiata bark extract. Food Chem. Toxicol. 2012, 50, 4316–4324. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Feng, J.; Zhang, X.-L.; Cui, Y.-Y. Pine bark extracts: Nutraceutical, pharmacological, and toxicological evaluation. J. Pharmacol. Exp. Ther. 2015, 353, 9–16. [Google Scholar] [CrossRef]
- Bayer, J.; Högger, P. Review of the pharmacokinetics of French maritime pine bark extract (Pycnogenol®) in humans. Front. Nutr. 2024, 11, 1389422. [Google Scholar] [CrossRef]
- Lim, W.X.J.; Gammon, C.S.; von Hurst, P.; Chepulis, L.; Page, R.A. The Inhibitory Effects of New Zealand Pine Bark (Enzogenol (R)) on alpha-Amylase, alpha-Glucosidase, and Dipeptidyl Peptidase-4 (DPP-4) Enzymes. Nutrients 2022, 14, 1596. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as alpha-Amylase and alpha-Glucosidase Inhibitors and their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini-Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 541–555. [Google Scholar] [CrossRef]
- Nyambe-Silavwe, H.; Villa-Rodriguez, J.A.; Ifie, I.; Holmes, M.; Aydin, E.; Jensen, J.M.; Williamson, G. Inhibition of human alpha-amylase by dietary polyphenols. J. Funct. Foods 2015, 19, 723–732. [Google Scholar] [CrossRef]
- Lin, S.R.; Chang, C.H.; Tsai, M.J.; Cheng, H.; Chen, J.C.; Leong, M.K.; Weng, C.F. The perceptions of natural compounds against dipeptidyl peptidase 4 in diabetes: From in silico to in vivo. Ther. Adv. Chronic Dis. 2019, 10, 16. [Google Scholar] [CrossRef]
- Huang, P.K.; Lin, S.R.; Chang, C.H.; Tsai, M.J.; Lee, D.N.; Weng, C.F. Natural phenolic compounds potentiate hypoglycemia via inhibition of Dipeptidyl peptidase IV. Sci. Rep. 2019, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B.; Högger, P. Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Cires, M.J.; Wong, X.; Carrasco-Pozo, C.; Gotteland, M. The gastrointestinal tract as a key target organ for the health-promoting effects of dietary proanthocyanidins. Front. Nutr. 2017, 3, 57. [Google Scholar] [CrossRef]
- Lown, M.; Fuller, R.; Lightowler, H.; Fraser, A.; Gallagher, A.; Stuart, B.; Byrne, C.; Lewith, G. Mulberry-extract improves glucose tolerance and decreases insulin concentrations in normoglycaemic adults: Results of a randomised double-blind placebo-controlled study. PLoS ONE 2017, 12, e0172239. [Google Scholar] [CrossRef]
- Wang, R.H.; Li, Y.F.; Mu, W.; Li, Z.Q.; Sun, J.X.; Mi, B.; Wang, B.H.; Zhong, Z.; Luo, X.Z.; Xie, C.; et al. Mulberry leaf extract reduces the glycemic indexes of four common dietary carbohydrates. Medicine 2018, 97, e11996. [Google Scholar] [CrossRef]
- Thondre, P.S.; Lightowler, H.; Ahlstrom, L.; Gallagher, A. Mulberry leaf extract improves glycaemic response and insulaemic response to sucrose in healthy subjects: Results of a randomized, double blind, placebo-controlled study. Nutr. Metab. 2021, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- El Kenz, H.; Bergmann, P. Evaluation of immunochemiluminometric assays for the measurement of insulin and C-peptide using the ADVIA Centaur®. Clin. Lab. 2004, 50, 171–174. [Google Scholar] [PubMed]
- Liu, X.; Wei, J.; Tan, F.; Zhou, S.; Würthwein, G.; Rohdewald, P. Antidiabetic effect of Pycnogenol® French maritime pine bark extract in patients with diabetes type II. Life Sci. 2004, 75, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, H.-J.; Rohdewald, P. French maritime pine bark extract Pycnogenol dose-dependently lowers glucose in type 2 diabetic patients. Diabetes Care 2004, 27, 839–840. [Google Scholar] [CrossRef]
- Zibadi, S.; Rohdewald, P.J.; Park, D.; Watson, R.R. Reduction of cardiovascular risk factors in subjects with type 2 diabetes by Pycnogenol supplementation. Nutr. Res. 2008, 28, 315–320. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Jenkins, D.J.A. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am. J. Clin. Nutr. 1986, 43, 167–172. [Google Scholar] [CrossRef]
- Lefloch, J.P.; Escuyer, P.; Baudin, E.; Baudon, D.; Perlemuter, L. Blood glucose area under the curve: Methodological aspects. Diabetes Care 1990, 13, 172–175. [Google Scholar] [CrossRef]
- Yang, K.; Chan, C.B. Proposed mechanisms of the effects of proanthocyanidins on glucose homeostasis. Nutr. Rev. 2017, 75, 642–657. [Google Scholar] [CrossRef]
- Jerez, M.; Selga, A.; Sineiro, J.; Torres, J.L.; Núñez, M.J. A comparison between bark extracts from Pinus pinaster and Pinus radiata: Antioxidant activity and procyanidin composition. Food Chem. 2007, 100, 439–444. [Google Scholar] [CrossRef]
- Hulman, A.; Simmons, R.K.; Vistisen, D.; Tabák, A.G.; Dekker, J.M.; Alssema, M.; Rutters, F.; Koopman, A.D.; Solomon, T.P.; Kirwan, J.P. Heterogeneity in glucose response curves during an oral glucose tolerance test and associated cardiometabolic risk. Endocrine 2017, 55, 427–434. [Google Scholar] [CrossRef]
- Ceriello, A.; Esposito, K.; Piconi, L.; Ihnat, M.; Thorpe, J.; Testa, R.; Bonfigli, A.R.; Giugliano, D. Glucose “peak” and glucose “spike”: Impact on endothelial function and oxidative stress. Diabetes Res. Clin. Pract. 2008, 82, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Vuksan, V.; Choi, H.; Zinman, B.; Retnakaran, R. Emerging parameters of the insulin and glucose response on the oral glucose tolerance test: Reproducibility and implications for glucose homeostasis in individuals with and without diabetes. Diabetes Res. Clin. Pract. 2014, 105, 88–95. [Google Scholar] [CrossRef]
- Mela, D.J.; Cao, X.-Z.; Dobriyal, R.; Fowler, M.I.; Lin, L.; Joshi, M.; Mulder, T.J.; Murray, P.G.; Peters, H.P.; Vermeer, M.A. The effect of 8 plant extracts and combinations on post-prandial blood glucose and insulin responses in healthy adults: A randomized controlled trial. Nutr. Metab. 2020, 17, 51. [Google Scholar] [CrossRef]
- Aston-Mourney, K.; Proietto, J.; Morahan, G.; Andrikopoulos, S. Too much of a good thing: Why it is bad to stimulate the beta cell to secrete insulin. Diabetologia 2008, 51, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Bang, C.-Y.; Choung, S.-Y. Enzogenol improves diabetes-related metabolic change in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. J. Pharm. Pharmacol. 2014, 66, 875–885. [Google Scholar] [CrossRef]
- Janssen, J.A.M.J.L. Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, cardiovascular disease and cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Williams, K.; DeFronzo, R.; Stern, M. Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care 2006, 29, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Lyssenko, V.; Tuomi, T.; DeFronzo, R.A.; Groop, L. The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes. Diabetes/Metab. Res. Rev. 2010, 26, 280–286. [Google Scholar] [CrossRef]
- Tschritter, O.; Fritsche, A.; Shirkavand, F.; Machicao, F.; Haring, H.; Stumvoll, M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care 2003, 26, 1026–1033. [Google Scholar] [CrossRef]
- Kaga, H.; Tamura, Y.; Takeno, K.; Kakehi, S.; Someya, Y.; Suzuki, R.; Kadowaki, S.; Sugimoto, D.; Furukawa, Y.; Funayama, T.; et al. The shape of the glucose response curve during an oral glucose tolerance test was associated with muscle insulin sensitivity and visceral fat accumulation in nonobese healthy men. J. Diabetes Investig. 2020, 11, 874–877. [Google Scholar] [CrossRef]
- Manco, M.; Nolfe, G.; Pataky, Z.; Monti, L.; Porcellati, F.; Gabriel, R.; Mitrakou, A.; Mingrone, G. Shape of the OGTT glucose curve and risk of impaired glucose metabolism in the EGIR-RISC cohort. Metabolism 2017, 70, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, G.; Martel, F. The effect of dietary polyphenols on intestinal absorption of glucose and fructose: Relation with obesity and type 2 diabetes. Food Rev. Int. 2019, 35, 390–406. [Google Scholar] [CrossRef]
- Barrett, A.H.; Farhadi, N.F.; Smith, T.J. Slowing starch digestion and inhibiting digestive enzyme activity using plant flavanols/tannins—A review of efficacy and mechanisms. LWT 2018, 87, 394–399. [Google Scholar] [CrossRef]
- Lee, Y.A.; Cho, E.J.; Tanaka, T.; Yokozawa, T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J. Nutr. Sci. Vitaminol. 2007, 53, 287–292. [Google Scholar] [CrossRef]
- Williamson, G. Effects of Polyphenols on Glucose-Induced Metabolic Changes in Healthy Human Subjects and on Glucose Transporters. Mol. Nutr. Food Res. 2022, 66, 2101113. [Google Scholar] [CrossRef]
- Lee, B.; Woleve, T. Effect of glucose, sucrose and fructose on plasma glucose and insulin responses in normal humans: Comparison with white bread. Eur. J. Clin. Nutr. 1998, 52, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Ramdath, D.D.; Renwick, S.; Hawke, A.; Ramdath, D.G.; Wolever, T.M. Minimal Effective Dose of Beans Required to Elicit a Significantly Lower Glycemic Response Than Commonly Consumed Starchy Foods: Predictions Based on In Vitro Digestion and Carbohydrate Analysis. Nutrients 2023, 15, 4495. [Google Scholar] [CrossRef]
- Chang, C.R.; Francois, M.E.; Little, J.P. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am. J. Clin. Nutr. 2019, 109, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Ajjan, R. The clinical importance of measuring glycaemic variability: Utilising new metrics to optimise glycaemic control. Diabetes Obes. Metab. 2024, 26, 3–16. [Google Scholar] [CrossRef]
- Bermingham, K.M.; Smith, H.A.; Gonzalez, J.T.; Duncan, E.L.; Valdes, A.M.; Franks, P.W.; Delahanty, L.; Dashti, H.S.; Davies, R.; Hadjigeorgiou, G. Glycaemic variability, assessed with continuous glucose monitors, is associated with diet, lifestyle and health in people without diabetes. Res. Sq. 2023, 3, rs-3469475. [Google Scholar]
- Mudra, M.; Ercan-Fang, N.; Zhong, L.; Furne, J.; Levitt, M. Influence of mulberry leaf extract on the blood glucose and breath hydrogen response to ingestion of 75g sucrose by type 2 diabetic and control subjects. Diabetes Care 2007, 30, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- El-Zein, O.; Kreydiyyeh, S.I. Pine bark extract inhibits glucose transport in enterocytes via mitogen-activated kinase and phosphoinositol 3-kinase. Nutrition 2011, 27, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Zagury, R.L.; Bhaskaran, K.; Neutel, J.; Mohd Yusof, B.N.; Mooney, L.; Yeo, L.; Kirwan, B.-A.; Aprikian, O.; von Eynatten, M. A Randomized, Placebo-Controlled Crossover Study to Evaluate Postprandial Glucometabolic Effects of Mulberry Leaf Extract, Vitamin D, Chromium, and Fiber in People with Type 2 Diabetes. Diabetes Ther. 2023, 14, 749–766. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.-L.; Andújar, I.; Schinella, G.R.; Francini, F. Modulation of diabetes by natural products and medicinal plants via incretins. Planta Medica 2019, 85, 825–839. [Google Scholar] [CrossRef]
| Characteristics | Male (n = 12) | Female (n = 28) | Mean (n = 40) |
|---|---|---|---|
| Age (year) | 33.8 ± 2.2 | 30.1 ± 1.6 | 30.1 ± 1.3 |
| Body Mass Index (BMI, kg/m2) | 23.8 ± 0.8 | 23.2 ± 0.6 | 23.4 ± 0.5 |
| Waist circumference (cm) | 83.0 ± 2.2 | 74.5 ± 1.7 | 77.0 ± 1.5 |
| Hip circumference (cm) | 99.5 ± 2.3 | 98.7 ± 1.5 | 98.9 ± 1.2 |
| Systolic blood pressure (mmHg) | 113 ± 3.3 | 106 ± 1.7 | 108 ± 1.6 |
| Diastolic blood pressure (mmHg) | 74 ± 2.0 | 68 ± 1.1 | 70 ± 1.0 |
| Fasting glucose (mmol/L) | 4.5 ± 0.2 | 4.7 ± 0.1 | 4.7 ± 0.1 |
| Glycated haemoglobin A1c (HbA1c, mmol/mol) | 32.8 ± 1.0 | 32.3 ± 0.7 | 32.5 ± 0.6 |
| Total cholesterol (TC, mmol/L) | 4.7 ± 0.3 | 4.6 ± 0.2 | 4.7 ± 0.1 |
| Triglyceride (TG, mmol/L) | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 |
| High density lipoprotein (HDL, mmol/L) | 1.5 ± 0.1 | 1.7 ± 0.1 | 1.6 ± 0.1 |
| Low density lipoprotein (LDL, mmol/L) | 2.7 ± 0.2 | 2.3 ± 0.2 | 2.4 ± 0.1 |
| Non-high-density lipoprotein (non-HDL, mmol/L) | 3.2 ± 0.3 | 2.9 ± 0.2 | 3.0 ± 0.1 |
| TC/HDL ratio | 3.1 ± 0.2 | 2.9 ± 0.2 | 3.0 ± 0.1 |
| Control (Only Sucrose Solution) | 50 mg Pine Bark Extract | 400 mg Pine Bark Extract | |
|---|---|---|---|
| iAUC 30 | 62.2 ± 3.4 | 58.1 ± 3.5 | 62.3 ± 3.4 |
| iAUC 60 | 163.4 ± 8.1 | 152.7 ± 8.1 | 147.5 ± 8.0 |
| iAUC 90 | 223.1 ± 12.0 | 203.1 ± 12.1 | 192.3 ± 11.9 * |
| iAUC 120 | 247.5 ± 14.0 | 220.8 ± 14.2 | 211.8 ± 13.9 * |
| 1 h glucose | 7.5 ± 0.2 | 7.3 ± 0.2 | 7.0 ± 0.2 |
| Glucose peak | 9.1 ± 0.2 | 8.6 ± 0.2 * | 8.7 ± 0.2 |
| Glucose peak time | 35.8 ± 1.8 | 36.7 ± 1.9 | 31.3 ± 1.8 |
| Time (min) | Control (Only Sucrose Solution) | 50 mg Pine Bark Extract | 400 mg Pine Bark Extract |
|---|---|---|---|
| 0 | 4.9 ± 0.1 | 4.8 ± 0.1 | 4.9 ± 0.1 |
| 15 | 7.1 ± 0.2 | 7.0 ± 0.2 | 7.3 ± 0.2 |
| 30 | 8.7 ± 0.2 | 8.2 ± 0.2 | 8.4 ± 0.2 |
| 45 | 8.4 ± 0.2 | 8.1 ± 0.2 | 7.8 ± 0.2 * |
| 60 | 7.5 ± 0.2 | 7.3 ± 0.2 | 7.0 ± 0.2 |
| 90 | 6.1 ± 0.2 | 5.7 ± 0.2 * | 5.7 ± 0.2 |
| 120 | 4.8 ± 0.2 | 4.6 ± 0.2 | 4.9 ± 0.2 |
| Control (Only Sucrose Solution) | 50 mg Pine Bark Extract | 400 mg Pine Bark Extract | |
|---|---|---|---|
| iAUC 30 | 1094.11 ± 122.67 | 939.31 ± 124.86 | 1001.49 ± 123.54 |
| iAUC 60 | 2787.52 ± 288.11 | 2797.96 ± 290.89 | 2547.35 ± 287.53 |
| iAUC 90 | 4021.70 ± 413.63 | 4059.75 ± 417.78 | 3629.88 ± 412.78 |
| iAUC 120 | 4601.86 ± 499.39 | 4873.98 ± 504.54 | 4231.87 ± 498.35 |
| 1 h insulin | 60.26 ± 6.76 | 67.77 ± 6.70 | 50.65 ± 6.69 |
| Insulin peak | 81.74 ± 7.59 | 77.99 ± 7.63 | 76.19 ± 7.54 |
| Insulin peak time | 37.2 ± 2.8 | 35.6 ± 2.8 | 37.8 ± 2.8 |
| Time (min) | Control (Only Sucrose Solution) | 50 mg Pine Bark Extract | 400 mg Pine Bark Extract |
|---|---|---|---|
| 0 | 8.12 ± 0.79 | 7.97 ± 0.80 | 8.41 ± 0.79 |
| 15 | 53.42 ± 6.02 | 54.03 ± 6.15 | 55.10 ± 6.01 |
| 30 | 74.06 ± 7.59 | 64.29 ± 7.73 | 67.54 ± 7.63 |
| 45 | 66.34 ± 6.76 | 66.35 ± 6.80 | 61.32 ± 6.72 |
| 60 | 60.26 ± 6.76 | 67.77 ± 6.70 | 50.66 ± 6.69 |
| 90 | 38.86 ± 5.50 | 40.79 ± 5.61 | 34.28 ± 5.45 |
| 120 | 18.66 ± 3.57 | 18.08 ± 3.47 | 21.39 ± 3.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, W.X.J.; Page, R.A.; Gammon, C.S.; Moughan, P.J. The Hypoglycaemic Effects of the New Zealand Pine Bark Extract on Sucrose Uptake and Glycaemic Responses in Healthy Adults—A Single-Blind, Randomised, Placebo-Controlled, Crossover Trial. Nutrients 2025, 17, 2277. https://doi.org/10.3390/nu17142277
Lim WXJ, Page RA, Gammon CS, Moughan PJ. The Hypoglycaemic Effects of the New Zealand Pine Bark Extract on Sucrose Uptake and Glycaemic Responses in Healthy Adults—A Single-Blind, Randomised, Placebo-Controlled, Crossover Trial. Nutrients. 2025; 17(14):2277. https://doi.org/10.3390/nu17142277
Chicago/Turabian StyleLim, Wen Xin Janice, Rachel A. Page, Cheryl S. Gammon, and Paul J. Moughan. 2025. "The Hypoglycaemic Effects of the New Zealand Pine Bark Extract on Sucrose Uptake and Glycaemic Responses in Healthy Adults—A Single-Blind, Randomised, Placebo-Controlled, Crossover Trial" Nutrients 17, no. 14: 2277. https://doi.org/10.3390/nu17142277
APA StyleLim, W. X. J., Page, R. A., Gammon, C. S., & Moughan, P. J. (2025). The Hypoglycaemic Effects of the New Zealand Pine Bark Extract on Sucrose Uptake and Glycaemic Responses in Healthy Adults—A Single-Blind, Randomised, Placebo-Controlled, Crossover Trial. Nutrients, 17(14), 2277. https://doi.org/10.3390/nu17142277







