Abstract
Aim: This systematic review aims to evaluate the effectiveness of microbiota-modulating interventions (such as probiotics, prebiotics, and fecal microbiota transplantation) in reducing cognitive symptoms, pain, and neuroinflammation in human studies relevant to fibromyalgia (FM). The review will investigate the role of gut–brain axis modulation through these interventions and explore the potential therapeutic benefits for FM management. Materials and Methods: A comprehensive search was conducted in electronic databases including PubMed, Scopus, and the Cochrane Library for studies published from 1 January 2015 to 30 April 2025. Studies were eligible if they were randomized controlled trials (RCTs), pilot studies, or observational studies assessing the impact of microbiota-targeted interventions (probiotics, prebiotics, fecal microbiota transplantation) on cognitive function, pain, or neuroinflammation in patients with FM. Studies were excluded if they involved animal models, lacked relevant outcome measures, or were not peer-reviewed. Although only a subset of the included studies directly involved FM patients, all were selected for their relevance to symptom domains (e.g., pain, cognition, mood) and mechanisms (e.g., neuroinflammation, gut–brain axis dysfunction) that are central to FM. A total of 11 human studies were included in the final qualitative synthesis. Results: Preliminary findings from the included studies suggest that microbiota-targeted interventions, particularly probiotics and prebiotics, show promise in reducing cognitive symptoms, pain, and neuroinflammation in FM patients. Improvements in mood and quality of life were also reported, indicating potential benefits for overall well-being. However, heterogeneity in study designs, sample sizes, and outcome measures limit the ability to draw definitive conclusions. Conclusions: This systematic review highlights the potential of microbiota modulation as a therapeutic strategy for managing FM symptoms, particularly cognitive dysfunction and neuroinflammation.
1. Introduction
1.1. Clinical Context: Understanding Fibromyalgia and Its Symptomatology
Fibromyalgia (FM) is a chronic and complex disorder characterized primarily by widespread musculoskeletal pain, persistent fatigue, and heightened sensitivity to tactile stimuli. It affects approximately 2–4% of the general population, with a significantly higher prevalence in women [1,2,3,4,5]. Despite its high burden, FM remains one of the most poorly understood conditions within rheumatology and pain medicine [6,7,8,9,10]. Diagnosis is based on symptom criteria, including chronic pain, fatigue, unrefreshing sleep, cognitive difficulties (commonly termed “fibro fog”), and somatic symptoms such as irritable bowel syndrome (IBS), headaches, or bladder discomfort [11,12,13,14,15]. In addition to physical symptoms, FM is closely associated with a range of psychiatric comorbidities. A substantial proportion of patients suffer from anxiety, depression, post-traumatic stress disorder, and emotional dysregulation, all of which may exacerbate pain perception and functional disability [16,17,18,19,20]. These psychological dimensions not only complicate the clinical profile but also suggest a possible role for central nervous system (CNS) dysfunction in the pathogenesis of FM. Cognitive impairment in Fibromyalgia (FM), often described by patients as mental cloudiness, difficulty concentrating, and memory lapses (collectively referred to as “fibro fog”), represents a particularly disabling aspect of the disease [21,22,23,24,25]. These symptoms resemble those in chronic fatigue syndrome (CFS) and mild cognitive impairment (MCI), and may be linked to brain connectivity changes, neuroinflammation, or neurotransmitter imbalances.
Fatigue affects over 90% of FM patients and is often resistant to standard treatments. Gastrointestinal complaints such as bloating, constipation, diarrhea, or alternating bowel habits are also common. The frequent overlap between FM and IBS—a fellow functional somatic syndrome—suggests shared mechanisms, possibly involving gut–brain axis disruption [26,27,28,29,30,31].
1.2. Biological Rationale: The Gut–Brain Axis and Microbiota in FM
Over the last decade, the concept of the gut–brain axis has gained considerable attention in the context of chronic pain and neuropsychiatric conditions. This bidirectional communication network between the gastrointestinal tract and the CNS involves neural, endocrine, and immune pathways and is modulated in part by the gut microbiota (Figure 1) [32,33,34,35,36,37,38].
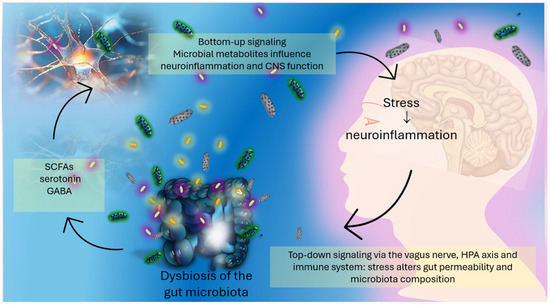
Figure 1.
The gut–brain axis: bidirectional communication between the gut microbiota and the central nervous system. Microbial metabolites such as Short-Chain Fatty Acids (SCFAs), gamma-aminobutyric acid (GABA), and serotonin are produced in the gut and influence brain function and neuroinflammation via bottom-up signaling. Conversely, stress-related brain signals modulate gut permeability and microbiota composition through top-down pathways involving the vagus nerve, the hypothalamic–pituitary–adrenal (HPA) axis, and immune regulation. Dysbiosis can amplify this cycle, contributing to neuropsychiatric and pain-related symptoms.
Emerging evidence points to the gut microbiome as a critical regulator of systemic inflammation, neuroimmune signaling, stress response, and even behavior [39,40,41,42,43,44,45]. In FM, proposed pathophysiological mechanisms include central sensitization, HPA axis dysfunction, mitochondrial impairment, and low-grade inflammation [46,47,48,49,50,51,52]. More recently, neuroinflammation, defined as chronic, low-level activation of glial cells in the brain and spinal cord, has been recognized as a potential contributor to the amplified pain and cognitive symptoms seen in FM [53,54,55,56,57,58,59]. Such states may be triggered by microbial products (e.g., lipopolysaccharide) or altered gut permeability, often described as “leaky gut.”
Dysbiosis, or microbial imbalance, has been consistently reported in FM patients [60,61,62,63,64,65,66]. For example, a landmark study by Minerbi et al. (2019) reported significant alterations in the relative abundance of specific bacterial taxa in FM compared to healthy controls [67,68,69,70,71,72,73]. These microbial differences correlated with symptom severity and pain scores, suggesting a potential causal or modulatory role [74,75,76,77,78,79,80]. Gut microbiota may influence CNS activity through the production of Short-Chain Fatty Acids (SCFAs), tryptophan metabolism, neuroactive compound synthesis (e.g., GABA, serotonin precursors), and immune modulation [81,82,83,84,85,86,87]. These pathways are intimately involved in maintaining the integrity of the blood–brain barrier, modulating microglial activation, and influencing the release of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α—molecules implicated in both mood disorders and chronic pain (Figure 2) [88,89,90,91,92,93,94].
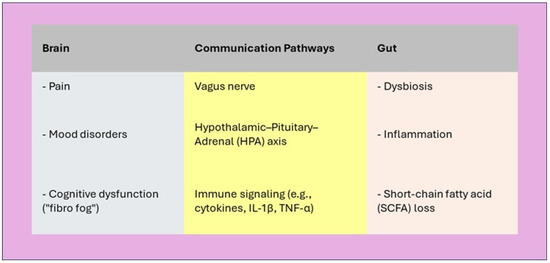
Figure 2.
Gut–Brain Axis in Fibromyalgia. The gut–brain axis is dysregulated in fibromyalgia, contributing to pain amplification, mood disturbances, and cognitive deficits through immune, endocrine, and neural pathways.
Another layer of complexity is introduced by dietary habits, which are powerful modulators of microbial ecology [95,96,97,98,99]. Diets rich in fiber, polyphenols, and fermented foods can promote a more anti-inflammatory microbiota profile, while Western-style diets high in saturated fats and refined sugars may foster dysbiosis and systemic inflammation [100,101,102,103,104,105,106]. Nutritional interventions that target the microbiome may therefore hold promise as adjunctive strategies in FM [107,108,109,110,111].
1.3. Literature Gap: Need for Human Clinical Evidence
Although preclinical studies have enhanced understanding of gut–brain interactions, their translation to clinical practice remains limited. Much of the existing FM literature on microbiota modulation is theoretical or based on animal and small-scale human studies. Animal models have shown that microbiota interventions—such as antibiotics, probiotics, or fecal microbiota transplantation—can alter pain and stress responses, but human data are sparse. Systematic reviews on FM typically focus on pharmacological (e.g., antidepressants, anticonvulsants), behavioral (e.g., CBT, exercise), or nutritional supplements (e.g., magnesium, vitamin D), with limited attention to the gut microbiome. Existing reviews on microbiota-based interventions are often narrative or include non-human studies, limiting clinical relevance. To date, no comprehensive synthesis has examined human interventional trials targeting the microbiota in FM, particularly regarding cognitive or neuroinflammatory outcomes.
Given the complex nature of FM—spanning somatic, emotional, and cognitive domains—integrative treatment approaches that also target peripheral systems like the gut and immune axis are increasingly needed [112]. Due to the limited number of interventional studies conducted in FM populations, this review also includes related clinical cohorts exhibiting symptom or mechanistic overlap, such as chronic pain or mood disorders, to extract insights relevant to FM care.
1.4. Objective of the Systematic Review
This systematic review aims to synthesize clinical evidence from human studies evaluating microbiota-modulating interventions—such as probiotics, prebiotics, selective antibiotics (e.g., rifaximin), and dietary strategies—on FM-related outcomes. Specifically, the review will assess:
- pain outcomes, including self-reported pain intensity and pain thresholds;
- cognitive function, particularly attention, working memory, and mental fatigue;
- psychological and affective symptoms, including anxiety and depression;
- markers of systemic or neuroinflammation, such as hs-CRP, IL-1β, and TNF-α.
Secondary objectives include examining the impact of these interventions on quality of life, sleep, gut symptoms, and potential side effects or adherence issues [113,114,115,116,117,118,119]. Where available, the review will also explore associations between symptom improvement and microbiota composition or metabolomic changes, as assessed by fecal or blood biomarkers [120,121,122,123,124,125,126]. By focusing exclusively on human interventional studies (e.g., RCTs, open-label trials) and excluding in vitro and animal research, this review seeks to provide clinically applicable insights into the potential of microbiota-targeted therapies in FM. It may also help identify microbial targets for future interventions and subgroups of FM patients—such as those with gastrointestinal comorbidities or elevated inflammation—who could particularly benefit from such approaches [127,128,129,130,131,132,133].
2. Materials and Methods
2.1. Search Processing
The literature search was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Three major databases—PubMed, Scopus, and Web of Science—were systematically searched using the following combination of keywords and MeSH terms: (“Neuroinflammation” OR “Fibromyalgia” OR “Nervous System Diseases”) AND (“Gastrointestinal Microbiome” OR “Microbiota” OR “Dysbiosis” OR “Probiotics”) AND (“Cognition” OR “Mood Disorders” OR “Depression” OR “Anxiety”). The search was limited to human studies published in English and covered the last 10 years, up to April 2025. This systematic review has been registered in the PROSPERO database (ID: 1064838) and is currently under review for formal approval. A total of 544 results were retrieved from PubMed, 478 from Scopus, and 303 from Web of Science, amounting to 1333 records. All search results were imported into a reference management software, and duplicates were removed. The remaining articles underwent an initial screening of titles and abstracts to assess relevance. Subsequently, full texts of potentially eligible studies were reviewed in detail. Studies were excluded if they were duplicates, not available as free full-text articles, conducted in vitro or on animal models, off-topic, or classified as reviews or meta-analyses. After applying these criteria, 11 articles met all inclusion criteria and were selected for the final analysis.
2.2. Inclusion and Exclusion Criteria
Studies were selected according to predefined criteria aligned with PRISMA guidelines to ensure clarity and reproducibility. Eligible studies were original research articles published in English, with freely accessible full texts, conducted on adult human participants (≥18 years old). Both randomized controlled trials (RCTs), controlled clinical trials, and well-designed observational studies were included. The review considered studies investigating the relationship between gut microbiota and neuroinflammatory or neuropsychological outcomes, including cognitive impairment, stress reactivity, mood disorders, or pain perception. Studies were included regardless of participant clinical condition (e.g., fibromyalgia, metabolic syndrome, IBS, MCI, or healthy), as long as relevant mechanisms involving the gut–brain–immune axis were explored. However, for studies specifically involving fibromyalgia patients, only those with a confirmed diagnosis according to the American College of Rheumatology (ACR) criteria were included. Although a minimum follow-up of four weeks was prioritized, cross-sectional or mechanistic studies with shorter durations were considered if they contributed relevant insights into microbiota-related pathways. Exclusion criteria encompassed in vitro or animal models, reviews, meta-analyses, conference abstracts, editorials, or commentary pieces. Studies were excluded if they lacked a focus on psychological, cognitive, or inflammatory outcomes in relation to gut microbiota, or if fibromyalgia diagnosis was not supported by validated clinical criteria when relevant. A schematic summary of the inclusion and exclusion criteria is presented in Figure 3, in accordance with PRISMA best practices.
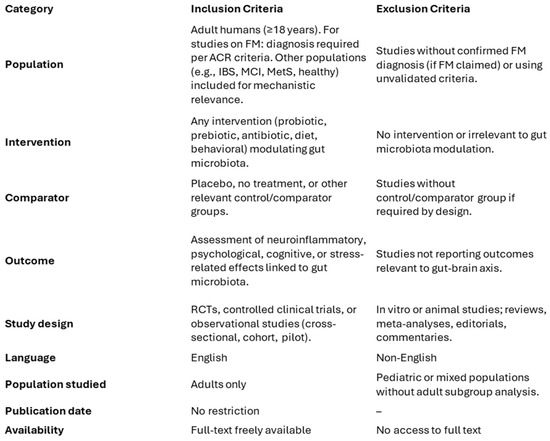
Figure 3.
Summary of Inclusion and Exclusion Criteria.
2.3. PICo Question
The systematic review was guided by the Population, Interest, and Context (PICo) framework. The population (P) comprised human subjects; the phenomenon of interest (I) was the gut microbiota; and the context (Co) involved neuroinflammatory processes and associated psychological outcomes. This framework facilitated the development of the search strategy and ensured a focused and relevant selection of studies addressing the central research question. The main outcomes evaluated across the included studies were: improvements in psychological well-being (e.g., reduced anxiety, stress, and depressive symptoms); enhancement of cognitive performance (e.g., attention, memory, concentration); reduction in systemic inflammation (e.g., lower levels of hs-CRP, IL-1β, TNF-α) and compositional and functional changes in the gut microbiota (e.g., increased SCFA-producing bacteria and microbial diversity).
These outcomes reflect the multifaceted impact of gut microbiota modulation on domains relevant to fibromyalgia and related conditions.
2.4. Data Processing
Data extraction was conducted using a pre-piloted spreadsheet in Excel, used by two independent reviewers. Extracted data included: author/year, study design, population characteristics, diagnostic criteria, intervention details, duration, outcome measures, analysis techniques (e.g., 16S rRNA), and key results. The ROBINS-I tool for non-randomized studies and the Cochrane RoB 2.0 tool for randomized controlled trials (RCTs) were used to evaluate the risk of bias. Key findings relevant to the link between gut microbiota and neuroinflammation were recorded. Any disagreements between reviewers were resolved through discussion and, when necessary, with input from a third reviewer. This process ensured the reliability and consistency of the data included in the final synthesis.
3. Results
3.1. Study Selection and Characteristics
The initial database search, conducted according to PRISMA guidelines, retrieved a total of 1333 records, 544 records from PubMed, 478 from Scopus, and 303 from Web of Science. After removing 346 duplicates, 987 records were assessed. Of these, 716 were excluded for being off-topic, reviews, meta-analyses, or non-human/in vitro studies. A total of 271 full-text articles were sought, of which 6 were not retrievable. Subsequently, 265 articles were reviewed for eligibility, and 254 were excluded for not meeting inclusion criteria, including lack of neuroinflammatory or psychological outcomes. Ultimately, 11 human studies, comprising randomized controlled trials and original research, were included in the qualitative synthesis. No quantitative synthesis (meta-analysis) was performed due to the high heterogeneity in study design, populations, interventions, and outcome measures, which precluded meaningful statistical pooling. The study selection process is illustrated in Figure 4, and the detailed characteristics of the included studies are summarized in Table 1.
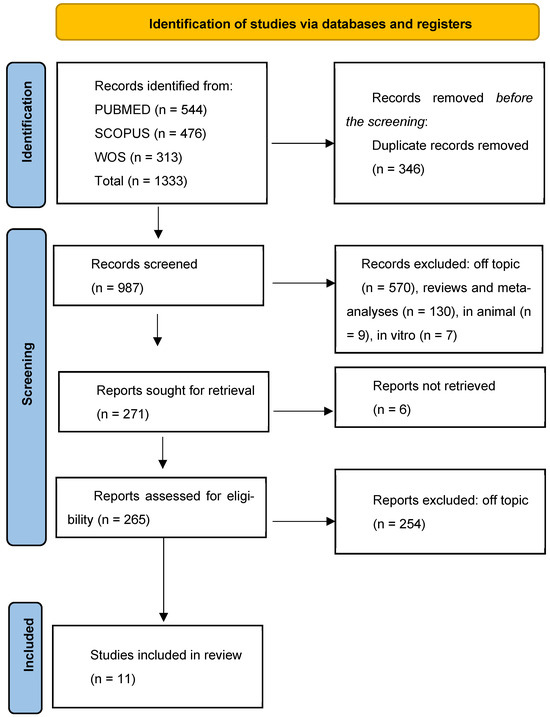
Figure 4.
PRISMA Flow diagram of study selection.

Table 1.
Summary of selected studies.
3.2. Risk of Bias Assessment
The risk of bias was assessed using two validated tools: the Cochrane RoB 2 tool for randomized controlled trials (RCTs), and the ROBINS-I tool for non-randomized studies of interventions. For RCTs, seven key domains were considered, including potential bias from the randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selective reporting. Additionally, attention was given to declared conflicts of interest and the accuracy of microbiological methodologies used to assess the gut microbiota. For non-randomized studies, the ROBINS-I framework enabled a comprehensive evaluation of bias occurring before, during, and after the intervention, with a particular focus on the presence of confounding variables and selection biases.
Overall, most randomized studies demonstrated a low to moderate risk of bias in the main methodological areas. However, some concerns emerged in domains related to randomization procedures—especially in pilot trials with small sample sizes—and in the selective reporting of results, particularly in cases where study protocols had not been pre-registered. Non-randomized studies showed a generally higher risk of bias, which was most pronounced in relation to potential confounders and outcome measurement limitations, consistent with the inherent weaknesses of their designs.
The results of this evaluation are summarized in Figure 5. They support the overall reliability of the body of evidence reviewed, while also underscoring the need for future studies with more robust designs and greater methodological transparency. Notably, the studies by Khine and Peter exhibited the highest levels of bias among the included non-randomized studies [10,136]. Among the domains assessed, randomization procedures and selective outcome reporting were the most frequently problematic areas across trials. While the microbiological methods employed were generally appropriate, the level of detail provided was sometimes insufficient to allow for a full assessment of methodological rigor.
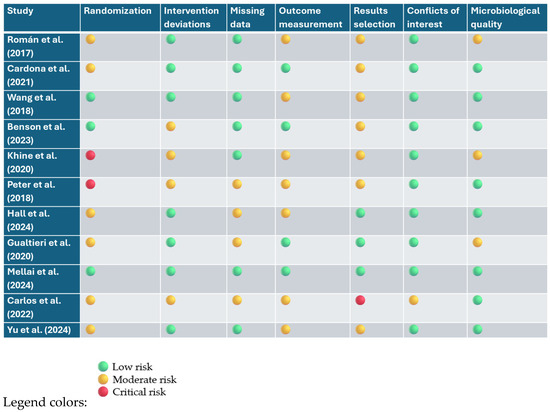
Figure 5.
Risk assessment of bias (RoB 2.0 and ROBINS-I) [10,35,37,41,68,134,135,136,137,138,139].
4. Discussion
4.1. Gut Microbiota in FM: A Growing Field of Interest
Recent studies have increasingly highlighted the gut microbiota’s involvement in systemic symptoms, mood disorders, and altered pain perception, underscoring its growing relevance in complex chronic conditions such as FM [140,141,142,143,144]. This multifactorial disorder, characterized by widespread musculoskeletal pain, fatigue, and neuropsychological dysfunction, is now being investigated through the lens of gut–brain–immune interactions [145,146,147,148,149].
4.2. Prebiotics and Immune-Mood Modulation
A notable contribution to this field is the pilot study by Hall et al., which evaluated the effects of prebiotic supplementation in individuals with metabolic syndrome (MetS), a condition often accompanied by low-grade systemic inflammation and psychological distress [137,150,151,152,153]. Over a 12-week intervention, participants receiving prebiotics exhibited a significant reduction in High-sensitivity C-reactive Protein (hs-CRP), a key biomarker of systemic inflammation. Additionally, marked improvements were reported in self-assessed stress, anxiety, and depressive symptoms [120,121,122,154,155]. However, these findings should be interpreted with caution due to the small sample size and the lack of microbiome sequencing data, which limit insights into mechanistic pathways. These findings suggest that modulating the gut microbiota through dietary fibers can influence not only immune function but also mental health, an effect likely mediated by the gut–brain axis, which may have clinical relevance in FM, where systemic inflammation and psychological symptoms often coexist. Although conducted in individuals with metabolic syndrome, the observed improvements in inflammation and psychological symptoms suggest gut-mediated mechanisms that may also be relevant to FM, where similar pathophysiological features are present.
4.3. IBS, Dysbiosis, and FM: Mechanistic Overlaps
Support for this hypothesis also comes from Peter et al., who analyzed the gut microbial composition in patients with IBS, identifying specific microbial signatures associated with psychological discomfort. Their study reinforces the emerging view that intestinal dysbiosis does not merely impact gastrointestinal function but plays a broader role in modulating emotional and cognitive states via neuroimmune pathways. These mechanisms are particularly relevant to FM, where chronic inflammation, psychological stress, and altered neuroimmune signaling are interwoven in symptom expression.
4.4. SCFA-Producing Bacteria and Neuroimmune Homeostasis
Further evidence for the microbiota’s therapeutic potential is provided by Mellai et al., who investigated the effects of Opuntia ficus-indica supplementation on gut microbiota in healthy subjects. Their results demonstrated an increased abundance of SCFA-producing bacteria, including Bifidobacterium and Parabacteroides, along with improvements in subjective well-being. Notably, these bacterial genera are known for their capacity to produce neuroactive metabolites like butyrate, which possess anti-inflammatory properties and are involved in supporting intestinal barrier integrity and central nervous system homeostasis.
4.5. Transgenerational Effects of Microbiota Modulation
The study by Yu et al. adds a transgenerational dimension to this discussion. In a cohort of mothers undergoing stress-reduction interventions during pregnancy, Yu and colleagues observed compositional changes in the maternal gut microbiota that were mirrored in their infants [136,156,157,158]. These alterations were accompanied by reductions in maternal stress levels, suggesting that psychosocial interventions can modulate gut microbial communities with downstream effects on offspring health.
4.6. Antibiotics, Brain Activity, and Social Stress
Expanding on this concept, Wang et al. explored the effects of short-term antibiotic administration on brain responses to social stress in healthy individuals. Participants treated with rifaximin, a non-absorbable antibiotic that alters gut microbiota, exhibited altered neural activity during a social exclusion task, particularly in prefrontal and cingulate regions. While the neuroimaging findings are compelling, the study’s short duration and small cohort limit its power to detect lasting clinical effects, particularly in patient populations. These changes were accompanied by a reduction in perceived social rejection, suggesting that gut microbiota modulation can influence emotional processing and stress reactivity.
4.7. Microglia, Inflammation, and Nociplastic Pain
Complementing these findings, Benson et al. provided a theoretical framework linking inflammation, mood disorders, and pain perception [134,159,160,161]. They emphasized how alterations in microbiota composition can modulate central nervous system processes, particularly through microglial activation and neurotransmitter imbalance, both of which are observed in FM [162,163,164,165,166].
4.8. Direct Evidence from FM Patients: Probiotic Interventions
Roman and Cardona investigated the clinical effects of multi-strain probiotics in FM patients. Their results showed a 15–20% reduction in pain scores compared to placebo, with parallel improvements in memory and concentration. As a randomized controlled trial in FM patients, this study provides stronger evidence, although the sample size was still limited, and microbiota changes were not directly analyzed. The response was particularly pronounced in patients with gastrointestinal comorbidities such as IBS, indicating that baseline microbiota profiles may influence therapeutic efficacy [167,168,169,170,171,172,173].
4.9. Host Genetics and Personalized Microbiota Therapies
Adding a genetic layer to this personalized approach, Gualtieri et al. examined the interaction between psychobiotic efficacy and host genetics. They found that individuals carrying the IL-1β A allele, a variant associated with hyperinflammatory responses, showed greater reductions in anxiety and IL-1β serum levels following probiotic intake [174,175,176,177]. Although promising, the study design was exploratory, and replication in larger, genetically stratified cohorts is needed.
4.10. Microbiota as Chronic Modulators of Inflammation
A more systemic perspective is offered by Carlos et al., who studied patients with schistosomiasis undergoing surgical correction of portal hypertension [137,178,179,180]. Despite anatomical resolution, persistent inflammation and fibrosis were observed postoperatively, pointing toward dysbiosis as a chronic driver of pathology [181,182,183,184,185]. This finding resonates with FM, where residual symptoms often persist despite standard care [186,187,188,189,190,191,192]. Even though the underlying disease is different, the persistent inflammation in the absence of active disease mirrors FM’s clinical trajectory, reinforcing the role of microbiota as a chronic modulator. This chronic inflammatory persistence despite resolution of primary insults mirrors the clinical trajectory seen in FM, reinforcing the need to consider microbiota as a long-term modulator rather than a transient player [138,193,194,195,196].
4.11. Mindfulness, SCFAs, and Cognitive Improvement
Finally, Khine et al. explored non-pharmacological strategies by analyzing the effects of mindfulness practices in patients with mild cognitive impairment. Their work linked these interventions to an increase in SCFA-producing bacteria and improvements in cognitive performance, likely via modulation of the hypothalamic–pituitary–adrenal (HPA) axis and enhancement of gut barrier function. Such approaches may be particularly valuable in FM, where cognitive symptoms (“fibro fog”) are prominent and pharmacological treatments often yield suboptimal results.
4.12. Synthesis of Evidence and Future Directions
In conclusion, the integrative perspective emerging from these eleven studies positions the gut microbiota as a central modulator of inflammation, mood, and pain, all key dimensions of FM [139,197,198,199]. The cumulative evidence suggests that targeted interventions, ranging from prebiotics and probiotics to stress reduction and lifestyle modifications, could offer multi-systemic benefits. However, several methodological limitations must be acknowledged, including small, heterogeneous samples, diverse intervention protocols, and variability in outcome measures [200,201,202,203,204,205,206]. This heterogeneity complicates direct comparisons and limits the generalizability of findings. Additionally, many of the included studies are preliminary or exploratory in nature, lacking long-term follow-up or placebo-controlled designs [207,208,209,210,211,212,213]. These factors should temper the interpretation of results and underscore the need for more standardized, large-scale investigations. Figure 6 summarizes the proposed mechanisms linking gut microbiota to key FM symptom domains, offering a visual framework for potential intervention points.
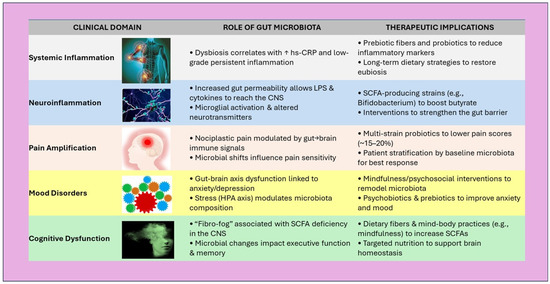
Figure 6.
Schematic overview of clinical domains in FM, illustrating gut microbiota contributions and potential interventional strategies. ↑ = improvement/increase. Abbreviations: SCFA—Short-Chain Fatty Acids, hs-CRP—High-Sensitivity C-Reactive Protein, LPS—Lipopolysaccharide, CNS—Central Nervous System.
Although this review primarily focuses on FMS and related neuroinflammatory conditions, several studies involving non-FMS populations (e.g., healthy individuals, patients with metabolic syndrome, irritable bowel syndrome, or mild cognitive impairment) were included due to overlapping pathophysiological mechanisms. Symptoms such as anxiety, visceral pain, cognitive impairment, and immune dysregulation strongly align with FM features, offering biologically plausible insights.
4.13. Toward Personalized, Microbiota-Based Therapies in FM
Future research should aim to delineate microbial signatures specific to FM and evaluate long-term outcomes through robust, longitudinal designs. Personalized strategies that integrate microbiota profiling, host genetics, and psychosocial context may ultimately redefine therapeutic paradigms for FM and related disorders (Figure 7) [67,135,214,215,216].
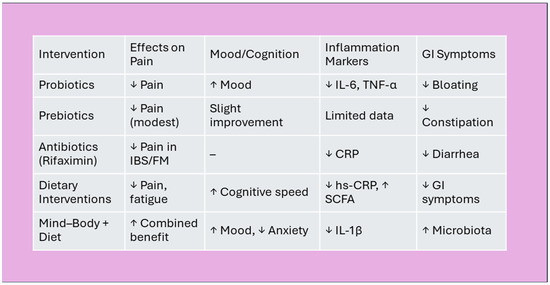
Figure 7.
Summary of microbiota-based interventions. Effects of microbiota interventions on FM symptoms. Legend: ↓ = reduction; ↑ = improvement/increase; “–” = no significant change. Abbreviations: IBS—Irritable Bowel Syndrome, FM—Fibromyalgia, CRP—C-reactive Protein, hs-CRP—High-sensitivity C-reactive Protein, SCFA—Short-Chain Fatty, IL-6—Interleukin-6, TNF-α—Tumor Necrosis Factor-alpha, IL-1β—Interleukin-1 beta.
5. Limitations of the Included Studies
Despite the promising findings highlighted in this review, several limitations must be acknowledged regarding the included studies. First, many of the trials were pilot or exploratory in nature, with small sample sizes that reduce statistical power and generalizability. Second, there was considerable heterogeneity in study design, population characteristics, intervention protocols (e.g., strain types, dosages, duration), and outcome measures, which precluded meaningful meta-analysis and limited direct comparisons across studies. Third, the majority of studies lacked long-term follow-up, making it difficult to assess the durability of observed effects. Moreover, in several cases, gut microbiota composition was inferred from limited sampling or reported without detailed taxonomic resolution, and microbiome sequencing techniques were inconsistently applied. Some studies also presented a moderate to high risk of bias, particularly in relation to randomization procedures, lack of blinding, or selective reporting. Lastly, only a few trials specifically targeted patients with fibromyalgia, while others included broader populations with symptom or mechanistic overlap, potentially diluting the specificity of the findings for FM. These limitations underscore the need for future high-quality, FM-specific clinical trials with standardized methodologies and rigorous reporting.
6. Conclusions
The current evidence suggests that modulation of the gut microbiota may influence key pathophysiological mechanisms relevant to fibromyalgia, particularly neuroinflammation, stress reactivity, and cognitive-emotional dysregulation. Although only a minority of the included studies directly investigated FM populations, consistent findings across diverse clinical contexts—such as improvements in attention, reductions in perceived stress, and shifts in microbial composition—support the hypothesis that targeting the gut–brain axis can impact symptom domains commonly affected in FM. Notably, several interventions, including probiotics and prebiotics, were associated with increased abundance of short-chain fatty acid–producing bacteria and reductions in inflammatory markers, highlighting potential biological pathways of relevance. However, due to the predominance of small, exploratory trials and the heterogeneity of study populations and methodologies, these findings remain preliminary. Robust, FM-specific clinical trials are required to validate the therapeutic relevance of microbiota-targeted strategies and to clarify their role within integrated, personalized treatment models.
Author Contributions
Conceptualization, L.F., A.D.N., A.M.I. and A.D.I.; methodology, L.F., M.D.F., G.D., A.P. and A.D.N.; software, A.P. and G.M.; validation, A.M.I. and A.P.; formal analysis, F.I.; resources, G.M.T., L.F. and A.D.N.; data curation, G.D. and G.M.; writing—original draft preparation, A.D.I., V.C., C.C., P.M. and F.I.; writing—review and editing, G.M., C.C., P.M. and V.C.; visualization, A.M.I., C.C. and P.M.; supervision, F.I.; project administration, G.M.T., G.D. and V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| BES | Binge Eating Scale |
| CFS | Chronic Fatigue Syndrome |
| CFU | Colony Forming Units |
| CNS | Central Nervous System |
| CRP | C-Reactive Protein |
| DMN | Default Mode Network (brain functional network) |
| E-Prime | E-Prime Software for Psychological Experiments |
| FM | Fibromyalgia |
| FMS | Fibromyalgia Syndrome |
| FMT | Fecal Microbiota Transplantation |
| FOS | Fructooligosaccharides (a type of prebiotic fiber) |
| GABA | Gamma-Aminobutyric Acid |
| GI | Gastrointestinal |
| GIQLI | Gastrointestinal Quality of Life Index |
| GSAS | Gastrointestinal Symptom Assessment Scale |
| HADS | Hospital Anxiety and Depression Scale |
| HE | Hepatic Encephalopathy |
| HPA | Hypothalamic–Pituitary–Adrenal |
| hs-CRP | High-Sensitivity C-Reactive Protein (marker of systemic inflammation) |
| IBS | Irritable Bowel Syndrome |
| IL-1β | Interleukin 1 Beta (Proinflammatory Cytokine) |
| LPS | Lipopolysaccharide |
| MCI | Mild Cognitive Impairment |
| MEG | Magnetoencephalography |
| MetS | Metabolic Syndrome |
| ML | Machine Learning |
| RCT | Randomized Controlled Trial |
| SCFA | Short-Chain Fatty Acids |
| SCFAs | Short-Chain Fatty Acids |
| YFAS | Yale Food Addiction Scale |
| fMRI | Functional Magnetic Resonance Imaging |
References
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Choi, S.B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Lactobacillus Plantarum DR7 Alleviates Stress and Anxiety in Adults: A Randomised, Double-Blind, Placebo-Controlled Study. Benef. Microbes 2019, 10, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136.e8. [Google Scholar] [CrossRef] [PubMed]
- Qadri, T.; Javed, F.; Poddani, P.; Tunér, J.; Gustafsson, A. Long-Term Effects of a Single Application of a Water-Cooled Pulsed Nd:YAG Laser in Supplement to Scaling and Root Planing in Patients with Periodontal Inflammation. Lasers Med. Sci. 2011, 26, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Zeger, S.L.; Liang, K.Y. Longitudinal Data Analysis for Discrete and Continuous Outcomes. Biometrics 1986, 42, 121–130. [Google Scholar]
- Kelly, J.R.; Allen, A.P.; Temko, A.; Hutch, W.; Kennedy, P.J.; Farid, N.; Murphy, E.; Boylan, G.; Bienenstock, J.; Cryan, J.F.; et al. Lost in Translation? The Potential Psychobiotic Lactobacillus Rhamnosus (JB-1) Fails to Modulate Stress or Cognitive Performance in Healthy Male Subjects. Brain. Behav. Immun. 2017, 61, 50–59. [Google Scholar] [CrossRef]
- Logan, A.C.; Katzman, M. Major Depressive Disorder: Probiotics May Be an Adjuvant Therapy. Med. Hypotheses 2005, 64, 533–538. [Google Scholar] [CrossRef]
- Alonso, C.; Guilarte, M.; Vicario, M.; Ramos, L.; Ramadan, Z.; Antolín, M.; Martínez, C.; Rezzi, S.; Saperas, E.; Kochhar, S.; et al. Maladaptive Intestinal Epithelial Responses to Life Stress May Predispose Healthy Women to Gut Mucosal Inflammation. Gastroenterology 2008, 135, 163–172.e1. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Tsilioni, I.; Bawazeer, M. Mast Cells, Neuroinflammation and Pain in Fibromyalgia Syndrome. Front. Cell. Neurosci. 2019, 13, 353. [Google Scholar] [CrossRef]
- Sherwin, E.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. May the Force Be with You: The Light and Dark Sides of the Microbiota-Gut-Brain Axis in Neuropsychiatry. CNS Drugs 2016, 30, 1019–1041. [Google Scholar] [CrossRef]
- Khine, W.W.T.; Voong, M.L.; Ng, T.K.S.; Feng, L.; Rane, G.A.; Kumar, A.P.; Kua, E.H.; Mahendran, R.; Mahendran, R.; Lee, Y.K. Mental Awareness Improved Mild Cognitive Impairment and Modulated Gut Microbiome. Aging 2020, 12, 24371–24393. [Google Scholar] [CrossRef]
- Hyland, N.P.; Cryan, J.F. Microbe-Host Interactions: Influence of the Gut Microbiota on the Enteric Nervous System. Dev. Biol. 2016, 417, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Dillon, J.F. Microbial Biofilms in the Human Gastrointestinal Tract. J. Appl. Microbiol. 2007, 102, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, A.; Allahyar, A.; Greiner, T.U.; Plovier, H.; Lundén, G.Ö.; Larsson, T.; Drucker, D.J.; Delzenne, N.M.; Cani, P.D.; Bäckhed, F. Microbial Modulation of Energy Availability in the Colon Regulates Intestinal Transit. Cell Host Microbe 2013, 14, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H. Microbiota and Healthy Ageing: Observational and Nutritional Intervention Studies. Microb. Biotechnol. 2013, 6, 326–334. [Google Scholar] [CrossRef]
- Heyck, M.; Ibarra, A. Microbiota and Memory: A Symbiotic Therapy to Counter Cognitive Decline? Brain Circ. 2019, 5, 124–129. [Google Scholar] [CrossRef]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota Revolution: How Gut Microbes Regulate Our Lives. World J. Gastroenterol. 2023, 29, 4368–4383. [Google Scholar] [CrossRef]
- Kursun, O.; Yemisci, M.; van den Maagdenberg, A.M.J.M.; Karatas, H. Migraine and Neuroinflammation: The Inflammasome Perspective. J. Headache Pain 2021, 22, 55. [Google Scholar] [CrossRef]
- Bertels, Z.; Mangutov, E.; Conway, C.; Siegersma, K.; Asif, S.; Shah, P.; Huck, N.; Tawfik, V.L.; Pradhan, A.A. Migraine and Peripheral Pain Models Show Differential Alterations in Neuronal Complexity. Headache 2022, 62, 780–791. [Google Scholar] [CrossRef]
- Fam, J.; Sun, Y.; Qi, P.; Lau, R.C.; Feng, L.; Kua, E.H.; Mahendran, R. Mindfulness Practice Alters Brain Connectivity in Community-Living Elders with Mild Cognitive Impairment. Psychiatr. Clin. Neurosci. 2020, 74, 257–262. [Google Scholar] [CrossRef]
- Jha, A.P.; Krompinger, J.; Baime, M.J. Mindfulness Training Modifies Subsystems of Attention. Cogn. Affect. Behav. Neurosci. 2007, 7, 109–119. [Google Scholar] [CrossRef]
- Creswell, J.D.; Irwin, M.R.; Burklund, L.J.; Lieberman, M.D.; Arevalo, J.M.G.; Ma, J.; Breen, E.C.; Cole, S.W. Mindfulness-Based Stress Reduction Training Reduces Loneliness and pro-Inflammatory Gene Expression in Older Adults: A Small Randomized Controlled Trial. Brain Behav. Immun. 2012, 26, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Cavada, C.; Goldman-Rakic, P.S. Multiple Visual Areas in the Posterior Parietal Cortex of Primates. Prog. Brain Res. 1993, 95, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Inchingolo, A.M.; Blasio, M.D.; Ruvo, E.D.; Noia, A.D.; Ferrante, L.; Vecchio, G.D.; Palermo, A.; Inchingolo, F.; Inchingolo, A.D.; et al. Neurological Complications Following Surgical Treatments of the Lower Molars. Int. J. Dent. 2024, 2024, 5415597. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, F.; Katz, R.S. Normalizing Memory Recall in Fibromyalgia with Rehearsal: A Distraction-Counteracting Effect. Arthritis Rheum. 2009, 61, 740–744. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, W.; Zhang, J.; Guo, Z.; Jiang, A.; Li, Q. Novel Coumarin-Functionalized Inulin Derivatives: Chemical Modification and Antioxidant Activity Assessment. Carbohydr. Res. 2022, 518, 108597. [Google Scholar] [CrossRef]
- Pagliai, G.; Giangrandi, I.; Dinu, M.; Sofi, F.; Colombini, B. Nutritional Interventions in the Management of Fibromyalgia Syndrome. Nutrients 2020, 12, 2525. [Google Scholar] [CrossRef]
- Dehghan, P.; Pourghassem Gargari, B.; Asghari Jafar-abadi, M. Oligofructose-Enriched Inulin Improves Some Inflammatory Markers and Metabolic Endotoxemia in Women with Type 2 Diabetes Mellitus: A Randomized Controlled Clinical Trial. Nutr. Burbank Los Angel. Cty. Calif 2014, 30, 418–423. [Google Scholar] [CrossRef]
- Amini-Khoei, H.; Haghani-Samani, E.; Beigi, M.; Soltani, A.; Mobini, G.R.; Balali-Dehkordi, S.; Haj-Mirzaian, A.; Rafieian-Kopaei, M.; Alizadeh, A.; Hojjati, M.R.; et al. On the Role of Corticosterone in Behavioral Disorders, Microbiota Composition Alteration and Neuroimmune Response in Adult Male Mice Subjected to Maternal Separation Stress. Int. Immunopharmacol. 2019, 66, 242–250. [Google Scholar] [CrossRef]
- Del Socorro Santos Díaz, M.; Barba de la Rosa, A.P.; Héliès-Toussaint, C.; Guéraud, F.; Nègre-Salvayre, A. Opuntia spp.: Characterization and Benefits in Chronic Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 8634249. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Moleculesd 2019, 24, 1583. [Google Scholar] [CrossRef]
- Holliday, R.S.; Campbell, J.; Preshaw, P.M. Effect of Nicotine on Human Gingival, Periodontal Ligament and Oral Epithelial Cells. A Systematic Review of the Literature. J. Dent. 2019, 86, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Reimer, R.A. Prebiotic Fiber Modulation of the Gut Microbiota Improves Risk Factors for Obesity and the Metabolic Syndrome. Gut Microbes 2012, 3, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Khodamorovati, M.; Vafaee, K.; Hemmati, M.; Darvishi, N.; Ghasemi, H. Prevalence of Migraine in Iran: A Systematic Review and Meta-Analysis. BMC Neurol. 2023, 23, 172. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v Decreases Kynurenine Concentration and Improves Cognitive Functions in Patients with Major Depression: A Double-Blind, Randomized, Placebo Controlled Study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Carlos, L.D.O.; Ramos, M.R.Z.; Wagner, N.R.F.; FREITAS, L.A.C.D.; Felicidade, I.; Campos, A.C.L. Probiotic supplementation attenuates binge eating and food addiction 1 year after Roux-en-Y gastric bypass: A randomized, double-blind, placebo-controlled trial. ABCD Arq. Bras. Cir. Dig. São Paulo 2022, 35, e1659. [Google Scholar] [CrossRef]
- Rezaei Asl, Z.; Sepehri, G.; Salami, M. Probiotic Treatment Improves the Impaired Spatial Cognitive Performance and Restores Synaptic Plasticity in an Animal Model of Alzheimer’s Disease. Behav. Brain Res. 2019, 376, 112183. [Google Scholar] [CrossRef]
- Roman, P.; Estévez, Á.F.; Sánchez-Labraca, N.; Cañadas, F.; Miras, A.; Cardona, D. Probiotics for Fibromyalgia: Study Design for a Pilot Double-Blind, Randomized Controlled Trial. Nutr. Hosp. 2017, 34, 1246–1251. [Google Scholar] [CrossRef]
- Lv, T.; Ye, M.; Luo, F.; Hu, B.; Wang, A.; Chen, J.; Yan, J.; He, Z.; Chen, F.; Qian, C.; et al. Probiotics Treatment Improves Cognitive Impairment in Patients and Animals: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2021, 120, 159–172. [Google Scholar] [CrossRef]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, Prebiotics and Immunomodulation of Gut Mucosal Defences: Homeostasis and Immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef]
- Ling, X.; Linglong, P.; Weixia, D.; Hong, W. Protective Effects of Bifidobacterium on Intestinal Barrier Function in LPS-Induced Enterocyte Barrier Injury of Caco-2 Monolayers and in a Rat NEC Model. PLoS ONE 2016, 11, e0161635. [Google Scholar] [CrossRef]
- Gualtieri, P.; Marchetti, M.; Cioccoloni, G.; De Lorenzo, A.; Romano, L.; Cammarano, A.; Colica, C.; Condò, R.; Di Renzo, L. Psychobiotics Regulate the Anxiety Symptoms in Carriers of Allele A of IL-1β Gene: A Randomized, Placebo-Controlled Clinical Trial. Mediat. Inflamm. 2020, 2020, 2346126. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Rawtaer, I.; Mahendran, R.; Yu, J.; Fam, J.; Feng, L.; Kua, E.H. Psychosocial Interventions with Art, Music, Tai Chi and Mindfulness for Subsyndromal Depression and Anxiety in Older Adults: A Naturalistic Study in Singapore. Asia-Pac. Psychiatr. Off. J. Pac. Rim Coll. Psychiatr. 2015, 7, 240–250. [Google Scholar] [CrossRef]
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced Anxiety-like Behavior and Central Neurochemical Change in Germ-Free Mice. Neurogastroenterol. Motil. 2011, 23, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Zandifar, A.; Banihashemi, M.; Haghdoost, F.; Masjedi, S.S.; Manouchehri, N.; Asgari, F.; Najafi, M.R.; Ghorbani, A.; Zandifar, S.; Saadatnia, M.; et al. Reliability and Validity of the Persian HIT-6 Questionnaire in Migraine and Tension-Type Headache. Pain Pract. Off. J. World Inst. Pain 2014, 14, 625–631. [Google Scholar] [CrossRef]
- Glass, J.M. Review of Cognitive Dysfunction in Fibromyalgia: A Convergence on Working Memory and Attentional Control Impairments. Rheum. Dis. Clin. N. Am. 2009, 35, 299–311. [Google Scholar] [CrossRef]
- Roy, S.; Dhaneshwar, S. Role of Prebiotics, Probiotics, and Synbiotics in Management of Inflammatory Bowel Disease: Current Perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef]
- Kaushik, R.; Yeltiwar, R.K.; Pushpanshu, K. Salivary Interleukin-1β Levels in Patients with Chronic Periodontitis before and after Periodontal Phase I Therapy and Healthy Controls: A Case-Control Study. J. Periodontol. 2011, 82, 1353–1359. [Google Scholar] [CrossRef]
- Derogatis, L.R.; Lipman, R.S.; Covi, L. SCL-90: An Outpatient Psychiatric Rating Scale—Preliminary Report. Psychopharmacol. Bull. 1973, 9, 13–28. [Google Scholar]
- Muñoz Gómez, E.; Aguilar Rodríguez, M.; Serra Añó, P.; Sempere Rubio, N.; Mollà Casanova, S.; Inglés, M. Sex-Related Differences in Migraine Clinical Features by Frequency of Occurrence: A Cross-Sectional Study. Scand. J. Pain 2023, 23, 553–562. [Google Scholar] [CrossRef]
- Majumdar, A.; Siva Venkatesh, I.P.; Basu, A. Short-Chain Fatty Acids in the Microbiota-Gut-Brain Axis: Role in Neurodegenerative Disorders and Viral Infections. ACS Chem. Neurosci. 2023, 14, 1045–1062. [Google Scholar] [CrossRef] [PubMed]
- Petkus, A.J.; Gatz, M.; Reynolds, C.A.; Kremen, W.S.; Wetherell, J.L. Stability of Genetic and Environmental Contributions to Anxiety Symptoms in Older Adulthood. Behav. Genet. 2016, 46, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the Gut-Brain Axis: Regulation by the Microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Tandon, R.K. Stress and the Gastrointestinal Tract. J. Gastroenterol. Hepatol. 2005, 20, 332–339. [Google Scholar] [CrossRef]
- Moloney, R.D.; Johnson, A.C.; O’Mahony, S.M.; Dinan, T.G.; Greenwood-Van Meerveld, B.; Cryan, J.F. Stress and the Microbiota-Gut-Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome. CNS Neurosci. Ther. 2016, 22, 102–117. [Google Scholar] [CrossRef]
- Jašarević, E.; Howard, C.D.; Misic, A.M.; Beiting, D.P.; Bale, T.L. Stress during Pregnancy Alters Temporal and Spatial Dynamics of the Maternal and Offspring Microbiome in a Sex-Specific Manner. Sci. Rep. 2017, 7, 44182. [Google Scholar] [CrossRef]
- Mayas, J.; Fuentes, L.J.; Ballesteros, S. Stroop Interference and Negative Priming (NP) Suppression in Normal Aging. Arch. Gerontol. Geriatr. 2012, 54, 333–338. [Google Scholar] [CrossRef]
- Fourie, N.H.; Wang, D.; Abey, S.K.; Creekmore, A.L.; Hong, S.; Martin, C.G.; Wiley, J.W.; Henderson, W.A. Structural and Functional Alterations in the Colonic Microbiome of the Rat in a Model of Stress Induced Irritable Bowel Syndrome. Gut Microbes 2017, 8, 33–45. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; Murray, K.; Gareau, M.G. Targeting the Microbiota, from Irritable Bowel Syndrome to Mood Disorders: Focus on Probiotics and Prebiotics. Curr. Pathobiol. Rep. 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Penfold, S.; St Denis, E.; Mazhar, M.N. The Association between Borderline Personality Disorder, Fibromyalgia and Chronic Fatigue Syndrome: Systematic Review. BJPsych Open 2016, 2, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Cuzzolaro, M.; Vetrone, G.; Marano, G.; Garfinkel, P.E. The Body Uneasiness Test (BUT): Development and Validation of a New Body Image Assessment Scale. Eat. Weight Disord. EWD 2006, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Wong, J.L.; Bagge, C.L.; Freedenthal, S.; Gutierrez, P.M.; Lozano, G. The Depression Anxiety Stress Scales-21 (DASS-21): Further Examination of Dimensions, Scale Reliability, and Correlates. J. Clin. Psychol. 2012, 68, 1322–1338. [Google Scholar] [CrossRef]
- Butel, M.J.; Waligora-Dupriet, A.J.; Wydau-Dematteis, S. The Developing Gut Microbiota and Its Consequences for Health. J. Dev. Orig. Health Dis. 2018, 9, 590–597. [Google Scholar] [CrossRef]
- Salli, K.; Hirvonen, J.; Anglenius, H.; Hibberd, A.A.; Ahonen, I.; Saarinen, M.T.; Maukonen, J.; Ouwehand, A.C. The Effect of Human Milk Oligosaccharides and Bifidobacterium Longum Subspecies Infantis Bi-26 on Simulated Infant Gut Microbiome and Metabolites. Microorganisms 2023, 11, 1553. [Google Scholar] [CrossRef]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.B.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.A.; Chevalier, S.; Shir, Y. Altered Microbiome Composition in Individuals with Fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef]
- Cardona, D.; Roman, P.; Cañadas, F.; Sánchez-Labraca, N. The Effect of Multiprobiotics on Memory and Attention in Fibromyalgia: A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 3543. [Google Scholar] [CrossRef]
- Mohan, R.; Varghese, J.; Bhat, V.; Chianeh, Y.R. The Effect of Nonsurgical Periodontal Therapy on Pentraxin 3 Levels in Smokers and Nonsmokers with Chronic Periodontitis. Gen. Dent. 2019, 67, e1–e6. [Google Scholar]
- Dipalma, G.; Inchingolo, A.M.; Latini, G.; Ferrante, L.; Nardelli, P.; Malcangi, G.; Trilli, I.; Inchingolo, F.; Palermo, A.; Inchingolo, A.D. The Effectiveness of Curcumin in Treating Oral Mucositis Related to Radiation and Chemotherapy: A Systematic Review. Antioxidants 2024, 13, 1160. [Google Scholar] [CrossRef]
- De Roos, N.M.; van Hemert, S.; Rovers, J.M.P.; Smits, M.G.; Witteman, B.J.M. The Effects of a Multispecies Probiotic on Migraine and Markers of Intestinal Permeability-Results of a Randomized Placebo-Controlled Study. Eur. J. Clin. Nutr. 2017, 71, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Martami, F.; Togha, M.; Seifishahpar, M.; Ghorbani, Z.; Ansari, H.; Karimi, T.; Jahromi, S.R. The Effects of a Multispecies Probiotic Supplement on Inflammatory Markers and Episodic and Chronic Migraine Characteristics: A Randomized Double-Blind Controlled Trial. Cephalalgia Int. J. Headache 2019, 39, 841–853. [Google Scholar] [CrossRef]
- Bercik, P.; Collins, S.M. The Effects of Inflammation, Infection and Antibiotics on the Microbiota-Gut-Brain Axis. Adv. Exp. Med. Biol. 2014, 817, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Rezaeiasl, Z.; Salami, M.; Sepehri, G. The Effects of Probiotic Lactobacillus and Bifidobacterium Strains on Memory and Learning Behavior, Long-Term Potentiation (LTP), and Some Biochemical Parameters in β-Amyloid-Induced Rat’s Model of Alzheimer’s Disease. Prev. Nutr. Food Sci. 2019, 24, 265–273. [Google Scholar] [CrossRef]
- Wallace, C.J.K.; Milev, R. The Effects of Probiotics on Depressive Symptoms in Humans: A Systematic Review. Ann. Gen. Psychiatr. 2017, 16, 14. [Google Scholar] [CrossRef]
- Ghavami, A.; Khorvash, F.; Khalesi, S.; Heidari, Z.; Askari, G. The Effects of Synbiotic Supplementation on Oxidative Stress and Clinical Symptoms in Women with Migraine: A Double-blind, Placebo-controlled, Randomized Trial. J. Funct. Foods 2021, 86, 104738. [Google Scholar] [CrossRef]
- De Roos, N.M.; Giezenaar, C.G.T.; Rovers, J.M.P.; Witteman, B.J.M.; Smits, M.G.; van Hemert, S. The Effects of the Multispecies Probiotic Mixture Ecologic®Barrier on Migraine: Results of an Open-Label Pilot Study. Benef. Microbes 2015, 6, 641–646. [Google Scholar] [CrossRef]
- Mollova, D.; Vasileva, T.; Bivolarski, V.; Iliev, I. The Enzymatic Hydrolysis of Human Milk Oligosaccharides and Prebiotic Sugars from LAB Isolated from Breast Milk. Microorganisms 2023, 11, 1904. [Google Scholar] [CrossRef]
- Blasco, G.; Moreno-Navarrete, J.M.; Rivero, M.; Pérez-Brocal, V.; Garre-Olmo, J.; Puig, J.; Daunis-I-Estadella, P.; Biarnés, C.; Gich, J.; Fernández-Aranda, F.; et al. The Gut Metagenome Changes in Parallel to Waist Circumference, Brain Iron Deposition, and Cognitive Function. J. Clin. Endocrinol. Metab. 2017, 102, 2962–2973. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Charitos, I.A.; Inchingolo, A.M.; Ferrante, L.; Inchingolo, F.; Inchingolo, A.D.; Castellaneta, F.; Cotoia, A.; Palermo, A.; Scacco, S.; Dipalma, G. The Gut Microbiota’s Role in Neurological, Psychiatric, and Neurodevelopmental Disorders. Nutrients 2024, 16, 4404. [Google Scholar] [CrossRef] [PubMed]
- Maslanik, T.; Mahaffey, L.; Tannura, K.; Beninson, L.; Greenwood, B.N.; Fleshner, M. The Inflammasome and Danger Associated Molecular Patterns (DAMPs) Are Implicated in Cytokine and Chemokine Responses Following Stressor Exposure. Brain Behav. Immun. 2013, 28, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.; Piras, F.; Ferrante, L.; Mancini, A.; Palermo, A.; Inchingolo, A.D.; Dipalma, G. The Interaction between Gut Microbiome and Bone Health. Curr. Opin. Endocrinol. Diabetes Obes. 2024, 31, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef]
- Wiley, N.C.; Dinan, T.G.; Ross, R.P.; Stanton, C.; Clarke, G.; Cryan, J.F. The Microbiota-Gut-Brain Axis as a Key Regulator of Neural Function and the Stress Response: Implications for Human and Animal Health. J. Anim. Sci. 2017, 95, 3225–3246. [Google Scholar] [CrossRef]
- De Palma, G.; Collins, S.M.; Bercik, P.; Verdu, E.F. The Microbiota-Gut-Brain Axis in Gastrointestinal Disorders: Stressed Bugs, Stressed Brain or Both? J. Physiol. 2014, 592, 2989–2997. [Google Scholar] [CrossRef]
- Burch, R.; Rizzoli, P.; Loder, E. The Prevalence and Impact of Migraine and Severe Headache in the United States: Updated Age, Sex, and Socioeconomic-Specific Estimates from Government Health Surveys. Headache 2021, 61, 60–68. [Google Scholar] [CrossRef]
- Bavetta, G.; Bavetta, G.; Randazzo, V.; Cavataio, A.; Paderni, C.; Grassia, V.; Dipalma, G.; Gargiulo Isacco, C.; Scarano, A.; De Vito, D.; et al. A Retrospective Study on Insertion Torque and Implant Stability Quotient (ISQ) as Stability Parameters for Immediate Loading of Implants in Fresh Extraction Sockets. BioMed Res. Int. 2019, 2019, 9720419. [Google Scholar] [CrossRef]
- Balzanelli, M.G.; Distratis, P.; Lazzaro, R.; Pham, V.H.; Tran, T.C.; Dipalma, G.; Bianco, A.; Serlenga, E.M.; Aityan, S.K.; Pierangeli, V.; et al. Analysis of Gene Single Nucleotide Polymorphisms in COVID-19 Disease Highlighting the Susceptibility and the Severity towards the Infection. Diagnostics 2022, 12, 2824. [Google Scholar] [CrossRef]
- Arrigoni, R.; Ballini, A.; Santacroce, L.; Cantore, S.; Inchingolo, A.; Inchingolo, F.; Di Domenico, M.; Quagliuolo, L.; Boccellino, M. Another Look at Dietary Polyphenols: Challenges in Cancer Prevention and Treatment. Curr. Med. Chem. 2022, 29, 1061–1082. [Google Scholar] [CrossRef]
- Malcangi, G.; Patano, A.; Ciocia, A.M.; Netti, A.; Viapiano, F.; Palumbo, I.; Trilli, I.; Guglielmo, M.; Inchingolo, A.D.; Dipalma, G.; et al. Benefits of Natural Antioxidants on Oral Health. Antioxidants 2023, 12, 1309. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Paracchini, L.; DE Angelis, F.; Cielo, A.; Orefici, A.; Spitaleri, D.; Santacroce, L.; Gheno, E.; Palermo, A. Biomechanical Behaviour of a Jawbone Loaded with a Prosthetic System Supported by Monophasic and Biphasic Implants. Oral Implantol. 2016, 9, 65–70. [Google Scholar] [CrossRef]
- Minetti, E.; Dipalma, G.; Palermo, A.; Patano, A.; Inchingolo, A.D.; Inchingolo, A.M.; Inchingolo, F. Biomolecular Mechanisms and Case Series Study of Socket Preservation with Tooth Grafts. J. Clin. Med. 2023, 12, 5611. [Google Scholar] [CrossRef]
- Dimonte, M.; Inchingolo, F.; Minonne, A.; Arditi, G.; Dipalma, G. Bone SPECT in Management of Mandibular Condyle Hyperplasia. Report of a Case and Review of Literature. Minerva Stomatol. 2004, 53, 281–285. [Google Scholar]
- Karimi, L.; Crewther, S.G.; Wijeratne, T.; Evans, A.E.; Afshari, L.; Khalil, H. The Prevalence of Migraine with Anxiety Among Genders. Front. Neurol. 2020, 11, 569405. [Google Scholar] [CrossRef]
- Zandifar, A.; Masjedi, S.S.; Haghdoost, F.; Asgari, F.; Manouchehri, N.; Banihashemi, M.; Najafi, M.R.; Ghorbani, A.; Zolfaghari, B.; Gholamrezaei, A.; et al. The Psychometric Properties of the Persian Migraine-Specific Quality of Life Questionnaire Version 2.1 in Episodic and Chronic Migraines. Sci. World J. 2013, 2013, 950245. [Google Scholar] [CrossRef]
- De Simone, R.; Sansone, M.; Russo, C.; Miele, A.; Stornaiuolo, A.; Braca, S. The Putative Role of Trigemino-Vascular System in Brain Perfusion Homeostasis and the Significance of the Migraine Attack. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2022, 43, 5665–5672. [Google Scholar] [CrossRef]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The Role of the Gut Microbiota in the Relationship Between Diet and Human Health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The Role of the Hypothalamic-Pituitary-Adrenal Axis in Neuroendocrine Responses to Stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Zhao, N.; Li, Q.; Wang, Y.; Qiao, Q.; Huang, H.; Guo, C.; Guo, Y. Anti-Angiogenic Drug Aggravates the Degree of Anti-Resorptive Drug-Based Medication-Related Osteonecrosis of the Jaw by Impairing the Proliferation and Migration Function of Gingival Fibroblasts. BMC Oral Health 2023, 23, 330. [Google Scholar] [CrossRef]
- Oneto, P.; Zubiry, P.R.; Schattner, M.; Etulain, J. Anticoagulants Interfere With the Angiogenic and Regenerative Responses Mediated by Platelets. Front. Bioeng. Biotechnol. 2020, 8, 223. [Google Scholar] [CrossRef]
- Shibahara, T. Antiresorptive Agent-Related Osteonecrosis of the Jaw (ARONJ): A Twist of Fate in the Bone. Tohoku J. Exp. Med. 2019, 247, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Mijiritsky, E.; Assaf, H.D.; Kolerman, R.; Mangani, L.; Ivanova, V.; Zlatev, S. Autologous Platelet Concentrates (APCs) for Hard Tissue Regeneration in Oral Implantology, Sinus Floor Elevation, Peri-Implantitis, Socket Preservation, and Medication-Related Osteonecrosis of the Jaw (MRONJ): A Literature Review. Biology 2022, 11, 1254. [Google Scholar] [CrossRef]
- Hellstein, J.W.; Marek, C.L. Bisphosphonate Osteochemonecrosis (Bis-Phossy Jaw): Is This Phossy Jaw of the 21st Century? J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2005, 63, 682–689. [Google Scholar] [CrossRef]
- Curi, M.M.; Cossolin, G.S.I.; Koga, D.H.; Zardetto, C.; Christianini, S.; Feher, O.; Cardoso, C.L.; dos Santos, M.O. Bisphosphonate-Related Osteonecrosis of the Jaws--an Initial Case Series Report of Treatment Combining Partial Bone Resection and Autologous Platelet-Rich Plasma. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2011, 69, 2465–2472. [Google Scholar] [CrossRef]
- Brady, D.; Parker, C.C.; O’Sullivan, J.M. Bone-Targeting Radiopharmaceuticals Including Radium-223. Cancer J. 2013, 19, 71. [Google Scholar] [CrossRef]
- Lovibond, P.F.; Lovibond, S.H. The Structure of Negative Emotional States: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther. 1995, 33, 335–343. [Google Scholar] [CrossRef]
- Middeldorp, C.M.; Wray, N.R. The Value of Polygenic Analyses in Psychiatry. World Psychiatr. Off. J. World Psychiatr. Assoc. WPA 2018, 17, 26–28. [Google Scholar] [CrossRef]
- Laforgia, A.; Inchingolo, A.D.; Piras, F.; Colonna, V.; Giorgio, R.V.; Carone, C.; Rapone, B.; Malcangi, G.; Inchingolo, A.M.; Inchingolo, F.; et al. Therapeutic Strategies and Genetic Implications for Periodontal Disease Management: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 7217. [Google Scholar] [CrossRef]
- Hochmeister, M.N.; Budowle, B.; Borer, U.V.; Eggmann, U.; Comey, C.T.; Dirnhofer, R. Typing of Deoxyribonucleic Acid (DNA) Extracted from Compact Bone from Human Remains. J. Forensic Sci. 1991, 36, 1649–1661. [Google Scholar]
- Chichlowski, M.; Rudolph, C. Visceral Pain and Gastrointestinal Microbiome. J. Neurogastroenterol. Motil. 2015, 21, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamoudi, N.; Alsahhaf, A.; Al Deeb, M.; Alrabiah, M.; Vohra, F.; Abduljabbar, T. Effect of Scaling and Root Planing on the Expression of Anti-Inflammatory Cytokines (IL-4, IL-9, IL-10, and IL-13) in the Gingival Crevicular Fluid of Electronic Cigarette Users and Non-Smokers with Moderate Chronic Periodontitis. J. Periodontal Implant Sci. 2020, 50, 74. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Sacchetti, L.; Napolitano, F.; De Chiara, V.; Motta, C.; Studer, V.; Musella, A.; Barbieri, F.; Bari, M.; Bernardi, G.; et al. Interleukin-1β Causes Anxiety by Interacting with the Endocannabinoid System. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 13896–13905. [Google Scholar] [CrossRef]
- Hewett, S.J.; Jackman, N.A.; Claycomb, R.J. Interleukin-1β in Central Nervous System Injury and Repair. Eur. J. Neurodegener. Dis. 2012, 1, 195–211. [Google Scholar]
- Agustí, A.; García-Pardo, M.P.; López-Almela, I.; Campillo, I.; Maes, M.; Romaní-Pérez, M.; Sanz, Y. Interplay Between the Gut-Brain Axis, Obesity and Cognitive Function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef]
- Wrigley-Carr, H.E.; van Dorst, J.M.; Ooi, C.Y. Intestinal Dysbiosis and Inflammation in Cystic Fibrosis Impacts Gut and Multi-Organ Axes. Med. Microecol. 2022, 13, 100057. [Google Scholar] [CrossRef]
- Ringel, Y.; Maharshak, N. Intestinal Microbiota and Immune Function in the Pathogenesis of Irritable Bowel Syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G529–G541. [Google Scholar] [CrossRef]
- Potrykus, M.; Czaja-Stolc, S.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Intestinal Microbiota as a Contributor to Chronic Inflammation and Its Potential Modifications. Nutrients 2021, 13, 3839. [Google Scholar] [CrossRef]
- Yanckello, L.M.; Fanelli, B.; McCulloch, S.; Xing, X.; Sun, M.; Hammond, T.C.; Colwell, R.; Gu, Z.; Ericsson, A.C.; Chang, Y.H.; et al. Inulin Supplementation Mitigates Gut Dysbiosis and Brain Impairment Induced by Mild Traumatic Brain Injury during Chronic Phase. J. Cell. Immunol. 2022, 4, 50–64. [Google Scholar] [CrossRef]
- Thompson, E. Hamilton Rating Scale for Anxiety (HAM-A). Occup. Med. Oxf. Engl. 2015, 65, 601. [Google Scholar] [CrossRef]
- Munoz-Ceron, J.; Marin-Careaga, V.; Peña, L.; Mutis, J.; Ortiz, G. Headache at the Emergency Room: Etiologies, Diagnostic Usefulness of the ICHD 3 Criteria, Red and Green Flags. PLoS ONE 2019, 14, e0208728. [Google Scholar] [CrossRef]
- Ferenci, P.; Lockwood, A.; Mullen, K.; Tarter, R.; Weissenborn, K.; Blei, A.T. Hepatic Encephalopathy—Definition, Nomenclature, Diagnosis, and Quantification: Final Report of the Working Party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002, 35, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Packard, A.E.B.; Egan, A.E.; Ulrich-Lai, Y.M. HPA Axis Interactions with Behavioral Systems. Compr. Physiol. 2016, 6, 1897–1934. [Google Scholar] [CrossRef] [PubMed]
- Alkasir, R.; Li, J.; Li, X.; Jin, M.; Zhu, B. Human Gut Microbiota: The Links with Dementia Development. Protein Cell 2017, 8, 90–102. [Google Scholar] [CrossRef]
- Rogus, J.; Beck, J.D.; Offenbacher, S.; Huttner, K.; Iacoviello, L.; Latella, M.C.; de Gaetano, M.; Wang, H.Y.; Kornman, K.S.; Duff, G.W. IL1B Gene Promoter Haplotype Pairs Predict Clinical Levels of Interleukin-1beta and C-Reactive Protein. Hum. Genet. 2008, 123, 387–398. [Google Scholar] [CrossRef]
- Capuron, L.; Miller, A.H. Immune System to Brain Signaling: Neuropsychopharmacological Implications. Pharmacol. Ther. 2011, 130, 226–238. [Google Scholar] [CrossRef]
- Sheng, W.; Ji, G.; Zhang, L. Immunomodulatory Effects of Inulin and Its Intestinal Metabolites. Front. Immunol. 2023, 14, 1224092. [Google Scholar] [CrossRef]
- Benton, D.; Williams, C.; Brown, A. Impact of Consuming a Milk Drink Containing a Probiotic on Mood and Cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef]
- Mady, E.A.; Doghish, A.S.; El-Dakroury, W.A.; Elkhawaga, S.Y.; Ismail, A.; El-Mahdy, H.A.; Elsakka, E.G.E.; El-Husseiny, H.M. Impact of the Mother’s Gut Microbiota on Infant Microbiome and Brain Development. Neurosci. Biobehav. Rev. 2023, 150, 105195. [Google Scholar] [CrossRef]
- Arranz, L.; Guayerbas, N.; De la Fuente, M. Impairment of Several Immune Functions in Anxious Women. J. Psychosom. Res. 2007, 62, 1–8. [Google Scholar] [CrossRef]
- Shinkov, A.; Borissova, A.M.; Kovatcheva, R.; Vlahov, J.; Dakovska, L.; Atanassova, I.; Petkova, P. Increased Prevalence of Depression and Anxiety among Subjects with Metabolic Syndrome and Known Type 2 Diabetes Mellitus—A Population-Based Study. Postgrad. Med. 2018, 130, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Chugh, G.; Asghar, M. Inflammation in Anxiety. Adv. Protein Chem. Struct. Biol. 2012, 88, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Peralta, V.; Papiol, S.; Cuesta, M.J.; Serrano, F.; Martínez-Larrea, A.; Fañanás, L. Interleukin-1beta (IL-1beta) Gene and Increased Risk for the Depressive Symptom-Dimension in Schizophrenia Spectrum Disorders. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2004, 124B, 10–14. [Google Scholar] [CrossRef]
- Wang, H.; Braun, C.; Enck, P. Effects of Rifaximin on Central Responses to Social Stress-a Pilot Experiment. Neurother. J. Am. Soc. Exp. Neurother. 2018, 15, 807–818. [Google Scholar] [CrossRef]
- Benson, S.; Labrenz, F.; Kotulla, S.; Brotte, L.; Rödder, P.; Tebbe, B.; Theysohn, N.; Engler, H.; Elsenbruch, S. Amplified Gut Feelings under Inflammation and Depressed Mood: A Randomized fMRI Trial on Interoceptive Pain in Healthy Volunteers. Brain Behav. Immun. 2023, 112, 132–137. [Google Scholar] [CrossRef]
- Peter, J.; Fournier, C.; Durdevic, M.; Knoblich, L.; Keip, B.; Dejaco, C.; Trauner, M.; Moser, G. A Microbial Signature of Psychological Distress in Irritable Bowel Syndrome. Psychosom. Med. 2018, 80, 698–709. [Google Scholar] [CrossRef]
- Hall, C.V.; Hepsomali, P.; Dalile, B.; Scapozza, L.; Gurry, T. Effects of a Diverse Prebiotic Fibre Blend on Inflammation, the Gut Microbiota and Affective Symptoms in Metabolic Syndrome: A Pilot Open-Label Randomised Controlled Trial. Br. J. Nutr. 2024, 132, 1002–1013. [Google Scholar] [CrossRef]
- Mellai, M.; Allesina, M.; Edoardo, B.; Cascella, F.; Nobile, V.; Spina, A.; Amone, F.; Zaccaria, V.; Insolia, V.; Perri, A.; et al. A Randomized, Double-Blind, Placebo-Controlled Trial: Efficacy of Opuntia Ficus-Indica Prebiotic Supplementation in Subjects with Gut Dysbiosis. Nutrients 2024, 16, 586. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Wells, J.C.K.; Wei, Z.; Bajaj-Elliott, M.; Nielsen, D.S.; Fewtrell, M.S. A Stress Reduction Intervention for Lactating Mothers Alters Maternal Gut, Breast Milk, and Infant Gut Microbiomes: Data from a Randomized Controlled Trial. Nutrients 2024, 16, 1074. [Google Scholar] [CrossRef]
- Colica, C.; Avolio, E.; Bollero, P.; Costa de Miranda, R.; Ferraro, S.; Sinibaldi Salimei, P.; De Lorenzo, A.; Di Renzo, L. Evidences of a New Psychobiotic Formulation on Body Composition and Anxiety. Mediat. Inflamm. 2017, 2017, 5650627. [Google Scholar] [CrossRef]
- Verdejo-García, A.; López-Torrecillas, F.; Calandre, E.P.; Delgado-Rodríguez, A.; Bechara, A. Executive Function and Decision-Making in Women with Fibromyalgia. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2009, 24, 113–122. [Google Scholar] [CrossRef]
- Glass, J.M.; Williams, D.A.; Fernandez-Sanchez, M.L.; Kairys, A.; Barjola, P.; Heitzeg, M.M.; Clauw, D.J.; Schmidt-Wilcke, T. Executive Function in Chronic Pain Patients and Healthy Controls: Different Cortical Activation during Response Inhibition in Fibromyalgia. J. Pain 2011, 12, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Farabegoli, F.; Santaclara, F.J.; Costas, D.; Alonso, M.; Abril, A.G.; Espiñeira, M.; Ortea, I.; Costas, C. Exploring the Anti-Inflammatory Effect of Inulin by Integrating Transcriptomic and Proteomic Analyses in a Murine Macrophage Cell Model. Nutrients 2023, 15, 859. [Google Scholar] [CrossRef] [PubMed]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Suda, K.; Kawai, M.; Shimizu, K.; Kushiro, A.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Fermented Milk Containing Lactobacillus Casei Strain Shirota Prevents the Onset of Physical Symptoms in Medical Students under Academic Examination Stress. Benef. Microbes 2016, 7, 153–156. [Google Scholar] [CrossRef]
- Glass, J.M. Fibromyalgia and Cognition. J. Clin. Psychiatry 2008, 69 (Suppl. S2), 20–24. [Google Scholar]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Cámara-Lemarroy, C.R.; Rodriguez-Gutierrez, R.; Monreal-Robles, R.; Marfil-Rivera, A. Gastrointestinal Disorders Associated with Migraine: A Comprehensive Review. World J. Gastroenterol. 2016, 22, 8149–8160. [Google Scholar] [CrossRef]
- Smoller, J.W.; Block, S.R.; Young, M.M. Genetics of Anxiety Disorders: The Complex Road from DSM to DNA. Depress. Anxiety 2009, 26, 965–975. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Mian, M.F.; Hossain, N.; Karimi, K.; Mao, Y.K.; Forsythe, P.; Min, K.K.; Stanisz, A.M.; Kunze, W.A.; Bienenstock, J. Gut Commensal Microvesicles Reproduce Parent Bacterial Signals to Host Immune and Enteric Nervous Systems. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 684–695. [Google Scholar] [CrossRef]
- Minerbi, A.; Fitzcharles, M.A. Gut Microbiome: Pertinence in Fibromyalgia. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S123), 99–104. [Google Scholar]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Mangiola, F.; Ianiro, G.; Franceschi, F.; Fagiuoli, S.; Gasbarrini, G.; Gasbarrini, A. Gut Microbiota in Autism and Mood Disorders. World J. Gastroenterol. 2016, 22, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M.; School of Advanced Studies of the European Headache Federation (EHF-SAS). Gut-Brain Axis Migraine Headache: A Comprehensive Review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.C.; Oliveira, L.F.; Noleto, F.M.; Gusmão, C.T.P.; de Castro Brito, G.A.; de Barros Viana, G.S. Gut-Microbiome-Brain Axis: The Crosstalk between the Vagus Nerve, Alpha-Synuclein and the Brain in Parkinson’s Disease. Neural Regen. Res. 2023, 18, 2611–2614. [Google Scholar] [CrossRef]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/Brain Axis and the Microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef]
- Casey, B.J.; Trainor, R.J.; Orendi, J.L.; Schubert, A.B.; Nystrom, L.E.; Giedd, J.N.; Castellanos, F.X.; Haxby, J.V.; Noll, D.C.; Cohen, J.D.; et al. A Developmental Functional MRI Study of Prefrontal Activation during Performance of a Go-No-Go Task. J. Cogn. Neurosci. 1997, 9, 835–847. [Google Scholar] [CrossRef]
- Goshen, I.; Kreisel, T.; Ounallah-Saad, H.; Renbaum, P.; Zalzstein, Y.; Ben-Hur, T.; Levy-Lahad, E.; Yirmiya, R. A Dual Role for Interleukin-1 in Hippocampal-Dependent Memory Processes. Psychoneuroendocrinology 2007, 32, 1106–1115. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Gillevet, P.M.; Patel, N.R.; Ahluwalia, V.; Ridlon, J.M.; Kettenmann, B.; Schubert, C.M.; Sikaroodi, M.; Heuman, D.M.; Crossey, M.M.E.; et al. A Longitudinal Systems Biology Analysis of Lactulose Withdrawal in Hepatic Encephalopathy. Metab. Brain Dis. 2012, 27, 205–215. [Google Scholar] [CrossRef]
- Ruiz-Gonzalez, C.; Roman, P.; Rueda-Ruzafa, L.; Rodriguez-Arrastia, M.; Cardona, D. Effects of Probiotics Supplementation on Dementia and Cognitive Impairment: A Systematic Review and Meta-Analysis of Preclinical and Clinical Studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 108, 110189. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Park, S.; Paik, J.W.; Chae, S.W.; Kim, D.H.; Jeong, D.G.; Ha, E.; Kim, M.; Hong, G.; Park, S.H.; et al. Efficacy and Safety of Lactobacillus Plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2019, 11, 305. [Google Scholar] [CrossRef]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the Role of Diet in Maintaining Gut Health to Reduce the Risk of Obesity, Cardiovascular and Other Age-Related Inflammatory Diseases: Recent Challenges and Future Recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.L.; Obiang, P.; Bannerman, D.; Cunningham, C. Endogenous IL-1 in Cognitive Function and Anxiety: A Study in IL-1RI-/- Mice. PLoS ONE 2013, 8, e78385. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Wade, J.B.; Heuman, D.M.; Hammeke, T.A.; Sanyal, A.J.; Sterling, R.K.; Stravitz, R.T.; Luketic, V.; Siddiqui, M.S.; Puri, P.; et al. Enhancement of Functional Connectivity, Working Memory and Inhibitory Control on Multi-Modal Brain MR Imaging with Rifaximin in Cirrhosis: Implications for the Gut-Liver-Brain Axis. Metab. Brain Dis. 2014, 29, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Martynowicz, H.; Smardz, J.; Michalek-Zrabkowska, M.; Gac, P.; Poreba, R.; Wojakowska, A.; Mazur, G.; Wieckiewicz, M. Evaluation of Relationship Between Sleep Bruxism and Headache Impact Test-6 (HIT-6) Scores: A Polysomnographic Study. Front. Neurol. 2019, 10, 487. [Google Scholar] [CrossRef]
- Prakasam, S.; Srinivasan, M. Evaluation of Salivary Biomarker Profiles Following Non-Surgical Management of Chronic Periodontitis. Oral Dis. 2014, 20, 171–177. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef]
- Cantore, S.; Ballini, A.; De Vito, D.; Abbinante, A.; Altini, V.; Dipalma, G.; Inchingolo, F.; Saini, R. Clinical Results of Improvement in Periodontal Condition by Administration of Oral Probiotics. J. Biol. Regul. Homeost. Agents 2018, 32, 1329–1334. [Google Scholar]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Tarullo, A.; Inchingolo, A.D.; Dipalma, G.; Podo Brunetti, S.; Tarullo, A.; Cagiano, R. Combined Occlusal and Pharmacological Therapy in the Treatment of Temporo-Mandibular Disorders. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1296–1300. [Google Scholar]
- Ceratti, C.; Maspero, C.; Consonni, D.; Caprioglio, A.; Connelly, S.T.; Inchingolo, F.; Tartaglia, G.M. Cone-Beam Computed Tomographic Assessment of the Mandibular Condylar Volume in Different Skeletal Patterns: A Retrospective Study in Adult Patients. Bioengineering 2022, 9, 102. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Saini, R.; Pettini, F.; Fotopoulou, E.A.; Saini, S.R.; Georgakopoulos, I.P.; Dipalma, G.; Gargiulo Isacco, C.; Inchingolo, F. Effect of Activated Charcoal Probiotic Toothpaste Containing Lactobacillus Paracasei and Xylitol on Dental Caries: A Randomized and Controlled Clinical Trial. J. Biol. Regul. Homeost. Agents 2019, 33, 977–981. [Google Scholar]
- Ballini, A.; Gnoni, A.; De Vito, D.; Dipalma, G.; Cantore, S.; Gargiulo Isacco, C.; Saini, R.; Santacroce, L.; Topi, S.; Scarano, A.; et al. Effect of Probiotics on the Occurrence of Nutrition Absorption Capacities in Healthy Children: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8645–8657. [Google Scholar] [CrossRef] [PubMed]
- Signorini, L.; Ballini, A.; Arrigoni, R.; De Leonardis, F.; Saini, R.; Cantore, S.; De Vito, D.; Coscia, M.F.; Dipalma, G.; Santacroce, L.; et al. Evaluation of a Nutraceutical Product with Probiotics, Vitamin D, Plus Banaba Leaf Extracts (Lagerstroemia Speciosa) in Glycemic Control. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Cantore, S.; Ballini, A.; Farronato, D.; Malcangi, G.; Dipalma, G.; Assandri, F.; Garagiola, U.; Inchingolo, F.; De Vito, D.; Cirulli, N. Evaluation of an Oral Appliance in Patients with Mild to Moderate Obstructive Sleep Apnea Syndrome Intolerant to Continuous Positive Airway Pressure Use: Preliminary Results. Int. J. Immunopathol. Pharmacol. 2016, 29, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Fila, M.; Jablkowska, A.; Pawlowska, E.; Blasiak, J. DNA Damage and Repair in Migraine: Oxidative Stress and Beyond. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2023, 29, 277–286. [Google Scholar] [CrossRef]
- Nelson, J.; Sjöblom, H.; Gjertsson, I.; Ulven, S.M.; Lindqvist, H.M.; Bärebring, L. Do Interventions with Diet or Dietary Supplements Reduce the Disease Activity Score in Rheumatoid Arthritis? A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 2991. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Marchesi, J.R.; Scully, P.; Codling, C.; Ceolho, A.M.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. Early Life Stress Alters Behavior, Immunity, and Microbiota in Rats: Implications for Irritable Bowel Syndrome and Psychiatric Illnesses. Biol. Psychiatry 2009, 65, 263–267. [Google Scholar] [CrossRef]
- Kapourchali, F.R.; Cresci, G.A.M. Early-Life Gut Microbiome-The Importance of Maternal and Infant Factors in Its Establishment. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2020, 35, 386–405. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kuhara, T.; Oki, M.; Xiao, J.Z. Effects of Bifidobacterium Breve A1 on the Cognitive Function of Older Adults with Memory Complaints: A Randomised, Double-Blind, Placebo-Controlled Trial. Benef. Microbes 2019, 10, 511–520. [Google Scholar] [CrossRef]
- Kovacs, D.; Eszlari, N.; Petschner, P.; Pap, D.; Vas, S.; Kovacs, P.; Gonda, X.; Juhasz, G.; Bagdy, G. Effects of IL1B Single Nucleotide Polymorphisms on Depressive and Anxiety Symptoms Are Determined by Severity and Type of Life Stress. Brain. Behav. Immun. 2016, 56, 96–104. [Google Scholar] [CrossRef]
- Ziaei, R.; Shahshahan, Z.; Ghasemi-Tehrani, H.; Heidari, Z.; Ghiasvand, R. Effects of Inulin-Type Fructans with Different Degrees of Polymerization on Inflammation, Oxidative Stress and Endothelial Dysfunction in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Endocrinol. 2022, 97, 319–330. [Google Scholar] [CrossRef]
- Inoue, T.; Kobayashi, Y.; Mori, N.; Sakagawa, M.; Xiao, J.Z.; Moritani, T.; Sakane, N.; Nagai, N. Effect of Combined Bifidobacteria Supplementation and Resistance Training on Cognitive Function, Body Composition and Bowel Habits of Healthy Elderly Subjects. Benef. Microbes 2018, 9, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Vajdi, M.; Khorvash, F.; Rouhani, M.H.; Ghavami, A.; Clark, C.C.T.; Askari, G. Effect of Inulin Supplementation on Clinical Symptoms, Inflammatory and Oxidative Stress Markers in Women with Migraine: Study Protocol for a Randomized Clinical Trial. Trials 2023, 24, 722. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Malignaggi, V.R.; Majoka, H.A.; Al-Kheraif, A.A.; Kellesarian, T.V.; Romanos, G.E.; Javed, F. Effect of Laser-Assisted Scaling and Root Planing on the Expression of pro-Inflammatory Cytokines in the Gingival Crevicular Fluid of Patients with Chronic Periodontitis: A Systematic Review. Photodiagnosis Photodyn. Ther. 2017, 18, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, L.; Blanco, I.; Bobes, J.; de Serres, F.J. Effect of One Year of a Gluten-Free Diet on the Clinical Evolution of Irritable Bowel Syndrome plus Fibromyalgia in Patients with Associated Lymphocytic Enteritis: A Case-Control Study. Arthritis Res. Ther. 2014, 16, 421. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, A.; Khorvash, F.; Heidari, Z.; Khalesi, S.; Askari, G. Effect of Synbiotic Supplementation on Migraine Characteristics and Inflammatory Biomarkers in Women with Migraine: Results of a Randomized Controlled Trial. Pharmacol. Res. 2021, 169, 105668. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.D.; Palumbo, I.; Guglielmo, M.; Balestriere, L.; Casamassima, L.; Ciccarese, D.; Marotti, P.; Mancini, A.; Palermo, A.; et al. Management of Physiological Gingival Melanosis by Diode Laser Depigmentation versus Surgical Scalpel: A Systematic Review. Dent. Rev. 2024, 4, 100146. [Google Scholar] [CrossRef]
- Malcangi, G.; Patano, A.; Palmieri, G.; Di Pede, C.; Latini, G.; Inchingolo, A.D.; Hazballa, D.; de Ruvo, E.; Garofoli, G.; Inchingolo, F.; et al. Maxillary Sinus Augmentation Using Autologous Platelet Concentrates (Platelet-Rich Plasma, Platelet-Rich Fibrin, and Concentrated Growth Factor) Combined with Bone Graft: A Systematic Review. Cells 2023, 12, 1797. [Google Scholar] [CrossRef]
- Balzanelli, M.G.; Distratis, P.; Catucci, O.; Cefalo, A.; Lazzaro, R.; Inchingolo, F.; Tomassone, D.; Aityan, S.K.; Ballini, A.; Nguyen, K.C.D.; et al. Mesenchymal Stem Cells: The Secret Children’s Weapons against the SARS-CoV-2 Lethal Infection. Appl. Sci. 2021, 11, 1696. [Google Scholar] [CrossRef]
- Casu, C.; Mosaico, G.; Natoli, V.; Scarano, A.; Lorusso, F.; Inchingolo, F. Microbiota of the Tongue and Systemic Connections: The Examination of the Tongue as an Integrated Approach in Oral Medicine. Hygiene 2021, 1, 56–68. [Google Scholar] [CrossRef]
- Campanella, V.; Syed, J.; Santacroce, L.; Saini, R.; Ballini, A.; Inchingolo, F. Oral Probiotics Influence Oral and Respiratory Tract Infections in Pediatric Population: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8034–8041. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Inchingolo, A.D.; Trilli, I.; Ferrante, L.; Di Noia, A.; de Ruvo, E.; Palermo, A.; Inchingolo, F.; Dipalma, G. Orthopedic Devices for Skeletal Class III Malocclusion Treatment in Growing Patients: A Comparative Effectiveness Systematic Review. J. Clin. Med. 2024, 13, 7141. [Google Scholar] [CrossRef]
- Rumpler, M.; Würger, T.; Roschger, P.; Zwettler, E.; Sturmlechner, I.; Altmann, P.; Fratzl, P.; Rogers, M.J.; Klaushofer, K. Osteoclasts on Bone and Dentin in Vitro: Mechanism of Trail Formation and Comparison of Resorption Behavior. Calcif. Tissue Int. 2013, 93, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Schrepf, A.; Kaplan, C.M.; Ichesco, E.; Larkin, T.; Harte, S.E.; Harris, R.E.; Murray, A.D.; Waiter, G.D.; Clauw, D.J.; Basu, N. A Multi-Modal MRI Study of the Central Response to Inflammation in Rheumatoid Arthritis. Nat. Commun. 2018, 9, 2243. [Google Scholar] [CrossRef]
- Kim, J.M.; Stewart, R.; Kim, S.Y.; Kang, H.J.; Jang, J.E.; Kim, S.W.; Shin, I.S.; Park, M.H.; Yoon, J.H.; Park, S.W.; et al. A One Year Longitudinal Study of Cytokine Genes and Depression in Breast Cancer. J. Affect. Disord. 2013, 148, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W. A Phase-by-Phase Review of Migraine Pathophysiology. Headache 2018, 58 (Suppl. S1), 4–16. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A Randomized Controlled Trial to Test the Effect of Multispecies Probiotics on Cognitive Reactivity to Sad Mood. Brain. Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Arikian, S.R.; Gorman, J.M. A Review of the Diagnosis, Pharmacologic Treatment, and Economic Aspects of Anxiety Disorders. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 110–117. [Google Scholar] [CrossRef]
- Malfa, G.A.; Di Giacomo, C.; Cardia, L.; Sorbara, E.E.; Mannucci, C.; Calapai, G. A Standardized Extract of Opuntia Ficus-Indica (L.) Mill and Olea Europaea L. Improves Gastrointestinal Discomfort: A Double-Blinded Randomized-Controlled Study. Phytother. Res. PTR 2021, 35, 3756–3768. [Google Scholar] [CrossRef]
- Soliman, M.L.; Smith, M.D.; Houdek, H.M.; Rosenberger, T.A. Acetate Supplementation Modulates Brain Histone Acetylation and Decreases Interleukin-1β Expression in a Rat Model of Neuroinflammation. J. Neuroinflammation 2012, 9, 51. [Google Scholar] [CrossRef]
- Ruggiero, S.L. Diagnosis and Staging of Medication-Related Osteonecrosis of the Jaw. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 479–487. [Google Scholar] [CrossRef]
- Fleisher, K.E.; Pham, S.; Raad, R.A.; Friedman, K.P.; Ghesani, M.; Chan, K.C.; Amintavakoli, N.; Janal, M.; Levine, J.P.; Glickman, R.S. Does Fluorodeoxyglucose Positron Emission Tomography with Computed Tomography Facilitate Treatment of Medication-Related Osteonecrosis of the Jaw? J. Oral Maxillofac. Surg. 2016, 74, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism-A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef]
- Pichardo, S.E.C.; Merkesteyn, J.P.R. van Evaluation of a Surgical Treatment of Denosumab-Related Osteonecrosis of the Jaws. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, S.; Soutome, S.; Yanamoto, S.; Fujita, S.; Hasegawa, T.; Komori, T.; Kojima, Y.; Miyamoto, H.; Shibuya, Y.; Ueda, N.; et al. Evaluation of the Treatment Strategies for Medication-Related Osteonecrosis of the Jaws (MRONJ) and the Factors Affecting Treatment Outcome: A Multicenter Retrospective Study with Propensity Score Matching Analysis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017, 32, 2022–2029. [Google Scholar] [CrossRef]
- Simon, M.J.K.; Niehoff, P.; Kimmig, B.; Wiltfang, J.; Açil, Y. Expression Profile and Synthesis of Different Collagen Types I, II, III, and V of Human Gingival Fibroblasts, Osteoblasts, and SaOS-2 Cells after Bisphosphonate Treatment. Clin. Oral Investig. 2010, 14, 51–58. [Google Scholar] [CrossRef]
- Klümpen, H.J.; Beijnen, J.H.; Gurney, H.; Schellens, J.H.M. Inhibitors of mTOR. Oncologist 2010, 15, 1262–1269. [Google Scholar] [CrossRef]
- Kroupova, K.; Palicka, V.; Rosa, J. Monoclonal Antibodies for Treatment of Osteoporosis. Drugs Today 2023, 59, 195–204. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Malcangi, G.; Ferrara, I.; Patano, A.; Viapiano, F.; Netti, A.; Azzollini, D.; Ciocia, A.M.; de Ruvo, E.; Campanelli, M.; et al. MRONJ Treatment Strategies: A Systematic Review and Two Case Reports. Appl. Sci. 2023, 13, 4370. [Google Scholar] [CrossRef]
- Tsao, C.; Darby, I.; Ebeling, P.R.; Walsh, K.; O’Brien-Simpson, N.; Reynolds, E.; Borromeo, G. Oral Health Risk Factors for Bisphosphonate-Associated Jaw Osteonecrosis. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2013, 71, 1360–1366. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Carpentiere, V.; Piras, F.; Netti, A.; Ferrara, I.; Campanelli, M.; Latini, G.; Viapiano, F.; Costa, S.; Malcangi, G.; et al. Orthodontic Surgical Treatment of Impacted Mandibular Canines: Systematic Review and Case Report. Appl. Sci. 2022, 12, 8008. [Google Scholar] [CrossRef]
- Lončar Brzak, B.; Horvat Aleksijević, L.; Vindiš, E.; Kordić, I.; Granić, M.; Vidović Juras, D.; Andabak Rogulj, A. Osteonecrosis of the Jaw. Dent. J. 2023, 11, 23. [Google Scholar] [CrossRef]
- Francini, F.; Pascucci, A.; Francini, E.; Miano, S.T.; Bargagli, G.; Ruggiero, G.; Petrioli, R. Osteonecrosis of the Jaw in Patients with Cancer Who Received Zoledronic Acid and Bevacizumab. J. Am. Dent. Assoc. 2011, 142, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Pamidronate (Aredia) and Zoledronate (Zometa) Induced Avascular Necrosis of the Jaws: A Growing Epidemic. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Freidin, M.B.; Stalteri, M.A.; Wells, P.M.; Lachance, G.; Baleanu, A.F.; Bowyer, R.C.E.; Kurilshikov, A.; Zhernakova, A.; Steves, C.J.; Williams, F.M.K. An Association between Chronic Widespread Pain and the Gut Microbiome. Rheumatol. Oxf. Engl. 2021, 60, 3727–3737. [Google Scholar] [CrossRef]
- Abbas, E.Y.; Ezzat, M.I.; El Hefnawy, H.M.; Abdel-Sattar, E. An Overview and Update on the Chemical Composition and Potential Health Benefits of Opuntia ficus-indica (L.) Miller. J. Food Biochem. 2022, 46, e14310. [Google Scholar] [CrossRef]
- Bamigbade, G.B.; Subhash, A.J.; Kamal-Eldin, A.; Nyström, L.; Ayyash, M. An Updated Review on Prebiotics: Insights on Potentials of Food Seeds Waste as Source of Potential Prebiotics. Molecules 2022, 27, 5947. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).