Fermented Milk Supplemented with Sodium Butyrate and Inulin: Physicochemical Characterization and Probiotic Viability Under In Vitro Simulated Gastrointestinal Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Acidity
2.4. Syneresis
2.5. Color
2.6. Organoleptic Evaluation
2.7. In Vitro Digestion Protocol
2.8. Microbiological Analysis
2.9. Statistical Analysis
3. Results and Discussion
| Probiotic Strains p-Values | Inulin p-Values | Inulin and Sodium Butyrate p-Values | Probiotic Strains* Inulin p-Values | Probiotic Strains*Inulin and Sodium Butyrate p-Values | Inulin*Inulin and Sodium Butyrate p-Values | Probiotic Strains*Inulin* Inulin and Sodium Butyrate p-Values | ||
|---|---|---|---|---|---|---|---|---|
| L* | 0.0000↑ | 0.0000↑ | 0.0000↑ | 0.1895 n.s. | 0.0076↑ | 0.0546 n.s. | 0.0141↑ | |
| a* | 0.0000↑ | 0.0000↑ | 0.0147↑ | 0.0083↑ | 0.0196↑ | 0.0492↑ | 0.0012↑ | |
| b* | 0.0000↑ | 0.0029↑ | 0.4285 n.s. | 0.0000↑ | 0.0039↑ | 0.3121 n.s. | 0.1281 n.s. | |
| C* | 0.0000↑ | 0.9633 n.s. | 0.5738 n.s. | 0.0766 n.s. | 0.0176↑ | 0.0512 n.s. | 0.4362 n.s. | |
| h° | 0.0000↑ | 0.0384↑ | 0.0001↑ | 0.0000↑ | 0.0000↑ | 0.0011↑ | 0.0123↑ | |
| pH | 0.0000↑ | 0.0000↑ | 0.0002↑ | 0.0000↑ | 0.0003↑ | 0.0021↑ | 0.0041↑ | |
| TA % | 0.0000↑ | 0.2531 n.s. | 0.0000↑ | 0.1118 n.s. | 0.0000↑ | 0.0002↑ | 0.0518 n.s. | |
| Syneresis | 0.0000↑ | 0.0001↑ | 0.0304↑ | 0.0007↑ | 0.2215 n.s. | 0.0041↑ | 0.0412↑ | |
| Consistency | 0.0000↑ | 0.0524 n.s. | 0.0501 n.s. | 0.4901 n.s. | 0.2111 n.s. | 0.8529 n.s. | 0.1653 n.s. | |
| Milky-creamy taste | 0.0025↑ | 0.1421 n.s. | 0.0868 n.s. | 0.5535 n.s. | 0.2405 n.s. | 0.5700 n.s. | 0.2032 n.s. | |
| Sour taste | 0.5283 | 0.1487 n.s. | 0.0491↑ | 0.1739 n.s. | 0.02170↑ | 0.4669 n.s. | 0.8357 n.s | |
| Sweet taste | 0.0000↑ | 0.1232 n.s. | 0.0432↑ | 0.1323 n.s. | 0.0510 n.s. | 0.7233 n.s. | 0.5349 n.s. | |
| Off-taste | 0.1210 n.s. | 0.0539 n.s. | 0.0511 n.s. | 0.6929 n.s. | 0.9231 n.s. | 0.3055 n.s. | 0.5797 n.s. | |
| Sour odor | 0.0166↑ | 0.8735 n.s. | 0.0001↑ | 0.0530 n.s. | 0.0490↑ | 0.0623 n.s. | 0.1818 n.s. | |
| Off-odor | 0.3047 n.s. | 0.7681 n.s. | 0.8153 n.s. | 0.2370 n.s. | 0.8460 n.s. | 0.3317 n.s. | 0.7190 n.s. | |
| Viable counts of bacteria in milk fermented by probiotic bacteria | Before digestion | 0.0000↑ | 0.0182↑ | 0.0000↑ | 0.0058↑ | 0.0000↑ | 0.0211↑ | 0.0011↑ |
| Oral cavity | 0.0000↑ | 0.0000↑ | 0.2060↑ | 0.0000↑ | 0.0070↑ | 0.0034↑ | 0.0003↑ | |
| Stomach | 0.0000↑ | 0.0485↑ | 0.0179↑ | 0.0103↑ | 0.0003↑ | 0.0211↑ | 0.0411↑ | |
| Small intestine | 0.0000↑ | 0.0002↑ | 0.0408 ↑. | 0.0064↑ | 0.0063 ↑. | 0.0044 ↑ | 0. 0101 ↑ | |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- en Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Duncan, S.H.; Barcenilla, A.; Stewart, C.S.; Pryde, S.E.; Flint, H.J. Acetate utilization and butyrate production by human colonic bacteria. Appl. Environ. Microbiol. 2002, 68, 518–524. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Luu, M.; Weigand, K.; Wed, F.; Breidenbend, C.; Leister, H.; Pautz, S.; Adhikary, T.; Visekruna, A. Regulation of the effector function of CD8⁺ T cells by gut microbiota-derived metabolite butyrate. Sci. Rep. 2018, 8, 14430. [Google Scholar] [CrossRef]
- Ma, L.; Yang, Y.; Liu, W.; Bu, D. Sodium butyrate supplementation impacts the gastrointestinal bacteria of dairy calves before weaning. Appl. Microbiol. Biotechnol. 2023, 107, 3291–3304. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Q.; Meng, M.; Chang, G.; Ma, N.; Shen, X. Butyrate Protects against γ-d-Glutamyl-meso-diaminopimelic Acid-Induced Inflammatory Response and Tight Junction Disruption through Histone Deacetylase 3 Inhibition in Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 2023, 71, 14638–14648. [Google Scholar] [CrossRef]
- Koruri, S.S.; Banerjee, D.; Chowdhury, R.; Bhattacharya, P. Studies on prebiotic food additive (inulin) in indian dietary fibre sources-garlic (Allium sativum), wheat (Triticum spp.), oat (Avena sativa) and dalia (Bulgur). Int. J. Pharm. Pharm. Sci. 2014, 6, 278–282. [Google Scholar]
- Paseephol, T.; Small, D.M.; Sherkat, F. Rheology and texture of set yogurt as affected by inulin addition. J. Texture Stud. 2008, 39, 617–634. [Google Scholar] [CrossRef]
- Pereira, E.; Barros, L.; Ferreira, I. Relevance of the mention of antioxidant properties in yogurt labels: In vitro evaluation and chromatographic analysis. Antioxidants 2013, 2, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Canbulat, Z.; Ozcan, T. Effects of short-chain and long-chain inulin on the quality of probiotic yogurt containing L actobacillus rhamnosus. J. Food Process. Preserv. 2015, 39, 1251–1260. [Google Scholar] [CrossRef]
- Mazloumi, S.M.; Shekarforoush, S.S.; Ebrahimnejad, H.; Sajedianfard, J. Effect of adding inulin on microbial and physico-chemical properties of low fat probiotic yogurt. Iran. J. Vet. Res. 2011, 12, 93–98. [Google Scholar]
- Zhong, H.; Yu, W.; Wang, M.; Lin, B.; Sun, X.; Zheng, N.; Wang, J.; Zhao, S. Sodium butyrate promotes gastrointestinal development of preweaning bull calves via inhibiting inflammation, balancing nutrient metabolism, and optimizing microbial community functions. Anim. Nutr. 2023, 14, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Chia, L.W.; Padam, B.S.; Chye, F.Y. Effect of Selected Oligosaccharides on the Viability and Fermentation Kinetics of Lactobacillus acidophilus and Lactobacillus casei in Cultured Milk. J. Pharm. Nutr. Sci. 2014, 4, 92–99. [Google Scholar] [CrossRef]
- Ziarno, M.; Zaręba, D.; Kowalska, E.; Florowski, T. A Study into the Effects of Chosen Lactic Acid Bacteria Cultures on the Quality Characteristics of Fermented Dairy, Dairy–Oat, and Oat Beverages. Appl. Sci. 2025, 15, 3714. [Google Scholar] [CrossRef]
- Jung, S.H.; Hong, D.K.; Bang, S.-J.; Heo, K.; Sim, J.-J.; Lee, J.-L. The Functional Properties of Lactobacillus casei HY2782 Are Affected by the Fermentation Time. Appl. Sci. 2021, 11, 2481. [Google Scholar] [CrossRef]
- Domingues, A.F.; Oliveira, L.G.S.D.; Sanches, A.; Maronesi, F.; Benis, C.M.; Spinosa, W.A.; Costa, G.N. Leite probiótico com adoçante: Desenvolvimento, caracterização e resistência gastrointestinal in vitro de Bifidobacterium lactis HN019. Res. Soc. Dev. 2022, 11, e34811528130. [Google Scholar] [CrossRef]
- Henrique-Bana, F.C.; Macedo, F., Jr.; Oliveira, L.G.S.; Takihara, A.M.; Miglioranza, L.H.S.; Spinosa, W.A.; Costa, G.N. Bifidobacterium lactis HN019: Survival, acid production and impact on sensory acceptance of fermented milk. Int. Food Res. J. 2019, 26, 695–703. [Google Scholar] [CrossRef]
- Dabaj, F.K.; Lasekan, O.; Manap, M.Y.A.; Ling, F.H. Evaluation of the volatilomic potentials of the Lactobacillus casei 431 and Lactobacillus acidophilus La-5 in fermented milk. CyTA J. Food 2020, 18, 291–300. [Google Scholar] [CrossRef]
- Speranza, B.; Bevilacqua, A.; Campaniello, D.; Sinigaglia, M.; Musaico, D.; Corbo, M.R.; Lamacchia, C. The Impact of Gluten Friendly Flour on the Functionality of an Active Drink: Viability of Lactobacillus acidophilus in a Fermented Milk. Front. Microbiol. 2018, 9, 2042. [Google Scholar] [CrossRef] [PubMed]
- Paraschiv, D.; Aida, V.; Constantin, M.; Ciobanu, A.; Bahrim, G.E. Study of physiological properties of some probiotics in multiple cultures with mesophilic lactic acid bacteria by Flora Danica Ch. Hansen Commer. Start. 2011, 35, 56–65. [Google Scholar]
- Lu, Y.; Tan, C.W.; Chen, D.; Liu, S.Q. Potential of three probiotic lactobacilli in transforming star fruit juice into functional beverages. Food Sci. Nutr. 2018, 6, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Vernazza, C.L.; Gibson, G.R.; Rastall, R.A. Carbohydrate preference, acid tolerance and bile tolerance in five strains of Bifidobacterium. J. Appl. Microbiol. 2006, 100, 846–853. [Google Scholar] [CrossRef]
- Jacobsen, C.N.; Nielsen, V.R.; Hayford, A.E.; Møller, P.L.; Michaelsen, K.F.; Pærregaard, A.; Sandström, B.; Tvede, M.; Jakobsen, M. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 1999, 65, 4949–4956. [Google Scholar] [CrossRef]
- Salgaço, M.K.; de Oliveira, F.L.; Sartoratto, A.; Mesa, V.; Mayer, M.P.A.; Sivieri, K. Impact of Lactobacillus acidophilus—La5 on Composition and Metabolism of the Intestinal Microbiota of Type 2 Diabetics (T2D) and Healthy Individuals Using a Microbiome Model. Fermentation 2023, 9, 740. [Google Scholar] [CrossRef]
- Nejati, R.; Gheisari, H.R.; Hosseinzadeh, S.; Behbod, M. Viability of encapsulated Lactobacillus acidophilus (LA-5) in UF cheese and its survival under in vitro simulated gastrointestinal conditions. Int. J. Dairy Technol. 2016, 70, 77–83. [Google Scholar] [CrossRef]
- Fávaro-Trindade, C.S.; Grosso, C.R.F. Microencapsulation of L. acidophilus (La-05) and B. lactis (Bb-12) and evaluation of their survival at the pH values of the stomach and in bile. J. Microencapsul. 2002, 19, 485–494. [Google Scholar] [CrossRef]
- Paturi, G. Probiotic characteristics of Lactobacillus acidophilus and Lactobacillus paracasei and their effects on immune response and gene expression in mice. Ph.D. Thesis, Western Sydney University, Sydney, NSW, Australia, 2007. Available online: http://handle.uws.edu.au:8081/1959.7/17783 (accessed on 9 August 2023).
- Wang, K.; Wang, Y.; Gu, L.; Yu, J.; Liu, Q.; Zhang, R.; Liang, G.; Chen, H.; Gu, F.; Liu, H.; et al. Characterization of Probiotic Properties and Whole-Genome Analysis of Lactobacillus johnsonii N5 and N7 Isolated from Swine. Microorganisms 2024, 12, 672. [Google Scholar] [CrossRef]
- Roy, C.C.; Kien, C.L.; Bouthillier, L.; Levy, E. Short-chain fatty acids: Ready for prime time? Nutr. Clin. Pract. 2006, 21, 351–366. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 11, 277. [Google Scholar] [CrossRef]
- Campos-Perez, W.; Martinez-Lopez, E. Effects of short chain fatty acids on metabolic and inflammatory processes in human health. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2021, 1866, 158900. [Google Scholar] [CrossRef] [PubMed]
- ADPI. Analytical Method #007 Titratable Acidity v2.0 Effective 9 August 2023. pp. 1–6. Available online: https://adpi.org/wp-content/uploads/2024/12/007-Titratable-Acidity-v2.0-Effective-09082023.pdf (accessed on 9 August 2023).
- Ziarno, M.; Zaręba, D. Tekstura Napojów Fermentowanych: Budowanie Tekstury Napojów [Texture of Fermented Beverages: Creating the Texture of Beverages]. Forum Mlecz. 2016, 3, 25. [Google Scholar]
- Szajnar, K.; Pawlos, M.; Znamirowska, A. The Effect of the Addition of Chokeberry Fiber on the Quality of Sheep’s Milk Fermented by Lactobacillus rhamnosus and Lactobacillus acidophilus. Int. J. Food Sci. 2021, 2021, 7928745. [Google Scholar] [CrossRef]
- Baryłko-Pikielna, N.; Matuszewska, I. Sensoryczne Badania Żywności. Podstawy—Metody—Zastosowania [Sensory Food Testing. Fundamentals-Methods-Applications]. Wyd. Nauk. PTTŻ Krakow 2014, 66, 150–157. [Google Scholar]
- PN-ISO 22935-2:2013-07; Milk and Milk Products—Sensory Analysis—Part 2: Recommended Methods for Sensory Evaluation. Polish Committee for Standardization: Warsaw, Poland, 2013.
- Znamirowska, A.; Szajnar, K.; Pawlos, M. Effect of Vitamin C Source on Its Stability during Storage and the Properties of Milk Fermented by Lactobacillus rhamnosus. Molecules 2021, 26, 6187. [Google Scholar] [CrossRef]
- Buniowska, M.; Carbonell-Capella, J.M.; Frigola, A.; Esteve, M.J. Bioaccessibility of bioactive compounds after non-thermal processing of an exotic fruit juice blend sweetened with Stevia rebaudiana. Food Chem. 2017, 221, 1834–1842. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Znamirowska-Piotrowska, A.; Buniowska-Olejnik, M.; Pawlos, M. Sheep Milk Symbiotic Ice Cream: Effect of Inulin and Apple Fiber on the Survival of Five Probiotic Bacterial Strains during Simulated In Vitro Digestion Conditions. Nutrients 2022, 14, 4454. [Google Scholar] [CrossRef]
- Khani, N.; Noorkhajavi, G.; Soleiman, R.A.; Raziabad, R.H.; Rad, A.H.; Akhlaghi, A.P. Aflatoxin Biodetoxification Strategies Based on Postbiotics. Probiotics Antimicrob. Proteins 2024, 16, 1673–1686. [Google Scholar] [CrossRef]
- Dunand, E.; Burns, P.; Binetti, A.; Bergamini, C.; Peralta, G.; Forzani, L.; Vinderola, G. Postbiotics produced at laboratory and industrial level as potential functional food ingredients with the capacity to protect mice against Salmonella infection. J. Appl. Microbiol. 2019, 127, 219–229. [Google Scholar] [CrossRef]
- Champagne, C.P.; da Cruz, A.G.; Daga, M. Strategies to improve the functionality of probiotics in supplements and foods. Curr. Opin. Food Sci. 2018, 22, 160–166. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Immune boosting functional foods and their mechanisms: A critical evaluation of probiotics and prebiotics. Biomed. Pharmacother. 2020, 130, 110625. [Google Scholar] [CrossRef] [PubMed]
- González-Tomás, L.; Bayarri, S.; Costell, E. Inulin-enriched dairy desserts: Physicochemical and sensory aspects. J. Dairy Sci. 2009, 92, 4188–4199. [Google Scholar] [CrossRef]
- Brodziak, A.; Król, J.; Barłowska, J.; Teter, A.; Florek, M. Changes in the Physicochemical Parameters of Yoghurts with Added Whey Protein in Relation to the Starter Bacteria Strains and Storage Time. Animals 2020, 10, 1350. [Google Scholar] [CrossRef]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef]
- Cais-Sokolińska, D.; Pikul, J. Evaluation of yoghurt quality changes using colour measurements [Ocena jakości jogurtu na podstawie instrumentalnego pomiaru barwy]. Apar. Badaw. I Dydakt. 2008, 13, 8–12. (In Polish) [Google Scholar]
- Cruz, A.G.; Cavalcanti, R.N.; Guerreiro, L.M.R.; Sant’Ana, A.; Nogueira, L.; Oliveira, C.; Deliza, R.; Cunha, R.; Faria, J.; Bolini, H. Developing a prebiotic yogurt: Rheological, physico-chemical and microbiological aspects and adequacy of survival analysis methodology. J. Food Eng. 2013, 114, 323–330. [Google Scholar] [CrossRef]
- Helal, A.; Rashid, N.N.; Dyab, N.E.; Otaibi, M.A.; Alnemr, T.M. Enhanced Functional, Sensory, Microbial and Texture Properties of Low-Fat Set Yogurt Supplemented With High-Density Inulin. J. Food Process. Beverages 2018, 6, 11. [Google Scholar]
- Gänzle, M.G.; Follador, R. Metabolism of oligosaccharides and starch in lactobacilli: A review. Front. Microbiol. 2012, 3, 340. [Google Scholar] [CrossRef]
- Hardi, J.; Slacanac, V. Examination of coagulation kinetics and rheological properties of fermented milk products: The influence of starter culture, milk fat content and addition of inulin. Mijekarstvo 2000, 50, 217–226. [Google Scholar]
- Kaplan, H.; Hutkins, R.W. Metabolism of fructooligosaccharides by Lactobacillus paracasei 1195. Appl. Environ. Microbiol. 2003, 69, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Guven, M.; Yasar, K.; Karaca, O.B.; Hayaloglu, A.A. The effect of inulin as a fat replacer on the quality of set-type low-fat yogurt manufacture. Int. J. Dairy Technol. 2005, 58, 180–184. [Google Scholar] [CrossRef]
- Wszołek, M. Influence of inulin addition on the quality parameters of bioyoghurts. Food Sci. Technol. Qual. 1999, 4, 176–183. (In Polish) [Google Scholar]
- Nastaj, M.; Gustaw, W. Effect of some selected prebiotics on rheological properties of set yoghurt. Food Sci. Technol. Qual. 2008, 60, 217–225. (In Polish) [Google Scholar]
- Zhang, J.; Bu, L.; Liu, Y.; Huo, W.; Xia, C.; Pei, C.; Liu, Q. Dietary supplementation of sodium butyrate enhances lactation performance by promoting nutrient digestion and mammary gland development in dairy cows. Anim. Nutr. 2023, 15, 137–148. [Google Scholar] [CrossRef]
- Chang, Y.H.; Jeong, C.H.; Cheng, W.N.; Choi, Y.; Shin, D.M.; Lee, S.; Han, S.G. Quality characteristics of yogurts fermented with short-chain fatty acid-producing probiotics and their effects on mucin production and probiotic adhesion onto human colon epithelial cells. J. Dairy Sci. 2021, 104, 7415–7425. [Google Scholar] [CrossRef]
- Ocak, E.; Köse, Ş. The Effects of Fortifying Milk with Cu, Fe and Zn Minerals on the Production and Texture of Yoghurt. J. Food Agric. Environ. 2010, 8, 122–125. [Google Scholar]
- Dmytrów, I. Wpływ probiotycznych bakterii kwasu mlekowego na stabilność przechowalniczą kwasowych serów twarogowych [Effect of lactic acid probiotic bacteria on storage stability of acid curd cheeses (tvarog)]. Żywność Nauka Technol. Jakość 2015, 5, 49–60. (In Polish) [Google Scholar] [CrossRef]
- Ipsen, R.; Otte, J.; Lozahic, G.; Qvist, K.B. Microstructure and viscosity of yoghurt with inulin added as a fat-replacer. Annu. Trans. Nord. Rheol. Soc. 2001, 9, 59–62. [Google Scholar]
- Kip, P.; Meyer, D.; Jellema, R.H. Inulins improve sensory and textural properties of low-fat yogurts. Int. Dairy J. 2006, 16, 1098–1103. [Google Scholar] [CrossRef]
- Aryana, K.J.; Mcgrew, P. Quality attributes of yogurt with Lactobacillus casei and various prebiotics. LWT-Food Sci. Technol. 2007, 40, 1808–1814. [Google Scholar] [CrossRef]
- Bisar, H.G.; El-Saadany, K.; Khattab AEl-Kholy, M.W. Implementing maltodextrin, polydextrose and inulin in making a synbiotic fermented dairy product. Br. Microbiol. Res. J. 2015, 8, 585–603. [Google Scholar] [CrossRef]
- Unal, B.; Metin, S.; Isikli, N.D. Use of response surface methodology to describe the combined effect of storage time, locust bean gum and dry matter of milk on the physical properties of low-fat set yoghurt. Int. Dairy J. 2003, 13, 909–916. [Google Scholar] [CrossRef]
- Srisuvor, N.; Chinprahast, N.; Prakitchaiwattana, C.; Subhimaros, S. Effects of inulin and polydextrose on physicochemical and sensory properties of low-fat set yoghurt with probiotic-cultured banana puree. LWT-Food Sci. Technol. 2013, 51, 30–36. [Google Scholar] [CrossRef]
- Qing, J.; Peng, C.; Chen, H.; Li, H.; Liu, X. Small molecule linoleic acid inhibiting whey syneresis via interact with milk proteins in the fermentation of set yogurt fortified with c9,t11-conjugated linoleic acid. Food Chem. 2023, 429, 136849. [Google Scholar] [CrossRef]
- Tungland BCMeyer, D. Non-digestible oligosaccharides (dietary fibre): Their physiology and role in human health and food. Compr. Rev. Food Sci. Food Saf. 2002, 3, 73–92. [Google Scholar] [CrossRef]
- Crispin-Isidro, G.; Lobato-Calleros, C.; Espinosa-Andrews, H.; AlvarezRamirez JVernon-Carter, J.E. Effect of inulin and agave fructans addition on the rheological, microstructural and sensory properties of reduced-fat stirred yogurt. LTW-Food Sci. Technol. 2015, 62, 438–444. [Google Scholar] [CrossRef]
- Villegas, B.; Carbonell, I.; Costell, E. Inulin Milk Beverages: Sensory differences in thickenss and creaminess using R-index analysis of the ranking data. J. Sens. Stud. 2007, 22, 377–393. [Google Scholar] [CrossRef]
- Tárrega, A.; Costell, E. Effect of inulin addition on rheological and sensory properties of fat-free starch-based dairy desserts. Int. Dairy J. 2006, 16, 1104–1112. [Google Scholar] [CrossRef]
- Cardarelli, H.R.; Buriti, F.C.A.; Castro, I.A.; Saad, S.M.I. Inulin and oligofructose improve sensory quality and increase the probiotic viable count in potentially synbiotic petit-suisse cheese. LWT-Food Sci. Technol. 2008, 41, 1037–1046. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Rastall, R.A.; Gibson, G.R. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr. Opin. Biotechnol. 2015, 32, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Bach Knudsen, K.E.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Fleming, D.; Chahin, L.; Rumbaugh, K. Glycoside Hydrolases Degrade Polymicrobial Bacterial Biofilms in Wounds. Antimicrob. Agents Chemother. 2017, 61, e01998-16. [Google Scholar] [CrossRef]
- Sheehan, V.M.; Sleator, R.D.; Hill, C.; Fitzgerald, G.F. Improving gastric transit, gastrointestinal persistence and therapeutic efficacy of the probiotic Bifidobacterium breve UCC2003. Microbiology 2007, 153, 3563–3571. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Abdella, S.; Youssef, S.H.; Song, Y.; Garg, S. Inulin and Its Application in Drug Delivery. Pharmaceuticals 2021, 14, 855. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 27, 837–890. [Google Scholar] [CrossRef]
- Santos, D.X.; Casazza, A.A.; Aliakbarian, B.; Bedani, R.; Saad, S.M.I.; Perego, P. Improved probiotic survival to in vitro gastrointestinal stress in a mousse containing Lactobacillus acidophilus La-5 microencapsulated with inulin by spray drying. LWT 2019, 99, 404–410. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, S.; Tang, C.; Li, D.; Kan, Y.; Yao, L. New insights into microbial bile salt hydrolases: From physiological roles to potential applications. Front. Microbiol. 2025, 16, 151354. [Google Scholar] [CrossRef] [PubMed]
- Ziarno, M.; Zaręba, D. Effects of milk components and food additives on survival of three bifidobacteria strains in fermented milk under simulated gastrointestinal tract conditions. Microb. Ecol. Health Dis. 2015, 26, 27812. [Google Scholar] [CrossRef]
- Talearngkul, R.; Sae-tan, S.; Sirivarasai, J. Effect of Yogurt Ice Cream on the Viability and Antidiabetic Potential of the Probiotics Lactobacillus acidophilus, Lacticaseibacillus rhamnosus, and Bifidobacterium animalis subsp. lactis after in vitro Digestion. Foods 2023, 12, 4373. [Google Scholar] [CrossRef]
- Rzepkowska, A.; Zielińska, D.; Kołożyn-Krajewska, D. Przeżywalność szczepów Lactobacillus wyizolowanych z żywności w warunkach modelowego przewodu pokarmowego (Survival of Lactobacillus strains isolated from food under conditions of simulated gastrointestinal tract model). Żywność Nauka Technol. Jakość 2015, 3, 42–52. (In Polish) [Google Scholar] [CrossRef]
- Kolida, S.; Gibson, G.R. Prebiotic capacity of inulin-type fructans. J. Nutr. 2007, 137, 2503–2506. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Szopa, K.; Szajnar, K.; Pawlos, M.; Znamirowska-Piotrowska, A. Probiotic Fermented Goat’s and Sheep’s Milk: Effect of Type and Dose of Collagen on Survival of Four Strains of Probiotic Bacteria during Simulated In Vitro Digestion Conditions. Nutrients 2023, 15, 3241. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Hayes, J.J.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Protection of Candidate Probiotic Lactobacilli by Cheddar Cheese Matrix during Simulated Gastrointestinal Digestion. J. Funct. Foods 2022, 92, 105042. [Google Scholar] [CrossRef]
- Prestes, A.A.; Verruck, S.; Vargas, M.O.; Canella, M.H.M.; Silva, C.C.; Barros, E.L.S.; Dantas, A.; Oliveira, L.V.A.; Maran, B.M.; Matos, M.; et al. Influence of guabiroba pulp (Campomanesia xanthocarpa o. berg) added to fermented milk on probiotic survival under in vitro simulated gastrointestinal conditions. Food Res. Int. 2021, 141, 110135. [Google Scholar] [CrossRef]
- Radicioni, M.; Koirala, R.; Fiore, W.; Leuratti, C.; Guglielmetti, S.; Arioli, S. Survival of L. casei DG® (Lactobacillus paracasei CNCMI1572) in the gastrointestinal tract of a healthy paediatric population. Eur. J. Nutr. 2019, 58, 3161–3170. [Google Scholar] [CrossRef] [PubMed]
- Zaręba, D.; Ziarno, M.; Strzelczyk, B. Przeżywalność bakterii fermentacji mlekowej w warunkach modelowych jelita cienkiego (Viability of lactic acid bacteria under the model conditions of small intestine). Żywność Nauka Technol. Jakość 2008, 5, 197–205. (In Polish) [Google Scholar]
- Ziarno, M. Survival of lactis acid bacteria in simulated duodenal fluid depending on cholesterol presence. Pol. J. Food Nutr. Sci. 2007, 57, 625–631. [Google Scholar]
- Matsumoto, M.; Ohishi, H.; Benno, Y. H+-ATPase activity in Bifidobacterium. Int. J. Food Microbiol. 2004, 93, 109–113. [Google Scholar] [CrossRef]
- Takahashi, N.; Xiao, J.Z.; Miyaji, K.; Yaeshiima, T.; Hiramatsu, A.; Iwatsuki, K. Selection of acid tolerant bifidobacteria and evidence for a low-pH-inducible acid tolerance response in Bifidobacterium longum. J. Dairy Res. 2004, 71, 340–345. [Google Scholar] [CrossRef]
- Liong, M.T.; Shah, N.P. Bile salt deconjugation and BSH activity of five bifidobacterial strains and their cholesterol co-precipitating properties. Food Res. Int. 2005, 38, 135–142. [Google Scholar] [CrossRef]
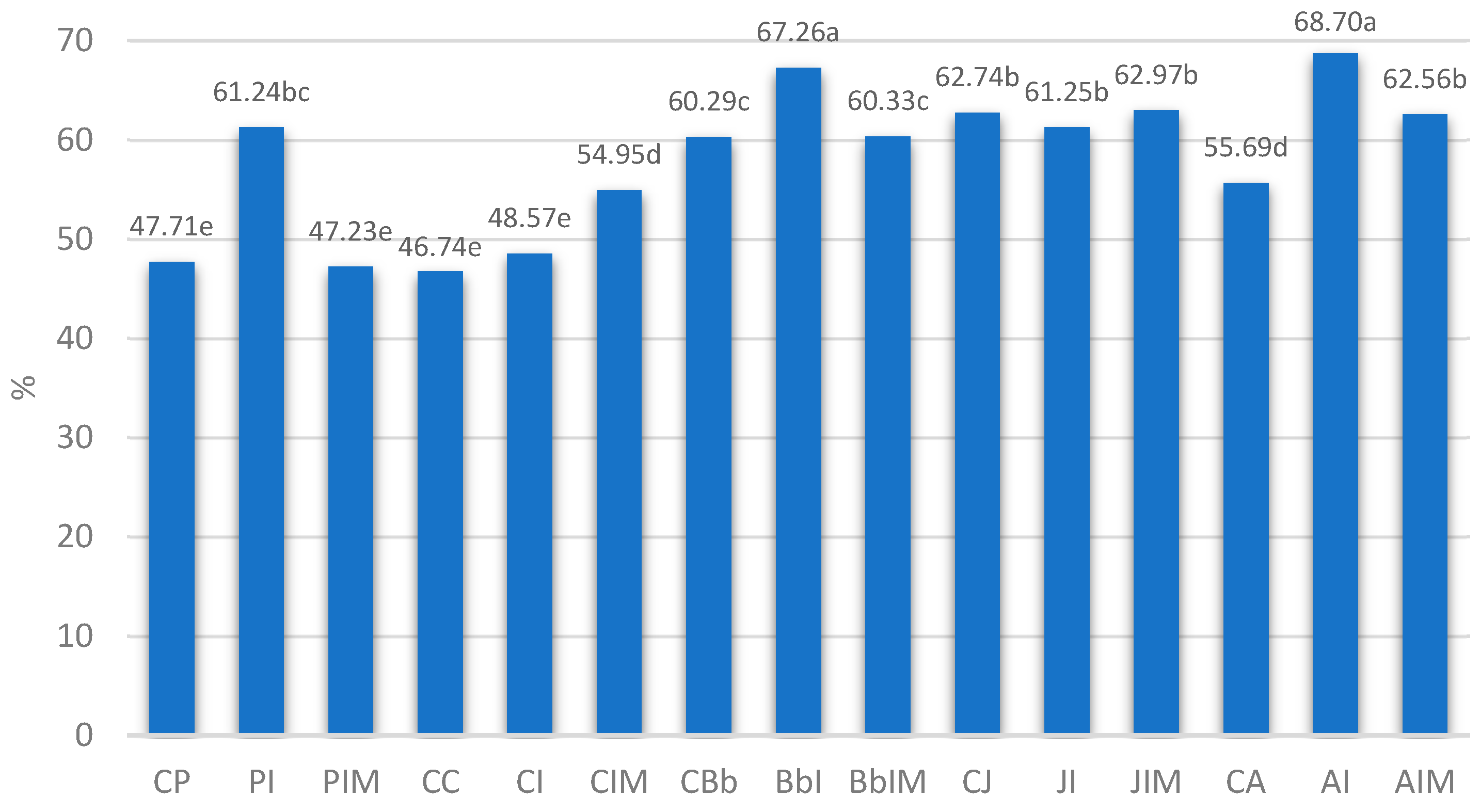
| Bacterial Strain | Control Group | Group with Inulin 4% | Group with Inulin 4% and Sodium Butyrate 0.06% |
|---|---|---|---|
| Lacticaseibacillus paracasei L26 | CP | PI | PIM |
| Lacticaseibacillus casei 431 | CC | CI | CIM |
| Bifidobacterium animalis ssp. Lactis BB-12 | CBb | BbI | BbIM |
| Lactobacillus johnsonii LJ | CJ | JI | JIM |
| Lactobacillus acidophilus LA-5 | CA | AI | AIM |
| Experimental Batch | L* | a* | b* | C* | h° |
|---|---|---|---|---|---|
| CP | 92.57 d ± 0.67 | −1.43 c ± 0.84 | 7.97 de ± 0.3 | 8.12 cd ± 0.34 | 101.71 cd ± 0.45 |
| PI | 90.72 e ± 1.11 | −1.55 c ± 0.09 | 8.46 d ± 0.38 | 8.66 c ± 0.51 | 100.40 d ± 0.71 |
| PIM | 91.19 e ± 1.24 | −1.71 c ± 0.07 | 8.29 d ± 0.37 | 8.45 c ± 0.37 | 101.67 d ± 0.41 |
| CC | 92.49 d ± 0.89 | −1.55 c ± 0.08 | 7.86 e ± 0.48 | 8.00 cd ± 0.45 | 101.20 cd ± 0.9 |
| CI | 91.97 de ± 0.67 | −1.54 c ± 0.14 | 7.74 e ± 0.49 | 7.88 d ± 0.49 | 101.03 d ± 0.76 |
| CIM | 91.45 e ± 0.97 | −1.46 c ± 0.08 | 8.10 de ± 0.34 | 8.23 c ± 0.34 | 100.21 e ± 0.72 |
| CBb | 97.14 a ± 0.24 | −2.58 b ± 0.09 | 11.28 a ± 0.33 | 11.57 a ± 0.32 | 102.85 c ± 0.62 |
| BbI | 94.40 c ± 0.07 | −3.10 a ± 0.01 | 10.86 b ± 0.04 | 11.30 a ± 0.04 | 105.91 a ± 0.05 |
| BbIM | 96.19 a ± 0.57 | −2.77 b ± 0.14 | 10.54 c ± 0.06 | 10.90 b ± 0.06 | 104.74 b ± 0.74 |
| CJ | 97.90 a ± 0.34 | −2.27 b ± 0.16 | 10.70 c ± 0.12 | 10.94 b ± 0.12 | 101.97 cd ± 0.9 |
| JI | 95.61 b ± 0.63 | −2.95 a ± 0.13 | 10.25 bc ± 0.24 | 10.66 b ± 0.27 | 106.05 a ± 0.37 |
| JIM | 96.63 a ± 0.42 | −2.84 ab ± 0.15 | 10.62 c ± 0.08 | 10.99 b ± 0.1 | 104.99 b ± 0.71 |
| CA | 98.45 a ± 1.07 | −1.89 c ± 0.48 | 11.36 a ± 0.28 | 11.52 a ± 0.21 | 99.47 e ± 1.55 |
| AI | 97.16 a ± 1.48 | −2.23 b ± 0.77 | 10.59 bc ± 0.21 | 10.87 b ± 0.13 | 102.63 cd ± 1.22 |
| AIM | 95.87 ab ± 0.64 | −2.72 b ± 0.41 | 10.58 c ± 0.08 | 10.95 b ± 0.06 | 105.35 ab ± 0.62 |
| Experimental Batch | pH | TA% | Syneresis, % |
|---|---|---|---|
| CP | 4.26 gh ± 0.02 | 0.93 ab ± 0.02 | 32.69 c ± 1.23 |
| PI | 4.23 h ± 0.01 | 0.88 c ± 0.02 | 26.34 d ± 1.38 |
| PIM | 4.25 g ± 0.01 | 0.90 b ± 0.02 | 23.96 e ± 1.06 |
| CC | 4.29 f ± 0.01 | 0.92 ab ± 0.03 | 28.53 d ± 1.82 |
| CI | 4.24 gh ± 0.01 | 0.89 c ± 0.01 | 25.72 e ± 1.22 |
| CIM | 4.28 f ± 0.01 | 0.93 b ± 0.01 | 20.43 f ± 1.77 |
| CBb | 4.63 a ± 0.01 | 0.76 f ± 0.01 | 41.30 a ± 1.54 |
| BbI | 4.51 b ± 0.03 | 0.79 e ± 0.01 | 40.7 a ± 1.73 |
| BbIM | 4.50 b ± 0.03 | 0.78 e ± 0.01 | 41.23 a ± 1.91 |
| CJ | 4.35 d ± 0.03 | 0.89 c ± 0.02 | 43.50 a ± 2.18 |
| JI | 4.33 e ± 0.01 | 0.96 a ± 0.01 | 26.07 d ± 1.41 |
| JIM | 4.31 de ± 0.03 | 0.92 b ± 0.02 | 27.97 d ± 1.3 |
| CA | 4.43 c ± 0.04 | 0.91 b ± 0.06 | 38.01 b ± 0.67 |
| AI | 4.37 d ± 0.03 | 0.94 ab ± 0.02 | 27.37 d ± 1.63 |
| AIM | 4.35 d ± 0.01 | 0.83 d ± 0.01 | 29.87 d ± 0.85 |
| Experimental Batch | Consistency | Milky-Creamy Taste | Sour Taste | Sweet Taste | Off-Taste | Sour Odor | Off-Odor |
|---|---|---|---|---|---|---|---|
| CP | 7.33 bc ± 0.78 | 6.67 a ± 0.66 | 4.25 c ± 0.76 | 5.58 a ± 0.64 | 1.42 a ± 0.40 | 3.58 b ± 0.07 | 1.00 a ± 0.00 |
| PI | 6.92 c ±0.78 | 5.33 b ± 0.27 | 5.50 b ± 0.83 | 3.92 bc ± 0.47 | 1.50 a ± 0.50 | 3.42 b ± 0.35 | 1.00 a ± 0.00 |
| PIM | 7.08 c ± 0.44 | 5.22 b ± 0.83 | 5.83 b ± 0.76 | 4.67 b ± 0.42 | 1.25 a ± 0.25 | 5.58 a ± 0.56 | 1.50 a ± 0.50 |
| CC | 7.87 b ± 0.25 | 6.53 a ± 0.50 | 4.67 c ± 0.53 | 4.40 c ± 0.35 | 1.67 a ± 0.64 | 3.27 b ± 0.87 | 1.00 a ± 0.00 |
| CI | 7.73 b ± 0.33 | 5.27 b ± 0.35 | 5.87 b ± 0.29 | 3.73 c ± 0.50 | 1.13 a ± 0.13 | 3.33 b ± 0.59 | 1.00 a ± 0.00 |
| CIM | 7.13 bc ± 0.92 | 5.27 b ± 0.34 | 6.00 a ± 0.93 | 3.47 d ± 0.55 | 1.67 a ± 0.60 | 4.67 a ± 1.09 | 1.47 a ± 0.70 |
| CBb | 6.80 c ± 0.84 | 5.20 b ± 0.43 | 4.80 c ± 0.84 | 5.20 a ± 0.89 | 1.00 a ± 0.00 | 2.60 c ± 0.14 | 1.00 a ± 0.00 |
| BbI | 6.40 d ± 0.55 | 5.40 b ± 0.67 | 5.20 b ± 0.52 | 4.80 b ± 0.59 | 1.00 a ± 0.00 | 2.80 c ± 0.55 | 1.00 a ± 0.00 |
| BbIM | 6.20 d ± 0.30 | 5.00 bc ± 0.58 | 5.40 b ± 0.61 | 4.40 c ± 0.90 | 1.40 a ± 0.40 | 3.60 b ± 0.52 | 1.00 a ± 0.00 |
| CJ | 7.75 b ± 0.500 | 4.50 c ± 0.91 | 4.75 c ± 0.31 | 6.25 a ± 0.92 | 1.25 a ± 0.25 | 2.25 c ± 0.50 | 1.00 a ± 0.00 |
| JI | 8.25 ab ± 0.96 | 4.50 c ± 2.08 | 4.25 c ± 0.80 | 5.25 a ± 0.81 | 1.25 a ± 0.25 | 2.00 c ± 0.15 | 1.00 a ± 0.00 |
| JIM | 8.75 a ± 0.50 | 5.00 bc ± 0.58 | 5.25 b ± 0.50 | 5.00 ab ± 0.71 | 1.75 a ± 0.75 | 3.50 b ± 0.58 | 1.00 a ± 0.00 |
| CA | 7.13 c ± 0.23 | 4.93 bc ± 0.83 | 4.60 c ± 0.45 | 4.40 b ± 0.23 | 1.67 a ± 0.60 | 3.27 b ± 0.87 | 1.00 a ± 0.00 |
| AI | 6.80 c ± 0.50 | 4.67 c ± 0.02 | 4.80 c ± 0.90 | 4.00 c ± 0.24 | 1.60 a ± 0.50 | 3.53 b ± 0.92 | 1.00 a ± 0.00 |
| AIM | 6.13 d ± 0.45 | 4.33 c ± 0.59 | 6.00 a ± 0.67 | 3.80 c ± 0.21 | 1.80 a ± 0.80 | 4.93 a ± 0.31 | 1.50 a ± 0.50 |
| Experimental Batches | Viable Counts of Probiotic Bacteria, log CFU g−1 | |||
|---|---|---|---|---|
| Digestion Phase | ||||
| Before Digestion | Oral Cavity | Stomach | Small Intestine | |
| CP | 10.50 eA ± 0.29 | 10.48 dA ± 0.14 | 3.73 fC ± 0.12 | 5.01 fB ± 0.05 |
| PI | 10.45 eA ± 0.23 | 10.43 dA ± 0.22 | 4.03 eC ± 0.25 | 6.40 cB ± 0.03 |
| PIM | 10.50 eA ± 0.14 | 10.52 dA ± 0.21 | 3.46 fC ± 0.32 | 5.01 fB ± 0.19 |
| CC | 11.06 cA ± 0.04 | 11.00 bA ± 0.14 | 1.43 hC ± 0.51 | 5.17 fB ± 0.15 |
| CI | 11.22 bA ± 0.06 | 11.16 bA ± 0.10 | 2.87 gC ± 0.08 | 5.45 eB ± 0.40 |
| CIM | 10.70 dA ± 0.08 | 10.70 cdA ± 0.16 | 2.80 gC ± 0.04 | 5.88 eB ± 0.70 |
| CBb | 10.78 dA ± 0.14 | 10.53 dA ± 0.12 | 6.23 dC ± 0.04 | 6.50 cB ± 0.16 |
| BbI | 10.63 dA ± 0.20 | 10.38 eA ± 0.28 | 6.55 cC ± 0.05 | 7.15 aB ± 0.22 |
| BbIM | 10.84 dA ± 0.41 | 10.68 cdA ± 0.08 | 6.40 cB ± 0.11 | 6.54 cB ± 0.25 |
| CJ | 11.97 aA ± 0.16 | 11.84 aA ± 0.08 | 6.49 cC ± 0.07 | 7.51 aB ± 0.29 |
| JI | 11.95 aA ± 0.07 | 11.04 bA ± 0.19 | 6.50 cC ± 0.01 | 7.32 aB ± 0.12 |
| JIM | 10.94 cA ± 0.16 | 10.76 cA ± 0.13 | 6.74 bB ± 0.03 | 6.89 bB ± 0.11 |
| CA | 10.88 dA ± 0.09 | 10.81 cA ± 0.18 | 4.44 eC ± 0.05 | 6.06 dB ± 0.07 |
| AI | 10.80 dA ± 0.10 | 10.33 eA ± 0.35 | 4.73 eC ± 0.06 | 7.42 aB ± 0.14 |
| AIM | 10.98 cdA ± 0.36 | 10.94 cA ± 0.08 | 6.85 aB ± 0.05 | 6.87 bB ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szajnar, K.; Pawlos, M.; Kowalczyk, M.; Drobniak, J.; Znamirowska-Piotrowska, A. Fermented Milk Supplemented with Sodium Butyrate and Inulin: Physicochemical Characterization and Probiotic Viability Under In Vitro Simulated Gastrointestinal Digestion. Nutrients 2025, 17, 2249. https://doi.org/10.3390/nu17132249
Szajnar K, Pawlos M, Kowalczyk M, Drobniak J, Znamirowska-Piotrowska A. Fermented Milk Supplemented with Sodium Butyrate and Inulin: Physicochemical Characterization and Probiotic Viability Under In Vitro Simulated Gastrointestinal Digestion. Nutrients. 2025; 17(13):2249. https://doi.org/10.3390/nu17132249
Chicago/Turabian StyleSzajnar, Katarzyna, Małgorzata Pawlos, Magdalena Kowalczyk, Julita Drobniak, and Agata Znamirowska-Piotrowska. 2025. "Fermented Milk Supplemented with Sodium Butyrate and Inulin: Physicochemical Characterization and Probiotic Viability Under In Vitro Simulated Gastrointestinal Digestion" Nutrients 17, no. 13: 2249. https://doi.org/10.3390/nu17132249
APA StyleSzajnar, K., Pawlos, M., Kowalczyk, M., Drobniak, J., & Znamirowska-Piotrowska, A. (2025). Fermented Milk Supplemented with Sodium Butyrate and Inulin: Physicochemical Characterization and Probiotic Viability Under In Vitro Simulated Gastrointestinal Digestion. Nutrients, 17(13), 2249. https://doi.org/10.3390/nu17132249







