Associations Between Dietary Amino Acid Intake and Elevated High-Sensitivity C-Reactive Protein in Children: Insights from a Cross-Sectional Machine Learning Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Operational Algorithm Model
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Participants
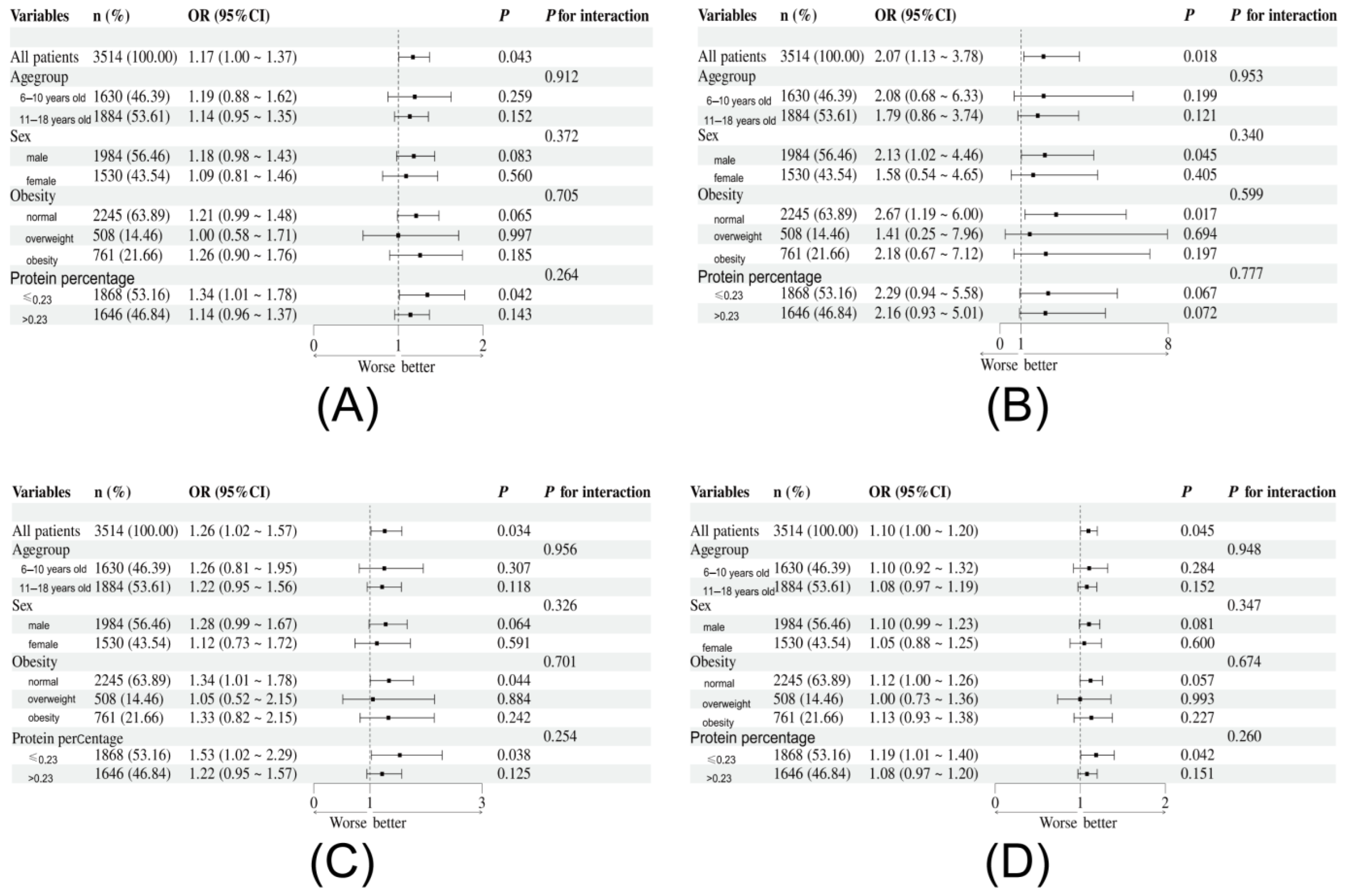
3.2. Association Between Dietary Amino Acids Intake and Elevated hs-CRP
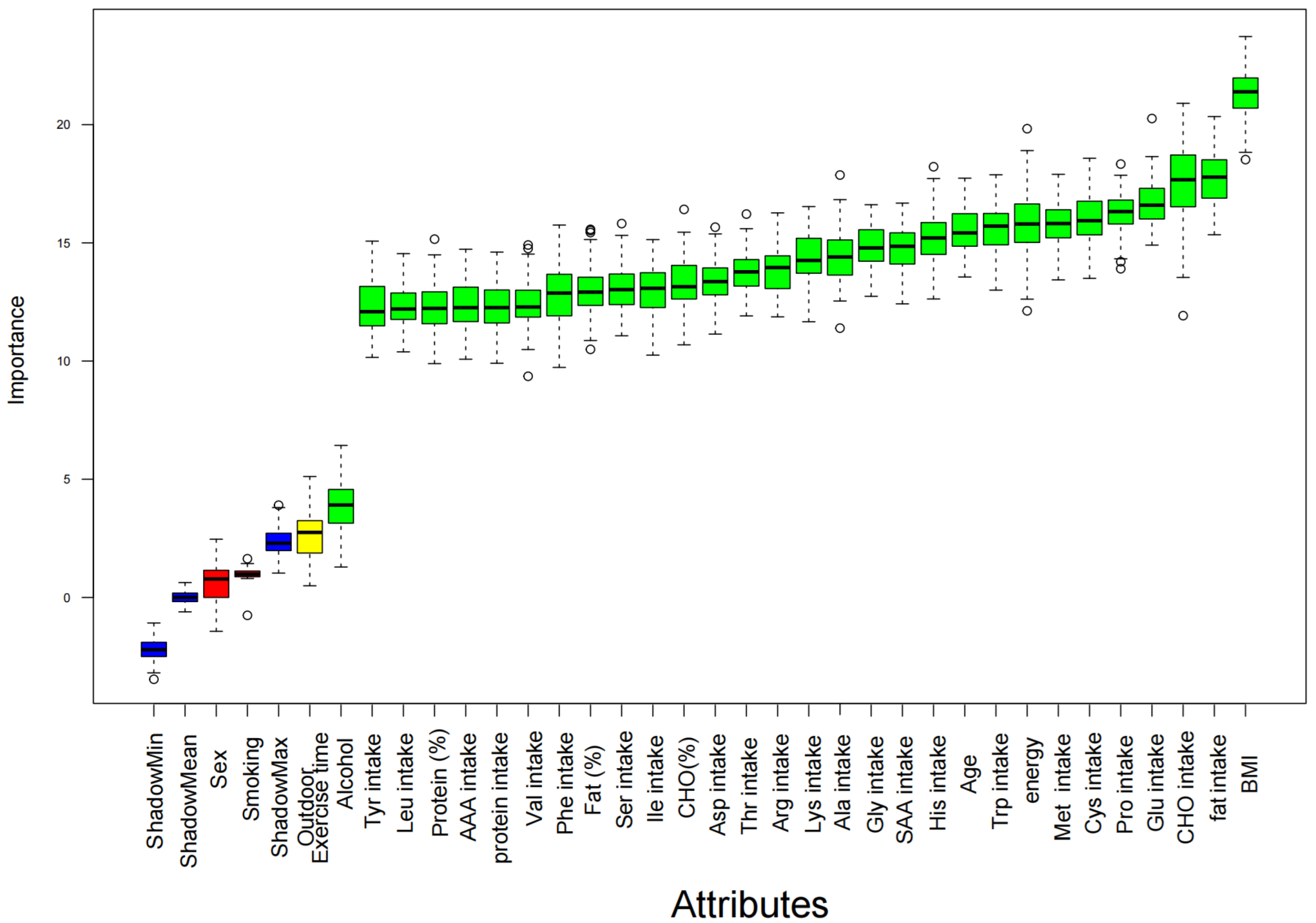
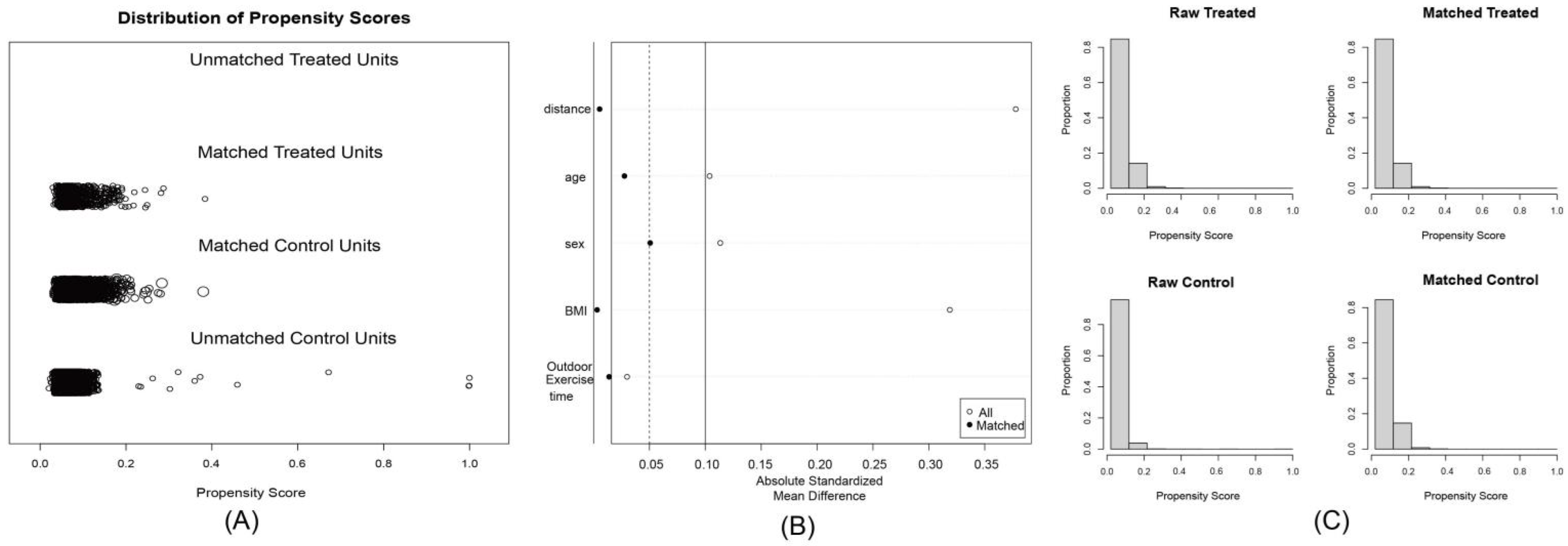
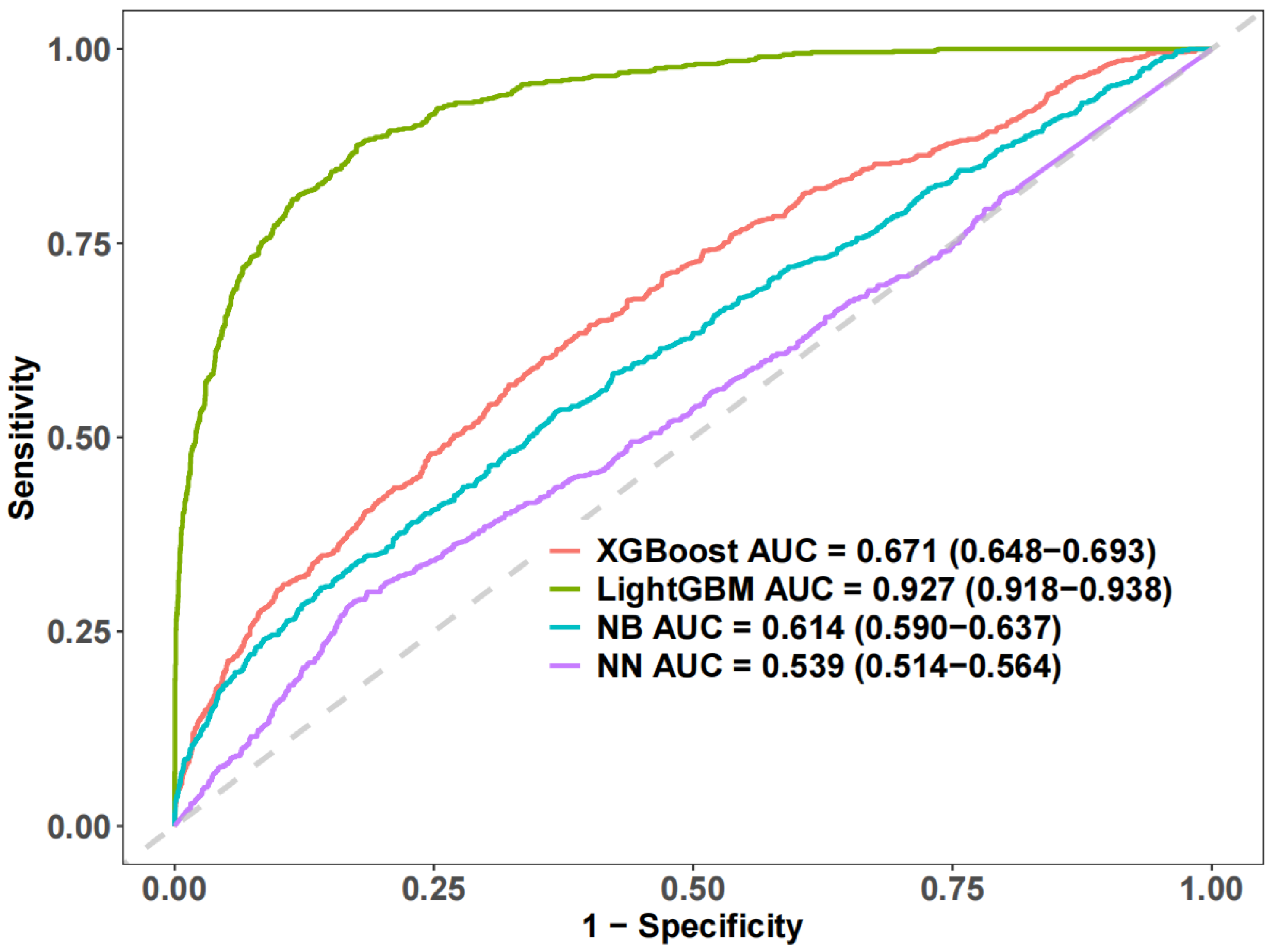
3.3. Machine Learning and Sensitivity Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamargo, J.A.; Hernandez-Boyer, J.; Teeman, C.; Martin, H.R.; Huang, Y.; Johnson, A.; Campa, A.; Martinez, S.S.; Li, T.; Rouster, S.D.; et al. Immune Activation: A Link Between Food Insecurity and Chronic Disease in People Living with Human Immunodeficiency Virus. J. Infect. Dis. 2021, 224, 2043–2052. [Google Scholar] [CrossRef]
- Albert, M.A.; Ridker, P.M. C-reactive protein as a risk predictor: Do race/ethnicity and gender make a difference? Circulation 2006, 114, e67–e74. [Google Scholar] [CrossRef] [PubMed]
- Pinter, A.; Schwarz, P.; Gerdes, S.; Simon, J.C.; Saalbach, A.; Rush, J.; Melzer, N.; Kramps, T.; Häberle, B.; Reinhardt, M. Biologic Treatment in Combination with Lifestyle Intervention in Moderate to Severe Plaque Psoriasis and Concomitant Metabolic Syndrome: Rationale and Methodology of the METABOLyx Randomized Controlled Clinical Trial. Nutrients 2021, 13, 3015. [Google Scholar] [CrossRef] [PubMed]
- Effoe, V.S.; Correa, A.; Chen, H.; Lacy, M.E.; Bertoni, A.G. High-Sensitivity C-Reactive Protein Is Associated with Incident Type 2 Diabetes Among African Americans: The Jackson Heart Study. Diabetes Care 2015, 38, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- den Engelsen, C.; Koekkoek, P.S.; Gorter, K.J.; van den Donk, M.; Salomé, P.L.; Rutten, G.E. High-sensitivity C-reactive protein to detect metabolic syndrome in a centrally obese population: A cross-sectional analysis. Cardiovasc. Diabetol. 2012, 11, 25. [Google Scholar] [CrossRef]
- Giannini, C.; de Giorgis, T.; Scarinci, A.; Ciampani, M.; Marcovecchio, M.L.; Chiarelli, F.; Mohn, A. Obese related effects of inflammatory markers and insulin resistance on increased carotid intima media thickness in pre-pubertal children. Atherosclerosis 2008, 197, 448–456. [Google Scholar] [CrossRef]
- Lucas, R.; Ramos, E.; Oliveira, A.; Monjardino, T.; Barros, H. Low-grade systemic inflammation and suboptimal bone mineral density throughout adolescence: A prospective study in girls. Clin. Endocrinol. 2012, 77, 665–671. [Google Scholar] [CrossRef]
- Wang, W.; Ren, D.; Wang, C.S.; Li, T.; Yao, H.C. High sensitivity C-reactive protein to prealbumin ratio measurement as a marker of the prognosis in acute coronary syndrome. Sci. Rep. 2019, 9, 11583. [Google Scholar] [CrossRef]
- Saijo, Y.; Utsugi, M.; Yoshioka, E.; Fukui, T.; Sata, F.; Nakagawa, N.; Hasebe, N.; Yoshida, T.; Kishi, R. Inflammation as a cardiovascular risk factor and pulse wave velocity as a marker of early-stage atherosclerosis in the Japanese population. Environ. Health Prev. 2009, 14, 159–164. [Google Scholar] [CrossRef]
- Martínez-Garza, Ú.; Torres-Oteros, D.; Yarritu-Gallego, A.; Marrero, P.F.; Haro, D.; Relat, J. Fibroblast Growth Factor 21 and the Adaptive Response to Nutritional Challenges. Int. J. Mol. Sci. 2019, 20, 4692. [Google Scholar] [CrossRef]
- Tang, Y.; Tan, B.; Xiong, X.; Li, F.; Ren, W.; Kong, X.; Qiu, W.; Hardwidge, P.R.; Yin, Y. Methionine deficiency reduces autophagy and accelerates death in intestinal epithelial cells infected with enterotoxigenic Escherichia coli. Amino Acids. 2015, 47, 2199–2204. [Google Scholar] [CrossRef]
- Zheng, A.; Zhang, A.; Chen, Z.; Pirzado, S.A.; Chang, W.; Cai, H.; Bryden, W.L.; Liu, G. Molecular mechanisms of growth depression in broiler chickens (Gallus Gallus domesticus) mediated by immune stress: A hepatic proteome study. J. Anim. Sci. Biotechnol. 2021, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Wang, Y.; Zhou, Z.; Sun, X.; Zhang, Y.; Zhang, F. Metabolic Regulation of Hepatitis B Virus Infection in HBV-Transgenic Mice. Metabolites 2022, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Bartali, B.; Frongillo, E.A.; Stipanuk, M.H.; Bandinelli, S.; Salvini, S.; Palli, D.; Morais, J.A.; Volpato, S.; Guralnik, J.M.; Ferrucci, L. Protein intake and muscle strength in older persons: Does inflammation matter? J. Am. Geriatr. Soc. 2012, 60, 480–484. [Google Scholar] [CrossRef]
- Yu, D.; Zhao, L.; Zhang, J.; Yang, Z.; Yang, L.; Huang, J.; Fang, H.; Guo, Q.; Xu, X.; Ju, L.; et al. China Nutrition and Health Surveys (1982–2017). China Cdc Wkly. 2021, 3, 193–195. [Google Scholar] [CrossRef]
- Yang, Y. Chinese Food Composition Table; Peking University Medical Press: Beijing, China, 2018. [Google Scholar]
- Lu, Y.; Wang, P.; Zhou, T.; Lu, J.; Spatz, E.S.; Nasir, K.; Jiang, L.; Krumholz, H.M. Comparison of Prevalence, Awareness, Treatment, and Control of Cardiovascular Risk Factors in China and the United States. J. Am. Heart Assoc. 2018, 7, e007462. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Park, K. Association between Phytochemical Index and Inflammation in Korean Adults. Antioxidants 2022, 11, 348. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China. Screening for Overweight and Obesity among School-Age Children and Adolescents. 2018. Available online: http://www.nhc.gov.cn/ewebeditor/uploadfile/2018/03/20180330094031236.pdf (accessed on 13 February 2024).
- John, E.R.; Abrams, K.R.; Brightling, C.E.; Sheehan, N.A. Assessing causal treatment effect estimation when using large observational datasets. Bmc Med. Res. Methodol. 2019, 19, 207. [Google Scholar] [CrossRef]
- Nugent, S.M.; Dobscha, S.K.; Morasco, B.J.; Demidenko, M.I.; Meath, T.; Frank, J.W.; Lovejoy, T.I. Substance Use Disorder Treatment Following Clinician-Initiated Discontinuation of Long-Term Opioid Therapy Resulting from an Aberrant Urine Drug Test. J. Gen. Intern. Med. 2017, 32, 1076–1082. [Google Scholar] [CrossRef]
- Maeda-Gutiérrez, V.; Galván-Tejada, C.E.; Cruz, M.; Galván-Tejada, J.I.; Gamboa-Rosales, H.; García-Hernández, A.; Luna-García, H.; Gonzalez-Curiel, I.; Martínez-Acuña, M. Risk-Profile and Feature Selection Comparison in Diabetic Retinopathy. J. Pers. Med. 2021, 11, 1327. [Google Scholar] [CrossRef]
- Onozato, Y.; Iwata, T.; Uematsu, Y.; Shimizu, D.; Yamamoto, T.; Matsui, Y.; Ogawa, K.; Kuyama, J.; Sakairi, Y.; Kawakami, E.; et al. Predicting pathological highly invasive lung cancer from preoperative [(18)F]FDG PET/CT with multiple machine learning models. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, C.; Mani, V.; Srividhya, S.R.; Khalaf, O.I.; Tavera, R.C. Early-Stage Alzheimer’s Disease Prediction Using Machine Learning Models. Front. Public Health 2022, 10, 853294. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Yang, N.B.; Fan, Y.; Dong, F.; Bo, Q.J.; Zhou, F.C.; Zhang, J.C.; Li, L.; Yin, G.Z.; Wang, C.Y.; et al. Detection of Schizophrenia Cases from Healthy Controls with Combination of Neurocognitive and Electrophysiological Features. Front. Psychiatry. 2022, 13, 810362. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, M.; Wang, S.; Chen, W. EFMSDTI: Drug-target interaction prediction based on an efficient fusion of multi-source data. Front. Pharmacol. 2022, 13, 1009996. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, C.; Mani, A.B.; Hong, L.E.; Kochunov, P.; Chen, S. Mediation Analysis for High-Dimensional Mediators and Outcomes with an Application to Multimodal Imaging Data. Comput. Stat. Data Anal. 2023, 185, 107765. [Google Scholar] [CrossRef]
- Shehzadi, S.; Hassan, M.A.; Rizwan, M.; Kryvinska, N.; Vincent, K. Diagnosis of Chronic Ischemic Heart Disease Using Machine Learning Techniques. Comput. Intell. Neurosci. 2022, 2022, 3823350. [Google Scholar] [CrossRef]
- Pang, X.; Fu, W.; Wang, J.; Kang, D.; Xu, L.; Zhao, Y.; Liu, A.L.; Du, G.H. Identification of Estrogen Receptor α Antagonists from Natural Products via In Vitro and In Silico Approaches. Oxidative Med. Cell. Longev. 2018, 2018, 6040149. [Google Scholar] [CrossRef]
- Zeng, P.; Li, X.; Wu, X.; Diao, Y.; Liu, Y.; Liu, P. Rapid Identification of Wild Gentiana Genus in Different Geographical Locations Based on FT-IR and an Improved Neural Network Structure Double-Net. Molecules 2022, 27, 5979. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K. The Effectiveness of Predicting Suicidal Ideation through Depressive Symptoms and Social Isolation Using Machine Learning Techniques. J. Pers. Med. 2022, 12, 516. [Google Scholar] [CrossRef]
- Luo, W.M.; Zhang, Z.P.; Zhang, W.; Su, J.Y.; Gao, X.Q.; Liu, X.; Wang, W.Y.; Jiang, C.T.; Fang, Z.Z. The Association of Homocysteine and Diabetic Retinopathy in Homocysteine Cycle in Chinese Patients with Type 2 Diabetes. Front. Endocrinol. 2022, 13, 883845. [Google Scholar] [CrossRef]
- Zeng, X.W.; Bloom, M.S.; Wei, F.; Liu, L.; Qin, J.; Xue, L.; Wang, S.; Huang, G.; Teng, M.; He, B.; et al. Perfluoroalkyl Acids in Follicular Fluid and Embryo Quality during IVF: A Prospective IVF Cohort in China. Environ. Health Perspect. 2023, 131, 27002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chang, K.; Ramkissoon, S.; Tanguturi, S.; Bi, W.L.; Reardon, D.A.; Ligon, K.L.; Alexander, B.M.; Wen, P.Y.; Huang, R.Y. Multimodal MRI features predict isocitrate dehydrogenase genotype in high-grade gliomas. Neuro-Oncology 2017, 19, 109–117. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, S.; Zhang, C.; Zhao, Y. Coordinated Modulation of Energy Metabolism and Inflammation by Branched-Chain Amino Acids and Fatty Acids. Front. Endocrinol. 2020, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.; Sandor, N.L.; Jang, H.; Lee, D.; Toraldo, G.; Guarneri, T.; Wong, S.; Zhang, A.; Guo, W.; Jasuja, R.; et al. The effects of testosterone deprivation and supplementation on proteasomal and autophagy activity in the skeletal muscle of the male mouse: Differential effects on high-androgen responder and low-androgen responder muscle groups. Endocrinology 2013, 154, 4594–4606. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Mohammad, G.; Sahajpal, N. Faulty homocysteine recycling in diabetic retinopathy. Eye Vis. 2020, 7, 4. [Google Scholar] [CrossRef]
- Callaghan, M.; Oyama, M.; Manary, M. Sufficient protein quality of food aid varies with the physiological status of recipients. J. Nutr. 2017, 147, 277–280. [Google Scholar] [CrossRef]
- Ghosh, S.; Suri, D.; Uauy, R. Assessment of protein adequacy in developing countries: Quality matters. Br. J. Nutr. 2012, 108, S77–S87. [Google Scholar] [CrossRef]



| Variables | Total (n = 3514) | hs-CRP Normal Group (n = 2790) | Elevated hs-CRP Group (n = 724) | Statistic | p 1,2 |
|---|---|---|---|---|---|
| Age (years) | 11.10 ± 3.16 | 11.12 ± 3.14 | 11.01 ± 3.24 | t = 0.79 | 0.43 |
| Sex, n (%) | χ2 = 0.43 | 0.513 | |||
| male | 1984 (56.46) | 1583 (56.74) | 401 (55.39) | ||
| female | 1530 (43.54) | 1207 (43.26) | 323 (44.61) | ||
| BMI (kg/m2) | 20.26 ± 4.90 | 20.18 ± 4.81 | 20.58 ± 5.26 | t = −1.84 | 0.066 |
| Alcohol, n (%) | χ2 = 5.63 | 0.06 | |||
| Yes, within 30 days | 139 (3.96) | 104 (3.73) | 35 (4.83) | ||
| Yes, before the last 30 days | 228 (6.49) | 170 (6.09) | 58 (8.01) | ||
| Never drank | 3147 (89.56) | 2516 (90.18) | 631 (87.15) | ||
| Secondhand smoke exposure, n (%) | χ2 = 9.88 | 0.042 | |||
| everyday | 334 (9.50) | 253 (9.07) | 81 (11.19) | ||
| 4–6 days per week | 137 (3.90) | 114 (4.09) | 23 (3.18) | ||
| 1–3 days per week | 402 (11.44) | 305 (10.93) | 97 (13.40) | ||
| less than one day a week | 511 (14.54) | 421 (15.09) | 90 (12.43) | ||
| no | 2130 (60.61) | 1697 (60.82) | 433 (59.81) | ||
| Outdoor exercise time (hours) | 1.96 ± 2.38 | 1.96 ± 2.42 | 1.95 ± 2.24 | t = 0.10 | 0.923 |
| Energy intake (kcal/day) | 2381.28 ± 1964.52 | 2352.34 ± 1721.57 | 2492.77 ± 2702.51 | t = −1.71 | 0.0866 |
| Protein intake (g/day) | 126.31 ± 121.35 | 124.26 ±95.01 | 134.22 ± 191.44 | t = −1.97 | 0.00491 |
| Carbohydrates intake (g/day) | 361.82 ± 281.18 | 358.29 ± 243.55 | 375.40 ± 393.83 | t = −1.46 | 0.145 |
| fat intake (g/day) | 47.64 ± 61.18 | 46.91 ± 60.19 | 50.48 ± 64.79 | t = −1.40 | 0.162 |
| Fat intake percentage | 0.17± 0.08 | 0.16 ± 0.08 | 0.17 ± 0.08 | t = −1.88 | 0.0601 |
| Carbohydrates intake percentage | 0.62 ± 0.09 | 0.62 ± 0.09 | 0.62 ± 0.10 | t = 1.70 | 0.0888 |
| Protein intake percentage | 0.21 ± 0.05 | 0.21 ± 0.05 | 0.21± 0.05 | t = −0.25 | 0.8034 |
| Ile intake (10 g/day) | 0.47 ± 0.41 | 0.46 ± 0.34 | 0.50 ± 0.61 | t = −2.34 | 0.019 |
| Leu intake (10 g/day) | 0.95 ± 0.90 | 0.93 ± 0.71 | 1.01 ± 1.40 | t = −2.22 | 0.027 |
| Lys intake (10 g/day) | 0.62 ± 0.61 | 0.61 ± 0.50 | 0.66 ± 0.91 | t = −2.25 | 0.024 |
| Ser intake (10 g/day) | 0.56 ± 0.53 | 0.55 ± 0.41 | 0.60 ± 0.83 | t = −2.23 | 0.026 |
| Cys intake (10 g/day) | 0.16 ± 0.13 | 0.16 ± 0.11 | 0.17 ± 0.17 | t = −1.93 | 0.054 |
| Tyr intake (10 g/day) | 0.41 ± 0.37 | 0.41 ± 0.30 | 0.44 ± 0.57 | t = −2.30 | 0.022 |
| Phe intake (10 g/day) | 0.57 ± 0.52 | 0.56 ± 0.42 | 0.61 ± 0.81 | t = −2.11 | 0.035 |
| Thr intake (10 g/day) | 0.49 ± 0.49 | 0.48 ± 0.38 | 0.53 ± 0.79 | t = −2.18 | 0.03 |
| Gly intake (10 g/day) | 0.60 ± 0.58 | 0.59 ± 0.45 | 0.63 ± 0.93 | t = −1.65 | 0.098 |
| Val intake (10 g/day) | 0.63 ± 0.62 | 0.62 ± 0.48 | 0.67 ± 0.99 | t = −2.15 | 0.031 |
| Arg intake (10 g/day) | 0.73 ± 0.67 | 0.72 ± 0.53 | 0.77 ± 1.04 | t = −1.71 | 0.088 |
| His intake (10 g/day) | 0.23 ± 0.20 | 0.23 ± 0.18 | 0.25 ± 0.28 | t = −1.82 | 0.07 |
| Ala intake (10 g/day) | 0.76 ± 0.84 | 0.75 ± 0.61 | 0.81 ± 1.41 | t = −1.68 | 0.092 |
| Asp intake (10 g/day) | 1.02 ± 0.98 | 1.00 ± 0.78 | 1.08 ± 1.53 | t = −2.01 | 0.044 |
| Glu intake (10 g/day) | 2.07 ± 1.64 | 2.04 ± 1.45 | 2.18 ± 2.22 | t = −1.56 | 0.12 |
| Met intake (10 g/day) | 0.26 ± 0.28 | 0.26 ± 0.21 | 0.28 ± 0.45 | t = −2.01 | 0.045 |
| Pro intake (10 g/day) | 0.68 ± 0.57 | 0.66 ± 0.48 | 0.72 ± 0.83 | t = −1.89 | 0.059 |
| Trp intake (10 g/day) | 0.16 ± 0.17 | 0.16 ± 0.13 | 0.18 ± 0.27 | t = −1.96 | 0.05 |
| SAA intake (10 g/day) | 0.42 ± 0.39 | 0.41 ± 0.31 | 0.45 ± 0.61 | t = −2.23 | 0.026 |
| AAA intake (10 g/day) | 0.98 ± 0.89 | 0.97 ± 0.71 | 1.05 ± 1.38 | t = −2.19 | 0.028 |
| Model 1 OR (95% CI) | p | Model 2 OR (95% CI) | p | Model 3 OR (95% CI) | p | |
|---|---|---|---|---|---|---|
| Ile | 1.22 (1.02~1.47) | 0.028 | 1.23 (1.02~1.48) | 0.027 | 1.24 (1.02~1.49) | 0.029 |
| Leu | 1.09 (1.01~1.19) | 0.044 | 1.09 (1.01~1.19) | 0.043 | 1.10 (1.01~1.20) | 0.045 |
| Lys | 1.14 (1.01~1.29) | 0.035 | 1.14 (1.01~1.29) | 0.035 | 1.15 (1.01~1.31) | 0.036 |
| Ser | 1.16 (1.01~1.34) | 0.043 | 1.16 (1.01~1.35) | 0.042 | 1.17 (1.01~1.37) | 0.043 |
| Cys | 2.05 (1.15~3.67) | 0.015 | 2.11 (1.17~3.82) | 0.014 | 2.07 (1.13~3.78) | 0.018 |
| Tyr | 1.25 (1.02~1.53) | 0.034 | 1.25 (1.02~1.54) | 0.032 | 1.26 (1.02~1.57) | 0.034 |
| Phe | 1.15 (1.00~1.33) | 0.052 | 1.16 (1.00~1.34) | 0.052 | 1.16 (1.00~1.36) | 0.054 |
| Thr | 1.17 (1.00~1.37) | 0.052 | 1.17 (1.00~1.37) | 0.051 | 1.18 (1.00~1.40) | 0.053 |
| Gly | 1.10 (0.97~1.26) | 0.130 | 1.10 (0.97~1.26) | 0.132 | 1.11 (0.97~1.28) | 0.133 |
| Val | 1.13 (1.00~1.28) | 0.053 | 1.13 (1.00~1.28) | 0.052 | 1.14 (1.00~1.30) | 0.053 |
| Arg | 1.09 (0.98~1.22) | 0.112 | 1.10 (0.98~1.22) | 0.111 | 1.10 (0.98~1.24) | 0.115 |
| His | 1.51 (1.06~2.16) | 0.023 | 1.53 (1.06~2.19) | 0.023 | 1.52 (1.05~2.22) | 0.028 |
| Ala | 1.07 (0.98~1.17) | 0.134 | 1.07 (0.98~1.17) | 0.133 | 1.08 (0.98~1.19) | 0.134 |
| Asp | 1.08 (1.00~1.16) | 0.065 | 1.08 (1.00~1.16) | 0.064 | 1.08 (1.00~1.17) | 0.065 |
| Glu | 1.05 (1.00~1.09) | 0.054 | 1.05 (1.00~1.10) | 0.056 | 1.04 (1.00~1.09) | 0.067 |
| Met | 1.29 (0.97~1.72) | 0.076 | 1.29 (0.97~1.72) | 0.075 | 1.32 (0.97~1.80) | 0.075 |
| Pro | 1.17 (1.03~1.33) | 0.015 | 1.18 (1.03~1.34) | 0.015 | 1.17 (1.03~1.34) | 0.018 |
| Trp | 1.51 (0.95~2.40) | 0.081 | 1.52 (0.95~2.41) | 0.079 | 1.55 (0.95~2.55) | 0.081 |
| GET | 1.23 (1.01~1.49) | 0.042 | 1.23 (1.01~1.50) | 0.041 | 1.24 (1.01~1.53) | 0.044 |
| AAA | 1.09 (1.01~1.19) | 0.044 | 1.09 (1.01~1.19) | 0.042 | 1.10 (1.01~1.20) | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Zheng, X.; Li, J.; Liu, C.; Liu, Y.; Tian, M.; Zhu, Q.; Tang, Z.; Wu, M. Associations Between Dietary Amino Acid Intake and Elevated High-Sensitivity C-Reactive Protein in Children: Insights from a Cross-Sectional Machine Learning Study. Nutrients 2025, 17, 2235. https://doi.org/10.3390/nu17132235
Yu L, Zheng X, Li J, Liu C, Liu Y, Tian M, Zhu Q, Tang Z, Wu M. Associations Between Dietary Amino Acid Intake and Elevated High-Sensitivity C-Reactive Protein in Children: Insights from a Cross-Sectional Machine Learning Study. Nutrients. 2025; 17(13):2235. https://doi.org/10.3390/nu17132235
Chicago/Turabian StyleYu, Lianlong, Xiaodong Zheng, Jilan Li, Changqing Liu, Yiya Liu, Meina Tian, Qianrang Zhu, Zhenchuang Tang, and Maoyu Wu. 2025. "Associations Between Dietary Amino Acid Intake and Elevated High-Sensitivity C-Reactive Protein in Children: Insights from a Cross-Sectional Machine Learning Study" Nutrients 17, no. 13: 2235. https://doi.org/10.3390/nu17132235
APA StyleYu, L., Zheng, X., Li, J., Liu, C., Liu, Y., Tian, M., Zhu, Q., Tang, Z., & Wu, M. (2025). Associations Between Dietary Amino Acid Intake and Elevated High-Sensitivity C-Reactive Protein in Children: Insights from a Cross-Sectional Machine Learning Study. Nutrients, 17(13), 2235. https://doi.org/10.3390/nu17132235






