A Community-Based Mixed-Methods Study: Fish Bycatch Protein Supplementation as a Sustainable Solution for Child Malnutrition in Bengaluru, India
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. The Feeding Approach of APP Supplementation and Control Diets
2.3. Indicators of Growth and Malnutrition Levels
2.4. Cognition Assessment
2.5. Dietary Intake Assessment
2.6. Statistics Analysis
2.7. Focus Group Discussions with Child Caregivers
3. Results
3.1. Baseline Measurement and Dietary Intervention
3.2. Impact of APP Supplementation Diets on Body Weight
3.3. Impact of APP Supplementation Diets on Height
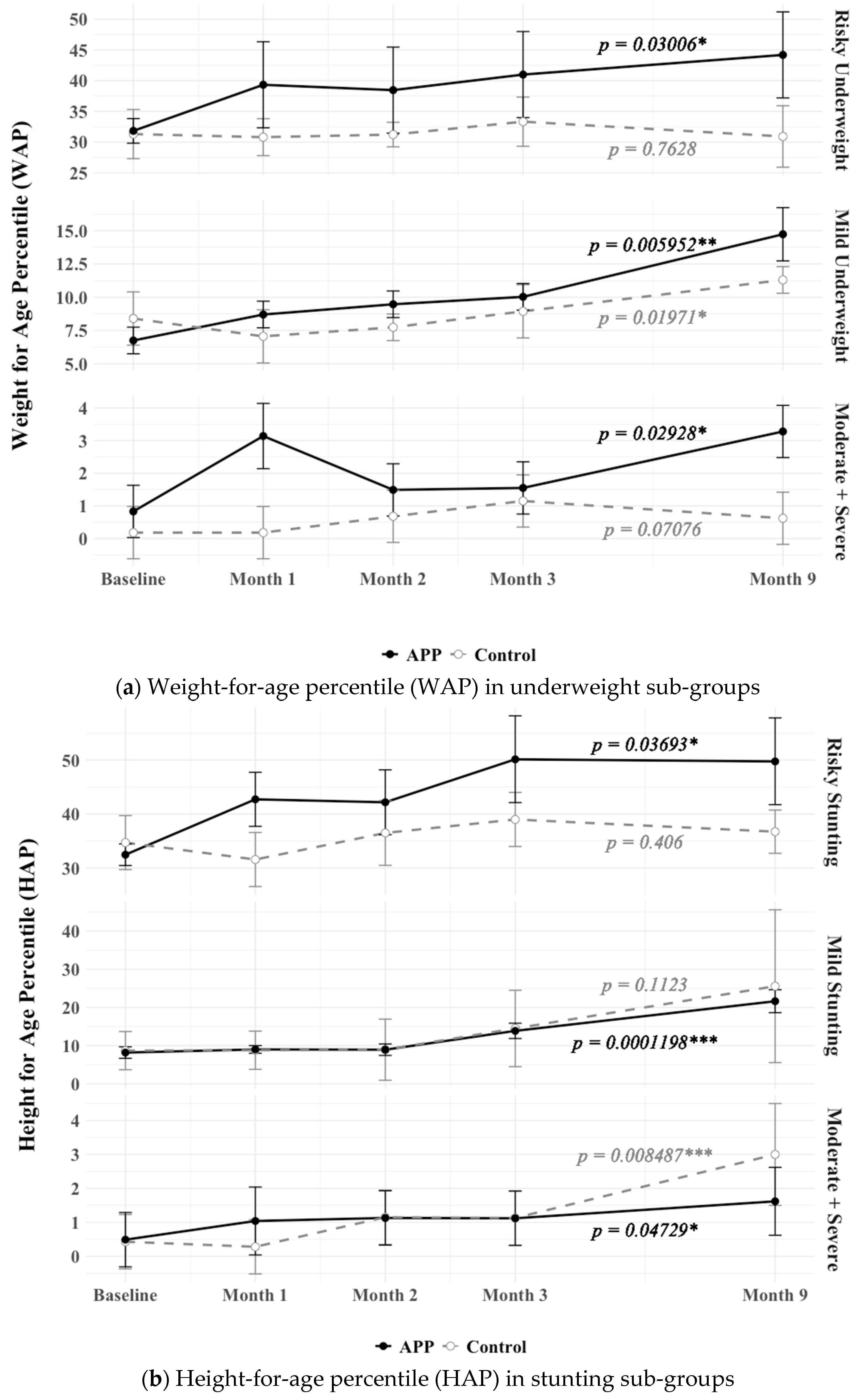
3.4. Impact of APP Supplementation Diets on Underweight and Stunting Improvement
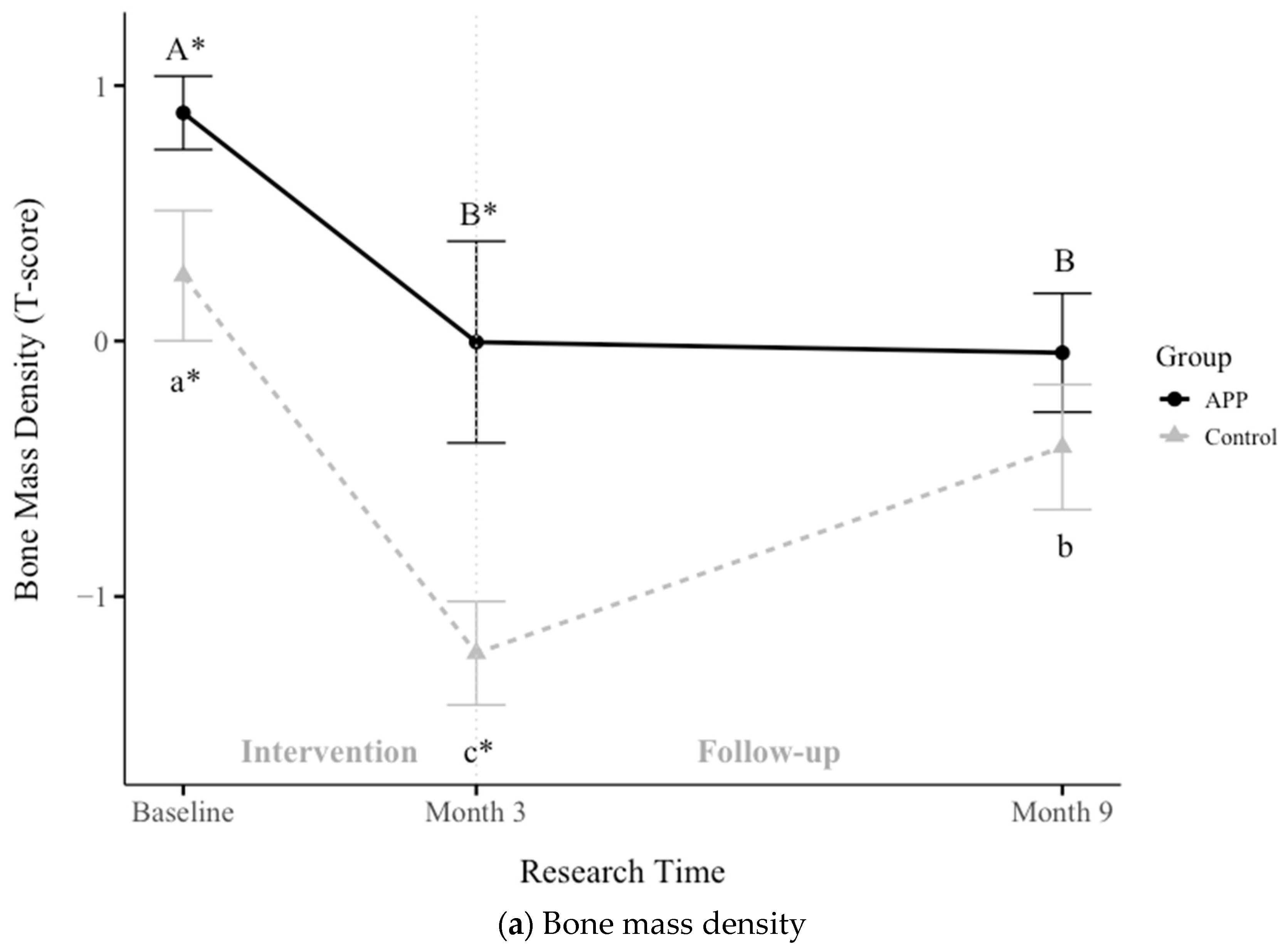
3.5. Impacts of APP Supplementation on Bone Mass Density, Muscle Mass Gain, and Hemoglobin
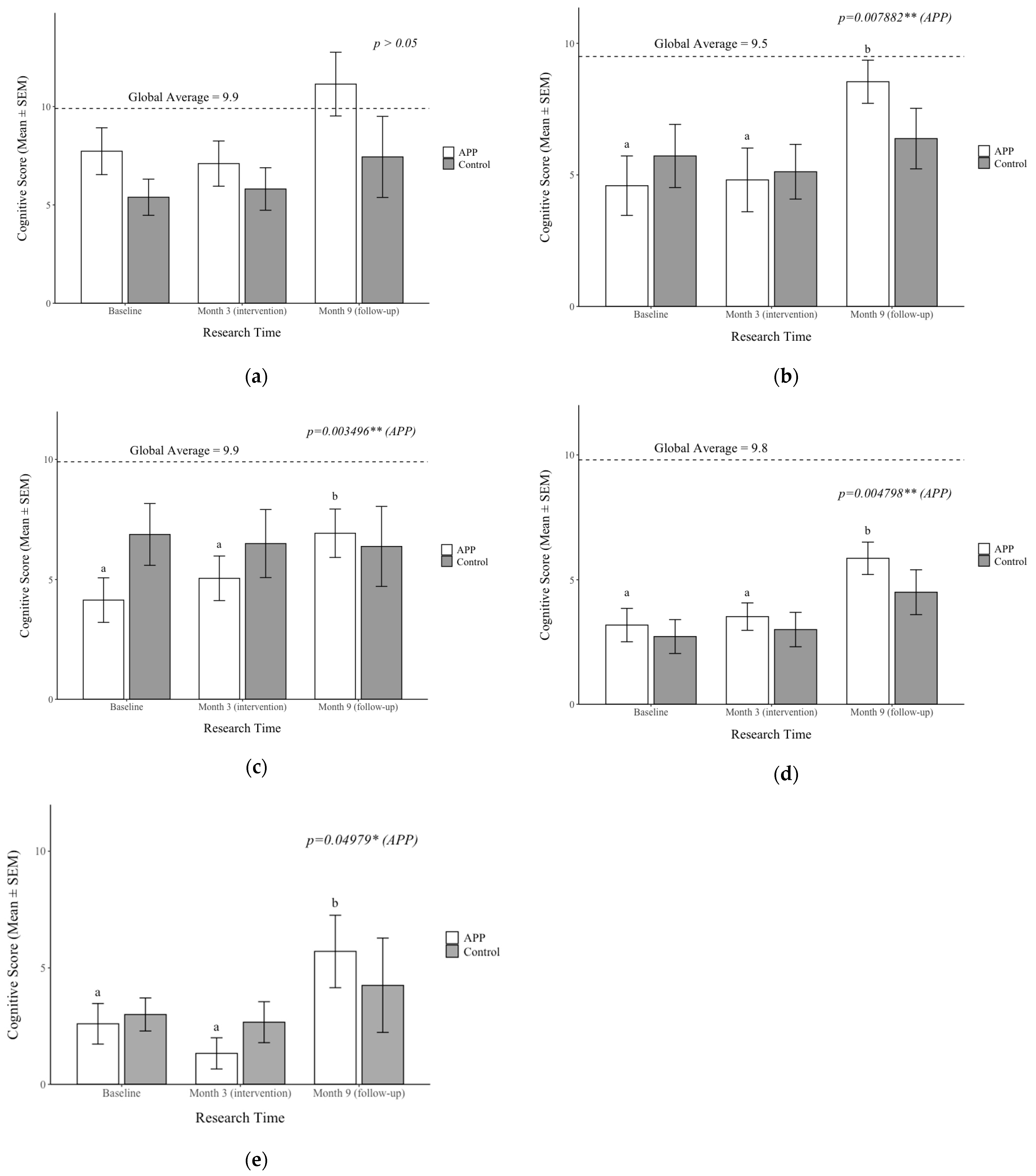
3.6. Impacts on APP Supplementation on Cognition
3.7. Caregiver’s Perspectives on the APP Supplementation and the SCOPE Approach
3.7.1. Physical Growth
“Facilities are good. Though we have not tasted the food yet, by the way the children are enjoying their meals, we can say that it is delicious. We have seen some positive changes in our children. They are growing. Height and weight are also improved. We are happy that the children have liked this food.”
“My husband has observed a lot of changes in our daughter. Initially, she was very weak. Now, everyone is surprised by her height and weight. Even the others have the same opinion about their children. Changes are observable and positive.
3.7.2. Eating Behavior Change
“If we give vegetables to our children at home, they won’t eat them. Here they are mixing carrots, potatoes, and tomatoes with food and boiling them. They simply eat here. If we give them vegetables at home, they just put them aside.”
“This place and the friends around give them a fresh air of change. Many times, my children were not able to eat, but this atmosphere has made it possible for them to have qualitative and nutritious food. I am happy to see them play here and eat well.”
“As we commenced our visits here, my son started to eat on his own. Earlier, he was using his mobile phone too much, but now he is engaged in activities and eats without distractions.”
“Children are given stars for finishing their meals. This is a motivating factor, which makes them fill their plates again.”
3.7.3. Cognitive Improvements
“Yes, a lot of changes have happened in her reading, which makes me happy. She is so brilliant now. Even her teacher has observed these changes.”
“Now my daughter understands almost everything and does her homework on her own. She even helps me with my routine and is able to take decisions responsibly.”
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APP | Advanced protein powder |
| BMD | Bone mass density |
| DRIs | Dietary reference intakes |
| FGD | Focus group discussion |
| HAP | Height-for-age percentile |
| Hb | Hemoglobin |
| MUAC | Mid-upper arm circumference |
| RDA | Recommended dietary allowance |
| SCOPE | Sustainable Community Partnership and Empowerment |
| SEM | Standard error of the mean |
| WAP | Weight-for-age percentile |
| WHO | World Health Organization |
Appendix A
| Nutrients | APP (10 g) | RDA 1 (%) |
|---|---|---|
| Energy (Kcal) | 36.9 Kcal | 2.7% |
| Protein (g) | 9.2 | 57.5% |
| Methionine (mg) | 388 | 124% |
| Cysteine (mg) | 113 | 36% |
| Phenylalanine (mg) | 423 | 77% |
| Tyrosine (mg) | 386 | 70% |
| Tryptophan (mg) | 127 | 145% |
| Leucine (mg) | 903 | 112% |
| Isoleucine (mg) | 533 | 127% |
| Valine (mg) | 550 | 104% |
| Histidine (mg) | 231 | 105% |
| Threonine (mg) | 495 | 150% |
| Lysine (mg) | 111 | 17% |
| Alanine (mg) | 650 | - |
| Arginine (mg) | 701 | - |
| Glycine (mg) | 402 | - |
| Serine (mg) | 463 | - |
| Proline (mg) | 345 | - |
| Aspartic acid (mg) | 115 | - |
| Glutamic acid (mg) | 1798 | - |
Appendix B
| Nutrients | Control Diet | Interventional Diet |
|---|---|---|
| Energy (Kcal/meal) | 450–500 Kcal | 450–500 Kcal |
| Carbohydrate (g/meal, %) | 80 g (70%) | 45 (40%) |
| Protein (g/meal, %) | 1–3 (<1%) | 11–13 (10%) |
| Fat (g/meal, %) | 15 (30%) | 15 (30%) |
References
- Katoch, O.R. Tackling Child Malnutrition and Food Security: Assessing Progress, Challenges, and Policies in Achieving SDG 2 in India. Nutr. Food Sci. 2024, 54, 349–365. [Google Scholar] [CrossRef]
- Walson, J.L.; Berkley, J.A. The Impact of Malnutrition on Childhood Infections. Curr. Opin. Infect. Dis 2018, 31, 231–236. [Google Scholar] [CrossRef]
- Sahu, S.K.; Kumar, S.G.; Bhat, B.V.; Premarajan, K.C.; Sarkar, S.; Roy, G.; Joseph, N. Malnutrition among Under-Five Children in India and Strategies for Control. J. Nat. Sci. Biol. Med. 2015, 6, 18–23. [Google Scholar] [CrossRef]
- International Institute for Population Sciences (IIPS). Key Indicators: 22 States/UTs from Phase I: National Family Health Survey (NFHS-5), 2019–2020: India. Mumbai: IIPS; International Institute for Population Sciences, Government of India: Mumbai, India, 2020. [Google Scholar]
- Vilar-Compte, M.; Burrola-Méndez, S.; Lozano-Marrufo, A.; Ferré-Eguiluz, I.; Flores, D.; Gaitán-Rossi, P.; Teruel, G.; Pérez-Escamilla, R. Urban poverty and nutrition challenges associated with accessibility to a healthy diet: A global systematic literature review. Int. J. Equity Health 2021, 20, 40. [Google Scholar] [CrossRef]
- Huey, S.L.; Finkelstein, J.L.; Venkatramanan, S.; Udipi, S.A.; Ghugre, P.; Thakker, V.; Thorat, A.; Potdar, R.D.; Chopra, H.V.; Kurpad, A.V.; et al. Prevalence and Correlates of Undernutrition in Young Children Living in Urban Slums of Mumbai, India: A Cross-Sectional Study. Front. Public Health 2019, 7, 191. [Google Scholar] [CrossRef]
- Shah More, N.; Waingankar, A.; Ramani, S.; Chanani, S.; D’Souza, V.; Pantvaidya, S.; Fernandez, A.; Jayaraman, A. Community-Based Management of Acute Malnutrition to Reduce Wasting in Urban Informal Settlements of Mumbai, India: A Mixed-Methods Evaluation. Glob. Health Sci. Pract. 2018, 6, 103–127. [Google Scholar] [CrossRef]
- George, C.E.; Norman, G.; Wadugodapitya, A.; Rao, S.V.; Nalige, S.; Radhakrishnan, V.; Behar, S.; de Witte, L. Health Issues in a Bangalore Slum: Findings from a Household Survey Using a Mobile Screening Toolkit in Devarajeevanahalli. BMC Public Health 2019, 19, 456. [Google Scholar] [CrossRef]
- Parikh, P.; Semba, R.; Manary, M.; Swaminathan, S.; Udomkesmalee, E.; Bos, R.; Poh, B.K.; Rojroongwasinkul, N.; Geurts, J.; Sekartini, R.; et al. Animal Source Foods, Rich in Essential Amino Acids, Are Important for Linear Growth and Development of Young Children in Low- and Middle-income Countries. Matern. Child Nutr. 2021, 18, e13264. [Google Scholar] [CrossRef]
- Petrikova, I. The role of complementary feeding in India’s high child malnutrition rates: Findings from a comprehensive analysis of NFHS IV (2015-2016) data. Food Secur. 2022, 14, 39–66. [Google Scholar] [CrossRef]
- Adesogan, A.T.; Havelaar, A.H.; McKune, S.L.; Eilittä, M.; Dahl, G.E. Animal Source Foods: Sustainability Problem or Malnutrition and Sustainability Solution? Perspective Matters. Global Food Sec. 2020, 25, 100325. [Google Scholar] [CrossRef]
- Singh, S.K.; Chauhan, A.; Sharma, S.K.; Puri, P.; Pedgaonkar, S.; Dwivedi, L.K.; Taillie, L.S. Cultural and Contextual Drivers of Triple Burden of Malnutrition among Children in India. Nutrients 2023, 15, 3478. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, I.; Trees; Suwarni; Pramono, M. Sago Worms as a Nutritious Traditional and Alternative Food for Rural Children in Southeast Sulawesi, Indonesia. Asia Pac. J. Clin. Nutr. 2017, 26, S40–S49. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ragone, D.; Murch, S. Breadfruit (Artocarpus Altilis): A Source of High-Quality Protein for Food Security and Novel Food Products. Amino Acids 2015, 47, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, R.S.; Nikodemova, M.; Stull, V.J.; Rapp, A.; Malecki, K.M.C. Comparison of Cricket Diet with Peanut-Based and Milk-Based Diets in the Recovery from Protein Malnutrition in Mice and the Impact on Growth, Metabolism and Immune Function. PLoS ONE 2020, 15, e0234559. [Google Scholar] [CrossRef] [PubMed]
- Maulu, S.; Nawanzi, K.; Abdel-Tawwab, M.; Khalil, H.S. Fish Nutritional Value as an Approach to Children’s Nutrition. Front. Nutr. 2021, 8, 780844. [Google Scholar] [CrossRef]
- Iannotti, L.L.; Blackmore, I.; Cohn, R.; Chen, F.; Gyimah, E.A.; Chapnick, M.; Humphries, A. Aquatic Animal Foods for Nutrition Security and Child Health. Food Nutr. Bull. 2022, 43, 127–147. [Google Scholar] [CrossRef]
- Sigh, S.; Roos, N.; Sok, D.; Borg, B.; Chamnan, C.; Laillou, A.; Dijkhuizen, M.; Wieringa, F. Development and Acceptability of Locally Made Fish-Based, Ready-to-Use Products for the Prevention and Treatment of Malnutrition in Cambodia. Food Nutr. Bull. 2018, 39, 420–434. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Yach, D.; Feldman, Z.A.; Bradley, D.G.; Khan, M. Can the Food Industry Help Tackle the Growing Global Burden of Undernutrition? Am. J. Public Health 2010, 100, 974–980. [Google Scholar] [CrossRef]
- Wong, C.P.; Bray, T.M.; Khanna, S.K. Growth, Bone Health, and Cognition: Nutritional Evaluation of a Sustainable Ocean-Based Advance Protein Powder (APP). Ecol. Food Nutr. 2019, 58, 80–92. [Google Scholar] [CrossRef]
- Yang, K.P.; Wong, C.P.; Khanna, S.K.; Bray, T.M. Supplementation of Ocean-Based Advance Protein Powder (APP) for Restoration of Body Growth, Bone Development and Immune Functions in Protein Malnourished Mice: Implications for Preventing Child Malnutrition. Ecol. Food Nutr. 2020, 59, 552–574. [Google Scholar] [CrossRef] [PubMed]
- Estrada, I.; Jost, C.; Kickner, I.; Lopez, K.; Kowal, M.; Reddy, D.; Khanna, S. Enhancing Health and Well-Being Through Sustainable Community Partnership and Empowerment (SCOPE). Report submitted to the Ministry of Health and the Ministry of Youth, Gender, Sport, and Culture, Government of Botswana. 2023. Available online: https://health.oregonstate.edu/sites/health.oregonstate.edu/files/2024-02/Botswana-Global-Health-Initiative-Report-2023.pdf (accessed on 18 May 2025).
- Jost, C.; MacDonald, M.; Khanna, S. A Community-Based Evaluation of Disability Resources and Inclusion Practices in Rural Botswana. Disabil. Health J 2022, 15, 101275. [Google Scholar] [CrossRef]
- Swasti It All Begins at Home. Available online: https://swasti.org/our_work/Primary-Health (accessed on 18 May 2025).
- Khadilkar, V.V.; Khadilkar, A.V.; Chiplonkar, S.A. Growth Performance of Affluent Indian Preschool Children: A Comparison with the New Who Growth Standard. Indian Pediatr. 2010, 47, 869–872. [Google Scholar] [CrossRef] [PubMed]
- ICMR-NIN. Revised Short Summary Report 2024, ICMR-NIN Expert Group on Nutrient Requirement for Indians: Recommended Dietary Allowances and Estimated Average Requirements-2020; ICMR-National Institute of Nutrition, Indian Council of Medical Research, Department of Health Research, Ministry of Health and Family Welfare, Government of India: Telangana, India, 2024. [Google Scholar]
- Golden, M.H. Proposed Recommended Nutrient Densities for Moderately Malnourished Children. Food Nutr. Bull. 2009, 30, S267–S342. [Google Scholar] [CrossRef]
- Becker, P.; Carney, L.N.; Corkins, M.R.; Monczka, J.; Smith, E.; Smith, S.E.; Spear, B.A.; White, J.V.; Academy of Nutrition and Dietetics; American Society for Parenteral and Enteral Nutrition. Consensus Statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition. Nutr. Clin. Pract. 2015, 30, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, A.S.; Lichtenberger, E.O.; Fletcher-Janzen, E.; Kaufman, N.L. Essentials of KABC-II Assessment; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 978-0-471-66733-9. [Google Scholar]
- Gopalan, C.; Rama Sastri, B.V.; Balasubrsmanian, S.C. Nutritive Value of Indian Food; National Institute of Nutrition (NIN), Indian council of Medical Research: Hyderabad, India, 2005. [Google Scholar]
- Advance International. Feeding the World and Protecting Our Planet. Available online: https://advanceprotein.com/ (accessed on 18 May 2025).
- van der Horst, K.; Deming, D.M.; Lesniauskas, R.; Carr, B.T.; Reidy, K.C. Picky Eating: Associations with Child Eating Characteristics and Food Intake. Appetite 2016, 103, 286–293. [Google Scholar] [CrossRef]
- Ragsdale, K.; Read-Wahidi, M.R.; Mudege, N.N.; Iannotti, L.L.; Muzungaire, L.; Funduluka, P. Sensory Panel Results of a Dried Fish Powder Supplement among Caregivers and Young Children in Zambia. Public Health Nutr. 2024, 27, e32. [Google Scholar] [CrossRef]
- Ip, P.; Ho, F.K.W.; Rao, N.; Sun, J.; Young, M.E.; Chow, C.B.; Tso, W.; Hon, K.L. Impact of Nutritional Supplements on Cognitive Development of Children in Developing Countries: A Meta-Analysis. Sci. Rep. 2017, 7, 10611. [Google Scholar] [CrossRef]
- Rao, N.; Sun, J.; Chen, E.; Ip, P. Effectiveness of Early Childhood Interventions in Promoting Cognitive Development in Developing Countries: A Systematic Review and Meta-Analysis. Hong Kong J. Paediatr. 2017, 22, 14–25. [Google Scholar]
- Semba, R.D. The Rise and Fall of Protein Malnutrition in Global Health. Ann. Nutr. Metab. 2016, 69, 79–88. [Google Scholar] [CrossRef]
- Semba, R.D.; Shardell, M.; Sakr Ashour, F.A.; Moaddel, R.; Trehan, I.; Maleta, K.M.; Ordiz, M.I.; Kraemer, K.; Khadeer, M.A.; Ferrucci, L.; et al. Child Stunting Is Associated with Low Circulating Essential Amino Acids. EBioMedicine 2016, 6, 246–252. [Google Scholar] [CrossRef]
- Manary, M.J.; Wegner, D.R.; Maleta, K. Protein quality malnutrition. Front. Nutr. 2024, 11, 1428810. [Google Scholar] [CrossRef] [PubMed]
- Goudet, S.M.; Bogin, B.A.; Madise, N.J.; Griffiths, P.L. Nutritional interventions for preventing stunting in children (birth to 59 months) living in urban slums in low- and middle-income countries (LMIC). Cochrane Database Syst. Rev. 2019, 6, CD011695. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, K.F.; Hoppe, C.; Roos, N.; Kaestel, P.; Stougaard, M.; Lauritzen, L.; Mølgaard, C.; Girma, T.; Friis, H. Choice of Foods and Ingredients for Moderately Malnourished Children 6 Months to 5 Years of Age. Food Nutri. Bull. 2009, 30, S343–S404. [Google Scholar] [CrossRef]
- Hauschild, D.B.; Oliveira, L.D.A.; Farias, M.S.; Barbosa, E.; Bresolin, N.L.; Mehta, N.M.; Moreno, Y.M.F. Enteral Protein Supplementation in Critically Ill Children: A Randomized Controlled Pilot and Feasibility Study. JPEN J. Parenter. Enteral. Nutr. 2019, 43, 281–289. [Google Scholar] [CrossRef]
- Schlossman, N. Higher Levels of Dairy Result in Improved Physical Outcomes: A Synthesis of 3 Randomized Controlled Trials in Guinea-Bissau Comparing Supplements with Different Levels of Dairy Ingredients Among Children 6 to 59 Months, 5 to 19 Year Olds, and Mothers in Preschools, Primary Schools, and Villages, and the Implications for Programs. Food Nutr. Bull. 2018, 39, S35–S44. [Google Scholar] [CrossRef] [PubMed]
- Sedlander, E.; Long, M.W.; Mohanty, S.; Munjral, A.; Bingenheimer, J.B.; Yilma, H.; Rimal, R.N. Moving beyond Individual Barriers and Identifying Multi-Level Strategies to Reduce Anemia in Odisha India. BMC Public Health 2020, 20, 457. [Google Scholar] [CrossRef]
- Sazawal, S.; Dhingra, U.; Dhingra, P.; Hiremath, G.; Sarkar, A.; Dutta, A.; Menon, V.P.; Black, R.E. Micronutrient Fortified Milk Improves Iron Status, Anemia and Growth among Children 1–4 Years: A Double Masked, Randomized, Controlled Trial. PLoS ONE 2010, 5, e12167. [Google Scholar] [CrossRef]
- Darling, A.L.; Millward, D.J.; Lanham-New, S.A. Dietary Protein and Bone Health: Towards a Synthesised View. Proc. Nutr. Soc. 2021, 80, 165–172. [Google Scholar] [CrossRef]
- Aggarwal, R.; Bains, K. Protein, Lysine and Vitamin D: Critical Role in Muscle and Bone Health. Crit. Rev. Food Sci. Nutr. 2022, 62, 2548–2559. [Google Scholar] [CrossRef]
- Galler, J.R.; Bringas-Vega, M.L.; Tang, Q.; Rabinowitz, A.G.; Musa, K.I.; Chai, W.J.; Omar, H.; Abdul Rahman, M.R.; Abd Hamid, A.I.; Abdullah, J.M.; et al. Neurodevelopmental Effects of Childhood Malnutrition: A Neuroimaging Perspective. NeuroImage 2021, 231, 117828. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.B.; Franceschini, M.A.; Silver, R.E.; Taylor, S.F.; De Sa, A.B.; Có, R.; Sonco, A.; Krauss, A.; Taetzsch, A.; Webb, P.; et al. Effects of Food Supplementation on Cognitive Function, Cerebral Blood Flow, and Nutritional Status in Young Children at Risk of Undernutrition: Randomized Controlled Trial. BMJ 2020, 370, m2397. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction. Rome. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/11f9288f-dc78-4171-8d02-92235b8d7dc7/content (accessed on 18 May 2025).


| Indicators | Total | Control | APP | p-Value |
|---|---|---|---|---|
| (n = 46) | (n = 19) | (n = 27) | ||
| Age (year) 1 | 4.31 ± 0.11 | 4.6 ± 0.28 | 4 ± 0.2 | 0.05792 |
| Gender (Boy/Girl, n) 2 | 28:20 | 12:7 | 14:13 | 0.6458 |
| Growth indicators | ||||
| Weight (kg) 1 | 14.77 ± 0.61 | 15.03 ± 0.68 | 14.58 ± 0.94 | 0.1291 |
| WAP 1 | 24.97 ± 4.1 | 25.18 ± 5.92 | 24.83 ± 5.71 | 0.9644 |
| Underweight (n, %) 2 | 12 (26.09) | 4 (21.05) | 8 (29.62) | 0.7556 |
| Height (cm) 1 | 97.55 ± 1.41 | 100.03 ± 2.16 | 95.8 ± 1.8 | 0.1132 |
| HAP 1 | 19.82 ± 3.58 | 24.78 ± 6.9 | 16.34 ± 3.66 | 0.7037 |
| Stunting (n, %) 2 | 18 (39.13) | 8 (42.1) | 10 (37.03) | 0.9681 |
| MUAC (cm) 1 | 15.97 ± 0.3 | 15.88 ± 0.27 | 16.03 ± 0.48 | 0.2323 |
| BMD (T-score) 1 | 0.63 ± 0.14 | 0.26 ± 0.25 | 0.89 ± 0.14 | 0.03903 * |
| Hemoglobin (%) 1 | 9.17 ± 0.34 | 9.29 ± 0.41 | 9.09 ± 0.51 | 0.8759 |
| Dietary intake | ||||
| Energy (Kcal/day) 1 | 908.38 ± 98.64 | 878.32 ± 65.26 | 929.53 ± 47.81 | 0.4129 |
| Carbohydrate (g/d) 1 | 116.22 ± 6.02 | 112.95 ± 9.89 | 118.52 ± 7.67 | 0.5506 |
| Protein (g/d) 1 | 21.16 ± 1.05 | 19.57 ± 1.41 | 22.28 ± 1.41 | 0.2676 |
| Fat (g/d) 1 | 35.11 ± 1.79 | 33.84 ± 3.26 | 36 ± 2.05 | 0.3759 |
| Baseline | Intervention | Follow up | p-Value | |||
|---|---|---|---|---|---|---|
| Month 0 | Month 1 | Month 2 | Month 3 | Month 9 | ||
| Control (n = 19) | 15.03 ± 0.68 a | 15.31 ± 0.7 b | 15.63 ± 0.71 c | 15.75 ± 0.67 c | 16.47 ± 0.76 d | <0.001 *** |
| Non-underweight (n = 3) | 18.2 ± 0.7 | 18.67 ± 1.13 | 18.9 ± 1.3 | 18.63 ± 0.99 | 20.13 ± 1.72 | 0.406 |
| Risky underweight (n = 7) | 16.46 ± 0.94 a | 16.74 ± 0.94 a | 17.1 ± 0.99 ab | 17.3 ± 0.95 b | 17.3 ± 1.29 ab | 0.008466 ** |
| Mild underweight (n = 5) | 14.1 ± 0.71 a | 14.36 ± 0.63 a | 14.48 ± 0.66 a | 14.74 ± 0.69 a | 15.88 ± 0.72 b | 0.001222 ** |
| Moderate + severe underweight (n = 4) | 11.33 ± 0.69 a | 11.48 ± 0.68 a | 12.05 ± 0.66 a | 12.15 ± 0.49 a | 13 ± 0.47 b | 0.008569 ** |
| APP (n = 27) | 14.58 ± 0.94 a | 15.28 ± 0.95 b | 15.42 ± 0.93 bc | 15.49 ± 0.97 c | 16.06 ± 1.01 d | <0.001 *** |
| Non-underweight (n = 4) | 24.2 ± 3.16 | 25.05 ± 2.84 | 25.3 ± 2.38 | 25.15 ± 3.1 | 25.48 ± 3.69 | 0.3007 |
| Riskily underweight (n = 9) | 14.24 ± 0.26 a | 15.1 ± 0.5 b | 15.26 ± 0.53 bc | 15.57 ± 0.52 c | 16.5 ± 0.54 d | <0.001 *** |
| Mildly underweight (n = 6) | 13.13 ± 0.49 a | 13.78 ± 0.42 b | 13.65 ± 0.35 bc | 13.98 ± 0.49 bc | 13.68 ± 1.12 bc | 0.01083 * |
| Moderately + severely underweight (n = 8) | 11.24 ± 0.44 a | 11.78 ± 0.55 b | 12 ± 0.56 b | 11.81 ± 0.51 b | 12.8 ± 0.53 c | <0.001 *** |
| Baseline | Intervention | Follow up | p-Value | |||
|---|---|---|---|---|---|---|
| Month 0 | Month 1 | Month 2 | Month 3 | Month 9 | ||
| Control (n = 19) | 101.04 ± 2.36 a | 101.19 ± 2.11 a | 102.16 ± 2.11 b | 103.25 ± 2.14 c | 107.04 ± 2.06 d | <0.0001 *** |
| Non-stunted (n = 3) | 107 ± 2.52 a | 108.23 ± 3.27 a | 109.1 ± 2.52 a | 109.83 ± 2.12 a | 114.33 ± 3.01 b | 0.04477 * |
| Risky stunting (n = 6) | 105.22 ± 2.42 a | 106.4 ± 2.29 a | 107.43 ± 2.09 a | 108.32 ± 2.18 ab | 111.12 ± 2.3 b | 0.000146 * |
| Mild stunting (n = 2) | 103.75 ± 14.25 | 104.7 ± 13.3 | 106.4 ± 14.1 | 107.5 ± 14.7 | 110.85 ± 13.95 | 0.0954 |
| Moderate + Severe (n = 8) | 95 ± 3.69 ab | 93.76 ± 1.76 a | 94.54 ± 1.64 a | 95.93 ± 1.97 b | 100.31 ± 1.87 c | <0.0001 *** |
| APP (n = 27) | 95.8 ± 1.81 a | 97.66 ± 1.84 b | 97.97 ± 1.86 b | 99.56 ± 1.88 c | 103.03 ± 1.8 d | <0.0001 *** |
| Non-stunted (n = 2) | 115.45 ± 3.45 | 117.25 ± 5.25 | 117.75 ± 4.75 | 118.45 ± 5.05 | 120.8 ± 4.6 | 0.1039 |
| Risky stunting (n = 8) | 100.84 ± 2.42 a | 103.08 ± 2.14 b | 103.28 ± 2.31 b | 105.88 ± 2.13 c | 109. 63 ± 1.92 d | <0.0001 *** |
| Mild stunting (n = 2) | 94.34 ± 2 a | 96.03 ± 2.26 b | 96.27 ± 2.34 b | 97.87 ± 2.21 c | 101.1 ± 2.22 d | <0.0001 *** |
| Moderate + Severe (n = 10) | 88. 87 ± 1.83 a | 90.56 ± 1.92 b | 90.95 ± 1.64 b | 91.9 ±1.89 c | 95.54 ± 1.73 d | <0.0001 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.P.; Khanna, S.K.; Chaudhuri, A.; Syam, S.B.; Bray, T.M. A Community-Based Mixed-Methods Study: Fish Bycatch Protein Supplementation as a Sustainable Solution for Child Malnutrition in Bengaluru, India. Nutrients 2025, 17, 1751. https://doi.org/10.3390/nu17111751
Yang KP, Khanna SK, Chaudhuri A, Syam SB, Bray TM. A Community-Based Mixed-Methods Study: Fish Bycatch Protein Supplementation as a Sustainable Solution for Child Malnutrition in Bengaluru, India. Nutrients. 2025; 17(11):1751. https://doi.org/10.3390/nu17111751
Chicago/Turabian StyleYang, Kristen P., Sunil K. Khanna, Angela Chaudhuri, Syama B. Syam, and Tammy M. Bray. 2025. "A Community-Based Mixed-Methods Study: Fish Bycatch Protein Supplementation as a Sustainable Solution for Child Malnutrition in Bengaluru, India" Nutrients 17, no. 11: 1751. https://doi.org/10.3390/nu17111751
APA StyleYang, K. P., Khanna, S. K., Chaudhuri, A., Syam, S. B., & Bray, T. M. (2025). A Community-Based Mixed-Methods Study: Fish Bycatch Protein Supplementation as a Sustainable Solution for Child Malnutrition in Bengaluru, India. Nutrients, 17(11), 1751. https://doi.org/10.3390/nu17111751






